Abstract
Invasive plants pose a significant threat to biodiversity, especially under the current unstable climatic conditions. This study aimed to test the salt and drought tolerance of two ornamental species of the genus Ipomoea during germination and vegetative growth. Germination tests were performed in the presence of increasing NaCl concentrations or iso-osmotic PEG concentrations—to mimic the osmotic stress caused by drought. Both species showed great invasive potential because of their high seed germination percentages and rapid germination under control (distilled water) and salt stress conditions, up to 200 mM NaCl. Germination and early seedling development were more affected in the presence of PEG. Subsequently, water stress (complete withholding of irrigation) and salt stress (watering with 100 mM and 200 mM NaCl) treatments were applied to young plants for three weeks, when all plants were harvested, to determine several morphological and biochemical parameters. Both species were sensitive to water deficit but relatively resistant to salt stress. Their salt stress responses were similar, based mainly on the inhibition of Na+ and the activation of K+ transport from roots to leaves and the uptake and accumulation of Ca2+; however, I. tricolor showed a slightly higher tolerance to salt stress than I. purpurea. Although I. tricolor has only been locally reported as invasive and is generally considered a ‘low-risk’ species, our results indicate that it may have an invasive potential even higher than I. purpurea, a recognised invasive weed, spread into areas with moderate salinity, affecting agricultural land or natural habitats of ecological interest.
1. Introduction
One of the most significant risks for biodiversity on a global scale is represented by the spread of invasive species, which is exacerbated by globalisation and climate change. They are often the result of intentional or accidental introductions of alien species into new territories, followed by their naturalisation and spread, which provokes a detriment to native species and ecosystems. Invasive species have been recognised for several decades as one of the main threats to endangered plants, exceeded only by habitat loss [1]. At the beginning of this century, 57% of threatened species were reported to be negatively affected by non-native competitors [2]. Invasive alien species compete with native species, alter food webs, nitrogen [3] and hydrological [4] cycles, and thus, disrupt the functioning of ecosystems and the services they provide [5]. In addition to these ecological effects, invasive species can have a negative economic impact [6] or affect human health [7,8]. The risks posed by invasive alien species are well understood [9,10], as reflected in a growing number of papers and metadata analyses [11,12,13,14,15,16]. However, the spread and establishment of alien species worldwide is not slowing down [17] and is expected to increase [18] despite prevention measures.
The leading cause of plant invasions is ornamental horticulture, as most invasive alien plants were either actively introduced for ornamental purposes or accidentally, such as seeds brought in by chance [19,20]. The economic importance of plants is a driver of their condition as potential invaders. In this sense, species used for ornamental, medicinal or culinary purposes or for fodder have the highest likelihood of naturalisation [2]. Horticulture might promote plant invasions by selecting species and genotypes of ornamental value based on features that inadvertently encourage spread [20]. Adaptability to the environment, quick germination and profusion of seedling emergence, rapid vegetative development, and early flowering or prolonged flowering periods are all characteristics of invasive plants that are also desirable as ornamental plants.
Plant invasions and climate change are closely linked [21]. Europe, the north-eastern United States, Central America, Africa, Indonesia, and Pacific Island regions are considered the areas with the highest susceptibility to invasion by alien species as their climatic conditions could be significantly altered [22]. Increasing temperatures promote the growth of introduced ornamental plants from warmer areas, allowing them to spread into temperate regions [23,24]. Before other species were forced to migrate due to climate change, these invasive species will have a “head start” in the new climatic conditions [25]. Moreover, abiotic stress-tolerant cultivars are preferred for ornamental horticulture in some regions to overcome problems derived from climate change and global warming [23]. Finally, the higher phenotypic plasticity of invasive plants, reported in many comparative studies on invasive and non-invasive taxonomically related species [26,27], favours its spread to new habitats.
With 600–700 species primarily found in tropical and warm temperate regions of the world and known as “morning glories”, Ipomoea L. is one of the prominent genera within the family Convolvulaceae. Most species in this genus are climbers, annual or perennial herbaceous plants, and shrubs [28]. The genus includes I. batatas (L.) Lam, the sweet potato, originated in America and is cultivated throughout the world where the climate conditions allow its growth [29], as well as many other species used for ornamental purposes due to the outstanding appearance of their flowers and their climbing habit, which makes them suitable for covering walls, fences, and pergolas. Plants of this genus have rapid growth and high seed production that confers them a high adaptability and microevolutionary capacity, which are typical “weedy traits” [30]. Around 170 species within the Ipomoea genus are listed in the Global Compendium of Weeds, and many are reported as invasive worldwide [31]. Two species of this genus, I. purpurea (L.) Roth and I. tricolor Cav. were selected for this study. The two are popular in temperate and warm regions of the world as cover plants for walls, fences and pergolas due to their large, showy flowers of beautiful colours, white to pink, blue and dark purple. Both have abundant seed production and fast growth, traits that favour invasiveness. Their ability to withstand abiotic stress has been analysed only in a few studies, and there is virtually no information on their biochemical responses to salinity and drought.
Ipomoea purpurea, the tall morning glory, is an annual vine first reported in England, where it was introduced via Spain from Central America. Nowadays, it is classified as a common weed rated with high global risk in many warm regions, including southern Europe [31,32]. Ipomoea purpurea affects different crops, orchards, and nursery production, inducing stunted growth, reduced yields, and hindering harvesting [33]. In warm, humid environments, the species outcompetes native plants, mainly invading riparian forests, wetlands, and coastal areas [32,34]. Once established in natural places, the tall morning glory can spread quickly by climbing on mature trees, shrubs, and other plant species, generating a dense canopy that competes with the supporting species for nutrients, water, and solar radiation [35,36].
Ipomoea tricolor, the Mexican morning glory, is an annual vine species native to Mexico and cultivated worldwide in mild climates. The species has been long cultivated in Spain and reported as naturalised in the last decades of the 19th century [37] but is generally not problematic as invasive. Although included in the Global Compendium of Weeds [31], it is rated as low risk. It has been reported as a weed for loofah, forage legumes, mango, okra, and sorghum fields in Mexico [38] and recently reported as naturalised in Turkey and predicted to spread to the areas near the Black Sea, Aegean, Mediterranean, and some parts of central Anatolia [39].
This study aimed to unveil the reason for the higher invasive risk of I. purpurea over I. tricolor by analysing their germination and growth under optimal control conditions and two common environmental stresses, salinity and water scarcity. Osmolyte synthesis and ion accumulation, two main mechanisms of stress responses, were also analysed to better comprehend the differences between the two species. The working hypothesis is that I. purpurea, with a higher invasive potential, will show a broader tolerance to stress based on more efficient biochemical responses.
2. Materials and Methods
2.1. Plant Material
Plants were obtained by germinating seeds purchased from Vilmorin Seed Generation, Paris, France.
2.2. Seed Germination
Seeds were placed in standard 90 mm diameter Petri dishes on two disks of filter paper moistened with 2.5 mL of distilled water for the control treatment or with increasing concentrations of NaCl or PEG 6000 (Polyethylene Glycol) for the stress treatments. Controls and treated seeds were covered with two other filter paper disks moistened with the same amount of distilled water or the respective stress treatment solutions. The germination assays were carried out with four replications per treatment and species, with ten seeds in each plate. The salt concentrations tested were 50, 100, 200 and 400 mM NaCl in aqueous solutions, and the corresponding iso-osmotic PEG concentrations ensuring osmotic potentials of −22, −44, −88, and −1.76 MPa, calculated by applying the Van’t Hoff equation [40]. Germination was performed in an EQUiTEC germination chamber (LAF Technologies, Bayswater North, VIC, Australia) at 30 °C for 16 h and at 20 °C for 8 h, with a relative humidity of 65%.
The number of germinated seeds was counted every two days for three weeks, considering germination as the emergence of a radicle of at least 2 mm. The germination capacity was expressed as the percentage of germination (GP), and the velocity of germination rate as mean germination time (MGT), calculated according to the formula by Ellis and Roberts [41]:
where D represents the number of days from the beginning of the germination test, and n is the number of seeds newly germinated on day D.
MGT = ∑Dn/∑n,
Lengths of the radicle and hypocotyl were measured at the end of the germination assay using Digimizer v.4.6.1 software (MedCalc Software, Ostend, Belgium, 2005–2016).
Other germination indexes calculated were: first germination day (FGD), last germination day (LGD), first day of germination (FDG), last day of germination (LDG), time spread of germination (TSG; differences in time between the last germination day and the first germination day), speed of emergence (SE), and seedling vigour index (SVI), calculated as follows [42]:
SVI = (Seedling length, in mm × Germination percentage)/100.
