Study of the Effect of Mowing and Drying on the Lipid Composition of Grass Leaves in Permafrost Ecosystems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Plant Species Composition
2.2. Plant Material
2.3. Determination of Lipids Content
2.4. Determination of FAs Content
2.5. Separation of Sterols and Their Esters
3. Results
3.1. Composition and Content of Lipids in Leaves of Plant Raw Material in Mowing and Drying
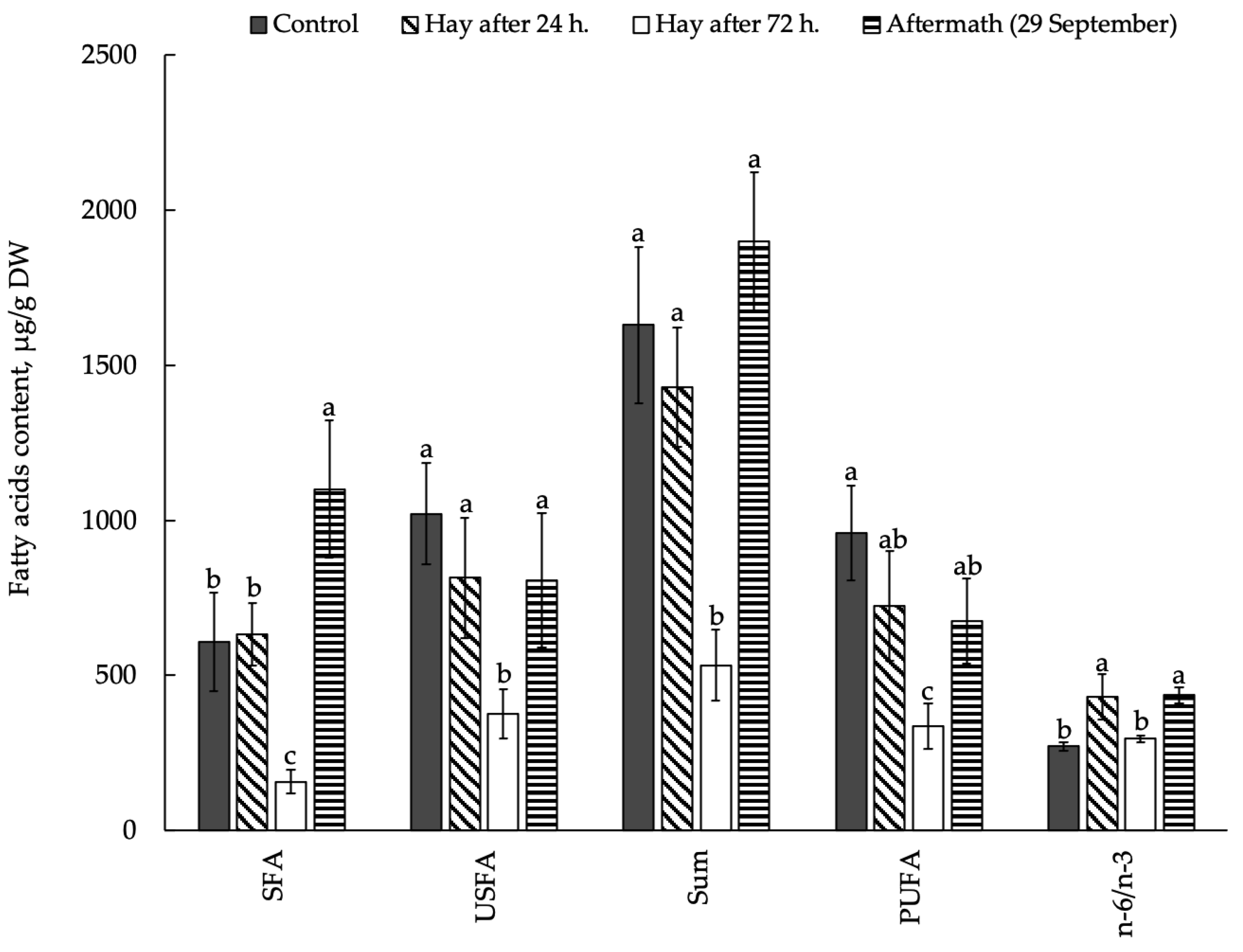
3.2. Influence of Mowing and Drying on the Composition and Content of FAs in Plant Raw Material Leaves
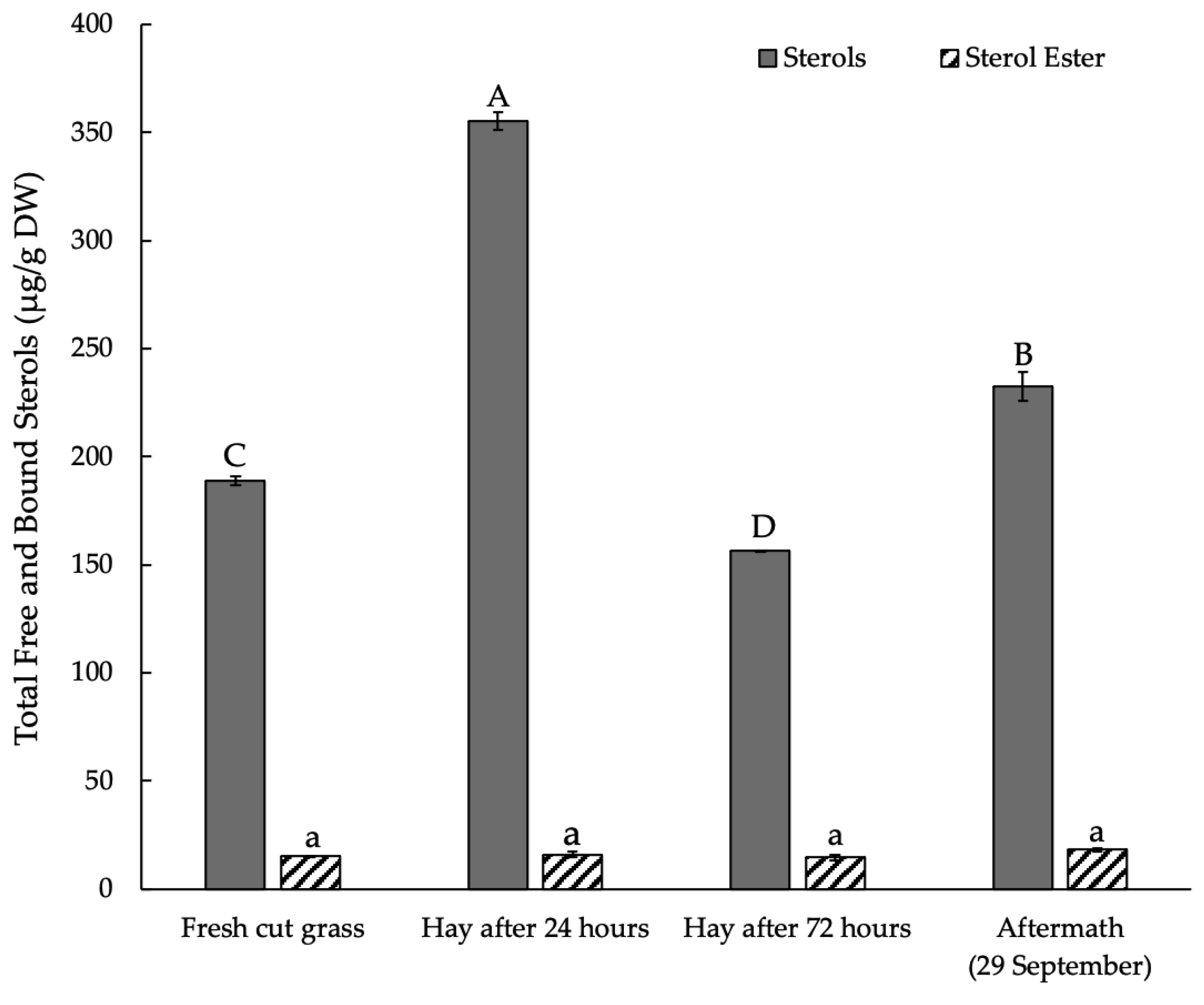
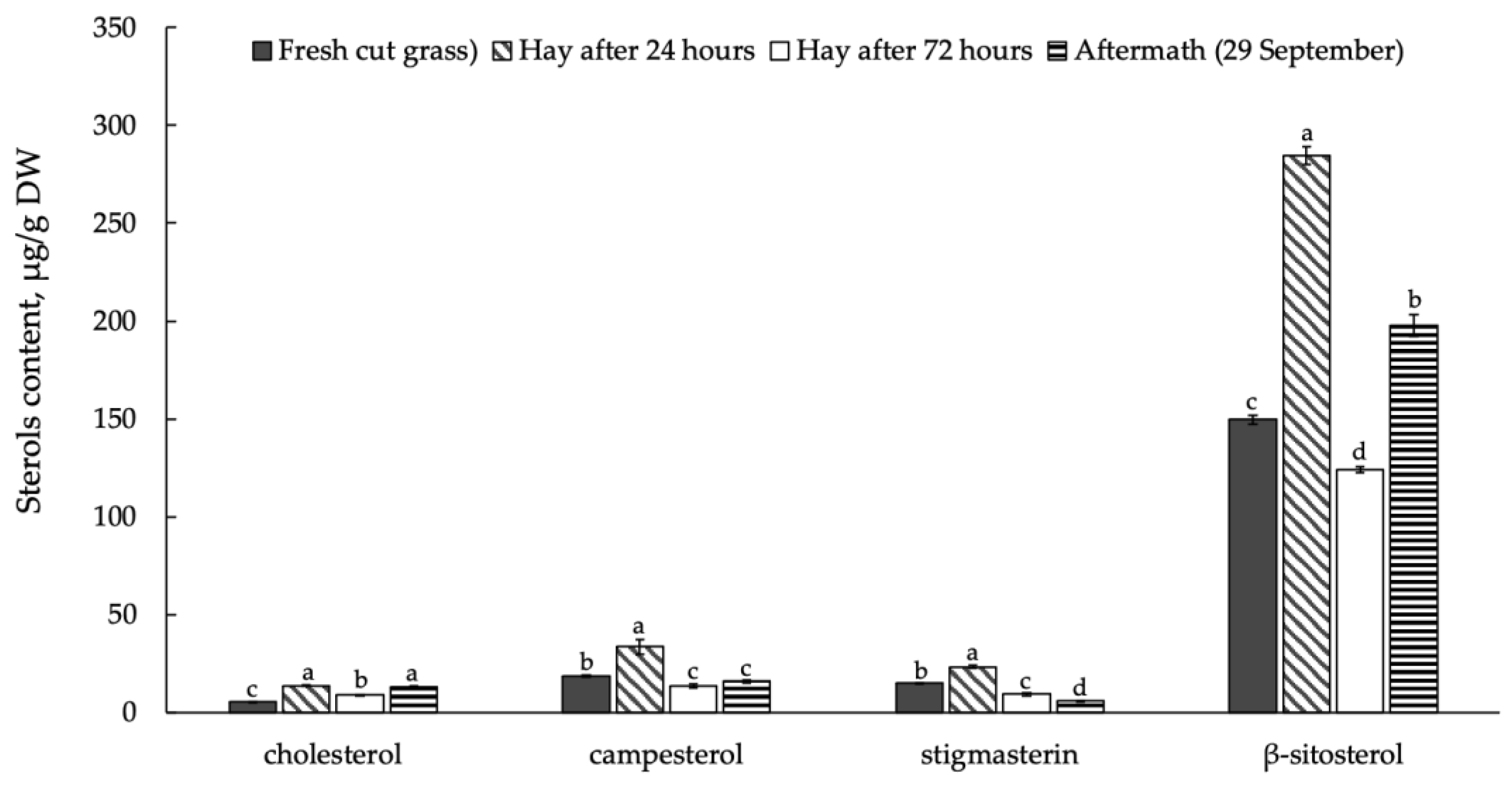
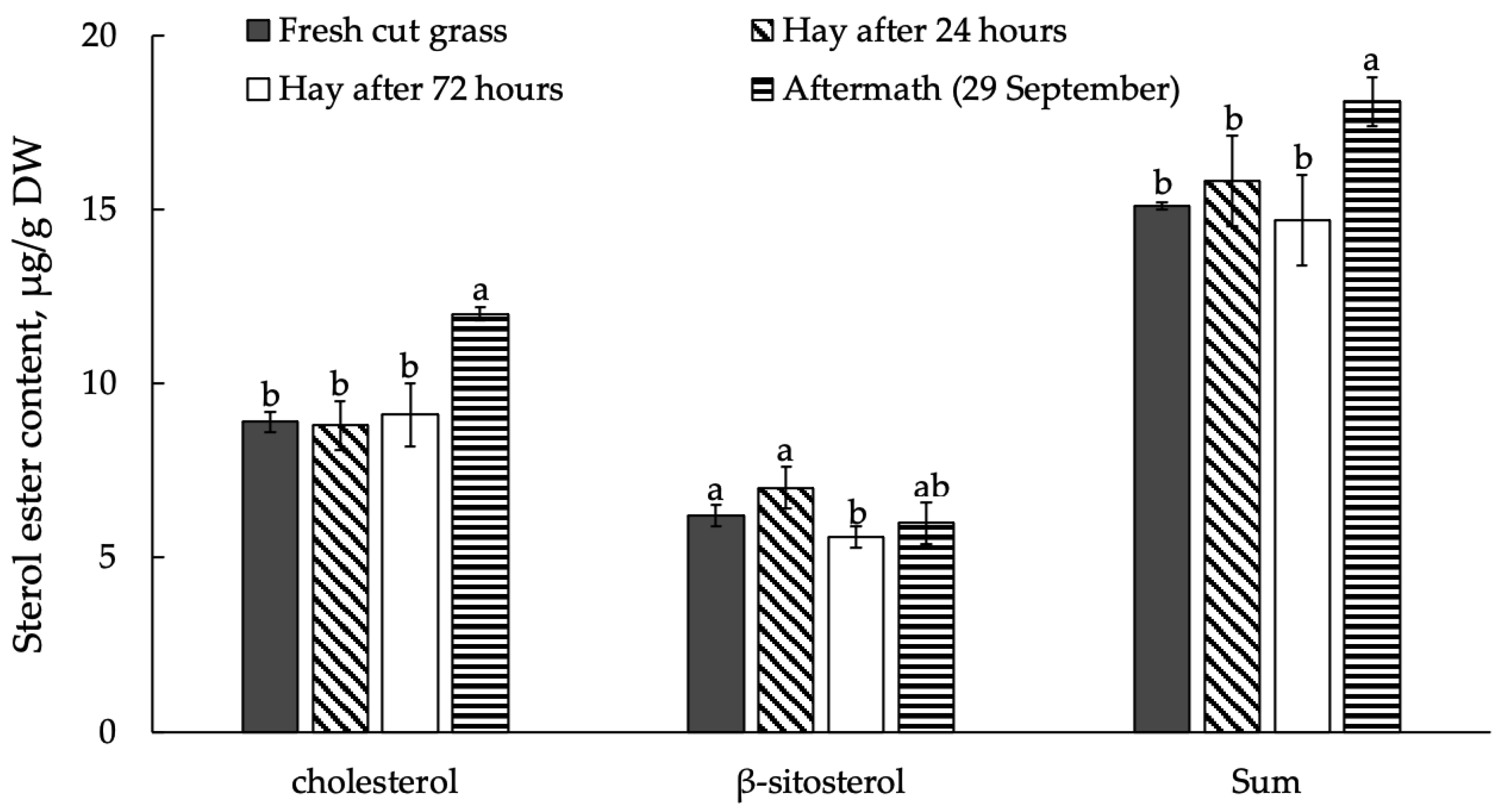
3.3. Sterols and Their Esters in Plant Raw Material Leaves in Mowing and Drying
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tran, T.L.C.; Callahan, D.L.; Islam, M.T.; Wang, Y.; Arioli, T.; Cahill, D. Comparative metabolomic profiling of Arabidopsis thaliana roots and leaves reveals complex response mechanisms induced by a seaweed extract. Front. Plant Sci. 2023, 14, 1114172. [Google Scholar] [CrossRef] [PubMed]
- Popova, L.; Avelova, S.; Gerasimova, A.; Lutz, J.; Mathiesen, S.V.; Moiakunova, A.; Petrova, A.; Pogodaev, M.; Shadrin, V.; Shishigina, A.N.; et al. Trends and Effects of Climate Change on Reindeer Husbandry in the Republic of Sakha (Yakutia). In Reindeer Husbandry; Mathiesen, S.D., Eira, I.M.G., Turi, E.I., Oskal, A., Pogodaev, M., Tonkopeeva, M., Eds.; Springer Polar Sciences; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Petrov, K.A.; Sofronova, V.E.; Chepalov, V.A.; Perk, A.A.; Maksimov, T.C. Seasonal changes in the content of photosynthetic pigments in perennial grasses of cryolithic zone. Russ. J. Plant Physiol. 2010, 57, 181–188. [Google Scholar] [CrossRef]
