Exogenous Silicon Application Improves Chilling Injury Tolerance and Photosynthetic Performance of Citrus
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Determination of Root Si Application Rates
2.3. Chilling Injury Experiment
3. Data Collection
3.1. Leaf Gas Exchange Measurements
3.2. Chlorophyll Fluorescence Measurements
4. Statistical Analysis
5. Results
5.1. Summary of the Analysis of Variance
5.2. Effect of Si Drenching on the Photosynthetic Performance of Citrus Trees after a Chilling Temperature Stress of 0 °C
5.2.1. Changes in Chlorophyll Fluorescence Parameters in Response to Chilling Stress and Si Drenching
5.2.2. Changes in Leaf Gas Exchange in Response to Chilling Stress and Si Drenching
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hajizadeh, S.; Azizi, S.; Rasouli, F.; Kaya, O. Evaluation of nano-silicon efficiency on compatible solutes and nutrient status of Damask rose affected by in vitro simulated drought stress. Chem. Biol. Technol. Agric. 2023, 10, 22. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R. Silicon (Si): Review and prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol. Environ. Saf. 2018, 147, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wong, J.; Wei, L. Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 2005, 58, 475–483. [Google Scholar] [CrossRef]
- Bilal, S.; Khan, T.; Asaf, S.; Khan, N.A.; Saad Jan, S.; Imran, M.; Al-Rawahi, A.; Khan, A.L.; Lee, I.-J.; Al-Harrasi, A. Silicon-induced morphological, biochemical and molecular regulation in Phoenix dactylifera L. under low-temperature stress. Int. J. Mol. Sci. 2023, 24, 6036. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R. Silicon-mediated cold stress tolerance in plants. In Silicon and Nano-Silicon in Environmental Stress Management and Crop Quality Improvement; Etesami, H., Al Saeedi, A.H., El-Ramady, H., Fujita, M., Pessarakli, M., Hossain, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 161–180. [Google Scholar]
- Zhu, J.; Liang, Y.; Ding, Y.; Li, Z. Effect of silicon on photosynthesis and its related physiological parameters in two winter wheat cultivars under cold stress. Zhongguo Nongye Kexue 2006, 39, 1780–1788. [Google Scholar]
- Zuccarini, P. Effects of silicon on photosynthesis, water relations and nutrient uptake of Phaseolus vulgaris L. under Na Cl stress. Biol. Plant. 2008, 52, 4. [Google Scholar] [CrossRef]
- Hattori, T.; Sonobe, K.; Inanaga, S.; An, P.; Morita, S. Effects of silicon on photosynthesis of young cucumber seedlings under osmotic stress. J. Plant Nutr. 2008, 31, 1046–1058. [Google Scholar] [CrossRef]
- Ma, C.C.; Li, Q.F.; Gao, Y.B.; Xin, T.R. Effects of silicon application on drought resistance of cucumber plants. Soil Sci. Plant Nutr. 2004, 50, 623–632. [Google Scholar] [CrossRef]
- Romero-Aranda, M.R.; Jurado, O.; Cuartero, J. Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status. J. Plant Physiol. 2006, 163, 847–855. [Google Scholar] [CrossRef]
- Gong, H.J.; Chen, K.M.; Chen, G.C.; Wang, S.M.; Zhang, C.L. Effects of silicon on growth of wheat under drought. J. Plant Nutr. 2003, 26, 1055–1063. [Google Scholar] [CrossRef]
- Matoh, T.; Murata, S.; Takahashi, E. Effect of silicate application on photosynthesis of rice [Oryza sativa] plants. Jpn. J. Soil Sci. Plant Nutr. 1991, 62, 248–251. [Google Scholar]
- FAO. Food and Agriculture Organization of the United Nations (FAO)—Statistical Pocketbook World Food and Agriculture. 2015. Available online: http://www.fao.org/3/i4691e/i4691e.pdf (accessed on 19 July 2023).
