Field Screening of Diverse Soybean Germplasm to Characterize Their Adaptability under Long-Day Condition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Morphological Trait Evaluation
2.3. Statistical Analysis
2.4. Variability Analysis
3. Results
3.1. Effects of Growing Conditions on Phenotypic Variation
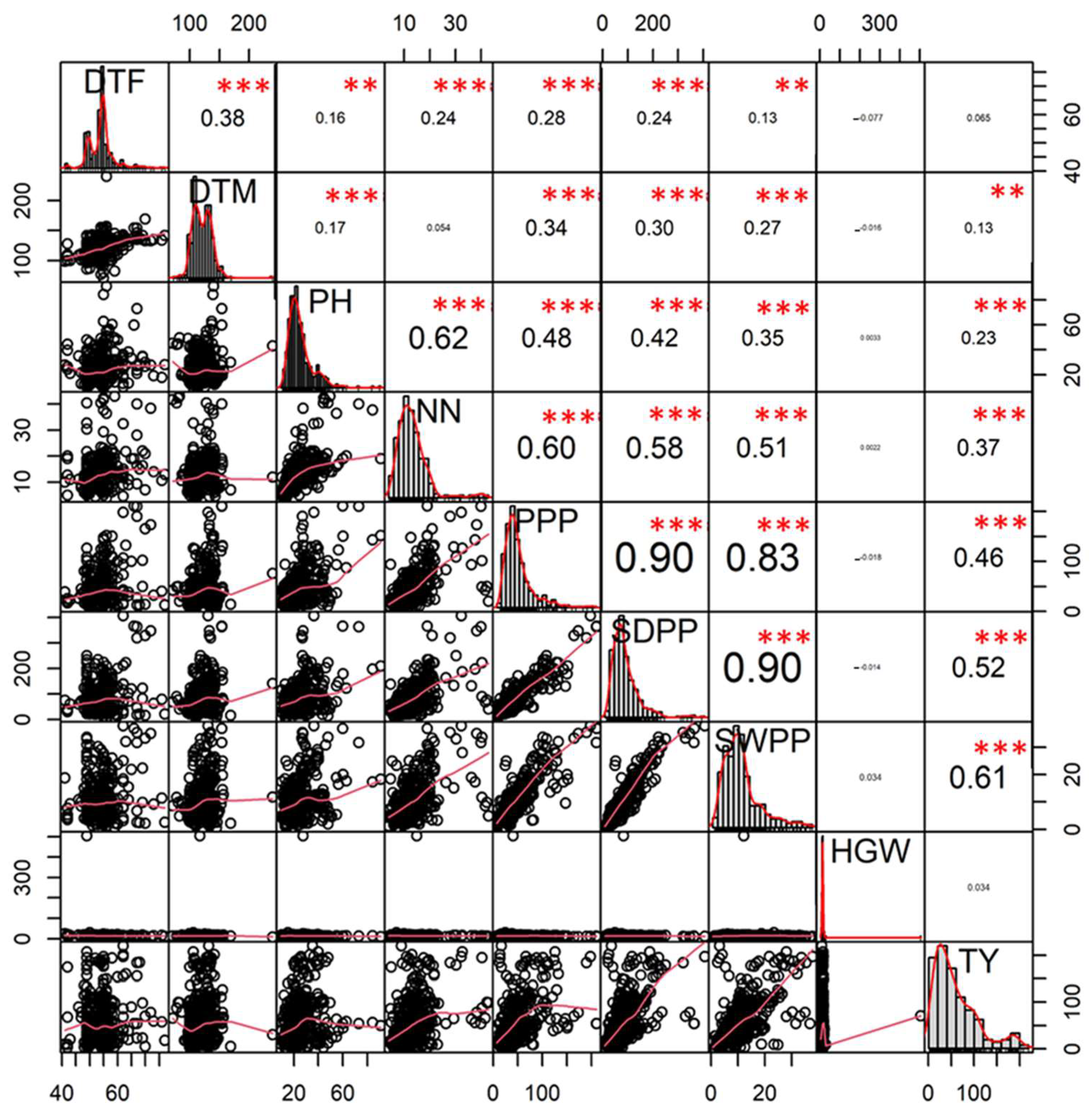
3.2. Correlation Analysis
3.3. Analysis of Variance of Agronomic Traits
3.4. Analysis of Variability, Heritability, and Genetic Advance
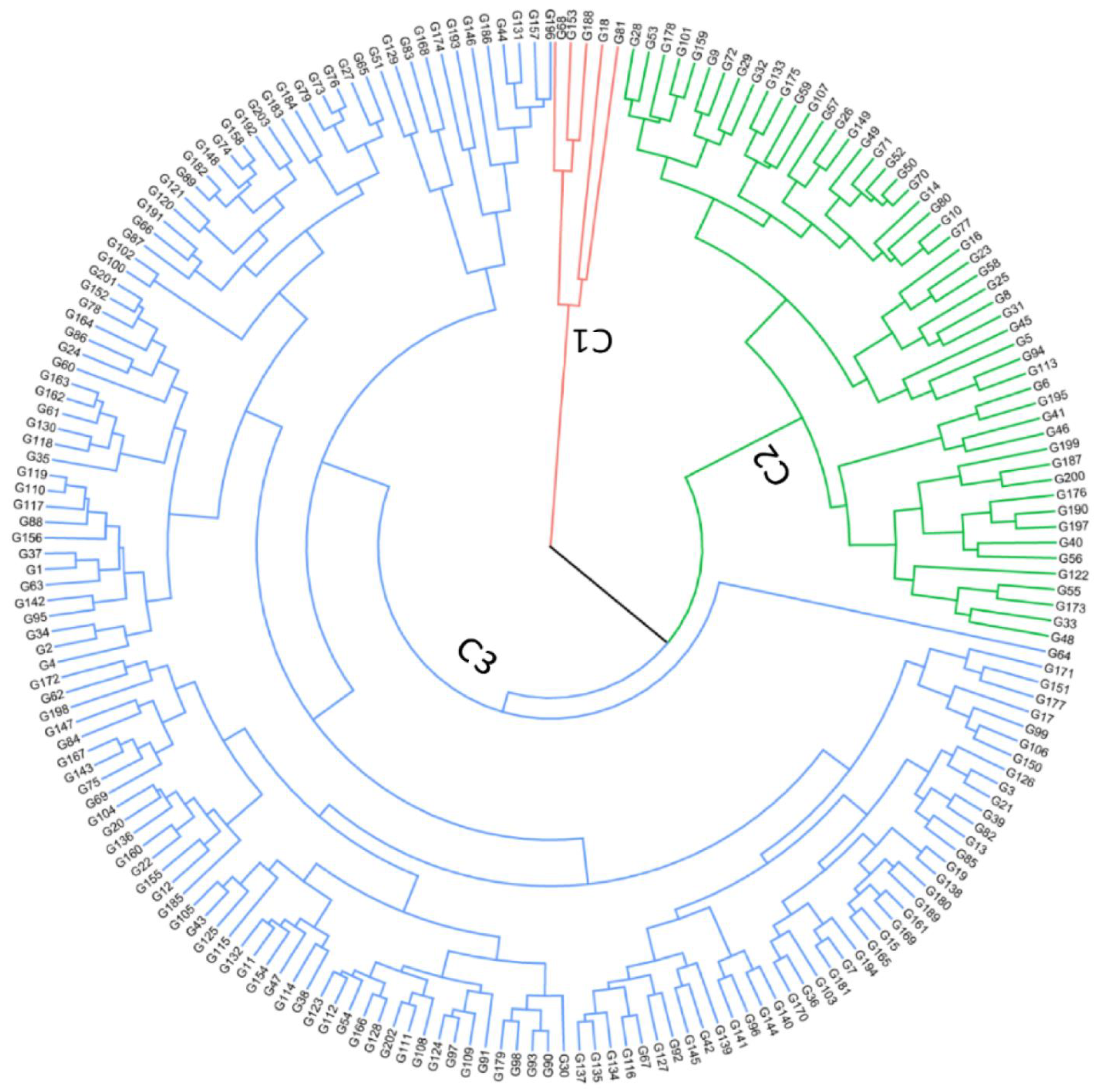
3.5. Phenotypic Variation Patterns in Germplasm Collection
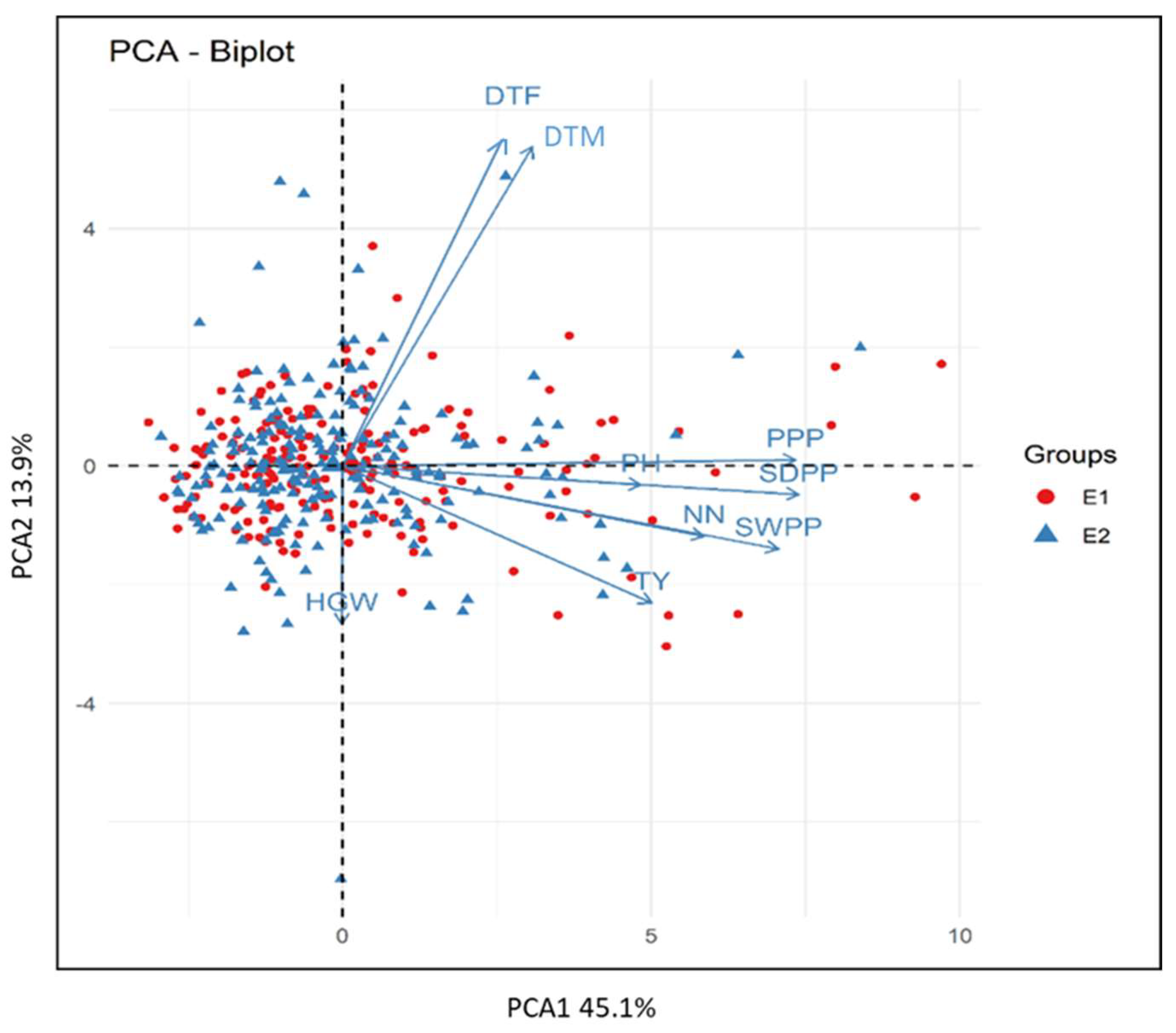
3.6. Biplot Analysis
3.7. Analysis of Agronomic Traits Based on BLUPs and BLUEs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, B.J.; Chang, S. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef]
- Raza, G.; Ahmad, N.; Hussain, M.; Zafar, Y.; Rahman, M. Role of genetics and genomics in mitigating abiotic stresses in soybeans. In Environmental Stresses in Soybean Production; Elsevier: Amsterdam, The Netherlands, 2016; pp. 205–228. [Google Scholar]
- Rani, R.; Raza, G.; Tung, M.H.; Rizwan, M.; Ashfaq, H.; Shimelis, H.; Razzaq, M.K.; Arif, M. Genetic diversity and population structure analysis in cultivated soybean (Glycine max [L.] Merr.) using SSR and EST-SSR markers. PLoS ONE 2023, 18, e0286099. [Google Scholar] [CrossRef] [PubMed]
- Faostat, F. New Food Balances. 2021. Available online: http://www.fao.org/faostat (accessed on 20 August 2023).
