Outcrossing Rate and Fruit Yield of Hass Avocado Trees Decline at Increasing Distance from a Polliniser Cultivar
Abstract
1. Introduction
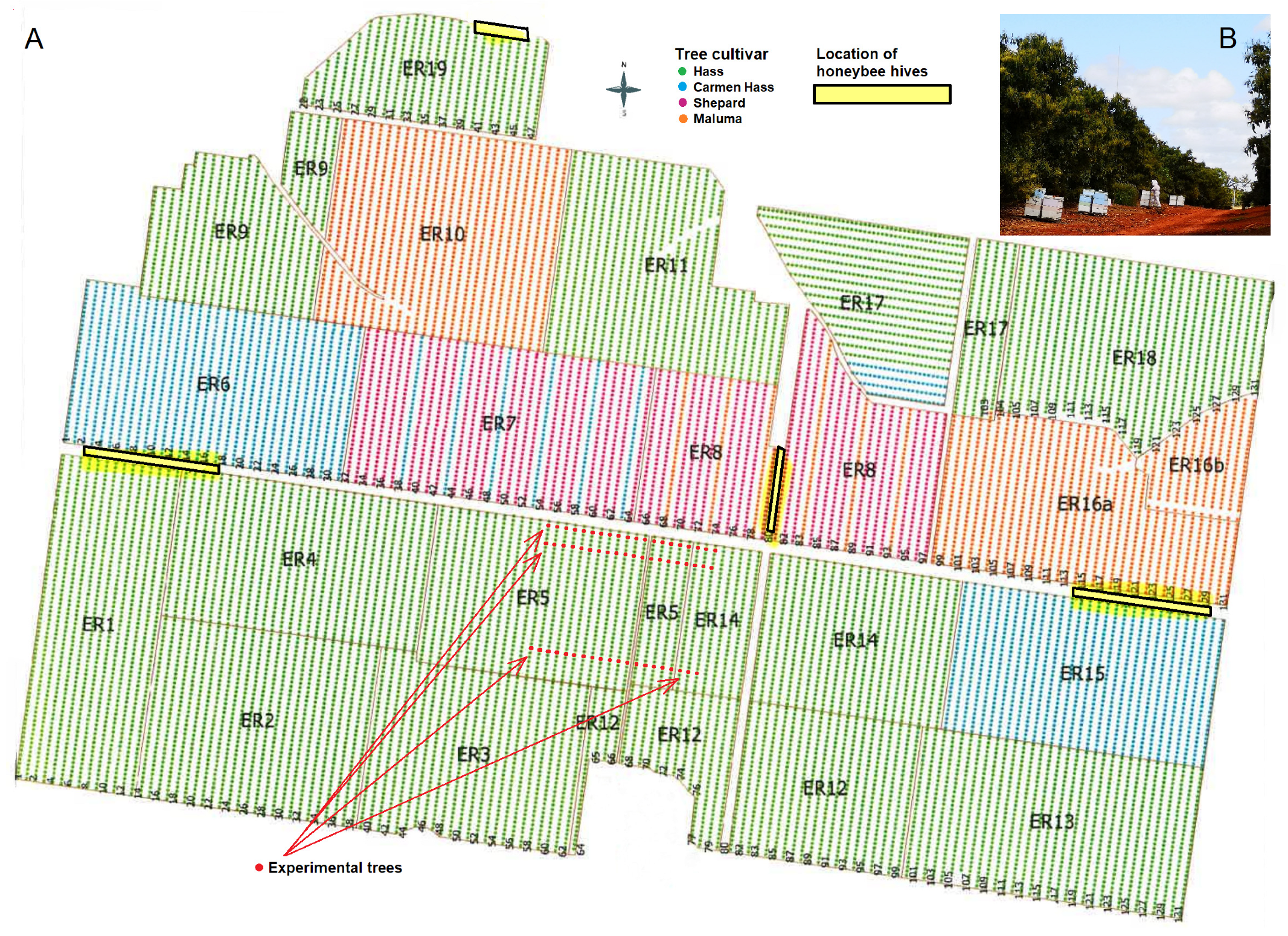
2. Materials and Methods
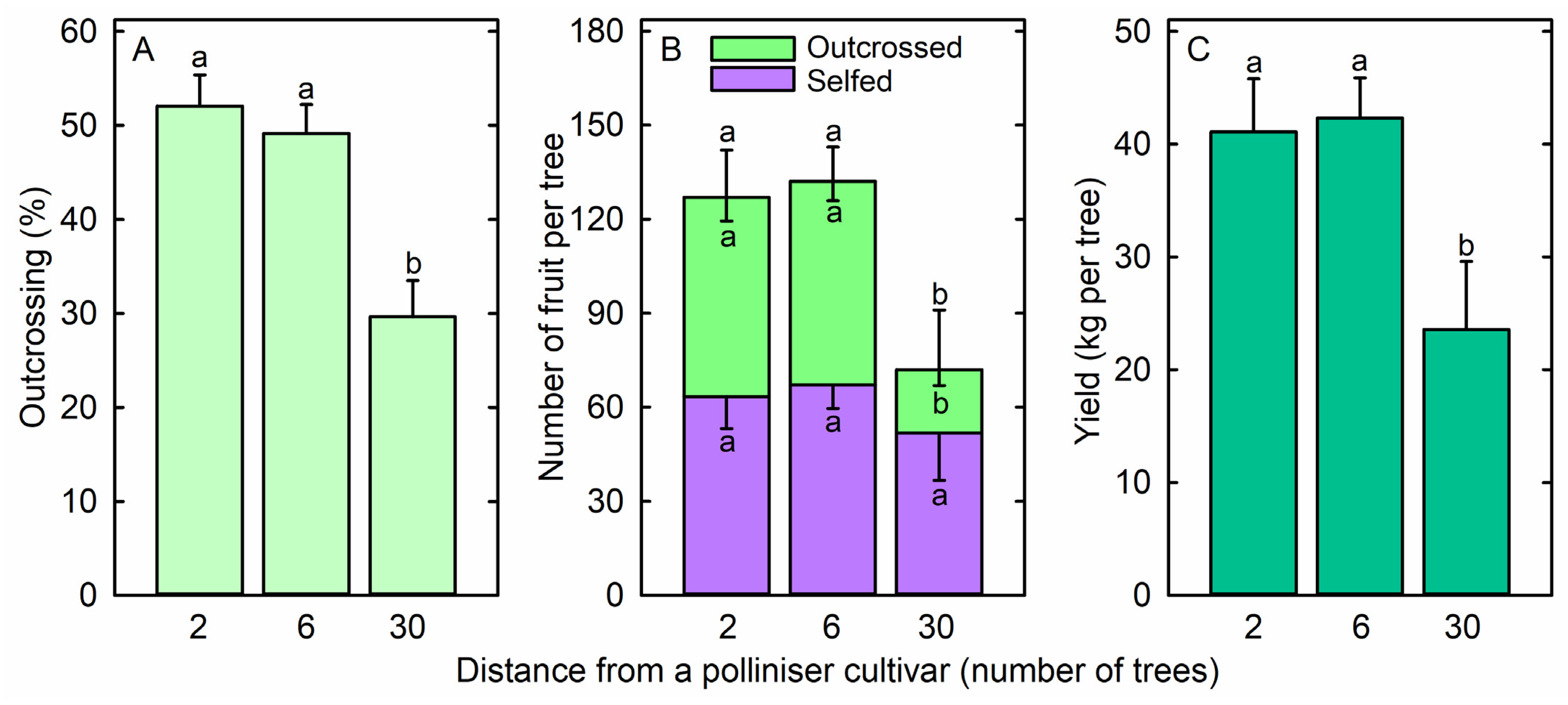
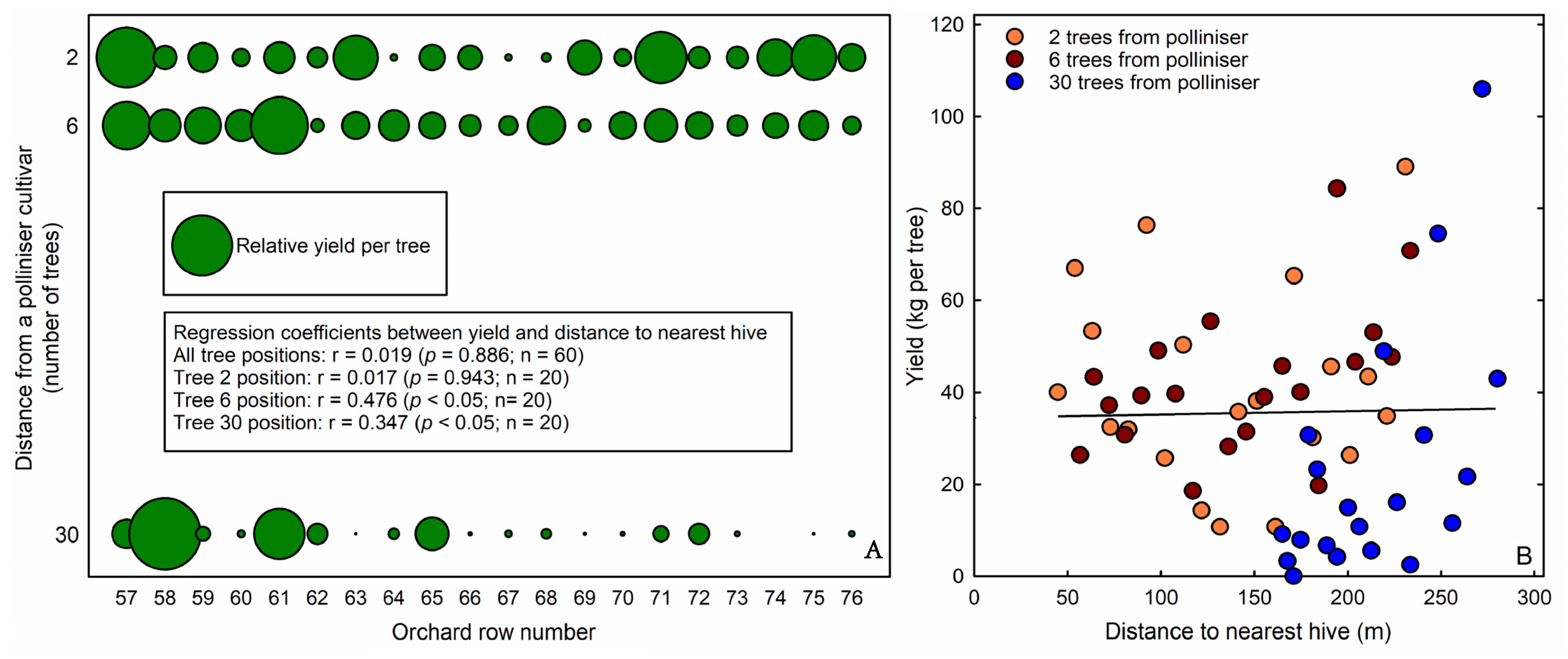
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. World Food and Agriculture–Statistical Yearbook 2022; FAO: Rome, Italy, 2008. [Google Scholar]
- Aizen, M.A.; Garibaldi, L.A.; Cunningham, S.A.; Klein, A.M. How much does agriculture depend on pollinators? Lessons from long-term trends in crop production. Ann. Bot. 2009, 103, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Stefan Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.M.; Steets, J.A.; Burns, J.H.; Burkle, L.A.; Vamosi, J.C.; Wolowski, M.; Arceo-Gómez, G.; Burd, M.; Durka, W.; Ellis, A.G.; et al. Land use and pollinator dependency drives global patterns of pollen limitation in the Anthropocene. Nat. Commun. 2020, 11, 3999. [Google Scholar] [CrossRef] [PubMed]
- Aizen, M.A.; Harder, L.D. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 2009, 19, 915–918. [Google Scholar] [CrossRef]
- Klein, A.-M.; Boreux, V.; Fornoff, F.; Mupepele, A.-C.; Pufal, G. Relevance of wild and managed bees for human well-being. Curr. Opin. Insect Sci. 2018, 26, 82–88. [Google Scholar] [CrossRef]
- Patel, V.; Pauli, N.; Biggs, E.; Barbour, L.; Boruff, B. Why bees are critical for achieving sustainable development. Ambio 2021, 50, 49–59. [Google Scholar] [CrossRef]
- Wallace, H.M.; Lee, L.S. Pollen source, fruit set and xenia in mandarins. J. Hortic. Sci. Biotechnol. 1999, 74, 82–86. [Google Scholar] [CrossRef]
- Trueman, S.J. The reproductive biology of macadamia. Sci. Hortic. 2013, 150, 354–359. [Google Scholar] [CrossRef]
- Hu, G.; Gao, C.; Fan, X.; Gong, W.; Yuan, D. Pollination compatibility and xenia in Camellia oleifera. HortScience 2020, 56, 898–905. [Google Scholar] [CrossRef]
- López, M.E.; Ramírez, O.A.; Dubón, A.; Ribeiro, T.H.C.; Díaz, F.J.; Chalfun, A., Jr. Sexual compatibility in cacao clones drives arrangements in the field leading to high yield. Sci. Hortic. 2021, 287, 110276. [Google Scholar] [CrossRef]
