Effects of Ionized Water Irrigation on Organic Nitrogen Mineralization in Saline-Alkali Soil in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sample Collection
2.2. Experimental Design and Treatments
2.2.1. Ionized Fresh Water Preparation
2.2.2. Experimental Design and Treatments
2.3. Determination of Ammonium Nitrogen and Nitrate Nitrogen Content
2.4. Characteristic Indicators of the Organic Nitrogen Mineralization
2.5. Organic Nitrogen Mineralization Model
- (1)
- One-pool model [18]:Nt = N0 (1 − exp(−k0 t))
- (2)
- Special model [19]:Nt = N1 (1 − exp(−k1 t)) + C2 t
- (3)
- EATM model [20]:Nt = K [(T − T0) t] n
2.6. Data Processing
3. Results
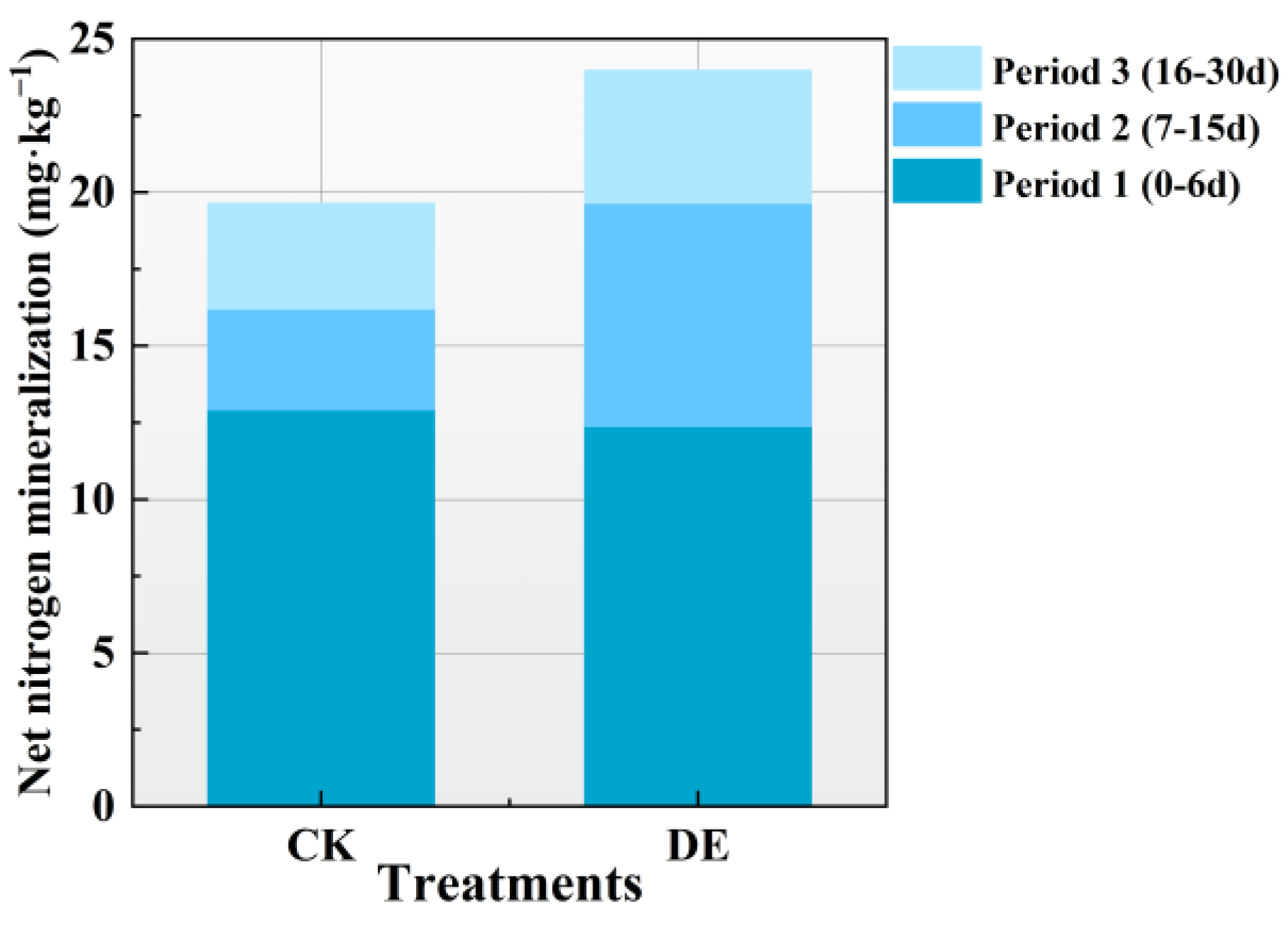
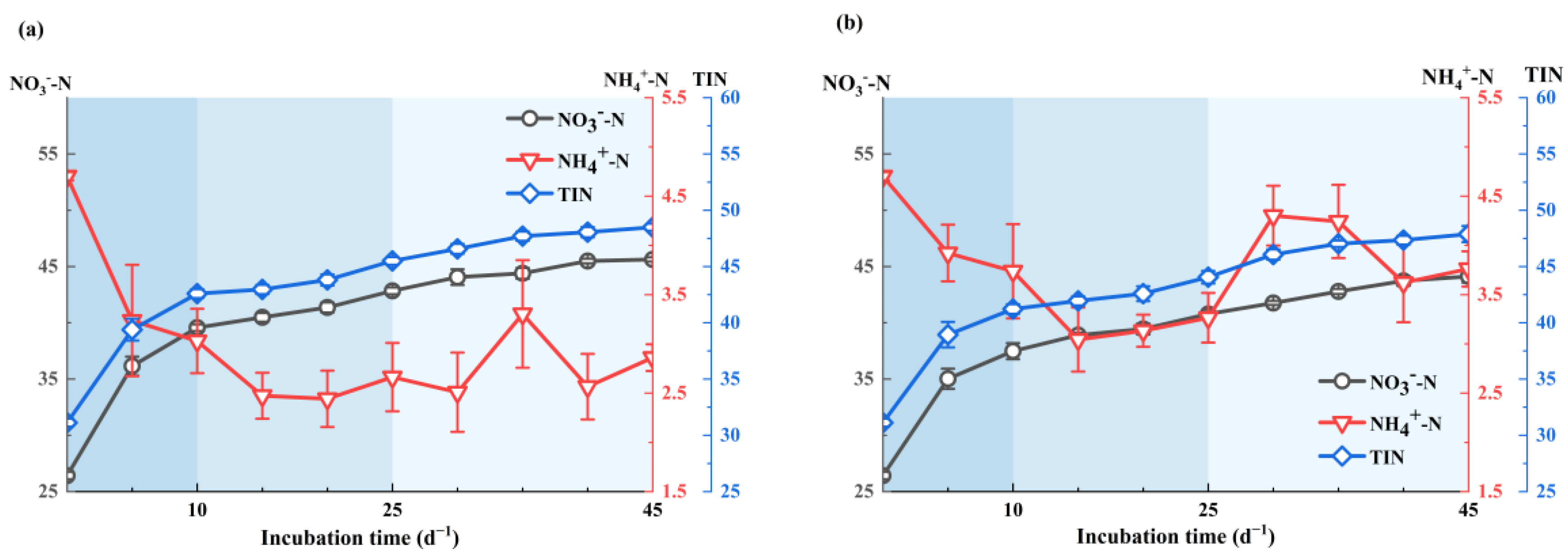
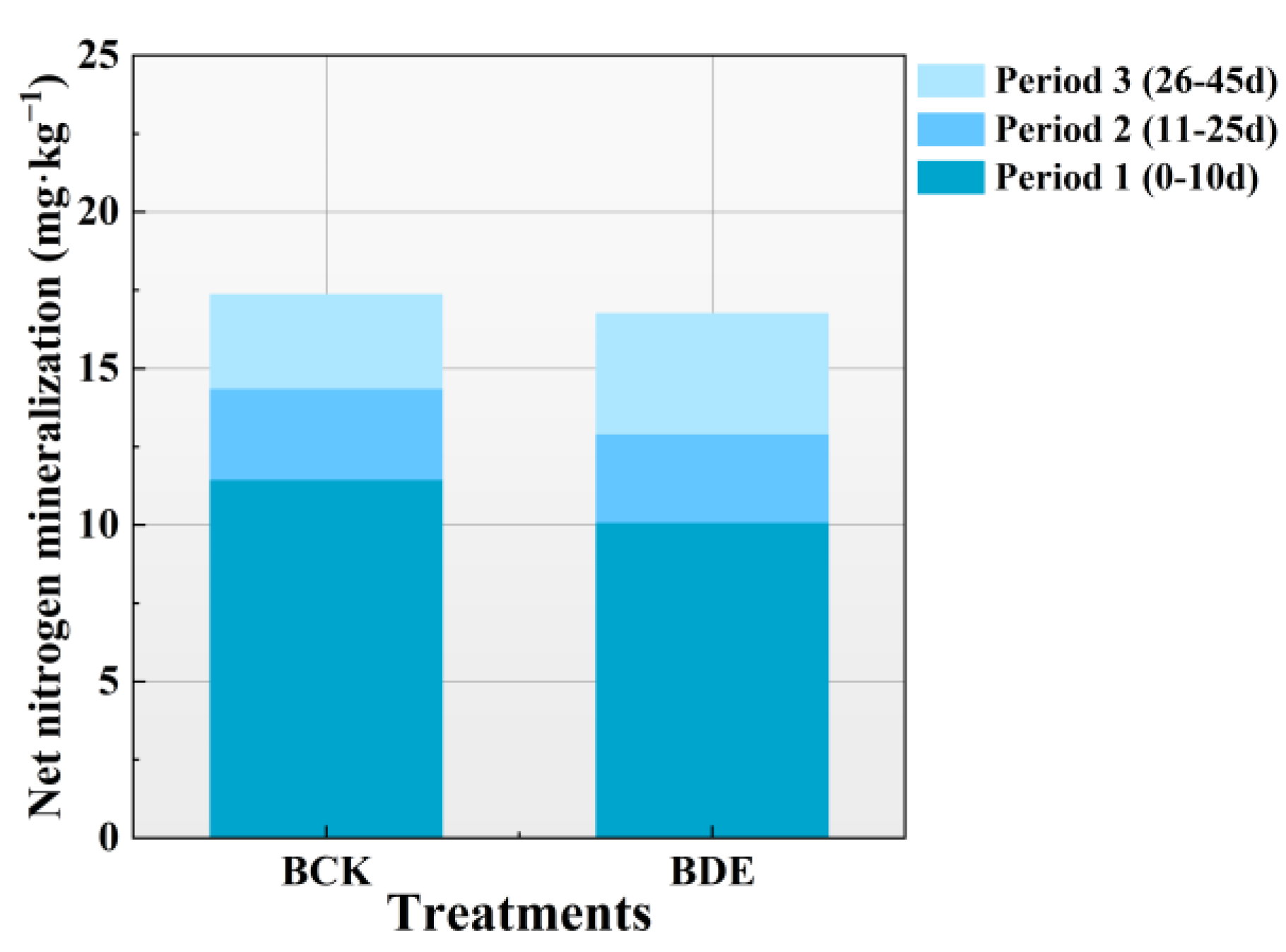
3.1. Dynamic Characteristics of Organic Nitrogen Mineralization
3.1.1. Characteristics of Soil Organic Nitrogen Mineralization under Fresh Water Irrigation
3.1.2. Characteristics of Soil Organic Nitrogen Mineralization under Brackish Water Irrigation
3.1.3. Differences in Soil Organic Nitrogen Mineralization under Fresh Water Irrigation and Brackish Water Irrigation
3.2. Comparison and Analysis of Three Organic Nitrogen Mineralization Models
4. Discussion
4.1. The Effects of Ionized Water on Organic Nitrogen Mineralization in Saline-Alkali Soil
4.2. Evaluation of Organic Nitrogen Mineralization Model
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mu, Y.; Zhao, G.Q.; Zhao, Q.Q.; Liu, H.; Wang, Q.J. Advances in the application of activated water irrigation. J. Agric. Resour. Environ. 2019, 36, 403–411. (In Chinese) [Google Scholar]
