The Effects of Long-Term Application of Stabilized and Coated Urea on Soil Chemical Properties, Microbial Community Structure, and Functional Genes in Paddy Fields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Location and Climatic
2.2. Experimental Design
2.3. Sample Collection and Measurement Methods
2.4. Data Analyses and Statistics
3. Results
3.1. Basic Chemical Properties of Soil
3.1.1. Differences in Soil Chemical Properties after and before the Long-Term Experiment
3.1.2. Characteristics of Changes in Soil Chemical Properties of Treatments after 16 Years of Application of Stabilized and Coated Urea Fertilizers
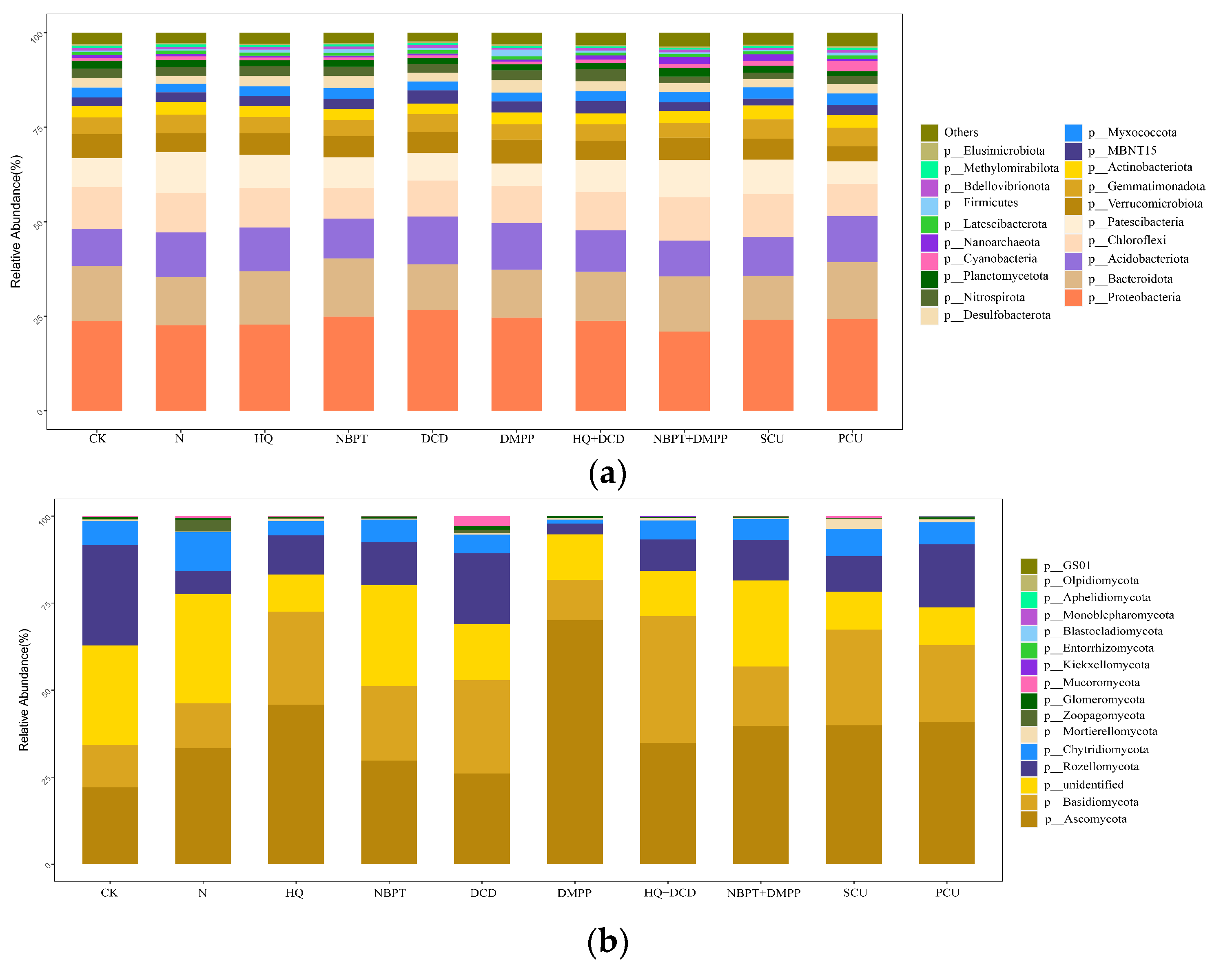
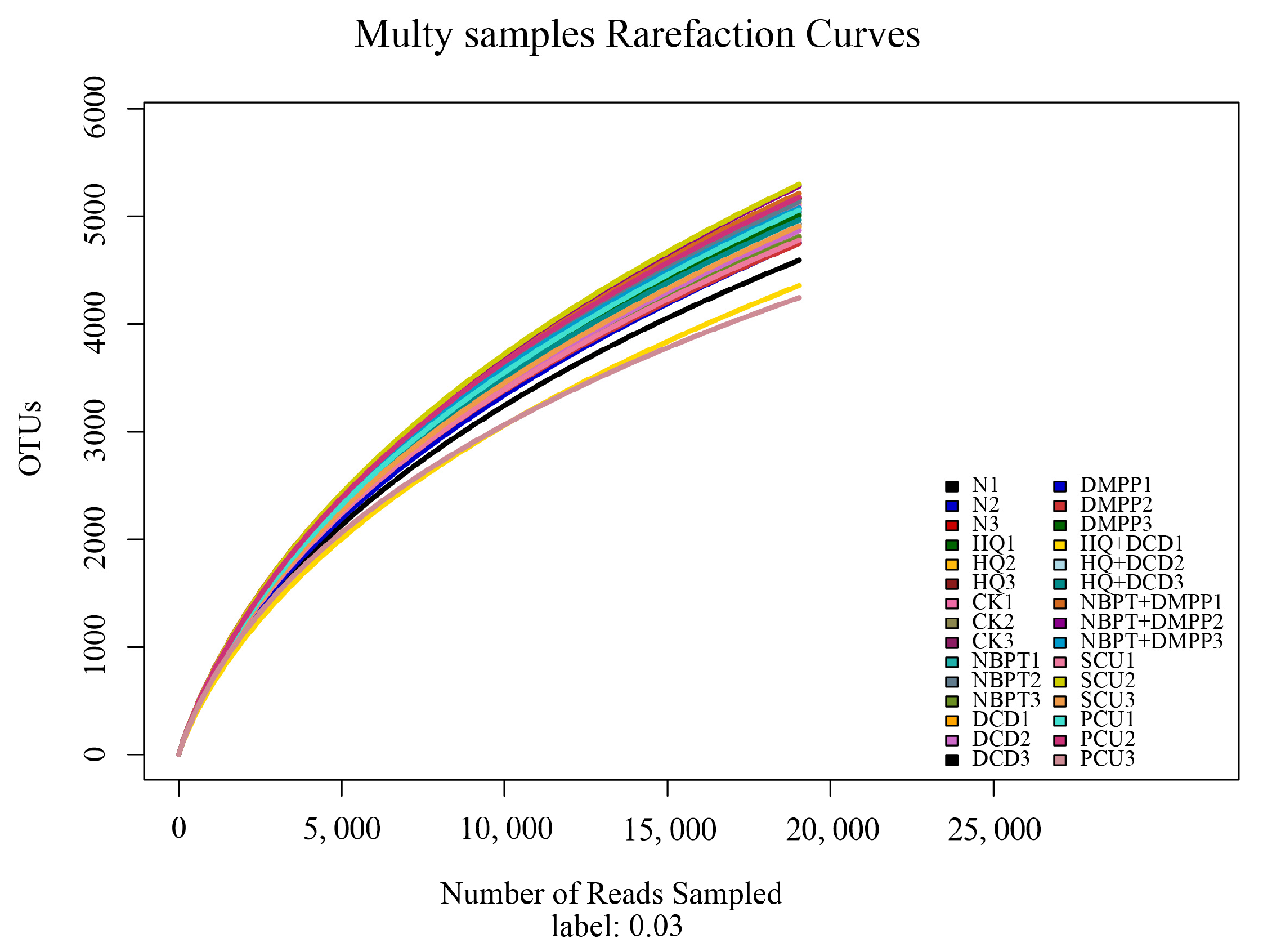
3.2. Soil Bacterial and Fungal Community Characteristics