- Vuletin Selak, G.; Baruca Arbeiter, A.; Cuevas, J.; Perica, S.; Pujic, P.; Raboteg Božiković, M.; Bandelj, D. Seed paternity analysis using SSR markers to assess successful pollen donors in mixed olive orchards. Plants 2021, 10, 2356. [Google Scholar] [CrossRef] [PubMed]
- Claessen, H.; Van de Poel, B.; Keulemans, W.; De Storme, N. A semi in vivo pollination technique to assess the level of gametophytic self incompatibility and pollen tube growth in pear (Pyrus communis L.). Plant Reprod. 2022, 35, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Masui, K.; Tao, R. Artificial control of the Prunus self-incompatibility system using antisense oligonucleotides against pollen genes. Hortic. J. 2022, 91, 437–447. [Google Scholar] [CrossRef]
- Degani, C.; Goldring, A.; Gazit, S. Pollen parent effect on outcrossing rate in ‘Hass’ and ‘Fuerte’ avocado plots during fruit development. J. Am. Soc. Hortic. Sci. 1989, 114, 106–111. [Google Scholar] [CrossRef]
- Degani, C.; El-Batsri, R.; Gazit, S. Outcrossing rate, yield, and selective fruit abscission in ‘Ettinger’ and ‘Ardith’ avocado plots. J. Am. Soc. Hortic. Sci. 1997, 122, 813–817. [Google Scholar] [CrossRef]
- Dag, A.; Eisenstein, D.; Gazit, S.; El-Batsri, R.; Degani, C. Effect of pollenizer distance and selective fruitlet abscission on outcrossing rate and yield in ‘Tommy Atkins’ mango. J. Am. Soc. Hortic. Sci. 1998, 123, 618–622. [Google Scholar] [CrossRef]
- Dag, A.; Degani, C.; Gazit, S. Gene flow in mango orchards and its impact on yield. Acta Hortic. 2009, 820, 347–350. [Google Scholar] [CrossRef]
- Trueman, S.; De Silva, A.; Kämper, W.; Nichols, J.; Hosseini Bai, S.; Wallace, H.; Royle, J.; Peters, T.; Ogbourne, S. Pollen parentage of nuts during premature nut drop: Do self-pollinated nuts drop and cross-pollinated nuts remain? Aust. Macadamia Soc. News Bull. 2022, 50, 19–20. [Google Scholar]
- Degani, C.; Stern, R.A.; El-Batsri, R.; Gazit, S. Pollen parent effect on the selective abscission of ‘Mauritius’ and ‘Floridian’ lychee fruitlets. J. Am. Soc. Hortic. Sci. 1995, 120, 523–526. [Google Scholar] [CrossRef]