2.3. Plant Growth and Stress Treatments
The seedlings from the controls in the germination experiments mentioned above were manually transferred into plastic pots (12 cm in diameter) filled with commercial peat (26% organic carbon, pH = 7.0, and EC = 0.6 dS m−1), placed in the greenhouse, and watered twice a week with tap water. Four weeks after transplanting, stress treatments were initiated, using five biological replicas (individual plants) per species and treatment. The pots were placed in plastic trays (10 pots per tray) and watered twice weekly, adding 1.5 L tap water to each tray for the control plants and the same amount of the corresponding NaCl solutions for the salt treatments. The water stress treatment consisted of total irrigation suppression. After three weeks of treatment, when the soil moisture of the water stress group reached 5–8%, plants were harvested, and the aerial part and roots were sampled and processed separately, the latter after being thoroughly cleaned with a brush. The following morphological parameters were registered: root length (RL), stem length (SL), number of leaves (LN), fresh weight of roots (RFW) and leaves (LFW), and water content of roots (RWC) and leaves (LWC). For the calculation of water content (WC), a fraction of the root and leaf material was weighed before (fresh weight, FW) and after drying at 65 °C for 72 h (dry weight, DW), and the following equation was used:
WC% = [(FW − DW)/FW] × 100
Fresh plant material was frozen in liquid N2 and stored at −75 °C, and dry material was kept at room temperature in tightly closed bags for further analysis.
2.4. Photosynthetic Pigments
Fresh shoot material (0.05 g) was ground and extracted overnight in ice-cold 80% acetone. The absorbance of the supernatant was then measured at 470 nm, 646 nm and 663 nm. Concentrations of chlorophyll a (Chl a), chlorophyll b (Chl b) and carotenoids (Caro) were calculated according to Lichtenthaler and Wellburn [43] and expressed in mg g−1 DW.
2.5. Ion Content Measurements
The concentrations of sodium (Na+), potassium (K+), calcium (Ca2+) and chloride (Cl−) were determined separately in roots and leaves following the method described by Weimberg [44]. Samples of 0.1 g ground dry material were extracted in boiling Milli-Q water, cooled on ice and filtered through a 0.45 µm Gelman nylon filter (Pall Corporation, Port Washington, NY, USA). The cations were quantified with a PFP7 flame photometer (Jenway Inc., Burlington, VT, USA), and Cl− was measured using a chlorimeter Sherwood 926 (Cambridge, UK).
2.6. Osmolyte Concentrations
Proline (Pro) concentration was quantified following the classical protocol by Bates et al., as previously described [45]. Fresh ground material (0.05 g) was extracted in 3% (w/v) aqueous sulphosalicylic acid. Samples were sequentially mixed with acid ninhydrin, incubated in a water bath for 1 h at 95 °C, cooled on ice, and then extracted with toluene. The absorbance of the organic phase was measured at 520 nm, using toluene as the blank. Samples of known Pro concentration were assayed in parallel to obtain a standard curve, and Pro concentrations were expressed as µmol g−1 DW.
Total soluble sugars (TSS) were determined following the method of Dubois et al. [46]. Samples of 0.05 g fresh ground material were extracted overnight with 80% (v/v) methanol, and the supernatant obtained upon centrifugation was mixed with 5% phenol and concentrated sulphuric acid. Spectrophotometric measurements were then performed at 490 nm. TSS concentrations were expressed as equivalents of glucose, used as the standard (mg eq. glucose g−1 DW).
2.7. Statistical Analysis
Statgraphics Centurion XVI (Statgraphics Technologies, The Plains, VA, USA) and SPSS Statistics statistical software, version 25.0.0 (IBM SPSS Statistics) were used to analyse the statistical data.
The effects of the stress treatments on the characteristics examined for each species were estimated using a one-way analysis of variance (ANOVA). If the null hypothesis was rejected, the Tukey test was employed as a post-hoc test using a 0.05 p-value to analyse the differences. Principal Component Analyses were carried out independently for plant growth and germination, considering the mean values of germination variables and significant biochemical and growth parameters.
3. Results
3.1. Seed Germination
Seeds of both, I. purpurea and I. tricolor, germinated up to concentrations of 400 mM NaCl, considering germination as radicle emergence. Seeds under PEG treatments showed a lower germination percentage and speed than the isosmotic solutions for both species; under the highest PEG concentration, equivalent to an osmotic potential of −1.76 MPa, no radicle emergence occurred (Figure 1). The pattern of germination evolution over 21 days was similar in the two species under NaCl and PEG treatments (compare Figure 1a,c, and Figure 1b,d).
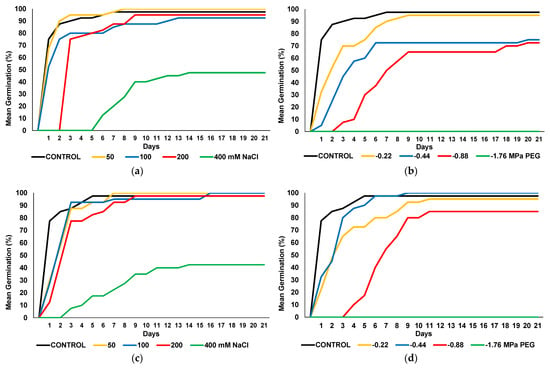
Figure 1.
Evolution of germination during 21 days in Ipomoea purpurea under increasing concentrations of NaCl (a) and polyethylene glycol PEG 6000 (b) and in Ipomoea tricolor under increasing concentrations of NaCl (c) and polyethylene glycol PEG 6000 (d).
The two species showed a very high germination percentage under control conditions in distilled water: 97.50% for I. purpurea (Figure 1a and Figure 2a) and 96.66% for I. tricolor (Figure 1c and Figure 2b). Under salt stress conditions, high germination percentages above 90% were recorded in all salt treatments, except for 400 mM NaCl, for which only 47.5% of I. purpurea seeds (Figure 1a and Figure 2a) and 42.5% of I. tricolor seeds (Figure 1c and Figure 2b) were able to germinate after 21 days of treatment. Under isosmotic PEG concentrations at −1.76 MPa, seeds did not germinate at all when PEG was applied (Figure 1b,d and Figure 2). At −0.44 and −0.88 MPa, mean germination percentages of 75% and 72.5% were recorded in I. purpurea and I. tricolor seeds, respectively, lower but not significantly different from the non-stressed controls (Figure 1b,d and Figure 2).
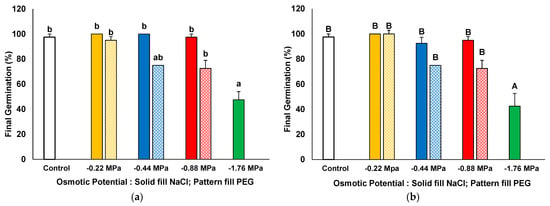
Figure 2.
Final germination percentages in Ipomoea purpurea (a) and I. tricolor (b) after 21 days of treatment with increasing iso-osmotic concentrations of NaCl and PEG. Control: germination in distilled water. The values plotted are the means ± SE (n = 4). Different lowercase and uppercase letters within the bars indicate significant differences between treatments, for each species, according to the Tukey post hoc test (p < 0.05).
In addition to germination percentages, germination speed was calculated as mean germination time (MGT). Both species had very fast germination in the absence of stress, as low as 1.5 days (Figure 3). For both species, MGT increased gradually in parallel to the increase in NaCl concentration in the germination medium. However, significant differences were only observed for 400 mM NaCl, reaching 8.7 and 7.8 days in I. purpurea (Figure 3a) and I. tricolor (Figure 3b), respectively. On the other hand, the effect of PEG on germination time was stronger than that of NaCl since a significant increase of close to 7 days in both species was registered at a PEG osmotic potential of −0.88 MPa (Figure 3).
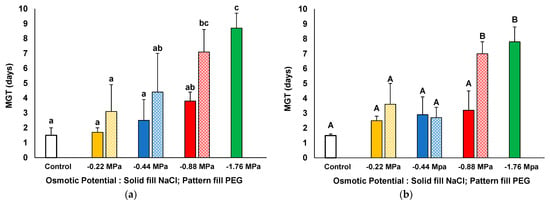
Figure 3.
Mean germination time (MGT) in I. purpurea (a) and I. tricolor (b) after 21 days of treatment with increasing iso-osmotic concentrations of NaCl and PEG. Control: germination in distilled water. The values plotted are the means ± SE (n = 4). Different lowercase and uppercase letters within the bars indicate significant differences between treatments, for each species, according to the Tukey post hoc test (p < 0.05). For both species, no germination was observed for PEG treatments equivalent to −1.79 MPa; thus, no MGT is measured and plotted for this condition.