- Ribeiro, R.V.; Machado, E.C.; Oliveira, R.F. Growth-and leaf-temperature effects on photosynthesis of sweet orange seedlings infected with Xylella fastidiosa. Plant Pathol. 2004, 53, 334–340. [Google Scholar] [CrossRef]
- Primo-Capella, A.; Martínez-Cuenca, M.-R.; Forner-Giner, M.Á. Cold stress in citrus: A molecular, physio-logical and biochemical perspective. Acta Hortic. 2021, 7, 340. [Google Scholar] [CrossRef]
- Abobatta, W.F. Potential impacts of global climate change on citrus cultivation. MOJ Ecol. Environ. Sci. 2019, 4, 308–312. [Google Scholar] [CrossRef]
- Lourkisti, R.; Froelicher, Y.; Herbette, S.; Morillon, R.; Tomi, F.; Gibernau, M.; Giannettini, J.; Berti, L.; Santini, J. Triploid citrus genotypes have a better tolerance to natural chilling conditions of photosynthetic capacities and specific leaf volatile organic compounds. Front. Plant Sci. 2020, 11, 330. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C. Some aspects of citrus ecophysiology in subtropical climates: Re-visiting photo-synthesis under natural conditions. Braz. J. Plant Physiol. 2007, 19, 393–411. [Google Scholar] [CrossRef]
- Kim, M.; Moon, Y.-E.; Han, S.G.; Yun, S.K.; Joa, J.-H.; Park, J.-S. Impact of cold stress on physiological responses and fruit quality of Shiranuhi mandarin in response to cold conditions. Acta Hortic. 2023, 9, 906. [Google Scholar] [CrossRef]
- Ladaniya, M. Citrus Fruit Biology, Technology and Evaluation; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Matichenkov, V.; Bocharnikova, E.; Calvert, D. Response of citrus to silicon soil amendments. Proc. Fla. State Hortic. Soc. 2001, 114, 94–97. [Google Scholar]
- Matichenkov, V.; Calvert, D.; Snyder, G. Silicon fertilizers for citrus in Florida. Proc. Fla. State Hortic. Soc. 1999, 112, 5–8. [Google Scholar]
- Wutscher, H. Growth and mineral nutrition of young orange trees grown with high levels of Si. Hortic. Sci. 1989, 24, 3. [Google Scholar]
- Liang, Y.; Zhu, J.; Li, Z.; Chu, G.; Ding, Y.; Zhang, J.; Sun, W. Role of silicon in enhancing resistance to freezing stress in two contrasting winter wheat cultivars. Environ. Exp. Bot. 2008, 64, 286–294. [Google Scholar] [CrossRef]
- Hazrati, S.; Tahmasebi-Sarvestani, Z.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A.; Nicola, S. Effects of water stress and light intensity on chlorophyll fluorescence parameters and pigments of Aloe vera L. Plant Physiol. Biochem. 2016, 106, 141–148. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K.; et al. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef]
- Reeksting, B.J.; Taylor, N.; Van den Berg, N. Flooding and Phytophthora cinnamomi: Effects on photosynthesis and chlorophyll fluorescence in shoots of non-grafted Persea americana (Mill.) rootstocks differing in tolerance to Phytophthora root rot. S. Afr. J. Bot. 2014, 95, 40–53. [Google Scholar] [CrossRef]
- Ribeiro, R.; Machado, E.; Santos, M.; Oliveira, R. Photosynthesis and water relations of well-watered orange plants as affected by winter and summer conditions. Photosynthetica 2009, 47, 215–222. [Google Scholar] [CrossRef]
- Sayed, O. Chlorophyll fluorescence as a tool in cereal crop research. Photosynthetica 2003, 41, 321–330. [Google Scholar] [CrossRef]
- Mashilo, J.; Odindo, A.O.; Shimelis, H.A.; Musenge, P.; Tesfay, S.Z.; Magwaza, L.S. Drought tolerance of selected bottle gourd [Lagenaria siceraria (Molina) Standl.] landraces assessed by leaf gas exchange and photosynthetic efficiency. Plant Physiol. Biochem. 2017, 120, 75–87. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Shen, X.; Zhou, Y.; Duan, L.; Li, Z.; Eneji, A.E.; Li, J. Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J. Plant Physiol. 2010, 167, 1248–1252. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Ashraf, U.; Khan, I.; Wang, L. Alteration in growth, leaf gas exchange, and photosynthetic pigments of maize plants under combined cadmium and arsenic stress. Water Air Soil Pollut. 2017, 228, 13. [Google Scholar] [CrossRef]
- Debona, D.; Rodrigues, F.Á.; Rios, J.A.; Martins, S.C.V.; Pereira, L.F.; Damatta, F.M. Limitations to photosynthesis in leaves of wheat plants infected by Pyricularia oryzae. Phytopathology 2014, 104, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Mvondo-She, M.A. A method for silicon analysis in citrus and horticulture leaf tissue. Commun. Soil Sci. Plant Anal. 2021, 52, 2089–2097. [Google Scholar] [CrossRef]
- Mvondo-She, M.A. Studies of Silicon Fertilization in Citrus to Enhance Chilling Injury Resistance. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2020. Available online: http://hdl.handle.net/2263/75621 (accessed on 10 July 2023).