- Zakharova, V.I.; Kuznetsova, L.V.; Ivanova, E.I.; Vasilyeva-Kralina, I.I.; Gabyshev, V.A.; Egorova, A.A.; Zolotov, V.I.; Ivanova, A.P.; Ignatov, M.S.; Ignatova, E.A.; et al. Raznoobrazie Rastitel’nogo Mira Yakutii [Diversity of the Flora of Yakutia]; Publishing House SO RAN: Novosibirsk, Russia, 2005; p. 328. [Google Scholar]
- Sidorov, R.A.; Tsydendambaev, V.D. Biosynthesis of fatty oils in higher plants. Russ. J. Plant Physiol. 2014, 61, 1–18. [Google Scholar] [CrossRef]
- Glasser, F.; Doreau, M.; Maxin, G.; Baumont, R. Fat and fatty acid content and composition of forages: A meta-analysis. Anim. Feed Sci. Technol. 2013, 185, 19–34. [Google Scholar] [CrossRef]
- Petrov, K.A. Kriorezistentnost’ Rastenii: Ekologo-Fiziologicheskie I Biokhemicheskie Aspekty [Cryoresistance of Plants: Ecological, Physiological and Biochemical Aspects]; SB RAS Publishing House: Novosibirsk, Russia, 2016; 276p. [Google Scholar]
- Sofronova, V.E.; Chepalov, V.A.; Dymova, O.V.; Golovko, T.K. Functional Condition of Photosystem II in Leaves of Spring Oats during Autumnal Decrease in Temperature. Russ. J. Plant Physiol. 2020, 67, 661–670. [Google Scholar] [CrossRef]
- Gabyshev, M.F. The Yakut Horse; Yakutsk Book Publishers: Yakutsk, Russia, 1957; p. 238. [Google Scholar]