- Wang, Q.J.; Zhang, J.H.; Men, Q.; Tan, S.Q.; Zhou, L.W.; Liu, X.Y. Experiment on physical and chemical characteristics of activated brackish water by magnetization or ionization. Trans. Chin. Soc. Agric. Eng. 2016, 32, 60–66. (In Chinese) [Google Scholar]
- Wang, Q.J.; Sun, Y.; Ning, S.R.; Zhang, J.H.; Zhou, B.B.; Su, L.J.; Shan, Y.Y. Effects of activated irrigation water on soil physicochemical properties and crop growth and analysis of the probable pathway. Adv. Earth Sci. 2019, 34, 660–670. (In Chinese) [Google Scholar]
- Zhao, G.Q.; Mu, Y.; Wang, Y.h.; Wang, L. Response of winter-wheat grain yield and water-use efficiency to irrigation with activated water on Guanzhong plain in China. Irrig. Sci. 2021, 39, 263–276. [Google Scholar] [CrossRef]
- Li, Z. Effects of the Different Water Treatment on Tomato Seedling Growth and Quality. Ph.D. Thesis, Shenyang Agricultural University, Shenyang, China, 2016. [Google Scholar]
- Li, Z.L.; Tian, D.S.; Wang, B.X.; Wang, J.S.; Wang, S.; Chen, H.Y.H.; Xu, X.F.; Wang, C.H.; He, N.P.; Niu, S.L. Microbes drive global soil nitrogen mineralization and availability. Glob. Chang. Biol. 2019, 25, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Elrys, A.S.; Ali, A.; Zhang, H.; Cheng, Y.; Zhang, J.; Cai, Z.C.; Müller, C.; Chang, S.X. Patterns and drivers of global gross nitrogen mineralization in soils. Glob. Chang. Biol. 2021, 27, 5950–5962. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.Z.; Duan, W.D.; Hu, K.; Wang, J.; Yang, H.J.; Li, H.L.; Shi, H.Z. Effects of temperature and moisture on soil nitrogen mineralization in typical aroma tobacco-growing areas. Soil 2019, 51, 442–450. (In Chinese) [Google Scholar]