Other germination parameters, such as the first day of germination (FGD), last day of germination (LGD), and total spread of germination (TSG), indicate that the two species have very rapid germination in the absence of stress. For both species under control conditions, germination started on the first day and finished before the fifth day (Table 1) of the trial. Indeed, a shorter TSG was found in I. purpurea (2.8) than in I. tricolor (3.5) (Table 1). A significant germination delay was recorded for I. purpurea at −0.88 MPa, either with salt or PEG treatments (Table 1). In contrast, for I. tricolor, it was only observed at −1.76 MPa (Table 1). Although a delay in the last day of germination and an extension of the germination spread were recorded in both species under NaCl and PEG, these were not significantly different from the control due to the large variability between replicates.

Table 1.
Germination parameters related to the velocity of germination. Control: germination in distilled water. Values are the means ± SE (n = 4). Different lowercase and uppercase letters indicate significant differences between treatments, for each species, according to the Tukey post hoc test (p < 0.05).
After 21 days, the seedlings’ length was analysed by measuring radicle and hypocotyl length separately (Table 2). Although radicle emergence was observed in seeds subjected to the highest NaCl concentration (400 mM) in the two species, the radicle did not grow over 2–3 mm, and seedlings were not viable. Thus, the osmotic potential of −1.76 MPa inhibited post-germination development under NaCl and PEG treatments in the two species. In I. purpurea, radicle length was significantly reduced, starting with the −0.88 MPa osmotic potential generated by PEG and NaCl, but hypocotyl length was significantly reduced in all stress treatments. The seedling vigour index (SVI) decreased significantly, starting with the −0.44 MPa osmotic potential treatment. Germination under 400 mM NaCl was blocked and was not even initiated in the PEG treatment at the same osmotic potential in I. purpurea and I. tricolor. However, in the latter species, radicle and hypocotyl length did not undergo significant reductions with respect to the control under all other experimental conditions tested; also, a significant reduction in SVI was only observed at an osmotic potential of -0.88 MPa (Table 2). Thus, I. tricolor showed better resistance to high osmotic pressure provoked by NaCl and PEG than I. purpurea.

Table 2.
Seedlings analysis after 21 days, at the end of the germination assays. Control: germination in distilled water. Values are the means ± SE (n = 4). Different lowercase and uppercase letters indicate significant differences between treatments, for each species, according to the Tukey post hoc test (p < 0.05).
3.2. Plant Growth
Both species had rapid growth, increasing during the three weeks of treatments by 105 cm in I. purpurea and 126 cm in I. tricolor (Figure 4b), reaching heights at the harvest date of 1.96 m for the former and 2.2 m for the latter. The roots were considerably shorter than the aerial part and showed a similar size in I. purpurea and I. tricolor under control conditions, circa 91 cm and 74 cm, respectively (Figure 4a). Plant growth was inhibited under stress conditions for both species, mainly by water stress followed by the higher salt concentration, but not so much by the 100 mM NaCl solution. For instance, the growth of I. purpurea roots was reduced 3-fold by water stress and high (i.e., 200 mM NaCl) salt concentration, whereas water stress but not salt stress treatment shortened I. tricolor roots (Figure 4a). On the other hand, the increase in stem length, calculated as the differences between final and initial stem length, revealed only a significant 1.6-fold reduction in the water-stressed I. tricolor plants (Figure 4b).
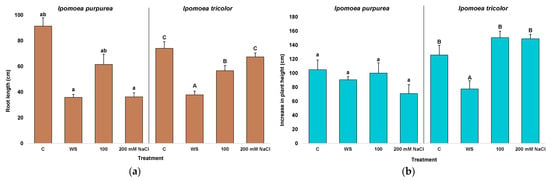
Figure 4.
Root length (a) and increase in stem length (b) after three weeks of treatment in the two Ipomoea species. The values plotted are the means ± SE (n = 5). Different lowercase and uppercase letters indicate significant differences between treatments, for each species, according to the Tukey post hoc test (p < 0.05).
The fresh weight (FW) of roots, stems, and leaves was registered. The highest FW was found in the two species in the absence of stress, with an average total FW of 37 g in I. purpurea and 42 g in I. tricolor. Under stress, a similar pattern of variation was found in the two species, with the strongest effect induced by the water stress treatment, followed by 200 mM NaCl (Figure 5a). Specifically, under water stress, there was a marked 14-fold reduction in I. purpurea root FW, an 11-fold reduction in I. tricolor root FW, a 3.2- and 4.3-fold reductions in leaf FW, and a 1.4- and a 2.2-fold in steam FW registered for I. purpurea and I. tricolor, respectively (Figure 5a). Only a small variation with respect to control plants was recorded in 100 mM NaCl growing plants, significant only in I. purpurea roots (Figure 5a).
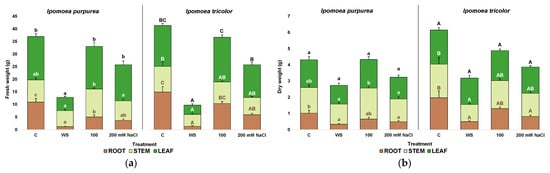
Figure 5.
Fresh weight (a) and dry weight (b) of roots, stems and leaves after three weeks of treatment in the two Ipomoea species. The values plotted are the means ± SE (n = 5). Different lowercase and uppercase letters indicate significant differences between treatments, for each species, according to the Tukey post hoc test (p < 0.05).
Similarly, the dry weight of roots, stems, and leaves was registered. The highest dry weight values for roots, stems, and leaves were found in plants grown under control conditions, slightly lower for I. purpurea than in I. tricolor plants (Figure 5b). These values agree with the fresh weight values observed (Figure 5a). The pattern of DW variation in plants subjected to stress treatments was similar to that of FW, although reductions between treatments were not as marked. The most significant DW decrease was recorded in water-stressed plants, especially in roots (3-fold in I. purpurea and 4-fold in I. tricolor, Figure 5b). Salt treatments had no effect in stem and leaf DW, neither in I. purpurea nor I. tricolor, and significant losses were only registered in the roots of plants treated with 200 mM NaCl for both species (Figure 5b).
The smaller reduction in dry weight than in fresh weight was related to the water loss under the stress treatment, shown in Figure 6. The strongest dehydration occurred in the plants of the water stress treatments, where significant variations from the control were recorded in the water content of roots and leaves but not of stems. I. purpurea plants under water stress showed a 1.6 and 1.1-fold reduction compared to the control in roots and leaves, respectively (Figure 6a), whereas in I. tricolor the water loss was more pronounced in leaves (1.6-fold) than in roots (1.4-fold; Figure 6b).
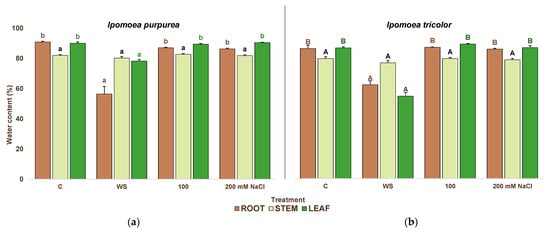
Figure 6.
Water content in roots, stems, and leaves in I. purpurea (a) and I. tricolor (b) after three weeks of treatment in the two Ipomoea species. The values plotted are the means ± SE (n = 5). Different lowercase and uppercase letters indicate significant differences between treatments, for each species, according to the Tukey post hoc test (p < 0.05).
3.3. Photosynthetic Pigments
The highest concentration of leaf photosynthetic pigments was recorded in plants grown under control conditions. Chlorophyll values were higher in I. purpurea than in I. tricolor (8.74 and 5.35 mg/g DW), whereas chlorophyll b concentrations were similar (Figure 7a). Carotenoid concentrations were also higher in I. purpurea (1.5 mg/g DW) than in I. tricolor (1.0 mg/g DW) (Figure 7b). Salinity but no water stress had a negative effect on chlorophyll a and carotenoid concentrations in I. purpurea, whereas I. tricolor accumulated similar pigment concentrations under all growing conditions (Figure 5a,b). Chlorophyll b concentrations were constant for both species in all treatments.
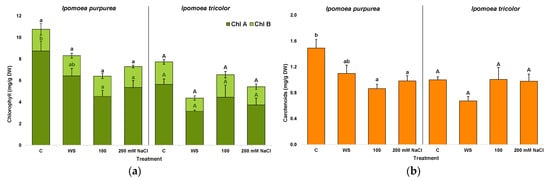
Figure 7.
Chlorophylls a and b (a) and carotenoids (b) after three weeks of treatment in the two Ipomoea species. The values plotted are the means ± SE (n = 5). Different lowercase and uppercase letters indicate significant differences between treatments, for each species, according to the Tukey post hoc test (p < 0.05).