- Martin, B.; Ruiz-Torres, N.A. Effects of water-deficit stress on photosynthesis, its components and component limitations, and on water use efficiency in wheat (Triticum aestivum L.). Plant Physiol. 1992, 100, 733–739. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA) Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Fryer, M.J.; Andrews, J.R.; Oxborough, K.; Blowers, D.A.; Baker, N.R. Relationship between CO2 assimilation, photosynthetic electron transport, and active O2 metabolism in leaves of maize in the field during periods of low temperature. Plant Physiol. 1998, 116, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Al-Aghabary, K.; Zhu, Z.; Shi, Q. Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. J. Plant Physiol. 2005, 27, 2101–2115. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Ashraf, M. Influence of foliar application of silicon on chlorophyll fluorescence, photo-synthetic pigments, and growth in water-stressed wheat cultivars differing in drought tolerance. Turk. J. Bot. 2015, 39, 625–634. [Google Scholar] [CrossRef]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon improves water use efficiency in maize plants. J. Plant Nutr. 2005, 27, 1457–1470. [Google Scholar] [CrossRef]
- Saud, S.; Li, X.; Chen, Y.; Zhang, L.; Fahad, S.; Hussain, S.; Sadiq, A.; Chen, Y. Silicon application increases drought tolerance of Kentucky bluegrass by improving plant water relations and morphophysiological functions. Sci. World J. 2014, 2014, 368694. [Google Scholar] [CrossRef]
- Feghhenabi, F.; Hadi, H.; Khodaverdiloo, H.; van Genuchten, M.T.; Lake, L. Quantitative evaluation of silicon applications on wheat response to salinity: Changes in photosynthetic pigments, chlorophyll fluorescence parameters, yield and yield components. Crop Pasture Sci. 2022, 73, 1118–1130. [Google Scholar] [CrossRef]
- Adams, W.W.; Demmig-Adams, B. Carotenoid composition and down regulation of photosystem II in three conifer species during the winter. Physiol. Plant. 1994, 92, 451–458. [Google Scholar] [CrossRef]