- Zheng, J.; Zhang, J.Z.; Zhang, L.M. Nitrogen mineralization and its influence factors in the farmland soils of Erhai Lake Basin. China Environ. Sci. 2010, 30, 35–40. (In Chinese) [Google Scholar]
- Davidson, E.A.; Hart, S.C.; Firestone, M.K. Internal cycling of nitrate in soils of a mature coniferous forest. Ecology 1992, 73, 1148–1156. [Google Scholar] [CrossRef]
- Li, W.J.; Zeng, X.M.; Peng, B.F.; Yang, J.F.; Zhao, D. Model simulation of paddy soil organic nitrogen mineralization in Dongting lake area. Chin. J. Ecol. 2019, 38, 1392–1401. (In Chinese) [Google Scholar]
- Qu, Z.; Li, M.J.; Wang, Q.J.I.; Sun, Y.; Wang, Y.; Li, J. Effects of Ionized Water Addition on Soil Nitrification Activity and Nitrifier Community Structure. Agronomy 2022, 12, 1399. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhao, G.Q.; Wang, Q.J.; Wang, L. Effect of electron water irrigation on growth and water use of winter wheat. J. Agro-Environ. Sci. 2020, 39, 953–963. (In Chinese) [Google Scholar]
- Wang, Q.J.; Xu, Z.Y.; Shan, Y.Y.; Zhang, J.H. Effect of salinity of de-electronic brackish water on characteristics of water and salt movement in soil. Trans. Chin. Soc. Agric. Eng. 2018, 34, 125–132. (In Chinese) [Google Scholar]
- Wei, K.; Zhang, J.H.; Wang, Q.J.; Guo, Y.; Mu, W.Y. Irrigation with ionized brackish water affects cotton yield and water use efficiency. Ind. Crops Prod. 2022, 175, 114244. [Google Scholar] [CrossRef]