- Egorov, A.D.; Potapov, V.Y.; Romanov, P.A. Zonal’no-Biokhimicheskie Osobennosti Kormovykh Rastenii Iakutii i Nekotorye Prob-lemy Razvitiia Zhivotnovodstva [Zonal-Biochemical Characteristics of Fodder Plants in Yakutia and Some Problems in the Development of Animal Husbandry]; Yakutsk Book Publishing House: Yakutsk, Russia, 1962; p. 51. [Google Scholar]
- Petrov, K.A.; Dudareva, L.V.; Nokhsorov, V.V.; Stoyanov, K.N.; Makhutova, O.N. Fatty Acid Content and Composition of the Yakutian Horses and Their Main Food Source: Living in Extreme Winter Conditions. Biomolecules 2020, 10, 315. [Google Scholar] [CrossRef]
- Maron, J.L.; Jeffries, R.L. Restoring enriched grasslands: Effects of mowing on species richness, productivity, and nitrogen retention. Ecol. Appl. 2001, 11, 1088–1100. [Google Scholar] [CrossRef]
- Talle, M.; Deak, B.; Poschlod, P.; Valko, O.; Westerberg, L.; Milberg, P. Grazing vs. mowing: A meta-analysis of biodiversity benefits for grassland management. Agric. Ecosyst. Environ. 2016, 222, 200–212. [Google Scholar] [CrossRef]
- Mikhailova, E.A.; Bryant, R.B.; Cherney, D.J.R.; Post, C.J.; Vassenev, I.I. Botanical composition, soil and forage quality under different management regimes in Russian grasslands. Agric. Ecosyst. Environ. 2000, 80, 213–226. [Google Scholar] [CrossRef]
- Yang, Z.; Minggagud, H.; Baoyin, T.; Li, F.Y. Plant production decreases whereas nutrients concentration increases in response to the decrease of mowing stubble height. J. Environ. Manag. 2020, 253, 109745. [Google Scholar] [CrossRef]
- Petrov, K.A.; Perk, A.A.; Chepalov, V.A.; Sofronova, V.E.; Ilyin, A.N.; Ivanov, R.V. Eco-physiological and biochemical bases of the green cryo-feed forming in Yakutia. Agric. Biol. 2017, 52, 1129–1138. [Google Scholar] [CrossRef]