- Ali, L.; Mi, J.; Shah, M.; Shah, S.J.; BiBi, K. The potential socio-economic impact of china Pakistan economic corridor. Asian Dev. Policy Rev. 2017, 5, 191–198. [Google Scholar] [CrossRef]
- Mustafa, H.S.B.; Sarwar, S.; Aftab, M.; Hassan, A. Crop Diversification Through Soybean Cultivation in Punjab; Oilseeds Research Institute: Faisalabad, Pakistan, 2021. [Google Scholar]
- Khurshid, H.; Baig, D.; Jan, S.; Arshad, M.; Khan, M. Miracle crop: The present and future of soybean production in Pakistan. MOJ Biol. Med. 2017, 2, 189–191. [Google Scholar] [CrossRef]
- Baig, D.; Khurshid, H.; Arshad, M.; Jan, S.A.; Khan, M.A.; Nawaz, N. 1. Evaluation of Soybean genotypes for yield and other economically important traits under rainfed condition. Pure Appl. Biol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Asad, S.A.; Wahid, M.A.; Farina, S.; Ali, R.; Muhammad, F. Soybean production in Pakistan: Experiences, challenges and prospects. Int. J. Agri. Biol. 2020, 24, 995–1005. [Google Scholar]
- Han, Y.; Zhao, X.; Liu, D.; Li, Y.; Lightfoot, D.A.; Yang, Z.; Zhao, L.; Zhou, G.; Wang, Z.; Huang, L. Domestication footprints anchor genomic regions of agronomic importance in soybeans. New Phytol. 2016, 209, 871–884. [Google Scholar] [CrossRef]
- Song, Q.; Hyten, D.L.; Jia, G.; Quigley, C.V.; Fickus, E.W.; Nelson, R.L.; Cregan, P.B. Fingerprinting soybean germplasm and its utility in genomic research. G3 Genes Genom. Genet. 2015, 5, 1999–2006. [Google Scholar] [CrossRef]
- Arif, A.; Parveen, N.; Waheed, M.Q.; Atif, R.M.; Waqar, I.; Shah, T.M. A comparative study for assessing the drought-tolerance of chickpea under varying natural growth environments. Front. Plant Sci. 2021, 11, 607869. [Google Scholar] [CrossRef] [PubMed]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. BMJ Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Robinson, G.K. That BLUP is a good thing: The estimation of random effects. Stat. Sci. 1991, 6, 15–32. [Google Scholar]
- Piepho, H.P. Best linear unbiased prediction (BLUP) for regional yield trials: A comparison to additive main effects and multiplicative interaction (AMMI) analysis. Theor. Appl. Genet. 1994, 89, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Lafaye de Micheaux, P.; Drouilhet, R.; Liquet, B. The R Software: Fundamentals of Programming and Statistical Analysis; Springer Publishing Company: New York, NY, USA, 2013. [Google Scholar]
- Köhn, H.F.; Hubert, L.J. Hierarchical Cluster Analysis; Wiley: Hoboken, NJ, USA, 2014; pp. 1–13. [Google Scholar]
- Lush, J.L. Heritability of Quantitative Characters in Farm Animals. Hereditas 1949, 35, 356–375. [Google Scholar] [CrossRef]
- Robinson, H.; Comstock, R.E.; Harvey, P. Estimates of heritability and the degree of dominance in corn. Agron. J. 1949, 41, 353–359. [Google Scholar] [CrossRef]
- Rani, R.; Raza, G.; Ashfaq, H.; Rizwan, M.; Shimelis, H.; Tung, M.H.; Arif, M. Analysis of genotype× environment interactions for agronomic traits of soybean (Glycine max [L.] Merr.) using association mapping. Front. Genet. 2023, 13, 1090994. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Nelson, R.L. Genetic diversity among soybean accessions from three countries measured by RAPDs. Crop Sci. 2001, 41, 1337–1347. [Google Scholar] [CrossRef]
- Sharma, S.; Rao, S.; Goswami, U. Genetic variation, correlation and regression analysis and their implications in selection of exotic soybean. Mysore J. Agric. Sci. 1983, 17, 26–30. [Google Scholar]
- Zhang, Z.; Deng, Y.; Tan, J.; Hu, S.; Yu, J.; Xue, Q. A genome-wide microsatellite polymorphism database for the indica and japonica rice. DNA Res. 2007, 14, 37–45. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Q.; Cregan, P.B.; Jiang, G.L. Genome-wide association study, genomic prediction and marker-assisted selection for seed weight in soybean (Glycine max). Theor. Appl. Genet. 2016, 129, 117–130. [Google Scholar] [CrossRef]