- Denney, J.O. Xenia includes metaxenia. HortScience 1992, 27, 722–728. [Google Scholar] [CrossRef]
- Fattahi, R.; Mohammadzedeh, M.; Khadivi-Khub, A. Influence of different pollen sources on nut and kernel characteristics of hazelnut. Sci. Hortic. 2014, 173, 15–19. [Google Scholar] [CrossRef]
- Fountain, M.T.; Mateos-Fierro, Z.; Shaw, B.; Brain, P.; Delgado, A. Insect pollinators of Conference pear (Pyrus communis L.) and their contribution to fruit quality. J. Pollinat. Ecol. 2019, 25, 103–114. [Google Scholar] [CrossRef]
- Deng, L.; Wang, T.; Hu, J.; Yang, X.; Yao, Y.; Jin, Z.; Huang, Z.; Sun, G.; Xiong, B.; Liao, L.; et al. Effects of pollen sources on fruit set and fruit characteristics of ‘Fengtangli’ plum (Prunus salicina Lindl.) based on microscopic and transcriptomic analysis. Int. J. Mol. Sci. 2022, 23, 12959. [Google Scholar] [CrossRef] [PubMed]
- Trueman, S.J.; Kämper, W.; Nichols, J.; Ogbourne, S.M.; Hawkes, D.; Peters, T.; Hosseini Bai, S.; Wallace, H.M. Pollen limitation and xenia effects in a cultivated mass-flowering tree, Macadamia integrifolia (Proteaceae). Ann. Bot. 2022, 129, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Dung, C.D.; Wallace, H.M.; Hosseini Bai, S.; Ogbourne, S.M.; Trueman, S.J. Fruit size and quality attributes differ between competing self-pollinated and cross-pollinated strawberry fruit. Int. J. Fruit Sci. 2023, 23, 1–12. [Google Scholar] [CrossRef]
- Pérez, V.; Herrero, M.; Hormaza, J.I. Self-fertility and preferential cross-fertilization in mango (Mangifera indica). Sci. Hortic. 2016, 213, 373–378. [Google Scholar] [CrossRef]
- Kämper, W.; Ogbourne, S.M.; Hawkes, D.; Trueman, S.J. SNP markers reveal relationships between fruit paternity, fruit quality and distance from a cross-pollen source in avocado orchards. Sci. Rep. 2021, 11, 20043. [Google Scholar] [CrossRef]
- Kämper, W.; Nichols, J.; Tran, T.D.; Burwell, C.J.; Byrnes, S.; Trueman, S.J. Flower visitors, levels of cross-fertilisation, and pollen-parent effects on fruit quality in mango orchards. Agronomy 2023, 13, 2568. [Google Scholar] [CrossRef]