3.4. Ion Contents
As expected, an increase in Na+ and Cl− concentrations were only found in the salt-treated plants but not in the water stress treatment (Table 3). However, the pattern of Na+ accumulation was different in the two species. In I. purpurea roots, a significant 2.3- and 1.8-fold increase was measured in 100 and 200 mM NaCl-treated plants, respectively, but no variation was observed in leaves, neither in water-stressed nor salt-treated plants (Table 3). Surprisingly, an opposite pattern was observed for I. tricolor; Na+ concentrations increased significantly only in the leaves of salt-treated plants (1.7-fold higher in 200 mM NaCl-treated plants, compared to control plants) but not in the roots (Table 3). On the other hand, Cl− concentration measured in the two species increased significantly in roots and leaves in the salt stress treatments but not in the water-stressed plants. Only I. tricolor grown under water stress showed a significant decrease of Cl− in roots (Table 3). A difference in the accumulation pattern of these two monovalent ions was observed in the two species, Na+ concentrations were substantially higher in roots than in leaves, whereas Cl− concentrations were similar in both organs.

Table 3.
Root and leaf ion concentrations after three weeks of treatment in plants of the two Ipomoea species. The values are the means ± SE (n = 5). Different lowercase and uppercase letters indicate significant differences between treatments, for each species, according to the Tukey post hoc test (p < 0.05).
A significant decrease in K+ concentrations was measured in the roots of salt-treated plants of both species but not in those of water-stressed plants (Table 3). K+ concentration in the roots of 200 mM NaCl-treated I. purpurea plants was reduced by ca. 50% with respect to control plants, and an even more substantial decrease was observed in I. tricolor. Regarding leaf K+ levels, they were not affected by the water or salt stress treatments in I. purpurea but increased significantly in plants of I. tricolor grown in the presence of 200 mM NaCl (Table 3). In non-stressed control plants, K+ concentrations were similar in roots and leaves.
Finally, Ca2+ levels in control plants were higher in leaves than in roots, about 3.5- and 1.6-fold in I. purpurea and I. tricolor, respectively. In both species, Ca2+ root or leaf contents were not significantly affected by the water stress treatment but increased in response to salt stress (Table 3).
3.5. Osmolytes Contents
Proline (Pro) and total soluble sugars (TSS) were quantified in the leaf tissue of all plants harvested after the different treatments. In I. purpurea, no significant differences were found in Pro contents between control and water-stressed or salt-stressed plants (Figure 8a, left). On the contrary, in I. tricolor Pro increased significantly, ca. 18-fold over control values, in plants subjected to water stress; salt stress also induced the accumulation of Pro, although to a lesser extent and with significant differences with respect to non-stressed controls observed only in the 200 mM NaCl-treated plants (Figure 8a, right). In any case, it should be pointed out that absolute Pro values are too low to have any significant osmotic effect.
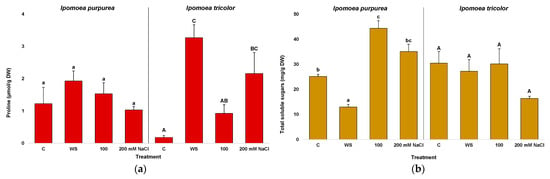
Figure 8.
Proline (a) and total soluble sugars (b) after three weeks of treatment in the two Ipomoea species. The values plotted are the means ± SE (n = 5). Different lowercase and uppercase letters indicate significant differences between treatments, for each species, according to the Tukey post hoc test (p < 0.05).
A different pattern was observed for TSS contents, which decreased significantly in I. purpurea plants subjected to water stress and increased in response to salt treatments, especially at 100 mM NaCl (Figure 8b, left). On the other hand, in I. tricolor, TSS levels did not vary significantly in the stressed plants with respect to those grown under control conditions (Figure 8b, right).
3.6. Multivariate Analysis
The mean values of the germination and seedling data were used in a Principal Component Analysis (PCA). The variables considered were clustered by the PCA and reduced to two main components with an eigenvalue greater than one, which together accounted for 86.65% of the total variability. The loading plots of the vectors and the scores of the two species in relation to these components are shown in Figure 9. Most of the overall variability of the analysed data was explained by the first component (72.74%). The variables with a positive correlation with the highest weight value in this component were the germination percentage, hypocotyl length (HL), and seedling vigour index (SVI). The variables related to the speed of germination, namely the first germination day (FGD), the last germination day (LGD), and the mean time of germination (MG), were negatively correlated. The first component separated the scores from the control treatments on its positive side and those of lower osmotic potential on the negative side. The second component, explaining an additional 13.90% of the total variability, was positively correlated with radicle length (RL) and the total spread of germination (TSG) and negatively correlated with the final percentage of germination (Germ %). The scores of I. purpurea at −0.88 MPa osmotic potential were separated along the OY axis, with the PEG score on the positive and the NaCl score on the negative extreme.
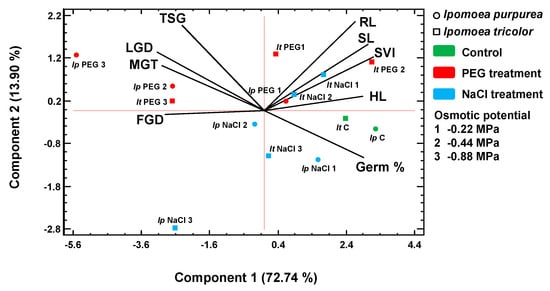
Figure 9.
Principal Component Analysis of germination data of I. purpurea and I. tricolor. Loading and scatter plots of the PCA scores were conducted with germination and seedling traits. Abbreviations: Germ %, final percentage of germination; MGT, mean germination time; FGD, first germination day; LGD, last germination day; TSG, total spread of germination; RL, radicle length, RL; HL, hypocotyl length; SL, seedling length; SVI, seedling vigour index.
Growth and biochemical parameters were combined in a second PCA (Figure 10). Only variables that changed significantly were taken into consideration. Four components had an eigenvalue higher than one, accounting for 95% of the total variability. The first, explaining 42.74% of the variation, was positively correlated with the fresh weight of leaves (FWl), the water content of leaves (WCl) and Ca2+ concentrations in roots and leaves, and negatively correlated with K+ in roots. The second axis, explaining 25.97% of the data variability, was positively related to root length (RL), root fresh weight (FWr), root dry weight (DWr) and chlorophyll a and negatively correlated with proline (Pro), Ca2+ and Cl− in roots.
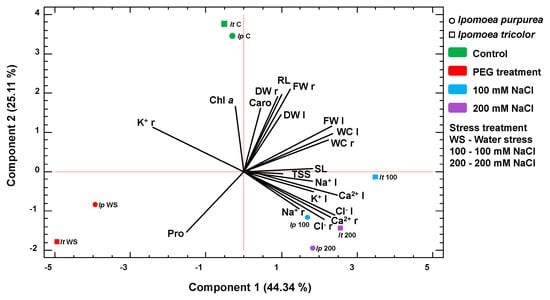
Figure 10.
Principal Component Analysis of growth and biochemical data of Ipomoea purpurea and I. tricolor. Loading and scatter plots of the PCA scores were conducted only with the parameters that showed a significant correlation. Abbreviations: C, control; 100, 100 mM NaCl; 200, 200 mM NaCl; WS, water stress; FW r, root fresh weight; FW l, leaf fresh weight; DW r, root dry weight; DW l, leaf dry weight; WC r, root water content; WC l, leaf water content; Chl a chlorophyll a; Caro, carotenoids; Pro, proline; TSS, total soluble sugars; Na r, root sodium content; Na l, leaf sodium content; K r, root potassium content; K l, leaf potassium content; Cl r, root chlorine content; Cl l, leaf chlorine content; Ca r, root calcium content; Ca l, leaf calcium content.