- Fu, J.; Huang, B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Khan, A.L.; Waqas, M.; Lee, I.-J. Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: A review. Front. Plant Sci. 2017, 8, 510. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.H. Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol. Plant. 1988, 74, 566–574. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Juurola, E.; Nikinmaa, E.; Berninger, F.; Ensminger, I.; Hari, P. Seasonal acclimation of photosystem II in Pinus sylvestris. I. Estimating the rate constants of sustained thermal energy dissipation and photochemistry. Tree Physiol. 2008, 28, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- HE, D.; EDWARDS, G.E. Evaluation of the potential to measure photosynthetic rates in C3 plants (Flaveria pringlei and Oryza sativa) by combining chlorophyll fluorescence analysis and a stomatal conductance model. Plant Cell Environ. 1996, 19, 1272–1280. [Google Scholar] [CrossRef]
- Kasajima, I.; Ebana, K.; Yamamoto, T.; Takahara, K.; Yano, M.; Kawai-Yamada, M.; Uchimiya, H. Molecular distinction in genetic regulation of nonphotochemical quenching in rice. Proc. Natl. Acad. Sci. USA 2011, 108, 13835–13840. [Google Scholar] [CrossRef]
- Aucique-Pérez, C.E.; de Menezes Silva, P.E.; Moreira, W.R.; DaMatta, F.M.; Rodrigues, F.Á. Photosynthesis impairments and excitation energy dissipation on wheat plants supplied with silicon and infected with Pyricularia oryzae. Plant Physiol. Biochem. 2017, 121, 196–205. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Winter, K. Oxygen-dependent electron transport and protection from photoinhibition in leaves of tropical tree species. Planta 1996, 198, 580–587. [Google Scholar] [CrossRef]
- Flexas, J.; Escalona, J.M.; Evain, S.; Gulías, J.; Moya, I.; Osmond, C.B.; Medrano, H. Steady-state chlorophyll fluorescence (Fs) measurements as a tool to follow variations of net CO2 assimilation and stomatal conductance during water-stress in C3 plants. Physiol. Plant. 2002, 114, 231–240. [Google Scholar] [CrossRef]