- Vithanage, V.; Meyers, N.; McConchie, C. Maximising the Benefits from Cross-Pollination in Macadamia Orchards; Horticulture Australia Ltd.: Sydney, Australia, 2002. [Google Scholar]
- Núñez-Elisea, R.; Cahn, H.; Caldeira, L.; Azarenko, A. Pollinizer distance affects crop load of young ‘Regina’ sweet cherry trees. Acta Hortic. 2008, 795, 537–540. [Google Scholar] [CrossRef]
- Sánchez-Estrada, A.; Cuevas, J. Profitability of artificial pollination in ‘Manzanillo’ olive orchards. Agronomy 2020, 10, 652. [Google Scholar] [CrossRef]
- Lucas-García, R.; Rosas-Guerrero, V.; Alemán-Figueroa, L.; Almazán-Núñez, R.C.; Violante-González, J.; Kuk-Dzul, J.G. Spatial proximity of ‘Ataulfo’ to ‘Haden’ cultivar increases mango yield and decreases incidence of nubbins. Agronomy 2021, 11, 450. [Google Scholar] [CrossRef]
- Kämper, W.; Trueman, S.J.; Ogbourne, S.M.; Wallace, H.M. Pollination services in a macadamia cultivar depend on across-orchard transport of cross pollen. J. Appl. Ecol. 2021, 58, 2529–2539. [Google Scholar] [CrossRef]
- Degani, C.; Goldring, A.; Adato, I.; El-Batsri, R.; Gazit, S. Pollen parent effect on outcrossing rate, yield, and fruit characteristics of ‘Fuerte’ avocado. HortScience 1990, 25, 471–473. [Google Scholar] [CrossRef]
- Schnell, R.J.; Tondo, C.L.; Brown, J.S.; Kuhn, D.N.; Ayala-Silva, T.; Borrone, J.W.; Davenport, T.L. Outcrossing between ‘Bacon’ pollinizers and adjacent ‘Hass’ avocado trees and the description of two new lethal mutants. HortScience 2009, 44, 1522–1526. [Google Scholar] [CrossRef]
- Hapuarachchi, N.S.; Kämper, W.; Wallace, H.M.; Hosseini Bai, S.; Ogbourne, S.M.; Nichols, J.; Trueman, S.J. Boron effects on fruit set, yield, quality and paternity of Hass avocado. Agronomy 2022, 12, 1479. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I. Fruit set in avocado: Pollen limitation, pollen load size, and selective fruit abortion. Agronomy 2021, 11, 1603. [Google Scholar] [CrossRef]
- Borrone, J.W.; Olano, C.T.; Kuhn, D.N.; Brown, J.S.; Schnell, R.J.; Violi, H.A. Outcrossing in Florida avocados as measured using microsatellite markers. J. Am. Soc. Hortic. Sci. 2008, 133, 255–261. [Google Scholar] [CrossRef]
