Noticeable Shifts in Soil Physicochemical and Biological Properties after Contrasting Tillage Management in Crop Rotations of Bean, Maize, and Amaranth in Ecuadorian Highland Soils
Abstract
:1. Introduction
2. Materials and Methods
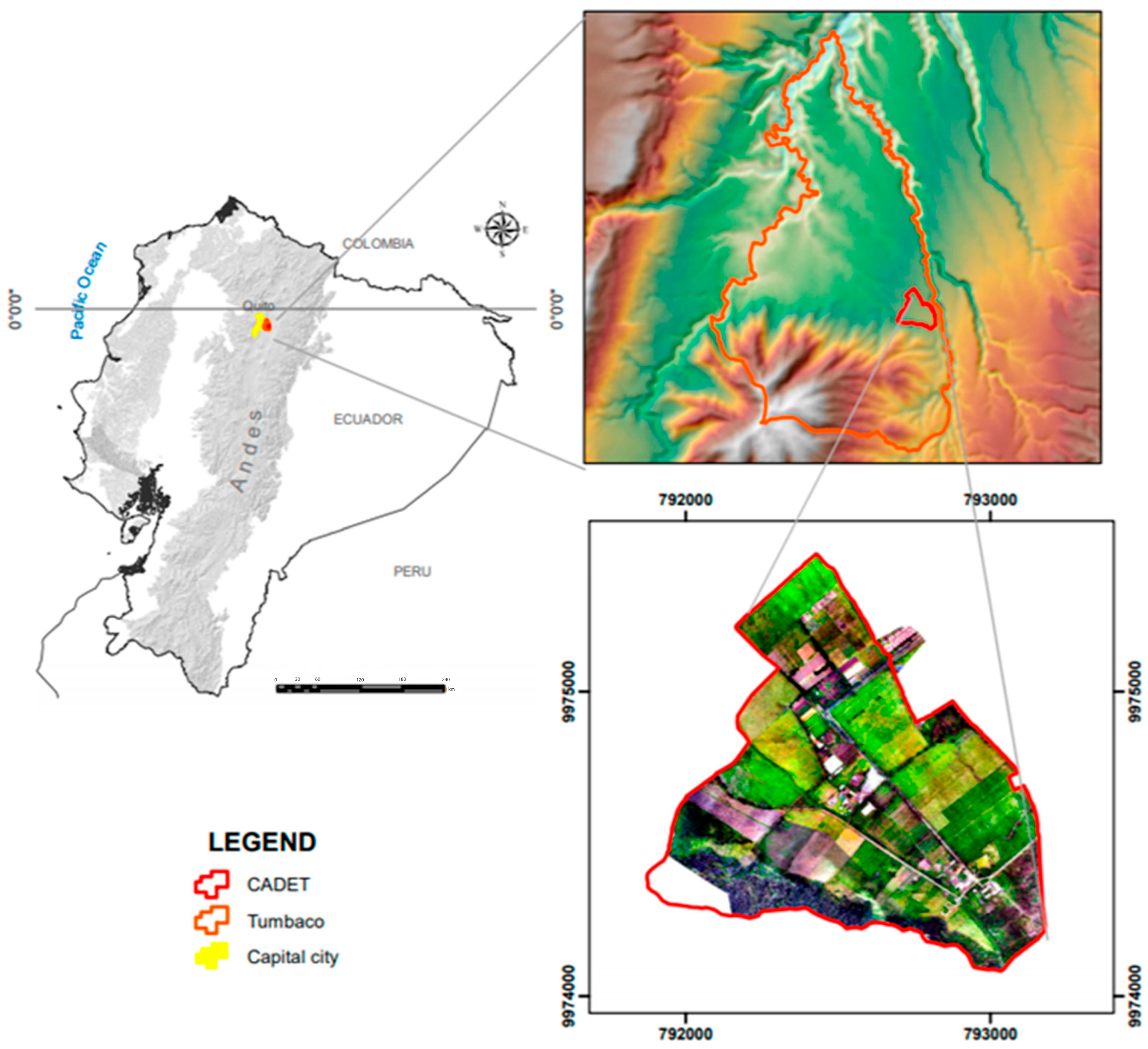
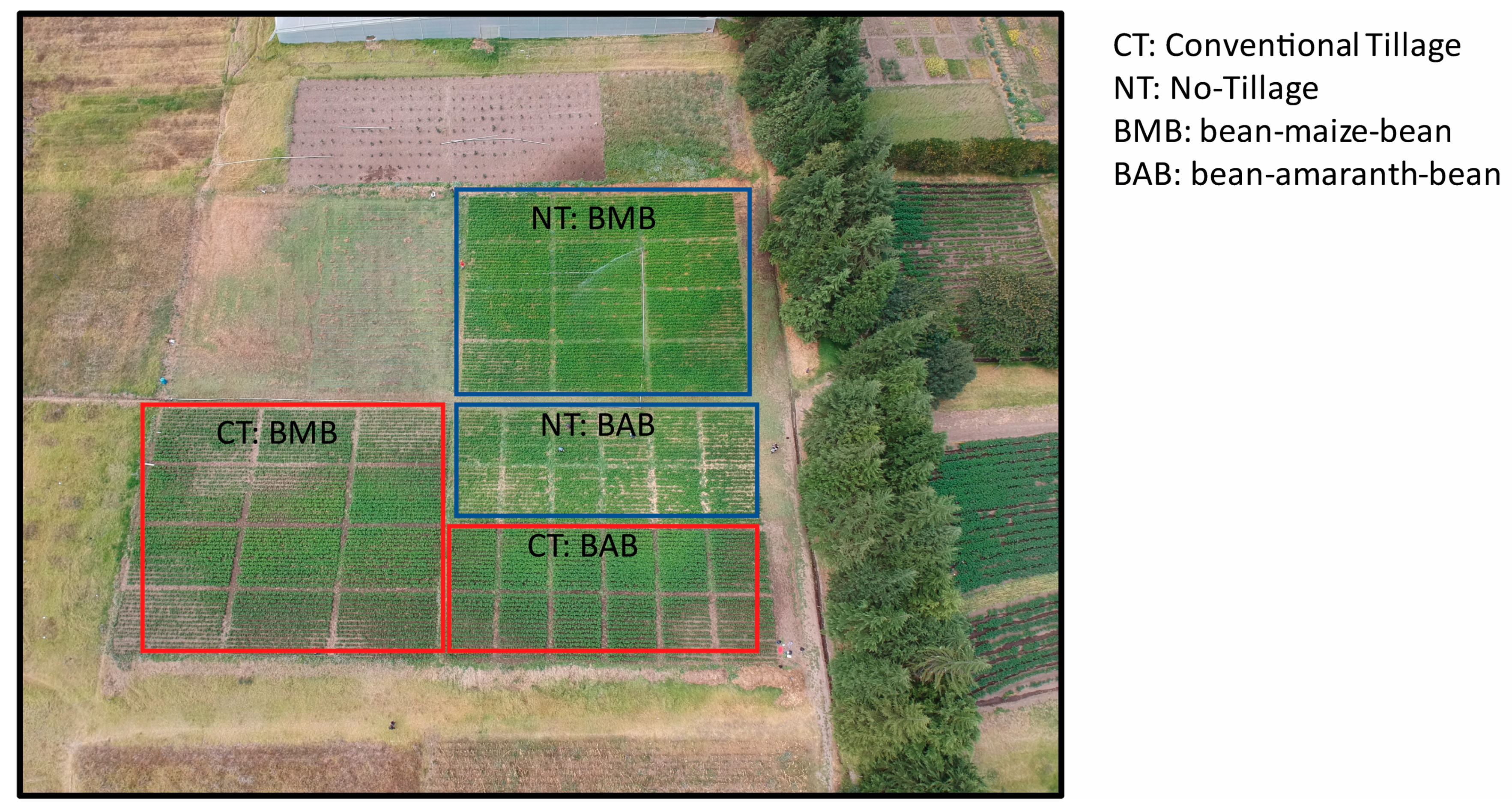
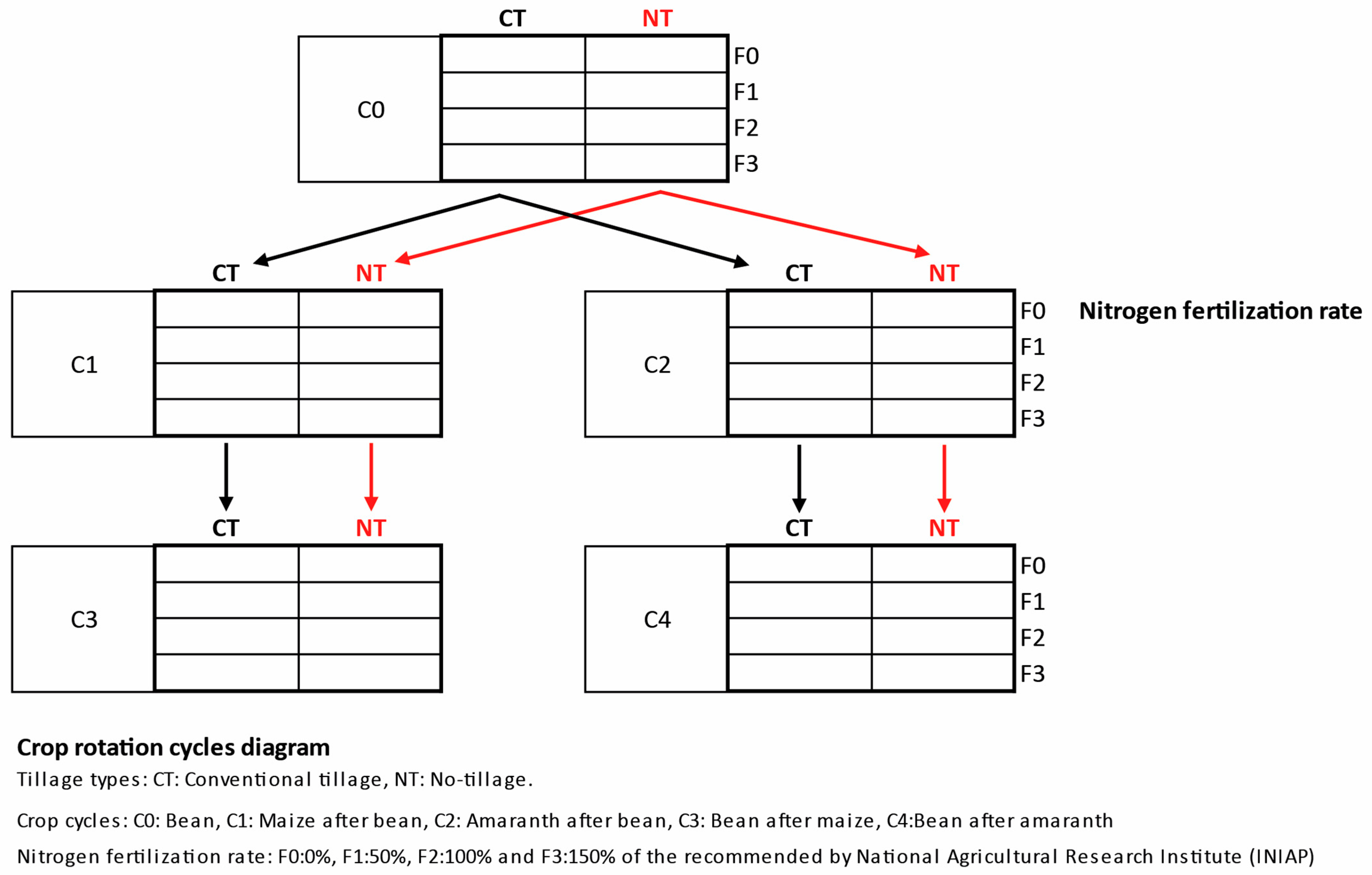
2.1. Site Description, Experimental Design, Soil and Plant Sampling
2.2. Soil Physical and Chemical Determinations
2.3. Soil Biological Determinations
2.4. Arbuscular Mycorrhizal Fungal Spores and Root Colonization
2.5. Total Glomalin-Related Soil Protein
2.6. Statistical Analysis
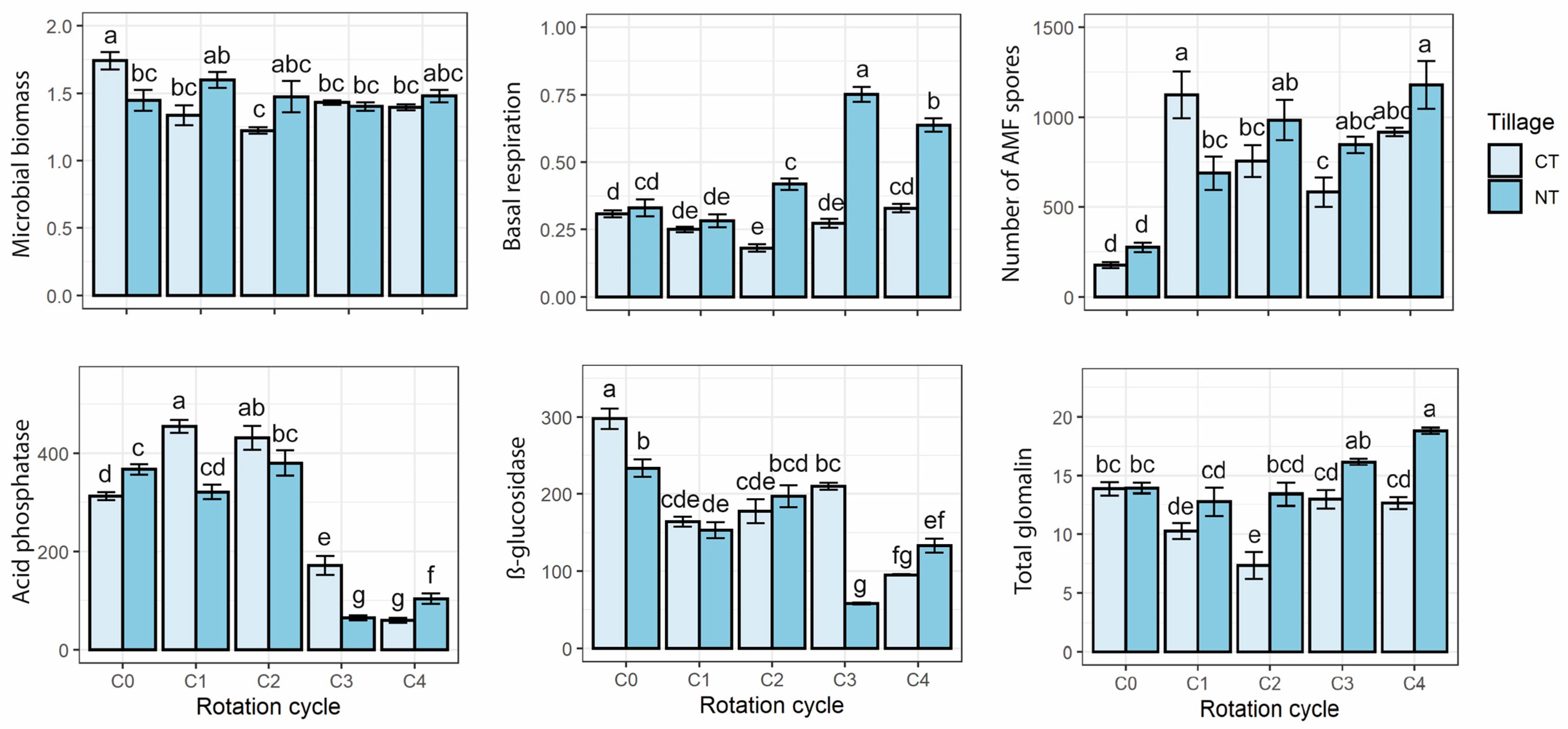
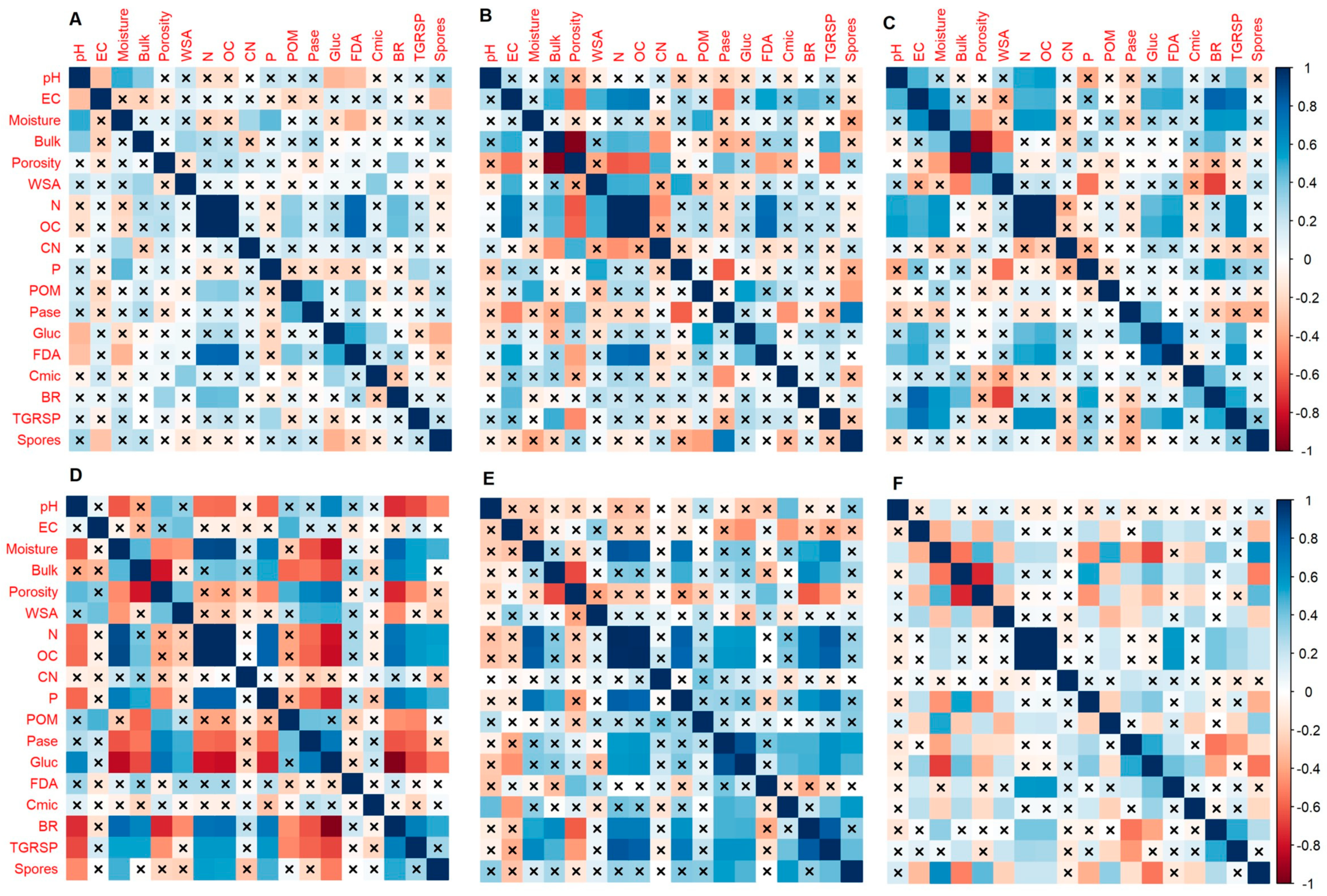
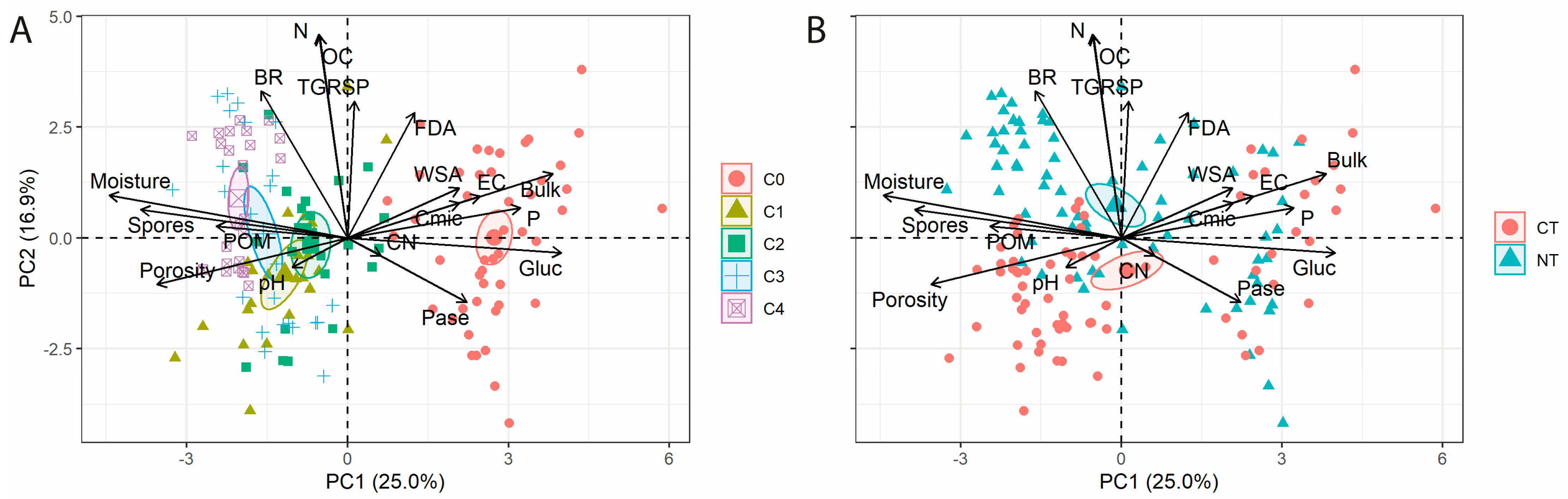
3. Results
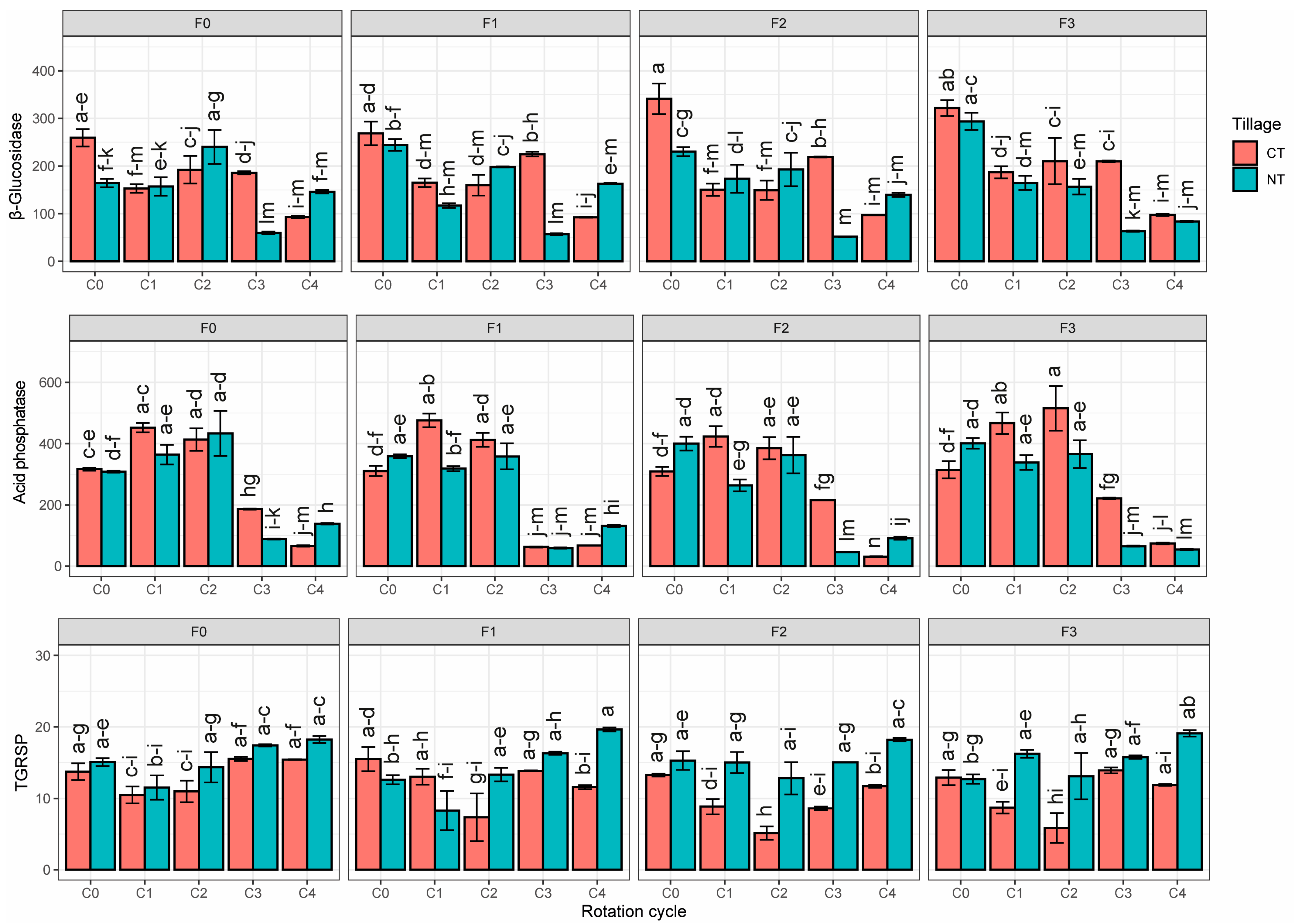
3.1. Biological Soil Traits
3.2. AMF Spores Density
3.3. Physicochemical Soil Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Arshad, M.A.; Martin, S. Identifying critical limits for soil quality indicators in agro-ecosystems. Agric. Ecosyst. Environ. 2002, 88, 153–160. [Google Scholar] [CrossRef]
- Panagos, P.; Ballabio, C.; Poesen, J.; Lugato, E.; Scarpa, S.; Montanarella, L.; Borrelli, P. A soil erosion indicator for supporting agricultural, environmental and climate policies in the European Union. Remote Sens. 2020, 12, 1365. [Google Scholar] [CrossRef]
- Allam, M.; Radicetti, E.; Quintarelli, V.; Petroselli, V.; Marinari, S.; Mancinelli, R. Influence of Organic and Mineral Fertilizers on Soil Organic Carbon and Crop Productivity under Different Tillage Systems: A Meta-Analysis. Agriculture 2022, 12, 464. [Google Scholar] [CrossRef]
- Rao, M.A.; Scelza, R.; Gianfreda, L. Soil enzymes. In Enzymes in Agricultural Sciences; OMICS Group eBooks: Foster City, FL, USA, 2014; pp. 10–43. [Google Scholar]
- D’Hose, T.; Cougnon, M.; De Vliegher, A.; Vandecasteele, B.; Viaene, N.; Cornelis, W.; Van Bockstaele, E.; Reheul, D. The positive relationship between soil quality and crop production: A case study on the effect of farm compost application. Appl. Soil Ecol. 2014, 75, 189–198. [Google Scholar] [CrossRef]