- Mashilo, J. Pre-Breeding of Bottle Gourd [Lagenaria siceraria (Molina) Standl.]. Ph.D. Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2016. [Google Scholar]
- Demmig-Adams, B.; Adams, W.W., III. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Nagao, M.; Minami, A.; Arakawa, K.; Fujikawa, S.; Takezawa, D. Rapid degradation of starch in chloroplasts and concomitant accumulation of soluble sugars associated with ABA-induced freezing tolerance in the moss Physcomitrella patens. J. Plant Physiol. 2005, 162, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Santini, J.; Giannettini, J.; Herbette, S.; Pailly, O.; Ollitrault, P.; Luro, F.; Berti, L. Physiological and biochemical response to photooxidative stress of the fundamental citrus species. Sci. Hortic. 2012, 147, 126–135. [Google Scholar] [CrossRef]
- Lobato AK, S.; Luz, L.M.; Costa RC, L.; Santos Filho, B.G.; Meirelles AC, S.; Oliveira Neto, C.F.; Laughinghouse, H.D.; Neto, M.A.M.; Alves, G.A.R.; Lopes, M.J.S.; et al. Silicon exercises influence on nitrogen compounds in pepper subjected to water deficit. Res. J. Biol. Sci. 2009, 4, 1048–1055. [Google Scholar]
- Mvondo-SHE, M.A.; Marais, D. The investigation of silicon localization and accumulation in citrus. Plants 2019, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon decreases transpiration rate and conductance from stomata of maize plants. J. Plant Nutr. 2006, 29, 1637–1647. [Google Scholar] [CrossRef]
- Ma, J.F.; Takahashi, E. Soil, Fertilizer, and Plant Silicon Research in Japan; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Dehghanipoodeh, S.; Ghobadi, C.; Baninasab, B.; Gheysari, M.; Shiranibidabadi, S. Effect of silicon on growth and development of strawberry under water deficit conditions. Hortic. Plant J. 2018, 4, 226–232. [Google Scholar] [CrossRef]