- Milchunas, D.G.; Mosier, A.R.; Morgan, J.A.; LeCain, D.R.; King, J.Y.; Nelson, J.A. Elevated CO2 and defoliation effects on a shortgrass steppe: Forage quality versus quantity for ruminants. Agric. Ecosyst. Environ. 2005, 111, 166–184. [Google Scholar] [CrossRef]
- Dickson, T.L. Burning and mowing similarly increase prairie plant production in the spring, but not due to increased soil temperatures. Ecosphere 2019, 10, e02606. [Google Scholar] [CrossRef]
- Venterink, H.O.; Kardel, I.; Kotowski, W.; Peeters, W.; Wassen, M.J. Long-term effects of drainage and hay-removal on nutrient dynamics and limitation in the Biebrza mires, Poland. Biogeochemistry 2009, 93, 235–252. [Google Scholar] [CrossRef]
- Nesterkina, I.S.; Gurina, V.V.; Ozolina, N.V.; Numinsky, V.N. Changes in Tonoplast Sterol Contents under Osmotic Stress. Biol. Membr. 2019, 36, 301–304. [Google Scholar] [CrossRef]
- Dudareva, L.V.; Semenova, N.V.; Nokhsorov, V.V.; Rudikovskaya, E.G.; Petrov, K.A. The component composition of the phytosterols of the aerial part of the horsetail variegated Equisétum variegatum Schleich. ex. Web. growing in north-east Yakutia. Khimiya Rastitel’nogo Syr’ya 2020, 2, 133–139. [Google Scholar] [CrossRef]
- Nokhsorov, V.V.; Dudareva, L.V.; Senik, S.V.; Chirikova, N.K.; Petrov, K.A. Influence of Extremely Low Temperatures of the Pole of Cold on the Lipid and Fatty-Acid Composition of Aerial Parts of the Horsetail Family (Equisetaceae). Plants 2021, 10, 996. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Scollan, N.D.; Youell, S.J.; Tweed, J.K.S.; Humphreys, M.O. Influence of species, cutting date and cutting interval on the fatty acid composition of grasses. Grass Forage Sci. 2001, 56, 68–74. [Google Scholar] [CrossRef]