- Wei, K. Research on Soil Water Salt Distribution and Cotton Growth Characteristics Using De-Electronic Brackish Water Drip Irrigation with Plastic. Ph.D. Thesis, Xi’an University of Technology, Xi’an, China, 2018. [Google Scholar]
- Wu, C.; Li, F.W.; Feng, P.; Liu, C.L.; Wang, X.L. Rainwater harvesting and tomato irrigation schemes optimization for facilities agriculture. Trans. Chin. Soc. Agric. Eng. 2021, 37, 153–162. (In Chinese) [Google Scholar]
- Zhu, M.; Li, B. Effects of irrigation with de-electronic water on yield, quality and water use efficiency of tomato grown in greenhouse. Water Sav. Irrig. 2020, 11, 20–24. (In Chinese) [Google Scholar]
- Jie, F.L.; Fei, L.J.; Li, S.; Zhu, H.Y.; Hao, K.; Liu, L.H. Calculation method for irrigation return flow in a water diversion irrigation district of arid areas. Trans. Chin. Soc. Agric. Eng. 2021, 37, 66–73. (In Chinese) [Google Scholar]
- Li, Z.H.; Zhan, C.S.; Hu, S.; Ning, L.K.; Wu, L.F.; Guo, H. Yield effects of irrigated acreage change under climate change in China. Trans. Chin. Soc. Agric. Eng. 2021, 37, 94–104. (In Chinese) [Google Scholar]
- Li, Y.; Xu, J.; Liu, S.; Qi, Z.; Wang, H.; Wei, Q.; Gu, Z.; Liu, X.; Hameed, F. Salinity-induced concomitant increases in soil ammonia volatilization and nitrous oxide emission. Geoderma 2020, 361, 114053. [Google Scholar] [CrossRef]
- Standford, G.; Smith, S.J. Nitrogen Mineralization Potentials of Soils. Soil Sci. Soc. Am. J. 1972, 36, 465–472. [Google Scholar] [CrossRef]