- Epstein, E. The anomaly of silicon in plant biology. Proc. Natl. Acad. Sci. USA 1994, 91, 11–17. [Google Scholar] [CrossRef]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Ma, J.F.; Miyake, Y.; Takahashi, E. Silicon as a beneficial element for crop plants. In Studies in Plant Science; Elsevier: Amsterdam, The Netherlands, 2001; pp. 17–39. [Google Scholar]
- Savant, N.K.; Korndörfer, G.H.; Datnoff, L.E. Silicon nutrition and sugarcane production: A review. J. Plant Nutr. 1999, 22, 1853–1903. [Google Scholar] [CrossRef]
- Li, Q.; Ma, C.; Shang, Q. Effects of silicon on photosynthesis and antioxidative enzymes of maize under drought stress. Ying yong sheng tai xue bao = (J. Appl. Ecol.) 2007, 18, 531–536. [Google Scholar]
- Peterhansel, C.; Horst, I.; Niessen, M.; Blume, C.; Kebeish, R.; Kürkcüoglu, S.; Kreuzaler, F. Photorespiration. Arab. Book/Am. Soc. Plant Biol. 2010, 8, e0130. [Google Scholar] [CrossRef] [PubMed]
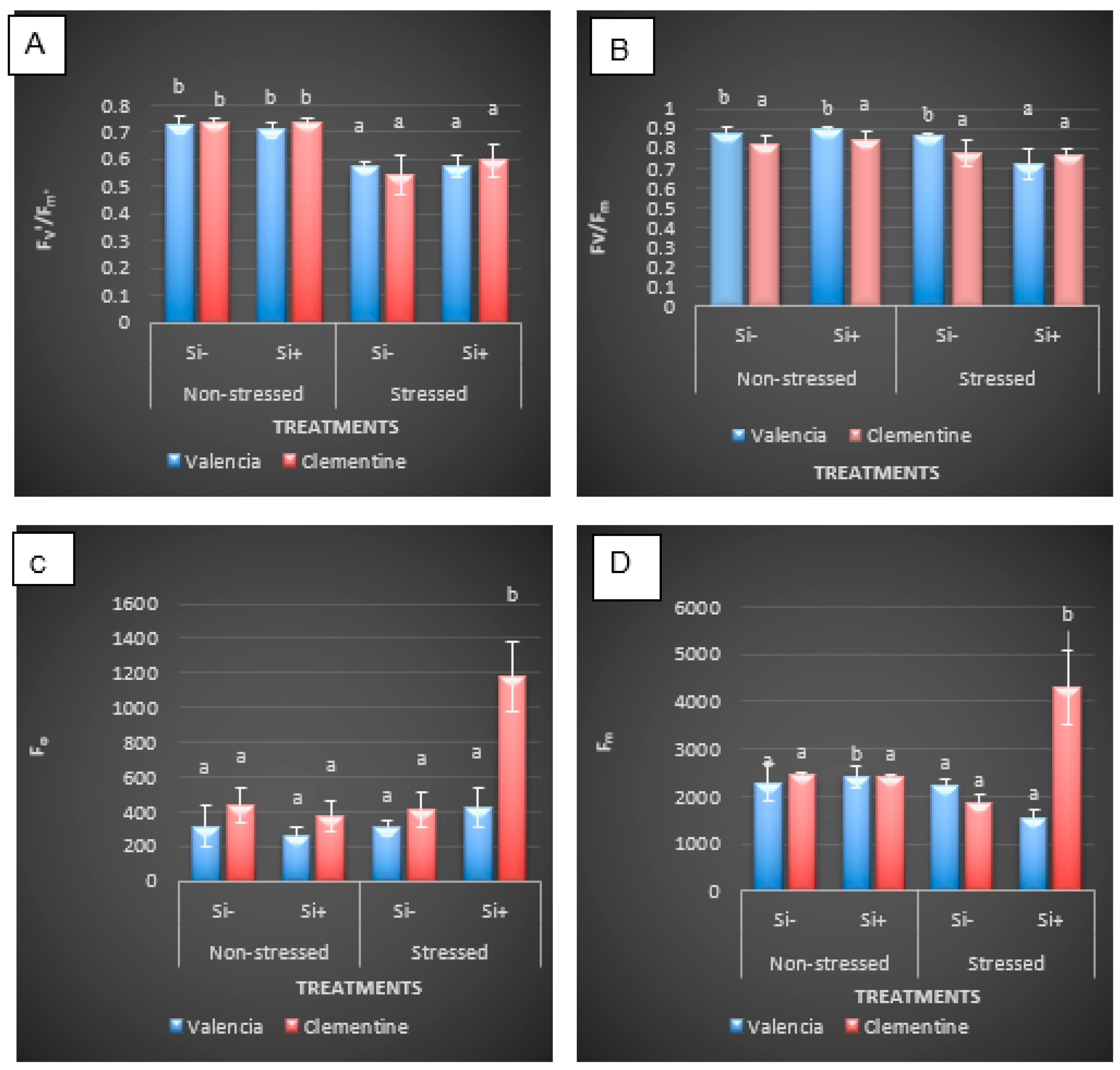
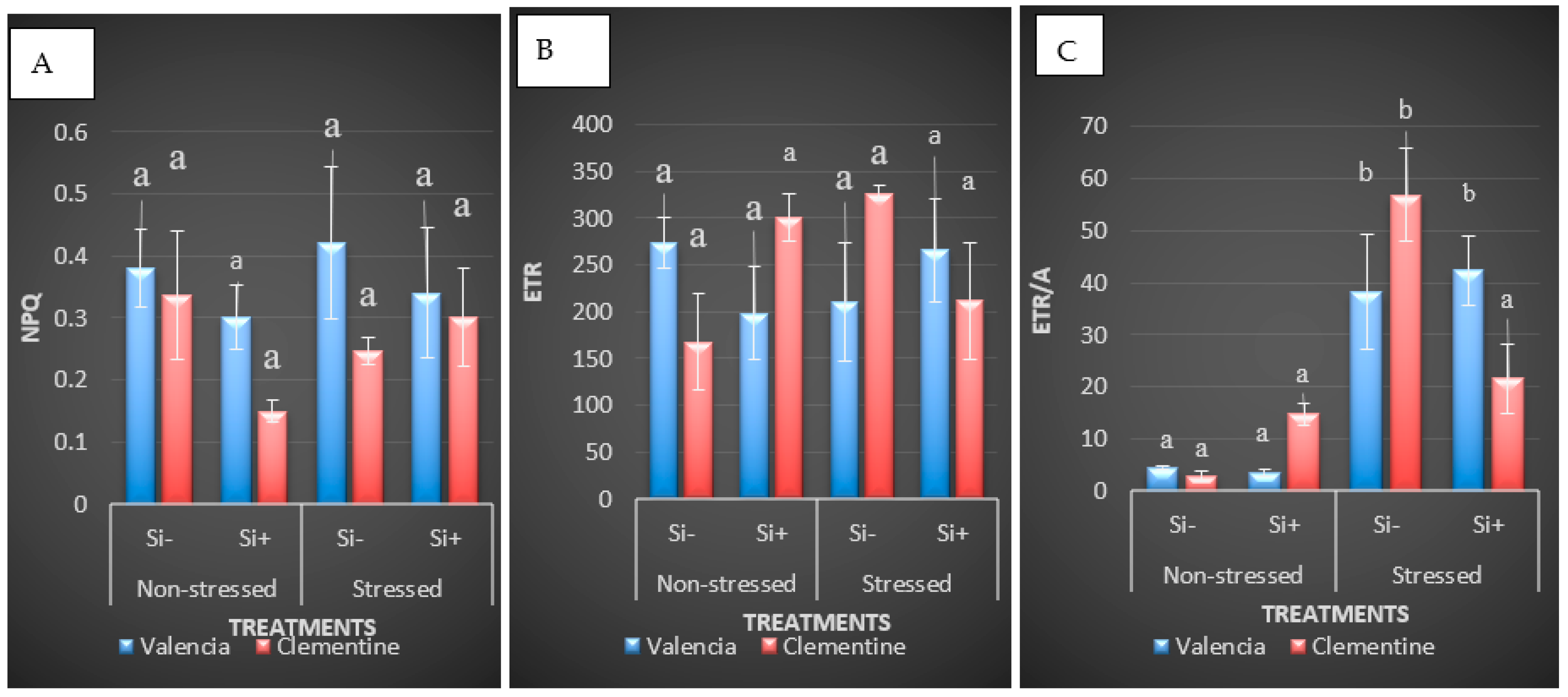

| Leaf Gas Exchange Measurements | ||||||
| Source of Variation | df | A | Cond | Tr | Ci | iWUE |
| Time | 1 | 3.9411 *** | 0.0586 *** | 0.4995 NS | 1437886 *** | 0.1806 NS |
| Variety | 1 | 0.0307 * | 0.0153 *** | 1.1836 ** | 5094 NS | 0.0948 NS |
| Si | 1 | 0.0253 NS | 0.0281 *** | 1.6567 *** | 116591 NS | 0.4679 * |
| Time × Variety | 1 | 0.0284 * | 0.0305 | 3.2384 *** | 20014 NS | 0.7024 ** |
| Time × Si | 1 | 0.032 * | 0.0305 *** | 2.2549 *** | 185324 NS | 0.3782 * |
| Variety × Si | 1 | 0.0073 NS | 0.0183 *** | 0.1378 NS | 65471 * | 0.2821 * |
| Time × Variety × Si | 1 | 0.0044 * | 0.0198 *** | 0.1171 NS | 17197 NS | 0.4193 ** |
| Residual | 35 | 0.228 | 0.001 | 0.1234 | 33019 | 0.0555 |
| Lsd Value | - | 0.0473 | 0.0189 | 0.2058 | 106.49 | 0.138 |
| CV Value | - | 7.3 | 46 | 34 | 63 | 17 |
| p Value | - | 0.01322 | 0.000106 | 0.00014 | 0.02348 | 0.009 |
| Chlorophyll Fluorescence Measurements | ||||||
| Source of Variation | F’v/F’m | Fv/Fm | Fo | F’m | NPQ | ETR |