- Watanabe, S.; Harada, K.; Abe, J. Genetic and molecular bases of photoperiod responses of flowering in soybean. Breed. Sci. 2012, 61, 531–543. [Google Scholar] [CrossRef]
- Contreras-Soto, R.I.; Mora, F.; de Oliveira, M.A.R.; Higashi, W.; Scapim, C.A.; Schuster, I. A genome-wide association study for agronomic traits in soybean using SNP markers and SNP-based haplotype analysis. PLoS ONE 2017, 12, e0171105. [Google Scholar] [CrossRef]
- Liu, B.; Kanazawa, A.; Matsumura, H.; Takahashi, R.; Harada, K.; Abe, J. Genetic redundancy in soybean photoresponses associated with duplication of the phytochrome A gene. Genetics 2008, 180, 995–1007. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, X.; Lee, R.; Li, Y.; Specht, J.E.; Nelson, R.L.; McClean, P.E.; Qiu, L.; Ma, J. Artificial selection for determinate growth habit in soybean. Proc. Nat. Acad. Sci. USA 2010, 107, 8563–8568. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Bashir, M. Evaluation of mid-season soybean varieties at Mansehra [Pakistan]. Sarhad J. Agric. 2005, 21, 531–533. [Google Scholar]
- Jiang, B.; Nan, H.; Gao, Y.; Tang, L.; Yue, Y.; Lu, S.; Ma, L.; Cao, D.; Sun, S.; Wang, J. Allelic combinations of soybean maturity loci E1, E2, E3 and E4 result in diversity of maturity and adaptation to different latitudes. PLoS ONE 2014, 9, e106042. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Lü, S.; Wang, Y.; Chen, X.; Ren, H.; Yang, J.; Cheng, W.; Zong, C.; Gu, H.; Qiu, H. Allelic variations at four major maturity E genes and transcriptional abundance of the E1 gene are associated with flowering time and maturity of soybean cultivars. PLoS ONE 2014, 9, e97636. [Google Scholar] [CrossRef]
- Zhang, L.; Kyei-Boahen, S.; Zhang, J.; Zhang, M.; Freeland, T.; Watson, C., Jr.; Liu, X. Modifications of optimum adaptation zones for soybean maturity groups in the USA. Crop Manag. 2007, 6, 1–11. [Google Scholar] [CrossRef]
- Jia, H.; Jiang, B.; Wu, C.; Lu, W.; Hou, W.; Sun, S.; Yan, H.; Han, T. Maturity group classification and maturity locus genotyping of early-maturing soybean varieties from high-latitude cold regions. PLoS ONE 2014, 9, e94139. [Google Scholar] [CrossRef]
- Josie, J.; Alcivar, A.; Rainho, J.; Kassem, M.A. Genomic regions containing QTL for plant height, internodes length, and flower color in soybean [Glycine max (L.) Merr]. Bios 2007, 78, 119–126. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, W.; Xu, X.; Wen, Z.; Li, H.; Li, J.; Lu, W. Relationship of dynamic plant height and its relative growth rate with yield using recombinant inbred lines of soybean. Acta Agron. Sin. 2011, 37, 559–562. [Google Scholar] [CrossRef]
- Guzman, P.; Diers, B.W.; Neece, D.; St. Martin, S.; LeRoy, A.; Grau, C.; Hughes, T.; Nelson, R.L. QTL associated with yield in three backcross-derived populations of soybean. Crop Sci. 2007, 47, 111–122. [Google Scholar] [CrossRef]
- Rehman, M.; Khaliq, T.; Ahmad, A.; Wajid, S.A.; Rasul, F.; Hussain, J.; Hussain, S. Effect of planting time and cultivar on soybean performance in Semi–Arid Punjab, Pakistan. Global J. Sci. Front. Res. Agric. Vet. 2014, 14, 41–45. [Google Scholar]
- Xavier, A.; Hall, B.; Casteel, S.; Muir, W.; Rainey, K.M. Using unsupervised learning techniques to assess interactions among complex traits in soybeans. Euphytica 2017, 213, 1–18. [Google Scholar] [CrossRef]
- Iqbal, Z.; Naeem, R.; Ashraf, M.; Arshad, M.; Afzal, A.; Shah, A.H.; Khan, M.S.; Farooq, M. Genetic diversity of soybean accessions using seed storage proteins. Pak. J. Bot. 2015, 47, 203–209. [Google Scholar]
- Liu, X.; Wu, J.-A.; Ren, H.; Qi, Y.; Li, C.; Cao, J.; Zhang, X.; Zhang, Z.; Cai, Z.; Gai, J. Genetic variation of world soybean maturity date and geographic distribution of maturity groups. Breed. Sci. 2017, 67, 221–232. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Purcell, L.C. Is a physiological perspective relevant in a ‘genocentric’age? J. Exp. Bot. 2005, 56, 2777–2782. [Google Scholar] [CrossRef] [PubMed]