- Seyfried, M.S.; Rao, P.S.C. Kinetics of nitrogen mineralization in Costa Rican soils: Model evaluation and pretreatment effects. Plant Soil 1988, 106, 159–169. [Google Scholar] [CrossRef]
- Cai, G.X.; Zhang, S.L.; Zhu, G.L. Experimental conditions for determining the nitrogen mineralization process during anaerobic incubation of paddy soil. Soils 1979, 6, 234–240. [Google Scholar]
- Chen, W.; Jin, M.; Ferr’e, T.P.A.; Liu, Y.; Xian, Y.; Shan, T.; Ping, X. Spatial distribution of soil moisture, soil salinity, and root density beneath a cotton field under mulched drip irrigation with brackish and fresh water. Field Crop Res. 2018, 215, 207–221. [Google Scholar] [CrossRef]
- Qu, Z.; Li, M.J.; Wang, Q.J.; Sun, Y.; Su, L.J.; Li, J. Effect of micro-nano water addition on nitration of sandy loam in Xinjiang. Trans. Chin. Soc. Agric. Eng. 2020, 36, 189–196. (In Chinese) [Google Scholar]
- Li, Y.; Li, T.L.; Jiao, H.; Gao, J.W.; He, B.; Li, S. Effect of different fertilizer cultivation measures on nitrogen mineralization of reclaimed soil in coal mining subsidence areas. J. Soil Water Conserv. 2018, 3204, 227–232+239. (In Chinese) [Google Scholar]
- Kieft, T.L.; Soroker, E.; Firstone, M.K. Microbial biomass response to a rapid increase in water potential when dry soil is wetted. Soil Biol. Biochem. 1987, 19, 119–126. [Google Scholar] [CrossRef]
- Qian, C.; Cai, Z.C. Response of Soil Nitrification Characteristics in Subtropical Area to Air-Drying. Environ. Sci. 2010, 31, 1379–1386. (In Chinese) [Google Scholar]