3.3. Changes in the Abundance of Microbial Functional Genes Involved in the N Cycle
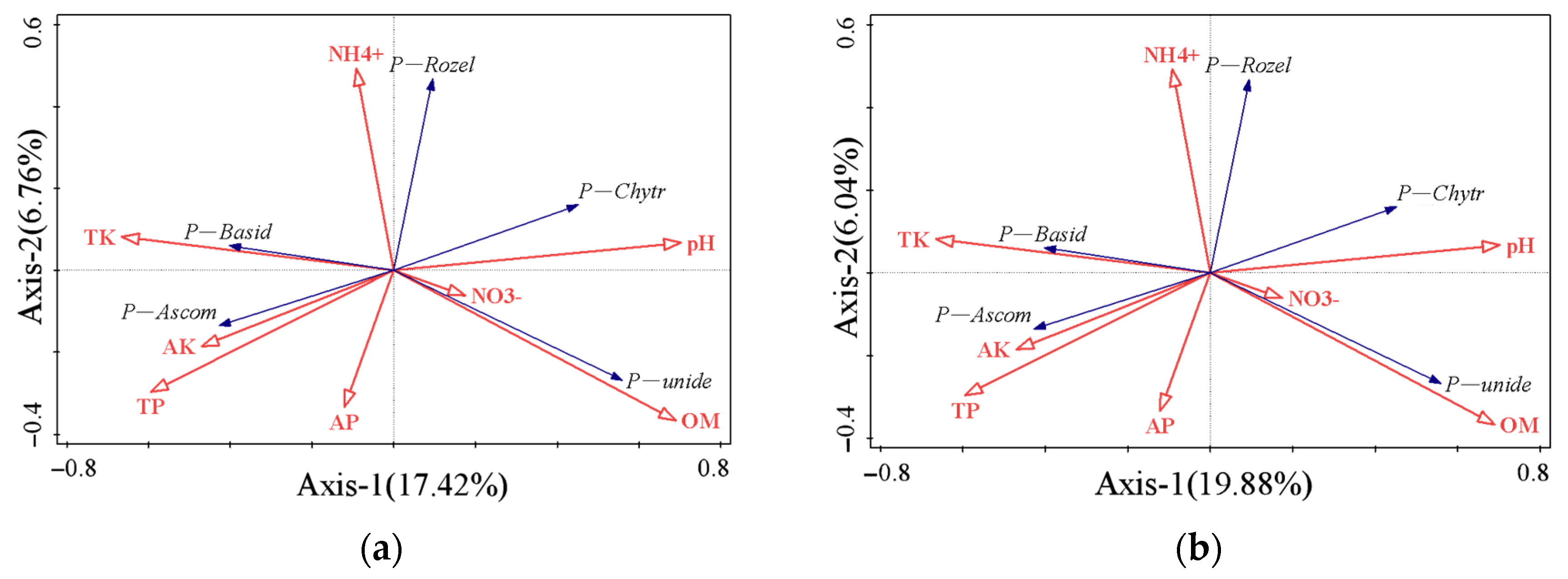
3.4. Relationship between Soil Microbial Composition and Soil Basic Chemistry
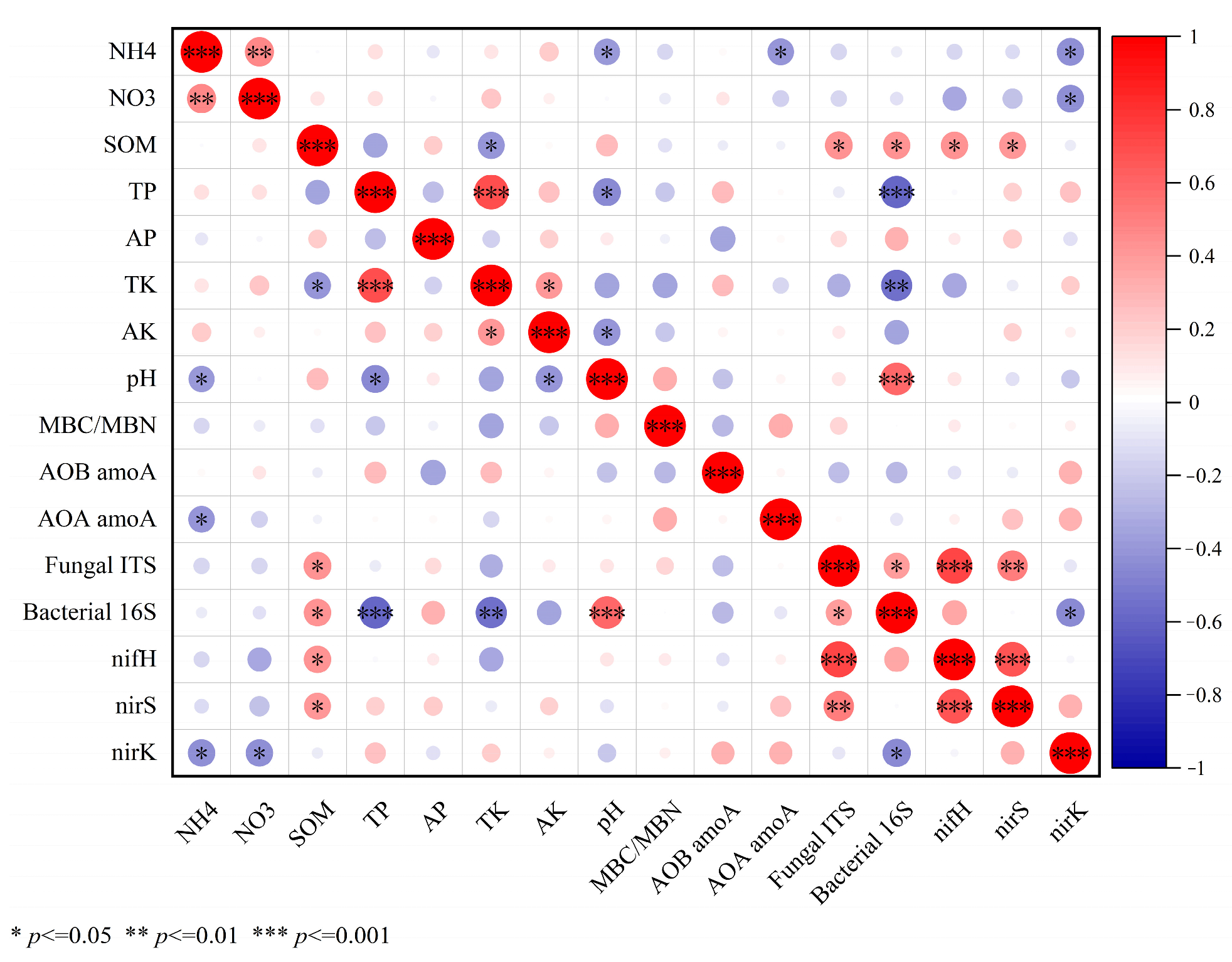
3.5. Correlation Analysis between Soil Basic Chemical Properties and Microbial Functional Gene Abundance
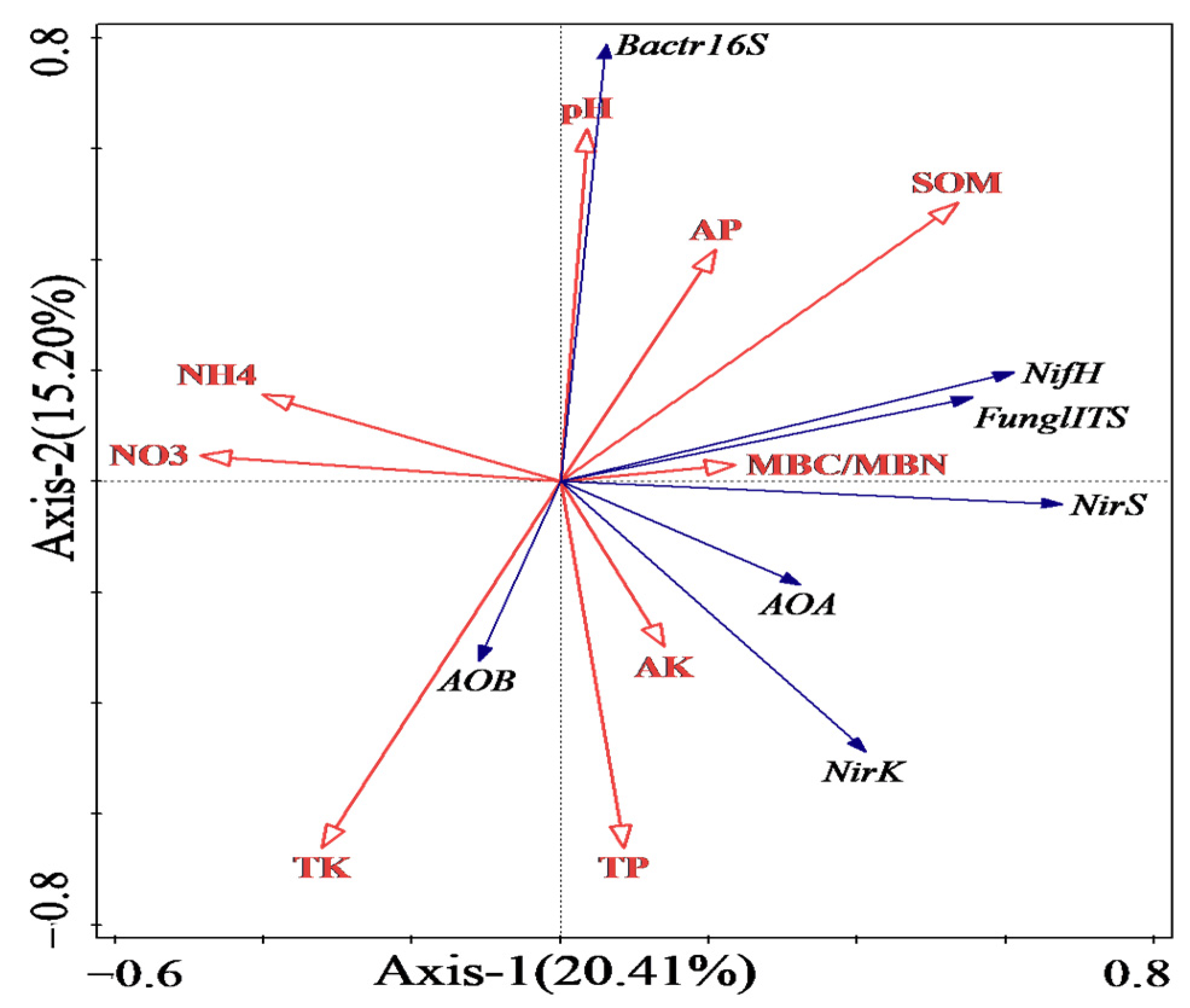
3.6. RDA Analysis of the Relationship between Soil Basic Chemical Properties and Microbial Functional Gene Abundance
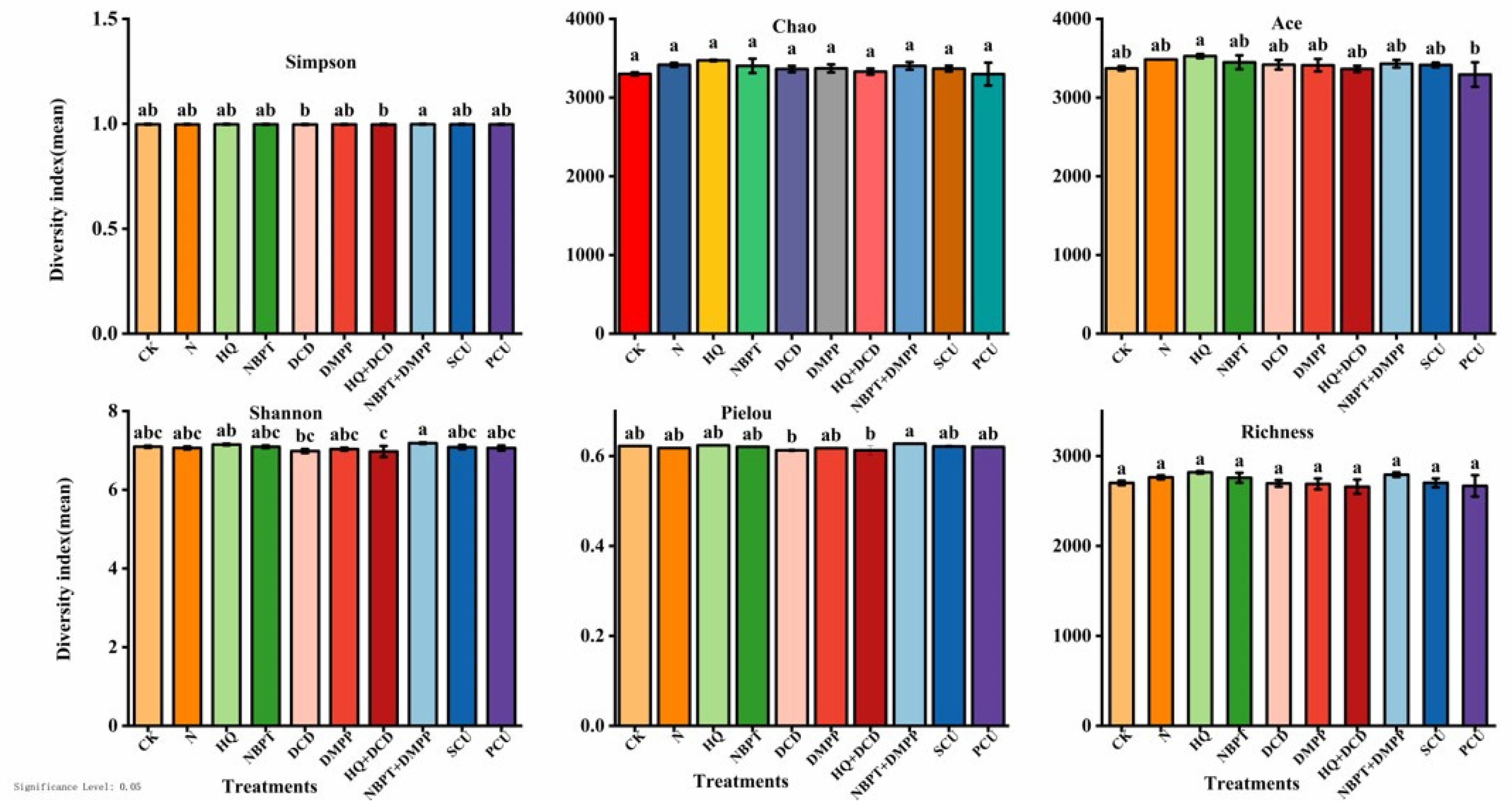
3.7. Alpha Diversity of Bacteria in Soil
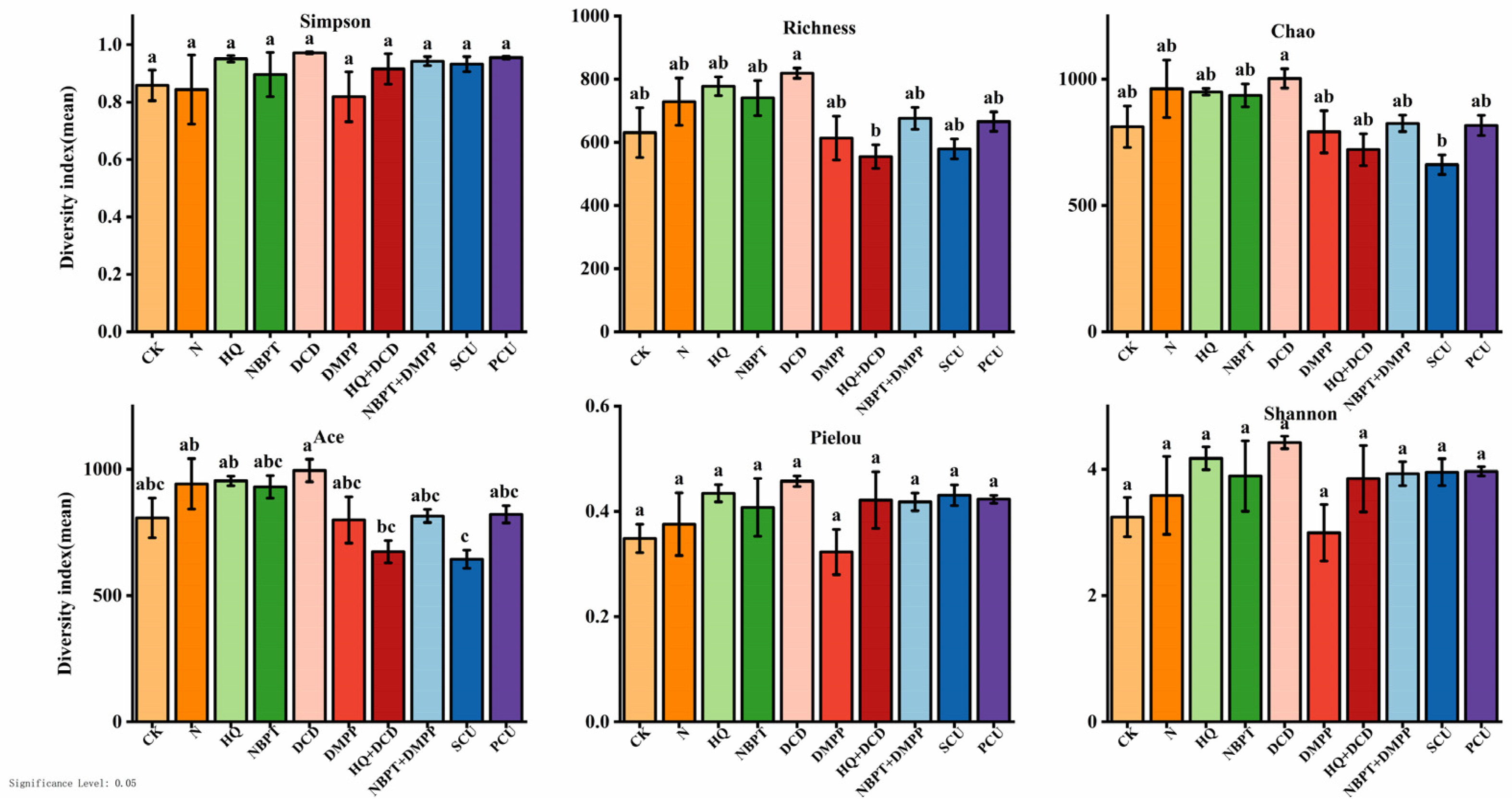
3.8. Alpha Diversity of Fungi in Soil
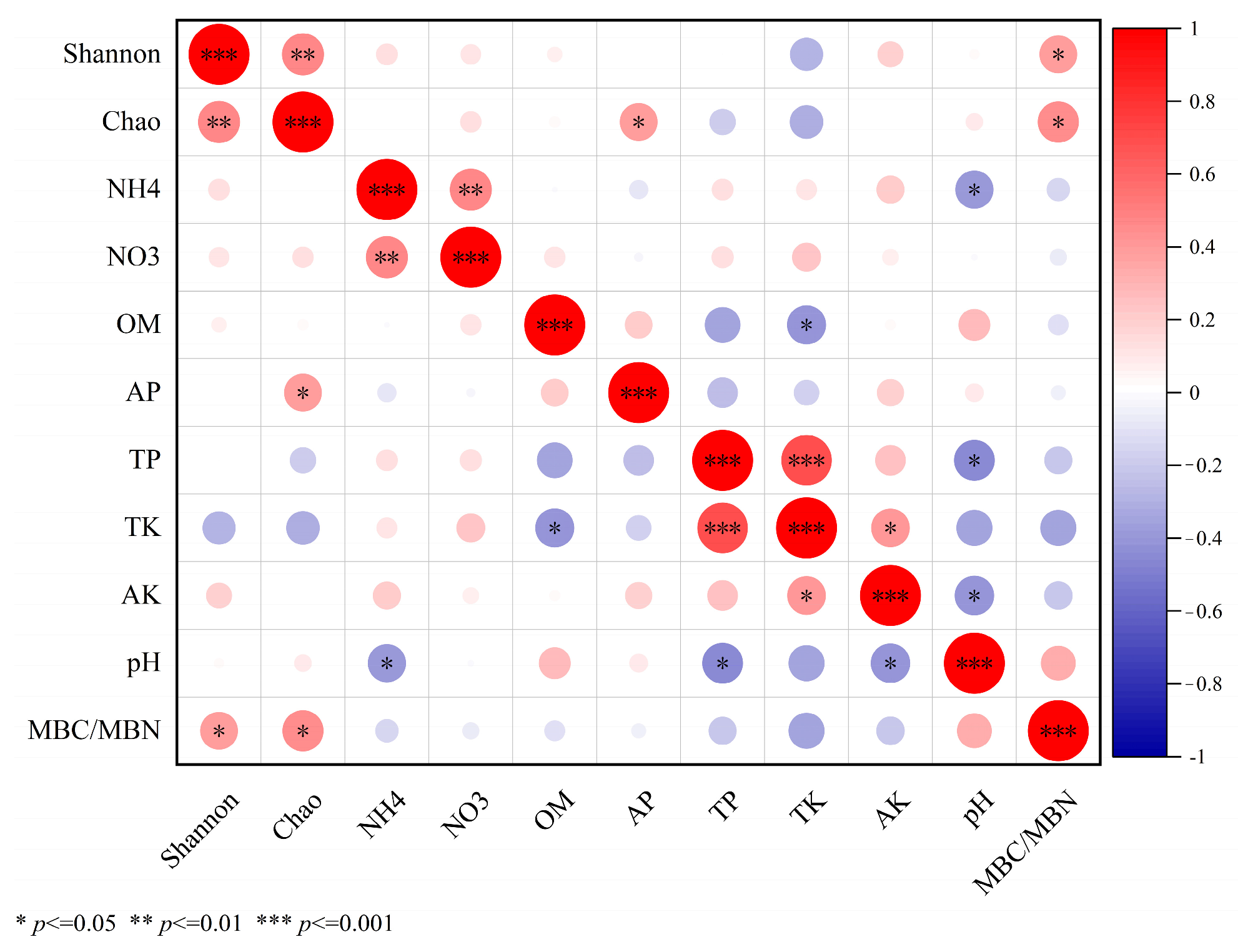
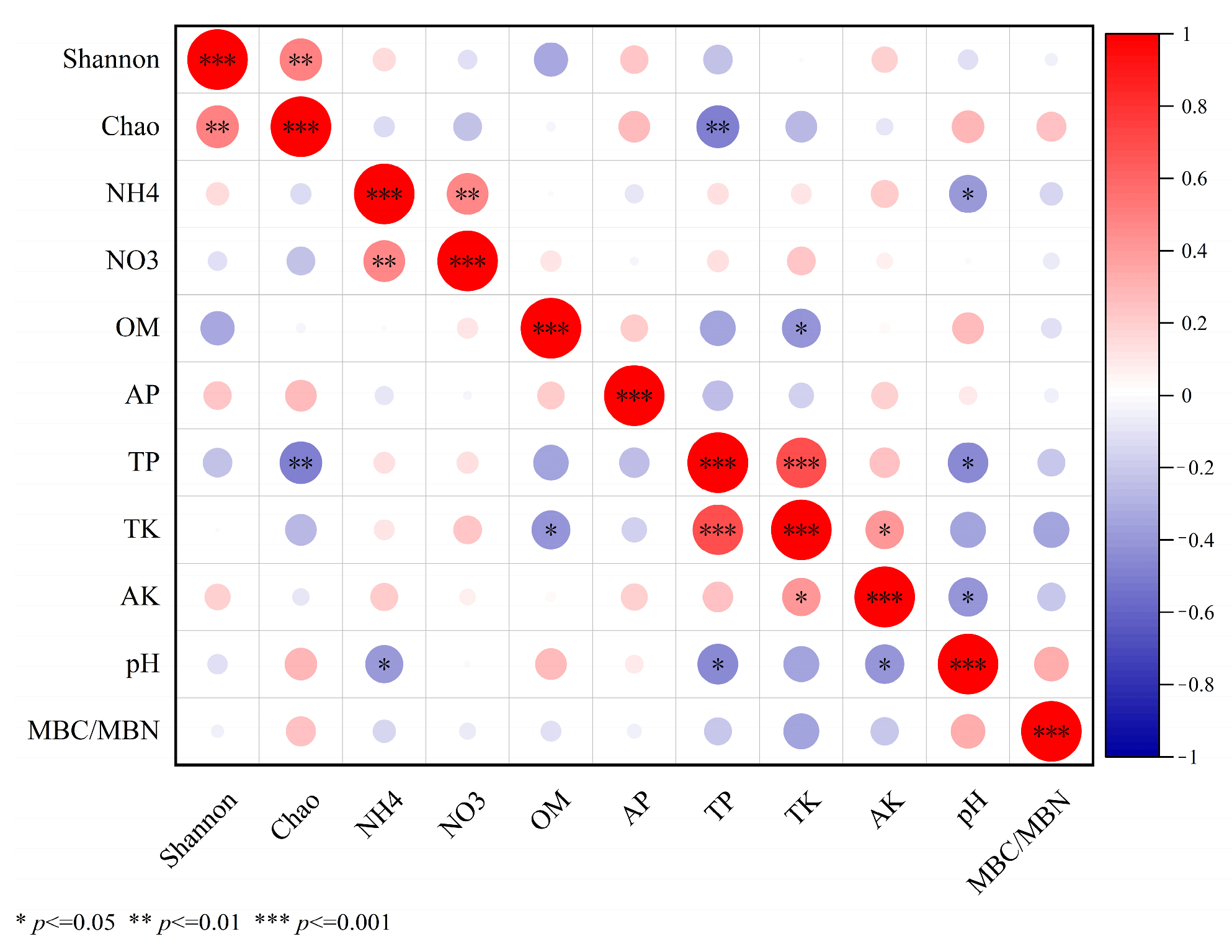