4. Discussion
High seed production and efficient vegetative propagation are common traits of invasive species, regardless of their phylogenetic relationships or ecology. Sexually reproducing invasive species usually produce a large number of seeds, ensuring a high rate of offspring. However, their ability to germinate earlier and faster is more relevant than their usually high germination rates [47,48,49]. The two Ipomoea species tested showed a very high germination percentage in the absence of stress, which is a common trait in commercial seeds of ornamental species. However, the most remarkable feature was their very rapid germination, with a high percentage of seeds already germinated on the first day, whereby a very short germination spread (TSG) was found, especially in I. purpurea, with an average of 2.8 days. Rapid germination is a functional trait that confers advantages in the early stages of interspecific competition [50,51]. Invasive species of ornamental origin are favoured by a selective introduction [51,52], with horticulture being the largest source of plant invasions [20]. The two Ipomoea species were also characterised by rapid seedling growth, which may play an additional role in outcompeting species with slower germination and seedling development [51]. Another exceptionally relevant trait characterising many invasive species is their ability to germinate in a wide range of environmental conditions [51,53,54,55]. Global warming is increasing the risk of exposure to unfavourable conditions and favours species with greater abiotic resistance. Comparison of germination success under environmentally constrained conditions between native and invasive species revealed in many cases that the latter have wider ranges of tolerance to temperatures and water potentials [56,57]. Low water availability delayed the germination of alien species less than that of native species coexisting in the same habitat in SW Australia [58], and salinity did not affect germination rates of woody invasive species in inland soils of the Mississippi region, contributing to the spread of these species [59]. The invasive Spartina densiflora in SW Spain had a broader range of salt tolerance than a cordgrass species native to the SW Iberian Peninsula [60], and its seeds germinated even at the hypersaline conditions of 0.75 M NaCl. The two Ipomoea species analysed here maintained over 90% germination in saline solutions up to 200 mM NaCl. In the treatment with 400 mM NaCl over 40% of seed-initiated germination was measured as radicle emergence, but their development was stopped immediately as this concentration was lethal for all seedlings. Seeds also germinated in the treatment with increasing concentrations of PEG, but in the two species, the percentage of germination at low osmotic potentials was reduced than in the salt treatments. Salinity affects germination due to the accumulation of toxic levels of Na+ and Cl− and to its osmotic component, as increased osmotic potential prevents water uptake and alters water imbibition by seeds [61,62]. Germination in polyethylene glycol (PEG) solutions is the standard method to test this osmotic effect, which mimics environmental drought conditions [54,63]. Similar findings indicating that germination is more affected by osmotic stress than by ionic toxicity have been previously reported in I. purpurea [64,65,66]. Additionally, in agreement with the results shown here, high germination percentages under salt stress conditions were found in this species [65,66], as in others of this genus [67,68]. A comparative study on several environmental constraints in I. purpurea revealed that germination was more affected by temperature than by salinity and seedling emergence by flooding and burial depths of over 13 cm [69]. The two species analysed here had similar germination patterns and percentages, except germination at −0.88 MPa osmotic potential in I. purpurea, which was more affected by stress than I. tricolor. The seedlings analysis also indicated a relatively higher tolerance to NaCl and PEG of the latter, as only in I. purpurea stress treatments significantly reduced the length of radicle and hypocotyl.
The two species have not only a high velocity of germination but also fast growth. I. purpurea has been reported to have a growth of about 20 cm per day under optimal conditions [70], although, under our growing conditions, an average increase of only 5 cm daily was recorded in plants from the control treatments. A higher growth rate of about 6 cm/day in control and 7 cm/day in plants from the 100 mM NaCl was found in I. tricolor. Quick growth is an essential trait of weeds [71] often associated with the species’ invasive potential [72]. In circumstances where there is competition, plants that grow faster have an advantage as they can emerge from the vegetation to exploit photosynthetic resources [73]. Both Ipomoea species are vining weeds, able to compete by “choking growth” [70]. Growth of the two species was not hampered at 100 mM NaCl and only a few parameters were significantly reduced at 200 mM, indicating that the two species are moderately salt-tolerant. Salt tolerance is a common trait in this genus, which has been reported in species such as the littoral or wetland species I. cairica [74], I. sagittata [75], I. pescaprae [76], or even in the sweet potato I. batatas [77]. However, the growth of the two species was severely affected by lack of irrigation, as reflected in the significant reduction of most of the traits analysed, as previously reported in I. purpurea [78]. Severe drought, in combination with leaf damage, had a drastic effect on the growth of this species [79], but other studies indicate a substantial plasticity of its ecophysiological traits [80].
Biochemical analysis indicated a variation of photosynthetic pigments in stressed plants, but significant reductions in chlorophyll a and carotenoids were only observed in I. purpurea, indicating a possible higher tolerance of I. tricolor, where only small, non-significant fluctuations were observed between treatments. Total chlorophyll and carotenoid concentrations correlated well with growth parameters and were recommended as reliable stress markers in multi-parameter assessments in the congener I. aquatica [81].
The compatible solutes analysed, proline (Pro) and total soluble sugars (TSS), showed a different pattern in the two species. Proline contents increased significantly, especially in I. tricolor plants subjected to water stress, followed by those subjected to 200 mM NaCl. A smaller and not significant increase was registered in I. purpurea, but its levels of Pro in control plants were considerably higher than in the other species, which had only a low content in the absence of stress. On the other hand, TSS increased significantly only in I. purpurea plants subjected to salt treatments. Proline, one of the most common osmolytes in plants, has an essential role in stress responses [82]. In addition to its function in osmotic adjustment, Pro plays multiple additional functions under stress, such as acting as a low-molecular-weight chaperone, metal chelator, ROS scavenger involved in antioxidant defence mechanisms, or signalling molecule [82,83,84]. The maximum absolute Pro concentrations reached in the two Ipomoea species are insufficient to produce a significant osmotic effect but are in the same range as those reported in the halophyte I. pescaprae [85]. However, there is evidence of Pro implication in stress tolerance in species of this genus, based on its additional biological functions. In a study on transgenic sweet potatoes, plants overexpressing IbSIMT1 accumulated more proline, which improved their salt tolerance not only by maintaining osmotic balance but also by activating SOD gene expression and enhancing ROS scavenging capacity [77]. Proline was reported to play an important role in drought resistance in Ipomoea, well documented in sweet potato and its hybrids [86,87,88]. Several publications also revealed the role of TSS in salt tolerance in sweet potatoes [87,89], although these compounds are involved in many physiological processes ranging from seed germination and flowering to plant senescence; therefore, variations in their concentrations are not always related to stress defence mechanisms [90,91].
Regulation of ion uptake and transport is of great importance in the response of plants to salinity stress. Halophytic dicots are generally salt includers, increasing the uptake and transport to the shoots of Na+ and Cl− where they are sequestered in vacuoles [92], whereas glycophytes and halophytic monocots are salt excluders. Their main mechanism of resistance to salt stress is to avoid the foliar accumulation of toxic ions, either by reducing the uptake by the roots or by blocking their transport to the aerial parts of the plant [93,94]. We detected differences between the two species in relation to the pattern of Na+ accumulation. Under salt stress, the root levels of this cation increased in I. purpurea, but not in I. tricolor plants; in leaves, on the contrary, they were maintained in I. purpurea and increased in I. tricolor plants, although only under 200 mM NaCl, the highest salinity tested. Most important, leaf Na+ concentrations were maintained lower in leaves than in roots under all tested experimental conditions. These data suggest the presence of mechanisms blocking Na+ transport to the aerial part of the plants, slightly more efficient in I. purpurea than in I. tricolor, which could contribute to salt tolerance in these species. In contrast, Cl− increased in both roots and leaves of the two species in response to salt and showed similar concentrations in roots and leaves.
Na+ accumulation is generally accompanied by decreased intracellular K+ levels, as the two cations compete for the same transporters, and increased Na+ concentrations inhibit K+-requiring enzymes [93]. K+ plays an essential role in multiple physiological processes in plants, and its homeostasis is a general adaptive trait to different environmental stresses [95]. The primary survival mechanism of many glycophytes under saline conditions is the regulation of Na+ transport and increased K+ uptake and accumulation [96]. Following the general pattern of response to salt stress, a significant decrease in root K+ content was observed in both Ipomoea species, somewhat more pronounced in I. tricolor. However, foliar K+ concentrations remained constant in I. purpurea and even increased in 200 mM NaCl-treated plants of I. tricolor. This finding indicates that K+ transport from the roots to the aerial part of the plants is activated under salt stress slightly more efficiently in I. tricolor than in I. purpurea, which probably represents a relevant tolerance mechanism in the two analysed species. Reports on other species of the genus support this idea. For example, a transcriptome profiling of salt-tolerant I. imperati revealed that one of the most promising genes for tolerance, HKT1 (high-affinity potassium transporter), was over-represented in salt-stressed tissue libraries [97]. Moreover, in a comparative analysis of salt tolerance of 12 sweet potato genotypes, the more tolerant ones retained higher K+ levels in their shoots under increasing salinity, revealing the importance of K+ as the “main driver of salinity tolerance” in this species [98]. Salt and drought tolerance in transgenic sweet potato was enhanced by IbNHX2, a vacuolar Na+/K+ antiporter gen [99], whereas NXH1 involved in the active transport of Na+ and/or K+ from the cytosol to the vacuoles was found to be responsible for an increased vacuolar pH in the petals of I. tricolor, which triggers a change in colour from purple-red to blue during flower opening [100].