- Masto, R.E.; Chhonkar, P.K.; Singh, D.; Patra, A.K. Soil quality response to long-term nutrient and crop management on a semi-arid Inceptisol. Agric. Ecosyst. Environ. 2007, 118, 130–142. [Google Scholar] [CrossRef]
- Sharma, K.; Mandal, U.K.; Srinivas, K.; Vittal, K.; Mandal, B.; Grace, J.K.; Ramesh, V. Long-term soil management effects on crop yields and soil quality in a dryland Alfisol. Soil Tillage Res. 2005, 83, 246–259. [Google Scholar] [CrossRef]
- Alves de Castro Lopes, A.; Gomes de Sousa, D.M.; Chaer, G.M.; Bueno dos Reis Junior, F.; Goedert, W.J.; de Carvalho Mendes, I. Interpretation of microbial soil indicators as a function of crop yield and organic carbon. Soil Sci. Soc. Am. J. 2013, 77, 461–472. [Google Scholar] [CrossRef]
- Qin, L.; Freeman, C.; Jia, X.; Zhang, Z.; Liu, B.; Zhang, S.; Jiang, M. Microbial enzyme activity and stoichiometry signal the effects of agricultural intervention on nutrient cycling in peatlands. Ecol. Indic. 2021, 122, 107242. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Al-Kaisi, M.; Yang, J.; Chen, Y.; Sui, P. Effects of seven diversified crop rotations on selected soil health indicators and wheat productivity. Agronomy 2020, 10, 235. [Google Scholar] [CrossRef]
- Yuan, J.; Yan, L.; Li, G.; Sadiq, M.; Rahim, N.; Wu, J.; Ma, W.; Xu, G.; Du, M. Effects of conservation tillage strategies on soil physicochemical indicators and N2O emission under spring wheat monocropping system conditions. Sci. Rep. 2022, 12, 7066. [Google Scholar] [CrossRef]
- Hassan, W.; Li, Y.; Saba, T.; Jabbi, F.; Wang, B.; Cai, A.; Wu, J. Improved and sustainable agroecosystem, food security and environmental resilience through zero tillage with emphasis on soils of temperate and subtropical climate regions: A review. Int. Soil Water Conserv. Res. 2022, 10, 530–545. [Google Scholar] [CrossRef]
- Zhang, H.; Hobbie, E.A.; Feng, P.; Hu, K. Can conservation agriculture mitigate climate change and reduce environmental impacts for intensive cropping systems in North China Plain? Sci. Total Environ. 2022, 806, 151194. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Desjardins, R.L.; Wang, S.; Worth, D.E.; Qian, B.; Shang, J. Climate impact from agricultural management practices in the Canadian Prairies: Carbon equivalence due to albedo change. J. Environ. Manag. 2022, 302, 113938. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, C.I.; Altieri, M.A. Plant biodiversity enhances bees and other insect pollinators in agroecosystems. A review. Agron. Sustain. Dev. 2013, 33, 257–274. [Google Scholar] [CrossRef]