3.9. Relationship between Soil Chemical Properties and Alpha Diversity of Microbial Communities
3.10. Soil Bacterial and Fungal Community β-Diversity Indices
4. Discussion
4.1. The Effect of Long-Term Application Stabilized and Coated Urea Fertilizers on pH in Rice Field Soils
4.2. The Effects of Long-Term Application Stabilized and Coated Urea Fertilizers on the Abundance of Functional Genes of Nitrogen-Cycling Microorganisms in Rice Field Soils
4.3. The Effects of Long-Term Application of Stabilized and Coated Urea Fertilizers on Microbial Community Succession in Rice Field Soils
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanz-Cobena, A.; Sanchez-Martin, L.; Garcia-Torres, L.; Vallejo, A. Gaseous emissions of N2O and NO and NO3− leaching from urea applied with urease and nitrification inhibitors to a maize (Zea mays) crop. Agric. Ecosyst. Environ. 2012, 149, 64–73. [Google Scholar] [CrossRef]
- Giordano, M.; Petropoulos, S.A.; Rouphael, Y. The Fate of Nitrogen from Soil to Plants: Influence of Agricultural Practices in Modern Agriculture. Agriculture 2021, 11, 944. [Google Scholar] [CrossRef]
- Abdo, A.I.I.; Sun, D.; Li, Y.; Yang, J.; Metwally, M.S.S.; Abdel-Hamed, E.M.W.; Wei, H.; Zhang, J. Coupling the environmental impacts of reactive nitrogen losses and yield responses of staple crops in China. Front. Plant Sci. 2022, 13, 927935. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Medhi, K.; Fagodiya, R.K.; Subrahmanyam, G.; Mondal, R.; Raja, P.; Malyan, S.K.; Gupta, D.K.; Gupta, C.K.; Pathak, H. Molecular and ecological perspectives of nitrous oxide producing microbial communities in agro-ecosystems. Rev. Environ. Sci. Bio-Technol. 