- Mir, P.S.; Bittman, S.; Hunt, D.; Entz, T.; Yip, B. Lipid content and fatty acid composition of grasses sampled on different dates through the early part of the growing season. Can. J. Anim. Sci. 2011, 86, 279–290. [Google Scholar] [CrossRef]
- Piironen, V.; Lindsay, D.G.; Miettinen, T.A.; Toivo, J.; Lampi, A.-M. Plant sterols: Biosynthesis, biological function and their importance to human nutrition. J. Sci. Food Agric. 2000, 80, 939–966. [Google Scholar] [CrossRef]
- Vezza, T.; Canet, F.; de Marañón, A.M.; Bañuls, C.; Rocha, M.; Víctor, V.M. Phytosterols: Nutritional Health Players in the Management of Obesity and Its Related Disorders. Antioxidants 2020, 9, 1266. [Google Scholar] [CrossRef]
- Benning, C.; Huang, Z.H.; Gage, D.A. Accumulation of a novel glycolipid and a betaine lipid in cell of Rhodobacter sphaeroides grown under phosphate limitation. Arch. Biochem. Biophys. 1995, 317, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Kates, M. Techniques of Lipidology: Isolation, Analysis and Identification of Lipids; North-Holland: Amsterdam, The Netherlands, 1972; pp. 269–610. [Google Scholar]
- Christie, W.W. Preparation of ester derivatives of fatty acids for chromatographic analysis. Adv. Lipid Methodol. 1993, 2, 69–111. [Google Scholar]
- Christie, W.W. The AOCS Lipid Library: Methyl Esters of Fatty Acids—Archive of Mass Spectra. Available online: https://www.lipidmaps.org/resources/lipidweb/index.php?page=ms/methesters/me-arch/index.htm (accessed on 31 March 2023).