- Nunes, M.R.; Karlen, D.L.; Veum, K.S.; Moorman, T.B.; Cambardella, C.A. Biological soil health indicators respond to tillage intensity: A US meta-analysis. Geoderma 2020, 369, 114335. [Google Scholar] [CrossRef]
- Aponte, H.; Medina, J.; Butler, B.; Meier, S.; Cornejo, P.; Kuzyakov, Y. Soil quality indices for metal (loid) contamination: An enzymatic perspective. Land Degrad. Dev. 2020, 31, 2700–2719. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Ncube, B.; Mulidzi, R.; Lewu, F.B. Potential use of soil enzymes as soil quality indicators in agriculture. In Frontiers in Soil and Environmental Microbiology, 1st ed.; Nayak, S.K., Mishra, B.B., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 57–64. [Google Scholar] [CrossRef]
- Gajda, A.; Przewloka, B.; Gawryjolek, K. Changes in soil quality associated with tillage system applied. Int. Agrophys. 2013, 27, 133–141. [Google Scholar] [CrossRef]
- Patle, P.; Navnage, N.; Barange, P.K. Fluorescein diacetate (FDA): Measure of total microbial activity and as indicator of soil quality. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2103–2107. [Google Scholar] [CrossRef]
- Selvaraj, A.; Thangavel, K. Arbuscular mycorrhizal fungi: Potential plant protective agent against herbivorous insect and its importance in sustainable agriculture. In Symbiotic Soil Microorganisms; Springer: Cham, Switzerland, 2021; pp. 319–337. [Google Scholar] [CrossRef]
- Cornejo, P.; Meier, S.; García, S.; Ferrol, N.; Durán, P.; Borie, F.; Seguel, A. Contribution of inoculation with arbuscular my-corrhizal fungi to the bioremediation of a copper contaminated soil using Oenothera picensis. J. Soil Sci. Plant Nutr. 2017, 17, 14–21. [Google Scholar] [CrossRef]
- Curaqueo, G.; Barea, J.M.; Acevedo, E.; Rubio, R.; Cornejo, P.; Borie, F. Effects of different tillage system on arbuscular my-corrhizal fungal propagules and physical properties in a Mediterranean agroecosystem in central Chile. Soil Tillage Res. 2011, 113, 11–18. [Google Scholar] [CrossRef]