2020, 19, 717–750. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Pan, S.-Y.; He, K.-H.; Lin, K.-T.; Fan, C.; Chang, C.-T. Addressing nitrogenous gases from croplands toward low-emission agriculture. Npj Clim. Atmos. Sci. 2022, 5, 43. [Google Scholar] [CrossRef]
- Arrobas, M.; Chiochetta, J.C.; Damo, L.; Julio, A.C.; Hendges, I.P.; Wagner, A.; Godoy, W.I.; Cassol, L.C.; Rodrigues, M.A. Controlled-release and stabilized fertilizers are equivalent options to split application of ammonium nitrate in a double maize-oats cropping system. J. Plant Nutr. 2022, 46, 996–1008. [Google Scholar] [CrossRef]
- Dalal, R.C.; Wang, W.J.; Robertson, G.P.; Parton, W.J. Nitrous oxide emission from Australian agricultural lands and mitigation options: A review. Aust. J. Soil Res. 2003, 41, 165–195. [Google Scholar] [CrossRef]
- Adu-Poku, D.; Ackerson, N.O.B.; Devine, R.N.O.A.; Addo, A.G. Climate mitigation efficiency of nitrification and urease inhibitors: Impact on N2O emission—A review. Sci. Afr. 2022, 16, e01170. [Google Scholar] [CrossRef]
- Dong, X.X.; Zhang, L.L.; Wu, Z.J.; Zhang, H.W.; Gong, P. The response of nitrifier, N-fixer and denitrifier gene copy numbers to the nitrification inhibitor 3,4-dimethylpyrazole phosphate. Plant Soil Environ. 2013, 59, 398–403. [Google Scholar] [CrossRef]
- Cassman, N.A.; Soares, J.R.; Pijl, A.; Lourenco, K.S.; van Veen, J.A.; Cantarella, H.; Kuramae, E.E. Nitrification inhibitors effectively target N2O-producing Nitrosospira spp. in tropical soil. Environ. Microbiol. 