- Scholefield, P.B. A scanning electron microscope study of flowers of avocado, litchi, macadamia and mango. Sci. Hortic. 1982, 16, 263–272. [Google Scholar] [CrossRef]
- Solares, E.; Morales-Cruz, A.; Figueroa Balderas, R.; Focht, E.; Ashworth, V.E.T.M.; Wyant, S.; Minio, A.; Cantu, D.; Arpaia, M.L.; Gaut, B.S. Insights into the domestication of avocado and potential genetic contributors to heterodichogamy. G3 Genes Genomes Gen. 2023, 13, jkac323. [Google Scholar] [CrossRef]
- Stahl, P.; Lev Mirom, Y.; Stern, R.A.; Goldway, M. Comparing ‘Iriet’ and ‘Ettinger’ avocado cultivars as pollinators of ‘Hass’ using SNPs for paternal identification. Sci. Hortic. 2019, 248, 50–57. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I. Influence of physical distance between cultivars on yield, outcrossing rate and selective fruit drop in avocado (Persea americana, Lauraceae). Ann. Appl. Biol. 2011, 158, 354–361. [Google Scholar] [CrossRef]
- Kobayashi, M.; Lin, J.-Z.; Davis, J.; Francis, L.; Clegg, M.T. Quantitative analysis of avocado outcrossing and yield in California using RAPD markers. Sci. Hortic. 2000, 86, 135–149. [Google Scholar] [CrossRef]
- Kämper, W.; Trueman, S.J.; Cooke, J.; Kasinadhuni, N.; Brunton, A.J.; Ogbourne, S.M. Single-nucleotide polymorphisms that uniquely identify cultivars of avocado (Persea americana). Appl. Plant Sci. 2021, 9, e11440. [Google Scholar] [CrossRef] [PubMed]
- Dymond, K.; Celis-Diez, J.L.; Potts, S.G.; Howlett, B.G.; Willcox, B.K.; Garratt, M.P.D. The role of insect pollinators in avocado production: A global review. J. Appl. Entomol. 2021, 145, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.E.; Plantegenest, M.; Amouroux, P.; Zaviezo, T. Native flower strips increase visitation by non-bee insects to avocado flowers and promote yield. Basic Appl. Ecol. 2021, 56, 369–378. [Google Scholar] [CrossRef]
- Stern, R.A.; Rozen, A.; Eshed, R.; Zviran, T.; Sisai, I.; Sherman, A.; Irihimovitch, V.; Sapir, G. Bumblebees (Bombus terrestris) improve ‘Hass’ avocado (Persea americana) pollination. Plants 2021, 10, 1372. [Google Scholar] [CrossRef]