- Säle, V.; Aguilera, P.; Laczko, E.; Mäder, P.; Berner, A.; Zihlmann, U.; van der Heijden, M.G.; Oehl, F. Impact of conservation tillage and organic farming on the diversity of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2015, 84, 38–52. [Google Scholar] [CrossRef]
- Nunes, M.R.; Karlen, D.L.; Moorman, T.B. Tillage intensity effects on soil structure indicators—A US meta-analysis. Sustainability 2020, 12, 2071. [Google Scholar] [CrossRef]
- Rovira, A. Biology of the soil-root interface. In The Soil–Root Interface; Elsevier: Amsterdam, The Netherlands, 1979; pp. 145–160. [Google Scholar] [CrossRef]
- Smith, J.L.; Doran, J.W. Measurement and use of pH and electrical conductivity for soil quality analysis. In Methods for Assessing Soil Quality; Soil Science Society of America: Madison, WI, USA, 1997; Volume 49, pp. 169–185. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Aziz, I.; Mahmood, T.; Islam, K.R. Effect of long term no-till and conventional tillage practices on soil quality. Soil Tillage Res. 2013, 131, 28–35. [Google Scholar] [CrossRef]
- Olsen, S.; Sommers, L. Phosphorous; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Kemper, W.; Rosenau, R. Aggregate stability and size distribution. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; Klute, A., Ed.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1986; Volume 5, pp. 425–442. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Weaver, R.; Angle, S.; Bottomley, P.; Bezdicek, D.; Smith, S.; Tabatabai, A.; Wollum, A. Methods of Soil Analysis: Part 2—Microbiological and Biochemical Properties; Soil Science Society of America: Madison, WI, USA, 1994; pp. 835–863. [Google Scholar]
- Vidal, I.; Etchevers, J.; Fischer, A. Biomasa microbiana en un suelo sometido a diferentes manejos de labranza y rotación. Agric. Técnica 1997, 57, 272–281. [Google Scholar]
- Jenkinson, D.S.; Powlson, D.S. Biochemistry. The effects of biocidal treatments on metabolism in soil—V: A method for measuring soil biomass. Soil Biol. 1976, 8, 209–213. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Ladd, J. Microbial biomass in soil: Measurement and turnover. In Soil Biochemistry; CRC Press: Boca Raton, FL, USA, 2021; pp. 415–472. [Google Scholar]