- Lin, J.H.; Li, H.X.; Hu, F.; Zhao, H.Y. Effect of dry soil effect on soil biological composition and mineralization and nitration. Soil J. 2004, 48, 924–930. (In Chinese) [Google Scholar]
- Ju, X.T.; Li, S.X. Effect of culture conditions on soil nitrogen mineralization. J. Northwest Agric. 1997, 2, 67–70. [Google Scholar]
- Wang, Q.J.; Li, Z.Y.; Zhang, J.H.; Xie, J.B.; Wei, K.; Sun, Y.A. Effect of magnetization intensity on characteristics of soil water and salt transport in magnetization-de-electronic activation water Trans. Trans. Chin. Soc. Agric. Mach. 2020, 51, 278–284. (In Chinese) [Google Scholar]
- Wang, X.P.; Wang, H.B.; Si, Z.Y.; Gao, Y.; Duan, A.W. Modelling responses of cotton growth and yield to pre-planting soil moisture with the CROPGRO-Cotton model for a mulched drip irrigation system in the Tarim Basin. Agric. Water Manag. 2020, 241, 106378. [Google Scholar] [CrossRef]
- Zhou, M.; Butterbach-Bahl, K.; Vereecken, H.; Brüggemann, N. A meta-analysis of soil salinization effects on nitrogen pools, cycles and fluxes in coastal ecosystems. Glob. Chang. Biol. 2017, 23, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.C.; Li, Z.Z.; Liu, H.; Gao, M.; Zhang, Y.Y. Microbial biomass and activity in salt affected soils under arid conditions. Appl. Soil. Ecol. 2007, 35, 319–328. [Google Scholar] [CrossRef]

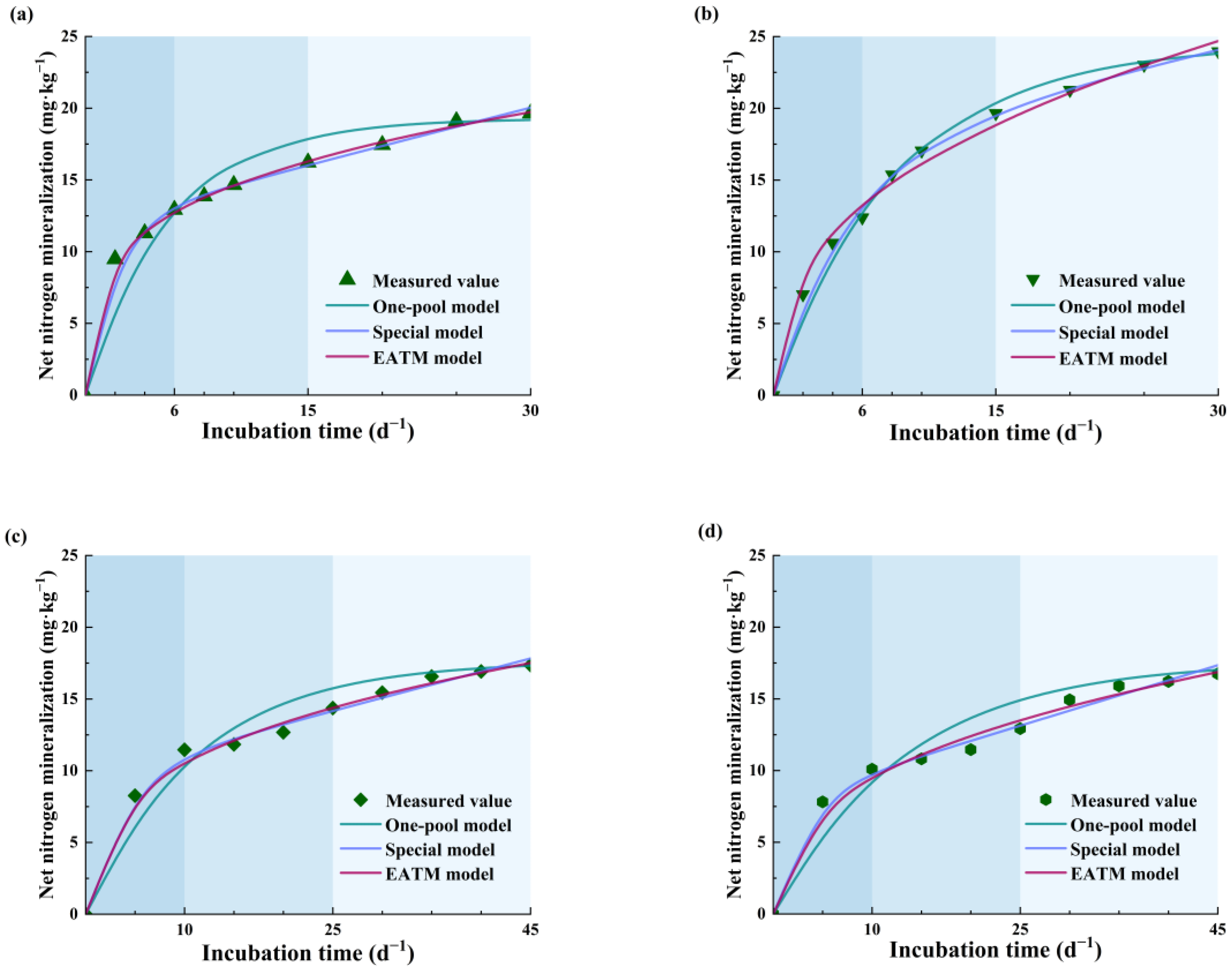
| Treatments | One-Pool | Special | EATM | |||
|---|---|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | R2 | RMSE | |
| CK | 0.992 | 1.514 | 0.999 | 0.416 | 0.999 | 0.154 |