- Sagwe, R.N.; Peters, M.K.; Dubois, T.; Steffan-Dewenter, I.; Latorff, H.M.G. Pollinator efficiency of avocado (Persea americana) flower insect visitors. Ecol. Solut. Evid. 2022, 3, e12178. [Google Scholar] [CrossRef]
- Beekman, M.; Ratnieks, F.L.W. Long-range foraging by the honey-bee, Apis mellifera L. Funct. Ecol. 2000, 14, 490–496. [Google Scholar] [CrossRef]
- Hagler, J.R.; Mueller, S.; Teuber, L.R.; Machtley, S.A.; Van Deynze, A. Foraging range of honey bees, Apis mellifera, in alfalfa seed production fields. J. Insect Sci. 2011, 11, 144. [Google Scholar] [CrossRef]
- Ratnieks, F.L.W.; Shackleton, K. Does the waggle dance help honey bees to forage at greater distances than expected for their body size? Front. Ecol. Evol. 2015, 3, 31. [Google Scholar] [CrossRef]
- Brittain, C.; Williams, N.; Kremen, C.; Klein, A.-M. Synergistic effects of non-Apis bees and honey bees for pollination services. Proc. R. Soc. B 2013, 280, 20122767. [Google Scholar] [CrossRef] [PubMed]
- Eeraerts, M.; Smagghe, G.; Meeus, I. Bumble bee abundance and richness improves honey bee pollination behaviour in sweet cherry. Basic Appl. Ecol. 2020, 43, 27–33. [Google Scholar] [CrossRef]
- Evans, L.J.; Jesson, L.; Read, S.F.J.; Jochym, M.; Cutting, B.T.; Gayrard, T.; Jammes, M.A.S.; Roumier, R.; Howlett, B.G. Key factors influencing forager distribution across macadamia orchards differ among species of managed bees. Basic Appl. Ecol. 2021, 53, 74–85. [Google Scholar] [CrossRef]
- Kron, P.; Husband, B.C.; Kevan, P.G.; Belaoussoff, S. Factors affecting pollen dispersal in high-density apple orchards. HortScience 2001, 36, 1039–1046. [Google Scholar] [CrossRef]
- Wallace, H.M.; King, B.J.; Lee, L.S. Pollen flow and the effect on fruit size in an ‘Imperial’ mandarin orchard. HortScience 2002, 37, 84–86. [Google Scholar] [CrossRef]
- Vrecenar-Gadus, M.; Ellstrand, N.C. The effect of planting design on out-crossing rate and yield in the ‘Hass’ avocado. Sci. Hortic. 1985, 27, 215–221. [Google Scholar] [CrossRef]
- Garner, L.C.; Ashworth, V.E.T.M.; Clegg, M.T.; Lovatt, C.J. The impact of outcrossing on yields of ‘Hass’ avocado. J. Am. Soc. Hortic. Sci. 2008, 133, 648–652. [Google Scholar] [CrossRef]