- Coradi, P.C.; Fernandes, C.H.; Helmich, J.C.; Goneli, A.L. Effects of drying air temperature and grain initial moisture content on soybean quality (Glycine max (L.) Merrill). Eng. Agríc. 2016, 36, 866–876. [Google Scholar] [CrossRef]
- Malik, M.F.A.; Ashraf, M.; Qureshi, A.S.; Ghafoor, A. Assessment of genetic variability, correlation and path analyses for yield and its components in soybean. Pak. J. Bot. 2007, 39, 405. [Google Scholar]
- Ashraf, M.; Iqbal, Z.; Arshad, M.; Waheed, A.; Glufran, M.A.; Chaudhry, Z.; Baig, D. Multi-environment response in seed yield of soybean [Glycine max (L.) Merrill], genotypes through GGE biplot technique. Pak. J. Bot. 2010, 42, 3899–3905. [Google Scholar]
- Cruz, C.; Regazzi, A.; Carneiro, P. Modelos biométricos Aplicados ao Melhoramento; UFV: Viçosa, Brazil, 2012. [Google Scholar]
- Aditya, J.; Bhartiya, P.; Bhartiya, A. Genetic variability, heritability and character association for yield and component characters in soybean (G. max (L.) Merrill). J. Cen. Eur. Agric. 2011, 12, 27–34. [Google Scholar] [CrossRef]
- Mudibu, J.; Nkongolo, K.; Kalonji-Mbuyi, A. Morphovariability and agronomic characteristics of soybean accessions from the Democratic Republic of Congo (DR-Congo) gene pool. J. Plant Breed. Crop Sci. 2011, 3, 260–268. [Google Scholar]
- Abady, S.; Merkeb, F.; Dilnesaw, Z. Heritability and path-coefficient analysis in soybean (Glycine Max L. Merrill) genotypes at Pawe, North Western Ethiopia. J. Environ. Sci. Water Resour. 2013, 2, 270–276. [Google Scholar]
- Ngalamu, T.; Ashraf, M.; Meseka, S. Soybean (Glycine max L.) genotype and environment interaction effect on yield and other related traits. J. Exp. Agric. Int. 2013, 3, 977–987. [Google Scholar] [CrossRef]
- Rodrigues, B.; Serafim, F.; Nogueira, A.; Hamawaki, O.; Sousa, L.; Hamawaki, R. Correlations between traits in soybean (Glycine max L.) naturally infected with Asian rust (Phakopsora pachyrhizi). Genet. Mol. Res. 2015, 14, 17718–17729. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Mahmood, T.; Ali, M.; Anwar, M.; Sarwar, M. Path coefficient analysis in different genotypes of soybean (Glycine max (L.) Merril). Pak. J. Biol. Sci. 2003, 6, 1085–1087. [Google Scholar] [CrossRef]
- Hatam, M. Performance of AVRDC vegetable soybean germplasm under Peshawar valley conditions [Pakistan]. Sarhad J. Agric. 2001, 17, 27–31. [Google Scholar]
- Liu, K.; Muse, S.V. PowerMarker: An integrated analysis environment for genetic marker analysis. Bioinformatics 2005, 21, 2128–2129. [Google Scholar] [CrossRef] [PubMed]
- Lyimo, L.D.; Tamba, M.R.; Madege, R.R. Effects of genotype on yield and yield component of soybean (Glycine max (L.) Merrill). Afr. J. Agric, Res. 2017, 12, 1930–1939. [Google Scholar]
- Abd El-Mohsen, A.A.; Mahmoud, G.O.; Safina, S.A. Agronomical evaluation of six soybean cultivars using correlation and regression analysis under different irrigation regime conditions. J. Plant Breed. Crop Sci. 2013, 5, 91–102. [Google Scholar] [CrossRef]
- Ajayi, A.; Adekola, M.; Taiwo, B.; Azuh, V. Character expression and differences in yield potential of ten genotypes of cowpea (Vigna unguiculata L. Walp). Int. J. Plant Res. 2014, 4, 63–71. [Google Scholar]
- Gohil, V.; Pandya, H.; Mehta, D. Genetic variability for seed yield and its component traits in soybean. Agric. Sci. Dig. 2006, 26, 73–74. [Google Scholar]
- Akram, S.; Hussain, B.N.; Al Bari, M.A.; Burritt, D.J.; Hossain, M.A. Genetic variability and association analysis of soybean (Glycine max (L.) Merrill) for yield and yield attributing traits. Plant Gene Trait 2016, 7, 13. [Google Scholar] [CrossRef]
- Bangar, N.; Mukhekar, G.; Lad, D.; Mukhekar, D. Genetic variability, correlation and regression studies in soybean. J. Maha-Rashtra Agric. Uni. 2003, 28, 320–321. [Google Scholar]