- Desyatkin, A.; Fedorov, P.; Filippov, N.; Desyatkin, R. Climate Change and Its Influence on the Active Layer Depth in Central Yakutia. Land 2021, 10, 3. [Google Scholar] [CrossRef]
- Obroucheva, N.V.; Sin’kevich, I.A. Aquaporins and cell growth. Russ. J. Plant Physiol. 2010, 57, 153–165. [Google Scholar] [CrossRef]
- Severin, F.F.; Severina, I.I.; Antonenko, Y.N.; Rokitskaya, T.I.; Cherepanov, D.A.; Mokhova, E.N.; Vyssokikh, M.Y.; Pustovidko, A.V.; Markova, O.V.; Yaguzhinsky, L.S.; et al. Penetrating cation/fatty acid anion pair as a mitochondria targeted protonophore. Proc. Natl. Acad. Sci. USA 2010, 107, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xu, Y.; Wang, J.; Singer, S.D.; Chen, G. The Role of Triacylglycerol in Plant Stress Response. Plants 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Park, M.-E.; Park, B.Y.; Kim, H.U.; Seo, P.J. The Arabidopsis MYB96 Transcription Factor Mediates ABA-Dependent Triacylglycerol Accumulation in Vegetative Tissues under Drought Stress Conditions. Plants 2019, 8, 296. [Google Scholar] [CrossRef]
- Angkawijaya, A.E.; Nguyen, V.C.; Nakamura, Y. Lysophosphatidic acid acyltransferases 4 and 5 are involved in glycerolipid metabolism and nitrogen starvation response in Arabidopsis. New Phytol. 2019, 224, 336–351. [Google Scholar] [CrossRef]
- Champeyroux, C.; Stoof, C.; Rodriguez-Villalon, A. Signaling phospholipids in plant development: Small couriers determining cell fate. Curr. Opin. Plant Biol. 2020, 57, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Garab, G.; Ughy, B.; Goss, R. Role of MGDG and Non-bilayer Lipid Phases in the Structure and Dynamics of Chloroplast Thylakoid Membranes. In Lipids in Plant and Algae Development; Springer: Cham, Switzerland, 2016; Volume 86, pp. 127–157. [Google Scholar] [CrossRef]
- Rozentsvet, O.A.; Nesterov, V.N.; Bogdanova, E.S. Membrane-forming lipids of wild halophytes growing under the conditions of Prieltonie of South Russia. Phytochemistry 2014, 105, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cang, J.; Zhou, Z.; Liu, L. Anatomical structure comparison between leaves of two winter wheat cultivars with different cold/freezing tolerance under low temperature stress. J. Northeast Agric. Univ. 2011, 18, 1–6. [Google Scholar] [CrossRef]
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef] [PubMed]
- Rozentsvet, O.A.; Bogdanova, E.S.; Nurminsky, V.N.; Nesterov, V.N.; Chernyshov, M.Y. Detergent-Resistant Membranes in Chloroplasts and Mitochondria of the Halophyte Salicornia perennans under Salt Stress. Plants 2023, 12, 1265. [Google Scholar] [CrossRef]
- Dawson, L.; Street, R. Relationship between dry matter, fibre and nitrogen degradation characteristics of silage and silage intake of steers. Anim. Sci. 2000, 70, 537–546. [Google Scholar] [CrossRef]
- Wright, D.A.; Gordon, F.J.; Steen, R.W.J.; Patterson, D.C. Factors influencing the response in intake of silage and animal performance after wilting of grass before ensiling: A review. Grass Forage Sci. 2000, 55, 1–13. [Google Scholar] [CrossRef]
- Van Ranst, G.; Fievez, V.; De Riek, J.; Van Bockstaele, E. Influence of ensiling forages at different dry matters and silage additives on lipid metabolism and fatty acid composition. Anim. Feed Sci. Technol. 2009, 150, 62–74. [Google Scholar] [CrossRef]
- Khan, N.A.; Cone, J.W.; Fievez, V.; Hendriks, W.H. Causes of variation in fatty acid content and composition in grass and maize silages. Anim. Feed Sci. Technol. 2012, 174, 36–45. [Google Scholar] [CrossRef]