- Oukabli, A.; Lansari, A.; Walali-Loudiyi, D.E.; Abousalim, A. Effects of controlled self-pollination and cross-pollination on fruit set, embryo viability and pomological traits in the self-compatible almond cv ‘Tuono’. Acta Hortic. 2002, 591, 429–435. [Google Scholar] [CrossRef]
- Kämper, W.; Thorp, G.; Wirthensohn, M.; Brooks, P.; Trueman, S.J. Pollen paternity can affect kernel size and nutritional composition of self-incompatible and new self-compatible almond cultivars. Agronomy 2021, 11, 326. [Google Scholar] [CrossRef]
- Stern, R.A.; Gazit, S.; El-Batsri, R.; Degani, C. Pollen parent effect on outcrossing rate, yield, and fruit characteristics of ‘Floridian’ and ‘Mauritius’ lychee. J. Am. Soc. Hortic. Sci. 1993, 118, 109–114. [Google Scholar] [CrossRef]
- Avocados Australia. Avocados Australia Best Practice Resource–Packaging for Export; Avocados Australia: Rocklea, Australia, 2018. [Google Scholar]
- Whiley, A.W. Crop management. In The Avocado: Botany, Production and Uses; Whiley, A.W., Schaffer, B., Wolstenholme, B.N., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 231–253. [Google Scholar]
- Lovatt, C.J. Hass Avocado Nutrition Research in California; University of California: Riverside, CA, USA, 2013. [Google Scholar]
- Lovatt, C.; Zheng, Y.; Khuong, T.; Campisi-Pinto, S.; Crowley, D.; Rolshausen, P. Yield characteristics of ‘Hass’ avocado trees under California growing conditions. In Proceedings of the VIII World Avocado Congress, Lima, Peru, 13–18 September 2015. [Google Scholar]
- Barrientos-Priego, A.F.; Martínez-Damián, M.T.; Vargas-Madríz, H.; Lázaro-Dzul, M.O. Effect of preharvest calcium spraying on ripening and chilling injury in ‘Hass’ (Persea americana Mill.) avocado. Rev. Chapingo Ser. Hortic. 2016, 22, 145–159. [Google Scholar] [CrossRef]
- Witney, G.W.; Hofman, P.J.; Wolstenholme, B.N. Effect of cultivar, tree vigour and fruit position on calcium accumulation in avocado fruit. Sci. Hortic. 1990, 44, 269–278. [Google Scholar] [CrossRef]
- Hofman, P.J.; Vuthapanich, S.; Whiley, A.W.; Klieber, A.; Simons, D.H. Tree yield and fruit minerals concentrations influence ‘Hass’ avocado fruit quality. Sci. Hortic. 2002, 92, 113–123. [Google Scholar] [CrossRef]