Finally, the bivalent cation Ca2+ showed substantially higher concentrations in leaves than in roots in both species. Regarding changes in Ca2+ contents in response to the salt stress treatments, they increased significantly in the roots of the two species and the leaves only of I. tricolor plants. The role of Ca2+ in salt tolerance mechanisms is well established and has been previously reported, for example, in the related species I. batatas [89]. Calcium is crucial for the structure and functional integrity of plants, as it is involved in the stabilisation of membrane and cell wall structures, regulating ion transport and selectivity, or cell wall enzyme activities. Under stress conditions, Ca2+ is a key component of stress signalling pathways that trigger essential stress tolerance mechanisms, including accumulation of osmoprotectants, stimulation of antioxidants, polyamines and nitric oxide machinery [101].
5. Conclusions
The knowledge of the limits of stress tolerance of invasive species is extremely relevant, as they can predict how specific invasive species may behave under an altered climate and which new species may emerge as invasive. Our results indicate that the two Ipomoea species are relatively tolerant to salinity but susceptible to water stress in the analysed developmental stages, seed germination and vegetative growth. Salt tolerance is based mainly on blocking Na+ while activating K+ transport from roots to shoots and the uptake and accumulation of Ca2+ in response to increased soil salinity. The two species responded similarly to salt stress, although these tolerance mechanisms appear to be more efficient in I. tricolor than in I. purpurea, so the former species is slightly more tolerant. Currently, I. purpurea is generally recognised as a common invasive weed, whereas I. tricolor is considered ‘low-risk’, only locally reported as invasive. However, our results indicate that I. tricolor may have an invasive potential as high, if not higher than I. purpurea, and spread into new areas, affecting cropland or natural habitats of ecological interest with moderate salinity. Such studies could be applied to practice when monitoring natural areas of high ecological value, such as wetlands, where early warning and eradication programmes against stress-tolerant invasive species are necessary under changing climatic conditions.
Author Contributions
M.B. and R.M.; methodology. D.-M.M., R.L. and L.B.G.; software. D.-M.M. and A.F.S.; validation. R.E.S., A.F.S. and R.M.; formal analysis. M.B.; investigation. D.-M.M., R.L. and L.B.G.; resources. R.M. and O.V.; data curation. D.-M.M. and R.L.; writing—original draft preparation. D.-M.M. and M.B.; writing—review and editing. R.M., R.E.S. and O.V.; visualisation. D.-M.M. and A.F.S.; supervision. M.B. and R.M.; project administration. M.B.; funding acquisition. O.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
D.-M.M. is supported by a pre-doctoral contract from the Polytechnic University of Valencia, Spain and R.M. by a CDEIGENT (2018/2023) grant from Generalitat Valenciana.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wilcove, D.S.; Rothstein, D.; Dubow, J.; Phillips, A.; Losos, E. Quantifying threats to imperiled species in the United States. Bioscience 1998, 48, 607–615. [Google Scholar] [CrossRef]
- Hayden Reichard, S.; White, P. Horticulture as a pathway of invasive plant introductions in the United States: Most invasive plants have been introduced for horticultural use by nurseries, botanical gardens, and individuals. Bioscience 2001, 51, 103–113. [Google Scholar] [CrossRef]
- Castro-Díez, P.; Alonso, Á. Alteration of Nitrogen cycling as a result of invasion. In Impact of Biological Invasions on Ecosystem Services; Springer International Publishing: Cham, Switzerland, 2017; pp. 49–62. [Google Scholar]
- Catford, J.A. Hydrological impacts of biological invasions. In Impact of Biological Invasions on Ecosystem Services; Springer International Publishing: Cham, Switzerland, 2017; pp. 63–80. [Google Scholar]
- Vilà, M.; Hulme, P.E. Non-Native species, ecosystem services, and human well-being. In Impact of Biological Invasions on Ecosystem Services; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–14. [Google Scholar]
- Bacher, S.; Blackburn, T.M.; Essl, F.; Genovesi, P.; Heikkilä, J.; Jeschke, J.M.; Jones, G.; Keller, R.; Kenis, M.; Kueffer, C.; et al. Socio-economic impact classification of alien taxa (SEICAT). Methods Ecol. Evol. 2018, 9, 159–168. [Google Scholar] [CrossRef]
- Neill, P.E.; Arim, M. Human health link to invasive species. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 570–578. [Google Scholar]
- Denóbile, C.; Chiba de Castro, W.A.; Silva Matos, D.M.d. Public health implications of invasive plants: A scientometric study. Plants 2023, 12, 661. [Google Scholar] [CrossRef] [PubMed]
- Bayón, Á.; Godoy, O.; Vilà, M. Invasion risks and social interest of non-native woody plants in urban parks of mainland Spain. Anales Jard. Bot. 2022, 79, e121. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Renteria, J.L.; Darin, G.M.S.; Grosholz, E.D. Assessing the risk of plant species invasion under different climate change scenarios in California. Invasive Plant Sci. Manag. 2021, 14, 172–182. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Mollot, G.; Pantel, J.H.; Romanuk, T.N. The effects of invasive species on the decline in species richness. In A Global Meta-Analysis; David, A., Bohan, A.J., Dumbrell, F.M., Eds.; Advances in ecological research; Academic Press: Cambridge, MA, USA, 2017; Volume 56, pp. 61–83. [Google Scholar]
- Kueffer, C.; Pyšek, P.; Richardson, D.M. Integrative invasion science: Model systems, multi-site studies, focused meta-analysis and invasion syndromes. New Phytol. 2013, 200, 615–633. [Google Scholar] [CrossRef]
- van Kleunen, M.; Weber, E.; Fischer, M. A Meta-Analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 2010, 13, 235–245. [Google Scholar] [CrossRef]
- Yang, B.; Cui, M.; Du, Y.; Ren, G.; Li, J.; Wang, C.; Li, G.; Dai, Z.; Rutherford, S.; Wan, J.S.H.; et al. Influence of multiple global change drivers on plant invasion: Additive effects are uncommon. Front. Plant. Sci. 2022, 13, 1020621. [Google Scholar] [CrossRef] [PubMed]
- Seebens, H.; Bacher, S.; Blackburn, T.M.; Capinha, C.; Dawson, W.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; Kleunen, M.; Kühn, I.; et al. Projecting the continental accumulation of alien species through to 2050. Glob. Chang. Biol. 2021, 27, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.E.; Brundu, G.; Carboni, M.; Dehnen-Schmutz, K.; Dullinger, S.; Early, R.; Essl, F.; González-Moreno, P.; Groom, Q.J.; Kueffer, C.; et al. Integrating invasive species policies across ornamental horticulture supply chains to prevent plant invasions. J. Appl. Ecol. 2018, 55, 92–98. [Google Scholar] [CrossRef]
- van Kleunen, M.; Essl, F.; Pergl, J.; Brundu, G.; Carboni, M.; Dullinger, S.; Early, R.; González-Moreno, P.; Groom, Q.J.; Hulme, P.E.; et al. The changing role of ornamental horticulture in alien plant invasions. Biol. Rev. 2018, 93, 1421–1437. [Google Scholar] [CrossRef]
- Dai, Z.-C.; Zhu, B.; Wan, J.S.H.; Rutherford, S. Editorial: Global changes and plant invasions. Front. Ecol. Evol. 2022, 10, 845816. [Google Scholar] [CrossRef]
- Bellard, C.; Thuiller, W.; Leroy, B.; Genovesi, P.; Bakkenes, M.; Courchamp, F. Will climate change promote future invasions? Glob. Chang. Biol. 2013, 19, 3740–3748. [Google Scholar] [CrossRef]
- Bradley, B.A.; Wilcove, D.S.; Oppenheimer, M. Climate change increases risk of plant invasion in the eastern United States. Biol. Invasions 2010, 12, 1855–1872. [Google Scholar] [CrossRef]
- Dullinger, I.; Wessely, J.; Bossdorf, O.; Dawson, W.; Essl, F.; Gattringer, A.; Klonner, G.; Kreft, H.; Kuttner, M.; Moser, D.; et al. Climate change will increase the naturalization risk from garden plants in Europe. Glob. Ecol. Biogeogr. 2017, 26, 43–53. [Google Scholar] [CrossRef]
- Van der Veken, S.; Hermy, M.; Vellend, M.; Knapen, A.; Verheyen, K. Garden plants get a head start on climate change. Front. Ecol. Environ. 2008, 6, 212–216. [Google Scholar] [CrossRef]
- Richards, C.L.; Bossdorf, O.; Muth, N.Z.; Gurevitch, J.; Pigliucci, M. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol. Lett. 2006, 9, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Zenni, R.D.; Lamy, J.-B.; Lamarque, L.J.; Porté, A.J. Adaptive evolution and phenotypic plasticity during naturalization and spread of invasive species: Implications for tree invasion biology. Biol. Invasions 2014, 16, 635–644. [Google Scholar] [CrossRef]
- Miller, R.E.; Rausher, M.D.; Manos, P.S. Phylogenetic systematics of Ipomoea (Convolvulaceae) based on ITS and Waxy sequences. Syst. Bot. 1999, 24, 209. [Google Scholar] [CrossRef]
- Morales Rodríguez, A.; Morales Tejón, A.; Rodríguez del Sol, D.; Javier, I.; Vargas, P.; Aracelis Méndez, C. Origen, evolución y distribución del boniato (Ipomoea batatas (L.) Lam.). Una revisión. Rev. Agric. Trop. 2017, 3, 1–13. [Google Scholar]
- Baucom, R.S.; Chang, S.M.; Kniskern, J.M.; Rausher, M.D.; Stinchcombe, J.R. Morning glory as a powerful model in ecological genomics: Tracing adaptation through both natural and artificial selection. Heredity 2011, 107, 377–385. [Google Scholar] [CrossRef]
- Randall, R.P. A Global Compendium of Weeds. Perth. Australia: Department of Agriculture and Food Western Australia. 2012. Available online: http://www.cabi.org/isc/FullTextPDF/2013/20133109119.pdf (accessed on 23 June 2023).