- Tabatabai, M. Soil enzymes. In Methods of Soil Analysis: Part 2 Microbiological Biochemical Properties; SSSA Book Series; Soil Science Society of America: Madison, WI, USA, 1994; Volume 5, pp. 775–833. [Google Scholar]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Schnrer, J.; Rosswall, T. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl. Environ. Microbiol. 1982, 43, 1256–1261. [Google Scholar] [CrossRef]
- Gerdemann, J.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Sieverding, E.; Friedrichsen, J.; Suden, W. Vesicular Arbuscular Mycorrhiza in Tropical Agrosystem; Deutsche Gesellsschaft fur techniische Zusammenarbeit (GTZ) GMBH: Eschborn, Germany, 1991. [Google Scholar]
- Phillips, J.M.; Hayman, D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161, IN16–IN18. [Google Scholar] [CrossRef]
- Koske, R.; Gemma, J. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 1989, 92, 486. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Moreno-Espíndola, I.P.; Ferrara-Guerrero, M.J.; León-González, F.; Rivera-Becerril, F.; Mayorga-Reyes, L.; Pérez, N.O. Enzymatic activity and culturable bacteria diversity in rhizosphere of amaranth, as indicators of crop phenological changes. Bot. Sci. 2018, 96, 640–649. [Google Scholar] [CrossRef]
- Bateman, A.; van der Horst, D.; Boardman, D.; Kansal, A.; Carliell-Marquet, C. Closing the phosphorus loop in England: The spatio-temporal balance of phosphorus capture from manure versus crop demand for fertiliser. Resour. Conserv. Recycl. 2011, 55, 1146–1153. [Google Scholar] [CrossRef]
- Zuber, S.M.; Villamil, M.B. Meta-analysis approach to assess effect of tillage on microbial biomass and enzyme activities. Soil Biol. Biochem. 2016, 97, 176–187. [Google Scholar] [CrossRef]
- Roldán, A.; Salinas-García, J.; Alguacil, M.; Díaz, E.; Caravaca, F. Soil enzyme activities suggest advantages of conservation tillage practices in sorghum cultivation under subtropical conditions. Geoderma 2005, 129, 178–185. [Google Scholar] [CrossRef]
- Alvear, M.; Pino, M.; Castillo, C.; Trasar-Cepeda, C.; Gil-Sotres, F. Efecto de la cero labranza sobre algunas actividades biológicas en un Alfisol del sur de Chile. R. C. Suelo Nutr. Veg. 2006, 6, 38–53. [Google Scholar] [CrossRef]