- Carisio, L.; Díaz, S.S.; Ponso, S.; Manino, A.; Porporato, M. Effects of pollinizer density and apple tree position on pollination efficiency in cv. Gala. Sci. Hortic. 2020, 273, 109629. [Google Scholar] [CrossRef]
- Sánchez-Estrada, A.; Cuevas, J. Pollination strategies to improve fruit set in orchards of ‘Manzanillo’ olive in a nontraditional producing country, Mexico. HortTechnology 2019, 29, 258–264. [Google Scholar] [CrossRef]
- Trueman, S.J.; McConchie, C.A.; Turnbull, C.G.N. Ethephon promotion of crop abscission for unshaken and mechanically shaken macadamia. Aust. J. Exp. Agric. 2002, 42, 1001–1008. [Google Scholar] [CrossRef]
| Parameter | Mother Cultivar × Father Cultivar | |
|---|---|---|
| Hass × Hass (Selfed) | Hass × Shepard (Outcrossed) | |
| Diameter (mm) | 77.8 ± 0.3 a | 80.4 ± 0.3 b |
| Length (mm) | 99.3 ± 0.5 a | 104.1 ± 0.6 b |
| Fresh mass (g) | 306 ± 3 a | 344 ± 4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trueman, S.J.; Nichols, J.; Farrar, M.B.; Wallace, H.M.; Hosseini Bai, S. Outcrossing Rate and Fruit Yield of Hass Avocado Trees Decline at Increasing Distance from a Polliniser Cultivar. Agronomy 2024, 14, 122. https://doi.org/10.3390/agronomy14010122
Trueman SJ, Nichols J, Farrar MB, Wallace HM, Hosseini Bai S. Outcrossing Rate and Fruit Yield of Hass Avocado Trees Decline at Increasing Distance from a Polliniser Cultivar. Agronomy. 2024; 14(1):122. https://doi.org/10.3390/agronomy14010122
Chicago/Turabian StyleTrueman, Stephen J., Joel Nichols, Michael B. Farrar, Helen M. Wallace, and Shahla Hosseini Bai. 2024. "Outcrossing Rate and Fruit Yield of Hass Avocado Trees Decline at Increasing Distance from a Polliniser Cultivar" Agronomy 14, no. 1: 122. https://doi.org/10.3390/agronomy14010122
APA StyleTrueman, S. J., Nichols, J., Farrar, M. B., Wallace, H. M., & Hosseini Bai, S. (2024). Outcrossing Rate and Fruit Yield of Hass Avocado Trees Decline at Increasing Distance from a Polliniser Cultivar. Agronomy, 14(1), 122. https://doi.org/10.3390/agronomy14010122