- Karnwal, M.K.; Singh, K. Studies on genetic variability, character association and path coefficient for seed yield and its contributing traits in soybean [Glycine max (L.) Merrill]. Legume Res. 2009, 32, 70–73. [Google Scholar]
- Neelima, G.; Mehtre, S.; Narkhede, G. Genetic variability, heritability and genetic advance in soybean. Int. J. Pure Appl. Biosci. 2018, 6, 1011–1017. [Google Scholar] [CrossRef]
- Reni, Y.P.; Rao, Y.K. Genetic variability in soybean [Glycine max (L.) Merrill]. Int. J. Plant Animal Environ. Sci. 2013, 3, 35–38. [Google Scholar]
- Baraskar, V.; Kachhadia, V.; VachhanI, J.; Barad, H.; Patel, M.; Darwankar, M. Genetic variability, heritability and genetic advance in soybean [Glycine max (L.) Merrill]. Electron. J. Plant Breed. 2014, 5, 802–806. [Google Scholar]
- Malek, M.; Rafii, M.Y.; Afroz, S.S.; Nath, U.K.; Mondal, M. Morphological characterization and assessment of genetic variability, character association, and divergence in soybean mutants. Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Stuti, M.; Pancheshwar, D.K.; Prateek, S.; Avinash, J. Study of genetic variability in recently evolved genotypes of soybean [Glycine max (L.) Merill]. Trends Biosci. 2015, 8, 5390–5393. [Google Scholar]
- Chandrawat, K.S.; Baig, K.; Hashmi, S.; Sarang, D.; Kumar, A.; Dumai, P.K. Study on genetic variability, heritability and genetic advance in soybean. Int. J. Pure Appl. Biosci. 2017, 5, 57–63. [Google Scholar] [CrossRef]
| Growing Season | Year | Temperature °C | Humidity (%) | Rain (mm) | Wind (km/h) | Pressure (mm) | Photoperiod | Cropping Stage | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Max | Min | Mean | h·min | |||||||
| February | 2016 | 28.21 | 13.25 | 20.73 | 30.92 | 0 | 9 | 1015.1 | 11–04 | Sowing |
| 2017 | 27.82 | 13.07 | 20.44 | 33.57 | 0.11 | 8.57 | 1015.5 | 11–04 | ||
| March | 2016 | 31.22 | 17.45 | 24.33 | 41.45 | 2.12 | 10.38 | 1011.96 | 12–00 | Vegetative stage |
| 2017 | 31.77 | 16.03 | 23.9 | 33.45 | 0.35 | 8.19 | 1010.87 | 11–59 | ||
| April | 2016 | 37.13 | 23.53 | 30.33 | 23.23 | 0.34 | 11.13 | 1005.7 | 12–57 | Reproductive Stage |
| 2017 | 40.33 | 25.33 | 32.83 | 21.46 | 1.29 | 9.93 | 1004.3 | 12–57 | ||
| May | 2016 | 43.58 | 31.45 | 37.51 | 19.45 | 0.4 | 9.25 | 999.25 | 13–45 | Reproductive Stage |
| 2017 | 44.16 | 30.87 | 37.51 | 18.83 | 0.16 | 8.12 | 1000.77 | 13–45 | ||
| June | 2016 | 46.03 | 34.93 | 40.48 | 22.27 | 0.07 | 8.8 | 996.64 | 14–09 | Harvesting |
| 2017 | 42.96 | 33.56 | 38.26 | 26.67 | 1.17 | 8.3 | 996.75 | 14–09 | ||
| July | 2016 | 42.51 | 33.61 | 38.06 | 35.48 | 0.69 | 7.32 | 994.96 | 13–57 | Harvesting |
| 2017 | 42.12 | 33.87 | 37.995 | 34.96 | 0.2 | 8.77 | 996.19 | 13–57 | ||
| Traits | X | δ2g | δ2p | δ2e | δ2g × δ2e | LSD | CV |
|---|---|---|---|---|---|---|---|
| DTF | 54.24 | 17.53 *** | 17.52 | 0 | 3.31 *** | 3.57 | 1.46 |
| DTM | 120 | 212.92 *** | 212.92 | 0 | 42.29 *** | 12.89 | 3.19 |
| PH | 24.6 | 90.83 *** | 94.68 | 3.85 *** | 19.39 *** | 8.92 | 11.98 |
| NN | 13.43 | 17.29 *** | 17.29 | 0 | 15.72 *** | 6.94 | 24.26 |
| PPP | 47.84 | 683.91 *** | 724.08 | 40.17 *** | 296.91 *** | 33.09 | 25.77 |
| SDPP | 85.26 | 2123.61 *** | 2235.6 | 111.99 *** | 817.39 *** | 55.87 | 25.01 |
| SWPP | 10.89 | 33.01 *** | 34.61 | 1.6 *** | 11.63 *** | 6.77 | 24.77 |
| TY | 58.53 | 2157.67 *** | 2177.4 | 19.73 *** | 60.91 *** | 16.87 | 5.23 |
| Traits | SEm | GCV (%) | PCV (%) | H2 (%) | GA |
|---|---|---|---|---|---|
| DTF | 0.598 | 7.8 | 8.03 | 91 | 8.46 |
| DTM | 0.1616 | 11.87 | 11.87 | 90 | 29.41 |
| PPP | 11.13 | 63.05 | 72.86 | 80 | 59.39 |
| PH | 1.9636 | 44.23 | 46.06 | 89 | 20.02 |
| NN | 1.92 | 47.2 | 53.45 | 64 | 11.41 |
| SDPP | 17.34 | 62.22 | 70.09 | 81 | 105.86 |
| SWPP | 2.19 | 59.12 | 67.47 | 82 | 12.46 |
| HGW | 0.39 | 4.97 | 18.72 | 92 | 4.91 |
| TY | 24.94 | 77.47 | 77.79 | 99 | 977.34 |