- Pittarello, M.; Dal Ferro, N.; Chiarini, F.; Morari, F.; Carletti, P. Influence of Tillage and Crop Rotations in Organic and Conventional Farming Systems on Soil Organic Matter, Bulk Density and Enzymatic Activities in a Short-Term Field Experiment. Agronomy 2021, 11, 724. [Google Scholar] [CrossRef]
- Pandey, D.; Agrawal, M.; Bohra, J.S. Effects of conventional tillage and no tillage permutations on extracellular soil enzyme activities and microbial biomass under rice cultivation. Soil Tillage Res. 2014, 136, 51–60. [Google Scholar] [CrossRef]
- De Almeida, R.F.; Naves, E.R.; da Mota, R.P. Soil quality: Enzymatic activity of soil β-glucosidase. Glob. J. Agric. Res. Rev. 2015, 3, 146–150. [Google Scholar]
- Espinoza, Y.; Lozano, Z.; Velásquez, L. Tillage system and crop rotation effects on soil organic matter fractions. Interciencia 2007, 32, 554–559. [Google Scholar]
- Bertin, C.; Yang, X.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- De Graaff, M.A.; Classen, A.T.; Castro, H.F.; Schadt, C.W. Labile soil carbon inputs mediate the soil microbial community composition and plant residue decomposition rates. New Phytol. 2010, 188, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Villanueva, D.A.; Bello-López, J.M.; Navarro-Noya, Y.E.; Luna-Guido, M.; Verhulst, N.; Govaerts, B.; Dendooven, L. Bacterial community structure in maize residue amended soil with contrasting management practices. Appl. Soil Ecol. 2015, 90, 49–59. [Google Scholar] [CrossRef]
- Bongiorno, G.; Bünemann, E.K.; Brussaard, L.; Mäder, P.; Oguejiofor, C.U.; de Goede, R.G. Soil management intensity shifts microbial catabolic profiles across a range of European long-term field experiments. Appl. Soil Ecol. 2020, 154, 103596. [Google Scholar] [CrossRef]
- Avila-Salem, M.E.; Montesdeoca, F.; Orellana, M.; Pacheco, K.; Alvarado, S.; Becerra, N.; Marín, C.; Borie, F.; Aguilera, P.; Cornejo, P. Soil Biological Properties and Arbuscular Mycorrhizal Fungal Communities of Representative Crops Established in the Andean Region from Ecuadorian Highlands. J. Soil Sci. Plant Nutr. 2020, 20, 2156–2163. [Google Scholar] [CrossRef]