- McNiven, M.A.; Duynisveld, J.L.; Turner, T.; Mitchell, A.W. Ratio of n-6/n-3 in the diets of beef cattle: Effect on growth, fatty acid composition, and taste of beef. Anim. Feed Sci. Technol. 2011, 170, 171–181. [Google Scholar] [CrossRef]
- Du, Y.; Fu, X.; Chu, Y.; Wu, P.; Liu, Y.; Ma, L.; Tian, H.; Zhu, B. Biosynthesis and the Roles of Plant Sterols in Development and Stress Responses. Int. J. Mol. Sci. 2022, 23, 2332. [Google Scholar] [CrossRef]
- Valitova, J.N.; Sulkarnayeva, A.G.; Minibayeva, F.V. Plant sterols: Diversity, biosynthesis, and physiological functions. Biochemistry 2016, 81, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Imai, H.; Kawamura, Y.; Uemura, M. Lipid profiles of detergent resistant fractions of the plasma membrane in oat and rye in association with cold acclimation and freezing tolerance. Cryobiology 2016, 72, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Rozentsvet, O.; Nesterkina, I.; Ozolina, N.; Nesterov, V. Detergent-resistant microdomains (lipid rafts) in endomembranes of wild halophytes. Funct. Plant Biol. 2019, 46, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Semenova, N.V.; Shmakov, V.; Park, M.E.; Tretyakova, I.N.; Konstantinov, Y.M.; Dudareva, L.V. Particularities of neutral lipid composition in embryogenic and non-embryogenic cell lines of Larix sibirica Ledeb. Biol. Membr. 2020, 37, 215–223. [Google Scholar] [CrossRef]
| № | Species | Stages of Development (1 July) | Stages of Development (29 September) | Sample | Percentage of Each Species (%) |
|---|---|---|---|---|---|
| 1 | Bromus inermis Leyss. | Stem elongation | Dough development | leaves | 30% |
| 2 | Calamagrostis langsdorffii (Link) Trin. | Tillering | Milk development | leaves | 20% |
| 3 | Beckmannia syzigachne (Steud.) Fernald | Stem elongation | Dough development | leaves | 20% |
| 4 | Elytrigia repens (L.) Nevski | Ear emergence | Milk development | leaves | 20% |
| 5 | Carex atherodes Spreng. | Tillering | Milk development | leaves | 10% |
| Sampling Date | Daily Average Air Temperature, °C * | Total Precipitation, mm ** | Photoperiod, h |
|---|---|---|---|
| 1 July (Fresh cut grass) | 24.9 ± 8.9 | 9.1 | 19.0 |
| 2 July (Hay after 24 h) | 23.0 ± 8.1 | 9.1 | 19.0 |
| 3 July | 24.4 ± 7.2 | 9.3 | 19.0 |
| 4 July (Hay after 72 h) | 26.4 ± 5.0 | 9.3 | 18.9 |
| 29 September (Aftergrass) | −0.2 ± 1.3 | 0.5 | 12.1 |
| Lipid Classes | Fresh Cut Grass | Hay after 24 h | Hay after 72 h | Aftergrass (29 September) |
|---|---|---|---|---|
| 1,2-DAG | 19.4 [17.3; 21.5] A | 11.3 [9.9; 12.7] B | 15.3 [12.8; 17.8] AB | 14.5 [12.6; 16.4] B |
| Sterols | 11.6 [9.5; 13.7] C | 16.6 [15.2; 18] B | 10.9 [9.2; 12.6] C | 20.9 [18.5; 23.3] A |
| FFA | 27.2 [24.7; 29.7] A | 23 [21.5; 24.5] A | 16 [15.1; 16.9] C | 20.5 [20.1; 20.9] B |
| TAG | 6.6 [6; 7.2] B | 21.4 [19.8; 23] A | 3.3 [2.4; 4.2] C | 4.1 [3.3; 4.9] C |
| FA | 14.4 [12; 16.8] B | 2.9 [2.5; 3.3] C | 3.4 [2.5; 4.3] C | 20 [19.4; 20.6] A |
| SE + Sq + Wax | 9.6 [9.1; 10.1] B | 10.4 [9.3; 11.5] B | 8.7 [8.1; 9.3] B | 15.8 [14.6; 17.0] A |
| Lipid Classes | Fresh Cut Grass | Hay after 24 h | Hay after 72 h | Aftergrass (29 September) |
|---|---|---|---|---|
| PS | 4.6 [3.7; 5.5] B | 6.5 [5.8; 7.1] A | 5.2 [4.4; 6.0] AB | 3.4 [2.8; 4.0] B |
| PI | 10.1 [9.0; 11.2] A | 4.3 [3.4; 5.2] C | 1.2 [0.9; 1.5] D | 7.2 [6.5; 8.0] B |
| PC | 12.7 [11.5; 13.9] B | 10.7 [9.7; 11.7] C | 7.1 [6.6; 7.6] D | 19.8 [18.4; 21.2] A |
| PG + DPG | 25.3 [23.9; 26.7] A | 10.5 [9.8; 11.1] C | 7.0 [6.3; 7.7] D | 13.8 [12.6; 15.0] B |
| DGDG | 19.6 [17.6; 21.6] B | 21.6 [20.6; 22.6] B | 10.5 [9.0; 12.0] C | 27.4 [25.7; 29.1] A |