| Soybean Germplasm | Days to Flowering (DTF) | Days to Maturity (DTM) | Pods per Plant (No.) | Plant Height (cm) | Nodes per Plant (No.) | Seeds per Plant (No.) | Seed Weight per Plant (g) | 100 Seed Weight (g) | Seed Yield (kg ha−1) |
|---|---|---|---|---|---|---|---|---|---|
| PI548271 | 62 ± 1.15 a | 131 ± 0.57 ab | 198.3 ± 17.63 a | 36.3 ± 0.88 cd | 32.3 ± 2.03 bc | 406 ± 73.33 a | 37.9 ± 1.54 a | 11.3 ± 0.61 h | 2150.6 ± 358.65 a |
| PI553039 | 58 ± 0.57 ab | 137 ± 0.57 ab | 89 ± 2.08 efg | 25 ± 2.89 efgh | 17.7 ± 2.34 efg | 155 ± 1.73 defg | 26.1 ± 0.20 bcdef | 15.3 ± 0.26 de | 2027.9 ± 228.33 ab |
| PI612608 | 49 ± 1.15 abc | 106 ± 0.57 abcde | 126.3 ± 5.85 cd | 40 ± 2.65 c | 32.7 ± 3.48 bc | 187 ± 45.94 cdef | 28.1 ± 5.12 abcd | 15.7 ± 0.09 cd | 1966.2 ± 258.93 ab |
| PI518664 | 55.33 ± 0.66 ab | 135 ± 0.57 ab | 77.3 ± 5.46 efgh | 23.3 ± 1.45 fghi | 12 ± 0.58 ghi | 135.3 ± 18.29 defgh | 25 ± 2.92 cdefg | 16.2 ± 0.20 bc | 1964.2 ± 171.86 ab |
| PI553042 | 49 ± 1.15 abc | 128 ± 0.57 abc | 101.7 ± 9.88 cdefg | 48 ± 2.31 b | 40.3 ± 3.18 a | 222.3 ± 24.86 cd | 33.7 ± 4.34 abc | 14 ± 0.20 f | 1927.4 ± 200.15 abc |
| PI591825 | 67 ± 0.57 a | 135 ± 0.57 ab | 190 ± 20.23 ab | 27 ± 1.53 efg | 28 ± 1.73 cd | 320 ± 52.74 ab | 35.4 ± 5.62 ab | 11.1 ± 0.19 h | 1915.1 ± 155.20 abc |
| PI548657 | 54 ± 0.57 ab | 113 ± 0.57 abcd | 63.3 ± 6.23 fghi | 20 ± 0 hi | 9.3 ± 1.45 hi | 123.3 ± 9.83 efgh | 16.8 ± 1.07 fgh | 14.6 ± 0.35 ef | 1888.4 ± 148.08 abcd |
| PI604464 | 55 ± 0.57 ab | 134 ± 0.57 ab | 60.7 ± 3.72 ghi | 21 ± 0.58 ghi | 15.3 ± 0.88 efgh | 97 ± 15.29 fgh | 16.1 ± 3.20 gh | 15.6 ± 0.19 cd | 1878.8 ± 178.09 abcd |
| Swat-20 | 55 ± 0.57 ab | 128 ± 0.57 abc | 129 ± 11.52 cd | 34.3 ± 0.67 cd | 21.3 ± 4.92 def | 224 ± 10.61 cd | 27.8 ± 2.07 bcd | 12.3 ± 0.06 g | 1856.3 ± 140.27 abcd |
| PI548533 | 54 ± 0.57 ab | 109 ± 0.57 abcde | 124.3 ± 44.48 cde | 39.3 ± 0.67 c | 19.7 ± 0.88 ef | 199 ± 36.06 cde | 29.1 ± 1.82 abcd | 16.8 ± 0.24 b | 1853.6 ± 80.76 abcd |
| PI518671 | 49 ± 1.15 abc | 106 ± 0.57 abcde | 85.3 ± 20.39 defg | 38.7 ± 1.86 c | 16.3 ± 2.03 efgh | 198.3 ± 48.40 cde | 26.9 ± 6.48 bcde | 14.3 ± 0.15 f | 1825.7 ± 33.40 abcd |
| PI522236 | 49 ± 0.57 abc | 118 ± 0.57 abcd | 109 ± 9.08 cdef | 27 ± 3.52 efg | 30.3 ± 2.61 c | 250 ± 13.52 bc | 33 ± 3.02 abc | 15.4 ± 0.40 d | 1743.5 ± 94.74 bcd |
| PI628837 | 63 ± 0.57 a | 137 ± 0.57 ab | 132.7 ± 32.16 cd | 27.7 ± 1.20 ef | 21.7 ± 2.91 de | 222 ± 5.20 cd | 32.1 ± 3.32 abc | 12.7 ± 0.21 g | 1600.7 ± 8.60 cd |
| PI548400 | 62 ± 1.15 a | 118 ± 0.57 abcd | 64.7 ± 7.89 fghi | 37.3 ± 1.45 c | 21.7 ± 1.86 de | 151.7 ± 6.67 defg | 21.2 ± 2.05 defg | 17.7 ±0.44 a | 1553.3 ± 45.82 d |
| PI548482 | 49 ± 1.15 abc | 122 ± 0.57 abc | 107 ± 20.33 cdefg | 31 ± 2.0 de | 15 ± 0.58 efghi | 212.3 ± 23.01 cde | 25.1 ± 2.49 cdefg | 14.3 ± 0.46 f | 1546.4 ± 12.96 d |
| Faisal | 55 ± 0.57 ab | 139 ± 0.57 ab | 143 ± 4.7 abc | 84.33 ± 4.7 a | 37.66 ± 4.17 ab | 197 ± 22.50 cde | 17.26 ± 3.3 defg | 14 ± 0.17 fg | 513.33 ± 85.96 e |
| NARC2 | 50 ± 0.57 ab | 132 ± 0.57 ab | 27.33 ± 1.45 h | 17.33 ± 1.45 h | 8 ± 1 i | 73.33 ± 12.4 fg | 9 ± 0.65 fg | 12.86 ± 0.08 gh | 235.80 ± 10.90 f |
| Ajmeri | 56 ± 0.57 ab | 143 ± 0.57 a | 30 ± 0.577 h | 21 ± 12.58 gh | 14.33 ± 1.45 fghi | 45.66 ± 11.85 g | 8.33 ± 3.74 g | 10.56 ± 0.14 j | 57.03 ± 9.40 g |
| Soybean Germplasm | Days to Flowering (DTF) | Days to Maturity (DTM) | Pods per Plant (No.) | Plant Height (cm) | Nodes per Plant (No.) | Seeds per Plant (No.) | Seed Weight per Plant (g) | 100 Seed Weight (g) | Seed Yield (kg ha−1) |
|---|---|---|---|---|---|---|---|---|---|
| PI548271 | 62 ± 0.57 a | 132 ± 0.57 ab | 190 ± 3.52 d | 35.33 ± 2.10 def | 29 ± 0.57 bcd | 384.11 ± 2.36 d | 33.18 ± 0.22 g | 9.32 ± 0.08 k | 2199.6 ± 16.62 a |
| PI553039 | 62 ± 0.57 a | 141 ± 0.57 a | 77.33 ± 2.02 d | 33.33 ± 1.37 defg | 16 ± 0.57 abcd | 131.045 ± 4.32 d | 24.11 ± 0.51 g | 16.81 ± 0.05 b | 2017.41 ± 23.09 ab |