- Dilly, O.; Winter, K.; Lang, A.; Munch, J.C. Energetic eco-physiology of the soil microbiota in two landscapes of southern and northern Germany. J. Plant. Nutr. Soil Sci. 2001, 164, 407–413. [Google Scholar] [CrossRef]
- Ghimire, R.; Norton, J.B.; Stahl, P.D.; Norton, U. Soil microbial substrate properties and microbial community responses under irrigated organic and reduced-tillage crop and forage production systems. PLoS ONE 2014, 9, e103901. [Google Scholar] [CrossRef]
- Schneider, F.; Don, A.; Hennings, I.; Schmittmann, O.; Seidel, S.J. The effect of deep tillage on crop yield–What do we really know? Soil Tillage Res. 2017, 174, 193–204. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Chandra, K. Soil moisture fluctuation influences AMF root colonization and spore population in tree species planted in degraded entisol soil. Int. J. Biosci. 2018, 13, 229–243. [Google Scholar] [CrossRef]
| Soil Depth (cm) | Texture | Color | pH | * EC (dS/cm) | ** OM (%) |
|---|---|---|---|---|---|
| 0–20 | Sandy loam Clay: 12–15% Sand: 53–58% Silt: 28–33% | 10YR2/2 Dark grayish brown | 6.94 | 0.26 | 3.29 |
| Factor | Pase | Gluc | FDA | Cmic | BR | TGRSP | AMF Spores |
|---|---|---|---|---|---|---|---|
| Cycle | 1061.2 *** | 94.5 *** | 2.6 * | 5.0 *** | 44.2 *** | 20.9 *** | 53.7 *** |
| Tillage | 17.1 *** | 40.8 *** | 0.2NS | 0.0NS | 165.8 *** | 56.7 *** | 3.8 ** |
| Fertilization | 11.7 *** | 3.8 * | 1.9NS | 1.8NS | 1.9NS | 3.0 * | 1.3NS |
| Cycle × Tillage | 115.5 *** | 26.9 *** | 1.4NS | 7.9 *** | 37.0 *** | 9.7 *** | 7.5 *** |
| Cycle × Fertilization | 17.4 *** | 3.9 *** | 4.3 *** | 2.2 * | 1.5NS | 1.6NS | 1.4NS |
| Tillage × Fertilization | 11.3 *** | 0.8NS | 0.6NS | 3.5 * | 1.5NS | 5.5 ** | 0.1NS |
| Cycle × Tillage × Fertilization | 16.7 *** | 2.3 * | 1.2NS | 1.0NS | 1.2NS | 2.2 * | 1.5NS |
| Factor | pH | EC | Moisture | Bulk | Porosity | WSA | N | OC | CN | P | POM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycle | 2.87 * | 103.34 *** | 465.84 *** | 56.70 *** | 27.93 *** | 14.20 *** | 1.7 | 1.98 | 2.43 | 76.10 *** | 78.77 *** |
| Tillage | 0.0049 | 6.36 ** | 22.59 *** | 33.67 *** | 17.23 *** | 0.60 | 12.82 *** | 11.57 ** | 0.54 | 27.19 *** | 1.02 |
| Fertilization | 8.57 *** | 3.10 * | 6.23 ** | 0.74 | 0.81 | 1.50 | 0.56 | 0.50 | 1.80 | 2.43 | 1.90 |