| Time | 0.0485 * | 0.195 *** | 440740 ** | 89612 NS | 0.005 NS | 2719 NS |
| Variety | 0.0091 NS | 0.0007 NS | 577593 ** | 3101270 * | 0.0668 NS | 1762 NS |
| Si | 0.0060 NS | 0.00068 NS | 290549 * | 1662320 NS | 0.032 NS | |
| Time × Variety | 0.0017 NS | 0.0009 NS | 198419 NS | 2535637 * | 0.0019 NS | 2079 NS |
| Time × Si | 0.019 NS | 0.0026 NS | 499401 ** | 1334069 NS | 0.0204 NS | 6861 NS |
| Variety × Si | 0.0096 NS | 0.0028 NS | 204565 NS | 4275174 ** | 0.0023 NS | 714 NS |
| Time × Variety × Si | 0.0082 NS | 0.0005 NS | 220400 NS | 5391827 ** | 0.0207 NS | 72197 NS |
| Residual | 0.0085 | 0.0051 NS | 53834 | 468052 | 0.0262 | 7080 ** |
| Lsd Value | 0.135 | 0.053 | 170.594 | 503.018 | 0.168 | 61.64 |
| CV Value | 11.27 | 11.075 | 50 | 28 | 51 | 34.33 |
| p Value | 0.0883 | 0.0094 | 0.788 | 0.0052 | 0.2967 | 0.8822 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mvondo-She, M.A.; Mashilo, J.; Gatabazi, A.; Ndhlala, A.R.; Laing, M.D. Exogenous Silicon Application Improves Chilling Injury Tolerance and Photosynthetic Performance of Citrus. Agronomy 2024, 14, 139. https://doi.org/10.3390/agronomy14010139
Mvondo-She MA, Mashilo J, Gatabazi A, Ndhlala AR, Laing MD. Exogenous Silicon Application Improves Chilling Injury Tolerance and Photosynthetic Performance of Citrus. Agronomy. 2024; 14(1):139. https://doi.org/10.3390/agronomy14010139
Chicago/Turabian StyleMvondo-She, Mireille Asanzi, Jacob Mashilo, Auges Gatabazi, Ashwell Rungano Ndhlala, and Mark Delmege Laing. 2024. "Exogenous Silicon Application Improves Chilling Injury Tolerance and Photosynthetic Performance of Citrus" Agronomy 14, no. 1: 139. https://doi.org/10.3390/agronomy14010139
APA StyleMvondo-She, M. A., Mashilo, J., Gatabazi, A., Ndhlala, A. R., & Laing, M. D. (2024). Exogenous Silicon Application Improves Chilling Injury Tolerance and Photosynthetic Performance of Citrus. Agronomy, 14(1), 139. https://doi.org/10.3390/agronomy14010139