- Sanz-Elorza, M.; Dana, E.D.; Sobrino Vesperinas, E. Atlas de las plantas alóctonas invasoras en España. In Dirección General para la Biodiversidad; Gobierno de España: Madrid, Spain, 2004. [Google Scholar]
- Defelice, M.S. Tall morning glory, Ipomoea purpurea (L.) Roth—Flower or foe? Weed Technol. 2001, 15, 601–606. [Google Scholar] [CrossRef]
- Rojas-Sandoval, J.; Acevedo-Rodríguez, P. Ipomoea purpurea (Tall Morning Glory). CABI Compendium 2022. CABI Compendium. 2014. [Google Scholar] [CrossRef]
- Halvorson, W.L.; Guertin, P. USGS Weeds in the West Project: Status of Introduced Plants in Southern Arizona Parks, Plant Fact Sheets Prepared for Tuzigoot National Monument, Tucson, AZ: U.S. Geological Survey. 2003. Available online: http://sdrsnet.srnr.arizona.edu/index.php?page=datamenu&lib=2&sublib=13 (accessed on 23 June 2023).
- Oviedo Prieto, R.; Herrera Oliver, P.; Caluff, M.G.; Regalado, L.; Ventosa Rodríguez, I.; Plasencia Fraga, J.M.; Baró Oviedo, I.; González Gutiérrez, P.A.; Pérez Camacho, J.; Hechavarría Schwesinger, L.; et al. National list of invasive and potentially invasive plants in the Republic of Cuba—2011. (Lista nacional de especies de plantas invasoras y potencialmente invasoras en la República de Cuba—2011). Bissea Boletín Sobre Conserv. Plantas Jardín Botánico Nac. Cuba. 2012, 6, 22–96. [Google Scholar]
- Guillot Ortiz, D. Ipomea nil (L.) Roth e I. hederacea (L.) Jacquin, Dos especies invasoras nuevas para la flora Valenciana. Acta Bot. 2006, 31, 153–156. [Google Scholar] [CrossRef]
- Villaseñor, R.J.L.; Espinosa, F.J.G. Catálogo de malezas de México. Universidad Nacional Autónoma de México. In Consejo Nacional Consultivo Fitosanitario; Fondo de Cultura Económica: Mexico City, Mexico, 1998. [Google Scholar]
- Onen, H.; Farooq, S.; Muñoz-Rodríguez, P.; Alharbi, S.A.; Alfarraj, S. Ipomoea tricolor (Convolvulaceae) in Turkey: New occurrence record and potential spread areas under current climatic conditions. J. King. Saud Univ. Sci. 2023, 35, 102543. [Google Scholar] [CrossRef]
- Ben-Gal, A.; Borochov-Neori, H.; Yermiyahu, U.; Shani, U. Is osmotic potential a more appropriate property than electrical conductivity for evaluating whole-plant response to salinity? Environ. Exp. Bot. 2009, 65, 232–237. [Google Scholar] [CrossRef]
- Ellis, R.A.; Roberts, E.H. The quantification of aging and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiple criteria. Crop. Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Weimberg, R. Solute adjustments in leaves of two species of wheat at two different stages of growth in response to salinity. Physiol. Plant 1987, 70, 381–388. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Baker, H.G. The evolution of weeds. Annu. Rev. Ecol. Syst. 1974, 5, 1–24. [Google Scholar] [CrossRef]
- Moravcová, L.; Pyšek, P.; Jarošík, V.; Pergl, J. Getting the right traits: Reproductive and dispersal characteristics predict the invasiveness of herbaceous plant species. PLoS ONE 2015, 10, e0123634. [Google Scholar] [CrossRef]
- Gioria, M.; Pyšek, P. Early bird catches the worm: Germination as a critical step in plant invasion. Biol. Invasions 2017, 19, 1055–1080. [Google Scholar] [CrossRef]
- Grime, J.P.; Mason, G.; Curtis, A.V.; Rodman, J.; Band, S.R. A comparative study of germination characteristics in a local flora. J. Ecol. 1981, 69, 1017. [Google Scholar] [CrossRef]
- Gioria, M.; Pyšek, P. The legacy of plant invasions: Changes in the soil seed bank of invaded plant communities. Bioscience 2016, 66, 40–53. [Google Scholar] [CrossRef]
- Chrobock, T.; Kempel, A.; Fischer, M.; van Kleunen, M. Introduction bias: Cultivated alien plant species germinate faster and more abundantly than native species in Switzerland. Basic Appl. Ecol. 2011, 12, 244–250. [Google Scholar] [CrossRef]
- Yuan, X.; Wen, B. Seed germination response to high temperature and water stress in three invasive Asteraceae weeds from Xishuangbanna, SW China. PLoS ONE 2018, 13, e0191710. [Google Scholar] [CrossRef] [PubMed]
- Bellache, M.; Moltó, N.; Benfekih, L.A.; Torres-Pagan, N.; Mir, R.; Verdeguer, M.; Boscaiu, M.; Vicente, O. Physiological and biochemical responses to water stress and salinity of the invasive moth plant, Araujia sericifera Brot., during seed germination and vegetative growth. Agronomy 2022, 12, 361. [Google Scholar] [CrossRef]
- Mircea, D.M.; Estrelles, E.; Al Hassan, M.; Soriano, P.; Sestras, R.E.; Boscaiu, M.; Sestras, A.F.; Vicente, O. Effect of water deficit on germination, growth and biochemical responses of four potentially invasive ornamental grass species. Plants 2023, 12, 1260. [Google Scholar] [CrossRef]
- Cervera, J.C.; Parra-Tabla, V. Seed germination and seedling survival traits of invasive and non-invasive congeneric Ruellia species (Acanthaceae) in Yucatan, Mexico. Plant. Ecol. 2009, 205, 285–293. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Cleland, E.E. Exotic species display greater germination plasticity and higher germination rates than native species across multiple cues. Biol. Invasions 2013, 15, 2253–2264. [Google Scholar] [CrossRef]
- Pérez-Fernández, M.A.; Lamont, B.B.; Marwick, A.L.; Lamont, W.G. Germination of seven exotic weeds and seven native species in South-Western Australia under steady and fluctuating water supply. Acta Oecol. 2000, 21, 323–336. [Google Scholar] [CrossRef]
- Paudel, S.; Battaglia, L.L. Germination responses of the invasive Triadica sebifera and two co-occurring native woody species to elevated salinity across a gulf coast transition ecosystem. Wetlands 2013, 33, 527–535. [Google Scholar] [CrossRef]
- Infante-Izquierdo, M.D.; Castillo, J.M.; Grewell, B.J.; Nieva, F.J.J.; Muñoz-Rodríguez, A.F. Differential effects of increasing salinity on germination and seedling growth of native and exotic invasive cordgrasses. Plants 2019, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Uçarlı, C. Effects of salinity on seed germination and early seedling stage. In Abiotic Stress in Plants; Intech Open: London, UK, 2020; p. 211. [Google Scholar]
- Hohl, M.; Schopfer, P. Water relations of growing maize coleoptiles: Comparison between mannitol and polyethylene glycol 6000 as external osmotica for adjusting turgor pressure. Plant Physiol. 1991, 95, 716–722. [Google Scholar] [CrossRef]
- Singh, M.; Ramirez, A.H.M.; Sharma, S.D.; Jhala, A.J. Factors affecting the germination of tall morning glory (Ipomoea purpurea). Weed Sci. 2012, 60, 64–68. [Google Scholar] [CrossRef]
- Kiani, A.; Siahmarguee, A.; Soltani, E. Effects of temperature, salinity, and planting depth on seed germination and emergence of tall morning glory (Ipomoea spp.). Iran. J. Plant Prot. Res. 2015, 29, 437–448. [Google Scholar] [CrossRef]
- Abbasi, I.; Zaefarian, F.; Younesabadi, M. Study of biological aspect of germination and emergence in morning glory (Ipomoea purpurea L.). Iran. J. Plant Prot. Res. 2022, 36, 125–139. [Google Scholar] [CrossRef]