| PI518664 | 55 ± 0.57 ab | 133 ± 0.57 ab | 67 ± 1.73 bcd | 25.89 ± 2.07 ghijk | 11 ± 0.58 abcd | 117.25 ± 3.03 bcd | 21.69 ± 0.56 abcd | 18.49 ± 0.01 a | 1968.89 ± 5.77 ab |
| PI612608 | 52 ± 0.57 ab | 107 ± 0.57 abcde | 37 ± 1.15 d | 43.5 ± 1.80 bc | 25 ± 0.57 abcd | 184.05 ± 4.99 d | 28.11 ± 2.58 g | 14.99 ± 0.05 c | 1932.12 ± 20.82 ab |
| PI604464 | 53 ± 0.57 ab | 132 ± 0.57 ab | 67 ± 1.15 bcd | 21.12 ± 1.54 ijk | 13 ± 2.08 abcd | 107.12 ± 1.84 bcd | 17.82 ± 0.3 bcdef | 16.63 ± 0.01 b | 1868.12 ± 17.29 abc |
| PI553042 | 50 ± 0.57 ab | 129 ± 0.57 abc | 93 ± 1.15 abc | 46.12 ± 2.08 b | 92.33 ± 3.38 a | 203.38 ± 2.52 ab | 30.79 ± 0.38 a | 14.80 ± 0.33 cd | 1867.81 ± 16.62 abc |
| Swat-20 | 54 ± 1.15 ab | 127 ± 1.15 abc | 110 ± 2.30 ab | 37.63 ± 1.15 cde | 19 ± 1.15 a | 191 ± 4.01 abc | 23.70 ± 0.49 abc | 12.34 ± 0.06 ghi | 1854.23 ± 9.23 abc |
| PI591825 | 68 ± 0.57 a | 136 ± 0.57 ab | 186.33 ± 3.52 d | 28 ± 2.08 fghij | 25 ± 0.57 abc | 301.89 ± 10.18 d | 32.41 ± 0.21 g | 11.02 ± 0.01 j | 1852.1 ± 17.21 abc |
| PI548533 | 55 ± 0.57 ab | 110 ± 0.57 abcde | 113 ± 1.73 ab | 40.23 ± 5.49 bcd | 20 ± 2.08 a | 180.86 ± 2.77 abc | 26.45 ± 0.40 ab | 14.62 ± 0.06 cde | 1789.98 ± 15.28 bcd |
| PI548657 | 55 ± 0.57 ab | 113 ± 0.57 abcd | 56 ± 1.73 bcd | 20.93 ± 1.64 jk | 11 ± 1.73 abcd | 109.05 ± 3.37 bcd | 14.85 ± 0.45 bcdef | 13.61 ± 0.05 def | 1778.98 ± 12.02 bcd |
| PI518671 | 42 ± 0.57 abc | 100 ± 0.57 qbcde | 73.66 ± 2.40 d | 24.33 ± 2.08 hijk | 13.66 ± 0.88 d | 154.18 ± 5.59 d | 25.36 ± 0.36 fg | 13.54 ± 001 efg | 1745.4 ± 7.51 bcde |
| PI522236 | 49 ± 0.57 bc | 117 ± 0.57 abcd | 102 ± 1.15 ab | 28.91 ± 1.73 fghi | 29 ± 1.53 a | 233.94 ± 2.64 a | 30.91 ± 0.34 a | 13.21 ± 0.06 fgh | 1693.45 ± 16.92 cd |
| PI628837 | 67 ± 0.57 a | 141 ± 0.57 a | 127.67 ± 1.73 d | 23.67 ± 0.57 ijk | 16.33 ± 1.53 abc | 196.30 ± 2.89 d | 26.70 ± 0.41 efg | 14.45 ± 0.05 cdef | 1560.89 ± 15.28 d |
| PI548482 | 60 ± 0.57 a | 117 ± 0.57 abcd | 101 ± 1.73 ab | 32.13 ± 2.14 efg | 14 ± 1.73 abcd | 200.42 ± 3.43 ab | 23.69 ± 0.4 abc | 11.82 ± 0.01 ij | 1535.65 ± 12.5 d |
| PI548400 | 56 ± 0.57 ab | 130 ± 0.57 ab | 57 ± 2.03 cd | 25.66 ± 0.57 ghijk | 23.33 ± 0.58 abcd | 168.97 ± 1.71 cd | 21.04 ± 0.57 cdefg | 13.64 ± 0.33 def | 1493.98 ± 10.15 ef |
| Faisal | 56 ± 0.57 ab | 140 ± 0.57 a | 152 ± 2.3 a | 91 ± 0.58 a | 32.33 ± 2.84 a | 208.91 ± 3.17 ab | 17.98 ± 0.17 abcde | 8.78 ± 0.06 k | 496 ± 18.04 e |
| NARC2 | 49 ± 1.15 abc | 130 ± 1.15 ab | 28 ± 2.3 d | 19.23 ± 1.53 k | 7 ± 1.15 cd | 76.05 ± 6.27 cd | 9.33 ± 0.76 defg | 12.27 ± 1.25 hij | 231.21 ± 13.11 f |
| Ajmeri | 54 ± 0.57 ab | 140 ± 0.57 a | 25.33 ± 1.76 d | 23.78 ± 1.15 ijk | 17 ± 1.15 ab | 40.16 ± 1.78 d | 7.78 ± 1.16 efg | 7.58 ± 0.67 a | 51.98 ± 9.24 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, R.; Arif, M.; Rahman, S.U.; Hammad, M.; Mukhtar, Z.; Rizwan, M.; Shimelis, H.; Raza, G. Field Screening of Diverse Soybean Germplasm to Characterize Their Adaptability under Long-Day Condition. Agronomy 2023, 13, 2317. https://doi.org/10.3390/agronomy13092317
Rani R, Arif M, Rahman SU, Hammad M, Mukhtar Z, Rizwan M, Shimelis H, Raza G. Field Screening of Diverse Soybean Germplasm to Characterize Their Adaptability under Long-Day Condition. Agronomy. 2023; 13(9):2317. https://doi.org/10.3390/agronomy13092317
Chicago/Turabian StyleRani, Reena, Muhammad Arif, Saleem Ur Rahman, Muhammad Hammad, Zahid Mukhtar, Muhammad Rizwan, Hussein Shimelis, and Ghulam Raza. 2023. "Field Screening of Diverse Soybean Germplasm to Characterize Their Adaptability under Long-Day Condition" Agronomy 13, no. 9: 2317. https://doi.org/10.3390/agronomy13092317
APA StyleRani, R., Arif, M., Rahman, S. U., Hammad, M., Mukhtar, Z., Rizwan, M., Shimelis, H., & Raza, G. (2023). Field Screening of Diverse Soybean Germplasm to Characterize Their Adaptability under Long-Day Condition. Agronomy, 13(9), 2317. https://doi.org/10.3390/agronomy13092317