| DE | 0.998 | 0.799 | 0.999 | 0.441 | 0.999 | 0.796 |
| BCK | 0.996 | 1.161 | 0.999 | 0.382 | 0.999 | 0.399 |
| BDE | 0.996 | 1.330 | 0.999 | 0.434 | 0.999 | 0.491 |
| Treatments | One-Pool | Special | EATM | ||||
|---|---|---|---|---|---|---|---|
| N0 | k0 | N1 | k1 | C2 | K | n | |
| (mg·kg−1) | (d−1) | (mg·kg−1) | (d−1) | (mg·kg−1·d−1) | |||
| CK | 19.266 | 0.183 | 12.002 | 0.531 | 0.268 | 3.768 | 0.271 |
| DE | 24.407 | 0.124 | 16.856 | 0.198 | 0.242 | 2.350 | 0.385 |
| BCK | 17.618 | 0.091 | 9.592 | 0.307 | 0.183 | 2.001 | 0.333 |
| BDE | 17.599 | 0.076 | 7.852 | 0.408 | 0.211 | 1.447 | 0.377 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Qu, Z.; Li, M.; Wang, Q. Effects of Ionized Water Irrigation on Organic Nitrogen Mineralization in Saline-Alkali Soil in China. Agronomy 2023, 13, 2285. https://doi.org/10.3390/agronomy13092285
Lu J, Qu Z, Li M, Wang Q. Effects of Ionized Water Irrigation on Organic Nitrogen Mineralization in Saline-Alkali Soil in China. Agronomy. 2023; 13(9):2285. https://doi.org/10.3390/agronomy13092285
Chicago/Turabian StyleLu, Jiangyue, Zhi Qu, Mingjiang Li, and Quanjiu Wang. 2023. "Effects of Ionized Water Irrigation on Organic Nitrogen Mineralization in Saline-Alkali Soil in China" Agronomy 13, no. 9: 2285. https://doi.org/10.3390/agronomy13092285
APA StyleLu, J., Qu, Z., Li, M., & Wang, Q. (2023). Effects of Ionized Water Irrigation on Organic Nitrogen Mineralization in Saline-Alkali Soil in China. Agronomy, 13(9), 2285. https://doi.org/10.3390/agronomy13092285