- Dehghan, S.; Siahmarguee, A.; Ghaderi Far, F.; Azimmohseni, M. Germination and emergence response of white morning glory (Ipomoea lacunose L.) to some environmental factors. Iran. J. Weed Sci. 2023, 19, 129111. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Abugho, S.B. Three lobe morning glory (Ipomoea triloba) germination and response to herbicides. Weed Sci. 2012, 60, 199–204. [Google Scholar] [CrossRef]
- Siahmarguee, A.; Taheri, M.; Ghaderi-Far, F.; Torabi, B. Germination ecology of invasive common morning-glory (Ipomoea purpurea L.) in Golestan province. J. Plant Prod. 2022, 29, 221–240. [Google Scholar]
- Chaney, L.; Baucom, R.S. The evolutionary potential of baker’s weediness traits in the common morning glory, Ipomoea purpurea (Convolvulaceae). Am. J. Bot. 2012, 99, 1524–1530. [Google Scholar] [CrossRef]
- Baker, H.G. Characteristics and mode of origin of weeds. In The Genetics of Colonizing Species; Baker, H.G., Stebbins, G.L., Eds.; Academic Press: New York, NY, USA, 1965; pp. 147–172. [Google Scholar]
- Graebner, R.C.; Callaway, R.M.; Montesinos, D. Invasive species grows faster, competes better, and shows greater evolution toward increased seed size and growth than exotic non-invasive congeners. Plant Ecol. 2012, 213, 545–553. [Google Scholar] [CrossRef]
- Chaney, L.; Baucom, R.S. The costs and benefits of tolerance to competition in Ipomoea purpurea, the common morning glory. Evolution 2014, 68, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Huang, Q.Q.; Lin, Z.G.; Huang, F.F.; Liao, H.X.; Peng, S.L. High tolerance to salinity and herbivory stresses may explain the expansion of Ipomoea cairica to salt marshes. PLoS ONE 2012, 7, e48829. [Google Scholar] [CrossRef]
- Huerta-Ramos, G.; Moreno-Casasola, P.; Sosa, V. Wetland conservation in the Gulf of Mexico: The example of the salt marsh morning glory, Ipomoea sagittata. Wetlands 2015, 35, 709–721. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, X.B.; Zhao, L.K.; Huo, K.S.; Jin, P.F.; Zhao, D.L.; Zhou, Z.L.; Tang, J.; Xiao, S.Z.; Cao, Q.H. RNA-Seq reveals the salt tolerance of Ipomoea pes-caprae, a wild relative of sweet potato. J. Plant Physiol. 2020, 255, 153276. [Google Scholar] [CrossRef]
- Liu, D.; He, S.; Song, X.; Zhai, H.; Liu, N.; Zhang, D.; Ren, Z.; Liu, Q. IbSIMT1, a novel salt-induced methyltransferase gene from Ipomoea batatas, is involved in salt tolerance. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 120, 701–715. [Google Scholar] [CrossRef]
- Atala, C.; Gianoli, E. Effect of water availability on tolerance of leaf damage in tall morning glory, Ipomoea purpurea. Acta Oecol. 2009, 35, 236–242. [Google Scholar] [CrossRef]
- Atala, C.; Cordero, C.; Gianoli, E. Drought and leaf damage limit the search for support in the climbing plant Ipomoea purpurea (L.) Roth (Convolvulaceae). Gayana Bot. 2011, 68, 207–212. [Google Scholar] [CrossRef][Green Version]
- Mason, C.M.; Christopher, D.A.; Rea, A.M.; Eserman, L.A.; Pilote, A.J.; Batora, N.l.; Chang, S.M. Low inbreeding depression and high plasticity in the tall morning glory (Ipomoea purpurea). Weed Sci. 2015, 63, 864–876. [Google Scholar] [CrossRef]
- Cha-Um, S.; Roytrakul, S.; Kirdmanee, C.; Akutagawa, I.; Takagaki, M. A rapid method for identifying salt tolerant water convolvulus (Ipomoea aquatica Forsk) under in vitro photoautotrophic conditions. Plant Stress 2007, 1, 228–234. [Google Scholar]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 1241–1257. [Google Scholar] [CrossRef]
- Venkatesan, A.; Chellappan, K.P. Accumulation of proline and glycine betaine in Ipomoea pes-caprae induced by NaCl. Biol. Plant 1998, 41, 271–276. [Google Scholar] [CrossRef]
- Yooyongwech, S.; Theerawitaya, C.; Samphumphuang, T.; Cha-um, S. Water-deficit tolerant identification in sweet potato genotypes (Ipomoea batatas (L.) Lam.) in vegetative developmental stage using multivariate physiological indices. Sci. Hortic. 2013, 162, 242–251. [Google Scholar] [CrossRef]
- Yooyongwech, S.; Samphumphung, T.; Tisaram, R.; Theerawitaya, C.; Cha-Um, S. Physiological, morphological changes and storage root yield of sweet potato (Ipomoea batatas (L.) Lam.) under PEG-Induced Water Stress. Not. Bot. Horti. Agrobot. Cluj Napoca 2017, 45, 164–171. [Google Scholar] [CrossRef]
- Jia, L.; Yang, Y.; Zhai, H.; He, S.; Xin, G.; Zhao, N.; Zhang, H.; Gao, S.; Liu, Q. Production and characterization of a novel interspecific somatic hybrid combining drought tolerance and high quality of sweet potato and Ipomoea triloba L. Plant Cell Rep. 2022, 41, 2159–2171. [Google Scholar] [CrossRef]
- Kitayama, M.; Samphumphuang, T.; Tisarum, R.; Theerawitaya, C.; Cha-um, K.; Takagaki, M.; Cha-um, S. Calcium and soluble sugar enrichments and physiological adaptation to mild NaCl salt stress in sweet potato (Ipomoea batatas) genotypes. J. Hortic. Sci. Biotechnol. 2020, 95, 782–793. [Google Scholar] [CrossRef]
- Gil, R.; Boscaiu, M.; Lull, C.; Bautista, I.; Lidón, A.; Vicente, O. Are soluble carbohydrates ecologically relevant for salt tolerance in halophytes? Funct. Plant Biol. 2013, 40, 805. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.M. Sodium in plants: Perception, signalling, and regulation of sodium fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Volkov, V. Salinity tolerance in plants. quantitative approach to ion transport starting from halophytes and stepping to genetic and protein engineering for manipulating ion fluxes. Front. Plant. Sci. 2015, 6, 873. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Pottosin, I. Regulation of potassium transport in plants under hostile conditions: Implications for abiotic and biotic stress tolerance. Physiol. Plant. 2014, 151, 257–279. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Solis, J.; Baisakh, N.; Brandt, S.R.; Villordon, A.; La Bonte, D. Transcriptome profiling of beach morning glory (Ipomoea imperati) under salinity and its comparative analysis with sweet potato. PLoS ONE 2016, 11, e0147398. [Google Scholar] [CrossRef]
- Mondal, S.; Rahaman, E.H.M.S.; Asch, F. Potassium content is the main driver for salinity tolerance in sweet potato before tuber formation. J. Agron. Crop. Sci. 2022, 208, 645–661. [Google Scholar] [CrossRef]
- Wang, B.; Zhai, H.; He, S.; Zhang, H.; Ren, Z.; Zhang, D.; Liu, Q. A vacuolar Na+/H+ antiporter gene, IbNHX2, enhances salt and drought tolerance in transgenic sweet potato. Sci. Hort. 2016, 30, 153–166. [Google Scholar] [CrossRef]
- Yoshida, K.; Kawachi, M.; Mori, M.; Maeshima, M.; Kondo, M.; Nishimura, M.; Kondo, T. The involvement of tonoplast proton pumps and Na+(K+)/H+ exchangers in the change of petal color during flower opening of morning glory, Ipomoea tricolor cv. Heavenly Blue. Plant Cell Physiol. 2005, 46, 407–415. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Shomali, A.; Azad, N.; Hassani, B.; Lastochkina, O.; Li, T. Calcium signaling and salt tolerance are diversely entwined in plants. Plant Signal. Behav. 2019, 14, 1665455. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).