| Cycle × Tillage | 11.69 *** | 15.09 ** | 1.97 | 3.98 ** | 1.07 | 3.68 ** | 8.30 *** | 8.48 *** | 1.18 | 14.99 *** | 3.25 * |
| Cycle × Fertilization | 2.77 ** | 1.40 | 6.05 *** | 0.36 | 0.59 | 0.89 | 1.09 | 0.98 | 0.55 | 2.76 * | 1.93 |
| Tillage × Fertilization | 5.25 ** | 0.82 | 4.91 ** | 0.18 | 0.23 | 1.01 | 0.65 | 0.64 | 0.44 | 6.86 *** | 2.11 |
| Cycle × Tillage × Fertilization | 1.95 | 1.21 | 2.90 ** | 2.54 ** | 0.92 | 2.24 * | 0.96 | 0.75 | 1.75 | 3.38 ** | 1.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila-Salem, M.E.; Aponte, H.; Montesdeoca, F.; Urgiles Gómez, N.; Cruz, D.; Orellana, M.; Pacheco, K.; Alvarado Ochoa, S.; Espinosa, J.; Borie, F.; et al. Noticeable Shifts in Soil Physicochemical and Biological Properties after Contrasting Tillage Management in Crop Rotations of Bean, Maize, and Amaranth in Ecuadorian Highland Soils. Agronomy 2023, 13, 2260. https://doi.org/10.3390/agronomy13092260
Avila-Salem ME, Aponte H, Montesdeoca F, Urgiles Gómez N, Cruz D, Orellana M, Pacheco K, Alvarado Ochoa S, Espinosa J, Borie F, et al. Noticeable Shifts in Soil Physicochemical and Biological Properties after Contrasting Tillage Management in Crop Rotations of Bean, Maize, and Amaranth in Ecuadorian Highland Soils. Agronomy. 2023; 13(9):2260. https://doi.org/10.3390/agronomy13092260
Chicago/Turabian StyleAvila-Salem, María Eugenia, Humberto Aponte, Fabián Montesdeoca, Narcisa Urgiles Gómez, Dayana Cruz, Marco Orellana, Katherine Pacheco, Soraya Alvarado Ochoa, José Espinosa, Fernando Borie, and et al. 2023. "Noticeable Shifts in Soil Physicochemical and Biological Properties after Contrasting Tillage Management in Crop Rotations of Bean, Maize, and Amaranth in Ecuadorian Highland Soils" Agronomy 13, no. 9: 2260. https://doi.org/10.3390/agronomy13092260
APA StyleAvila-Salem, M. E., Aponte, H., Montesdeoca, F., Urgiles Gómez, N., Cruz, D., Orellana, M., Pacheco, K., Alvarado Ochoa, S., Espinosa, J., Borie, F., & Cornejo, P. (2023). Noticeable Shifts in Soil Physicochemical and Biological Properties after Contrasting Tillage Management in Crop Rotations of Bean, Maize, and Amaranth in Ecuadorian Highland Soils. Agronomy, 13(9), 2260. https://doi.org/10.3390/agronomy13092260