| PE | 8.6 [7.6; 9.6] C | 13.3 [11.6; 14.9] B | 6.6 [5.5; 7.7] CD | 19.2 [17.3; 21.1] A |
| MGDG | 6.7 [6.0; 7.5] C | 15.7 [15.2; 16.1] B | 8.5 [7.4; 9.6] C | 17.8 [16.7; 18.9] A |
| Fatty Acids | Fresh Cut Grass | Hay after 24 h | Hay after 72 h | Aftergrass (29 September) | ||||
|---|---|---|---|---|---|---|---|---|
| % of Sum | µg/g DW | % of Sum | µg/g DW | % of Sum | µg/g DW | % of Sum | µg/g DW | |
| C12:0-i | 0.20 ± 0.16 | 3.18 ± 2.51 | - | - | - | - | - | - |
| C14:0 | 1.07 ± 0.25 a | 17.37 ± 5.94 B | 2.56 ± 1.94 a | 36.55 ± 17.63 A | 1.06 ± 0.38 a | 5.64 ± 3.01 C | 1.71 ± 0.33 a | 32.43 ± 14.86 A |
| C15:0 | 0.24 ± 0.05 b | 3.90 ± 0.54 B | 0.06 ± 0.00 c | 0.83 ± 0.67 C | 0.82 ± 0.00 a | 4.37 ± 2.85 B | 0.68 ± 0.16 a | 12.86 ± 7.78 A |
| C16:0 | 19.19 ± 2.94 b | 312.78 ± 74.28 B | 28.88 ± 4.44 a | 413.01 ± 68.52 B | 21.38 ± 0.63 b | 113.33 ± 24.58 C | 27.88 ± 2.87 a | 529.81 ± 22.61 A |
| C16:1n-9 | 0.32 ± 0.05 | 5.14 ± 0.97 | - | - | - | - | - | - |
| C16:1n-7 | 0.26 ± 0.07 c | 4.19 ± 0.96 C | 0.51 ± 0.00 b | 7.28 ± 1.88 B | 0.78 ± 0.10 a | 4.16 ± 2.12 BC | 1.26 ± 0.53 a | 23.85 ± 9.14 A |
| C16:1n-5 | 1.01 ± 0.11 a | 16.44 ± 3.13 B | 1.02 ± 0.62 a | 14.57 ± 3.74 B | 1.56 ± 0.05 a | 8.25 ± 1.72 C | 1.42 ± 0.28 a | 26.93 ± 2.37 A |
| C17:0 | 0.35 ± 0.10 b | 5.64 ± 1.95 B | 0.28 ± 0.12 b | 3.97 ± 1.38 B | 0.36 ± 0.07 b | 1.88 ± 0.42 C | 0.69 ± 0.14 a | 13.07 ± 3.78 A |
| C18:0 | 14.69 ± 3.84 b | 239.50 ± 45.37 B | 8.13 ± 1.64 c | 116.32 ± 12.65 C | 3.81 ± 0.32 d | 20.19 ± 3.93 D | 24.23 ± 2.47 a | 460.41 ± 32.19 A |
| C18:1n-9 | 1.67 ± 0.19 c | 27.22 ± 7.82 B | 3.45 ± 0.83 ab | 49.27 ± 6.89 A | 4.17 ± 0.42 a | 22.10 ± 3.12 B | 3.09 ± 0.05 b | 58.62 ± 9.65 A |
| C18:1n-7 | 0.52 ± 0.15 c | 8.43 ± 4.97 B | 1.33 ± 0.43 a | 19.02 ± 3.15 A | 0.97 ± 0.07 b | 5.12 ± 0.98 B | 1.13 ± 0.26 a | 21.46 ± 6.98 A |
| C18:2n-6 | 12.54 ± 2.01 a | 204.46 ± 30.13 A | 15.27 ± 2.13 a | 218.36 ± 45.32 A | 14.47 ± 0.84 a | 76.67 ± 18.18 B | 10.81 ± 2.13 ab | 205.32 ± 50.22 A |
| C18:3n-3 | 46.30 ± 6.55 a | 754.65 ± 131.74 A | 35.42 ± 3.59 b | 506.49 ± 131.65 AB | 48.92 ± 1.21 a | 259.27 ± 55.25 C | 24.76 ± 5.72 c | 470.52 ± 87.52 AB |
| C20:0 | 0.71 ± 0.17 b | 11.51 ± 2.42 B | 2.00 ± 0.42 a | 28.57 ± 5.96 A | 0.83 ± 0.06 b | 4.40 ± 1.12 C | 1.37 ± 0.33 a | 26.02 ± 9.01 A |
| C22:0 | 0.96 ± 0.18 b | 15.60 ± 2.16 B | 2.36 ± 0.83 a | 33.75 ± 8.45 A | 1.42 ± 0.35 ab | 7.53 ± 2.92 C | 1.40 ± 0.34 ab | 26.64 ± 8.99 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nokhsorov, V.V.; Dudareva, L.V.; Semenova, N.V.; Petrov, K.A. Study of the Effect of Mowing and Drying on the Lipid Composition of Grass Leaves in Permafrost Ecosystems. Agronomy 2023, 13, 2252. https://doi.org/10.3390/agronomy13092252
Nokhsorov VV, Dudareva LV, Semenova NV, Petrov KA. Study of the Effect of Mowing and Drying on the Lipid Composition of Grass Leaves in Permafrost Ecosystems. Agronomy. 2023; 13(9):2252. https://doi.org/10.3390/agronomy13092252
Chicago/Turabian StyleNokhsorov, Vasiliy V., Lyubov V. Dudareva, Natalia V. Semenova, and Klim A. Petrov. 2023. "Study of the Effect of Mowing and Drying on the Lipid Composition of Grass Leaves in Permafrost Ecosystems" Agronomy 13, no. 9: 2252. https://doi.org/10.3390/agronomy13092252
APA StyleNokhsorov, V. V., Dudareva, L. V., Semenova, N. V., & Petrov, K. A. (2023). Study of the Effect of Mowing and Drying on the Lipid Composition of Grass Leaves in Permafrost Ecosystems. Agronomy, 13(9), 2252. https://doi.org/10.3390/agronomy13092252