2019, 21, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Florio, A.; Clark, I.M.; Hirsch, P.R.; Jhurreea, D.; Benedetti, A. Effects of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on abundance and activity of ammonia oxidizers in soil. Biol. Fertil. Soils 2014, 50, 795–807. [Google Scholar] [CrossRef]
- Kong, X.; Duan, Y.; Schramm, A.; Eriksen, J.; Petersen, S.O. 3,4-Dimethylpyrazole phosphate (DMPP) reduces activity of ammonia oxidizers without adverse effects on non-target soil microorganisms and functions. Appl. Soil Ecol. 2016, 105, 67–75. [Google Scholar] [CrossRef]
- Sigurdarson, J.J.; Svane, S.; Karring, H. The molecular processes of urea hydrolysis in relation to ammonia emissions from agriculture. Rev. Environ. Sci. Bio-Technol. 2018, 17, 241–258. [Google Scholar] [CrossRef]
- Xi, R.; Long, X.-E.; Huang, S.; Yao, H. pH rather than nitrification and urease inhibitors determines the community of ammonia oxidizers in a vegetable soil. AMB Express 2017, 7, 129. [Google Scholar] [CrossRef]
- Fan, X.; Yin, C.; Yan, G.; Cui, P.; Shen, Q.; Wang, Q.; Chen, H.; Zhang, N.; Ye, M.; Zhao, Y.; et al. The contrasting effects of N-(n-butyl) thiophosphoric triamide (NBPT) on N2O emissions in arable soils differing in pH are underlain by complex microbial mechanisms. Sci. Total Environ. 2018, 642, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Sahrawat, K.L. Nitrification inhibitors for controlling methane emission from submerged rice soils. Curr. Sci. 2004, 87, 1084–1087. [Google Scholar]
- Silva, A.G.B.; Sequeira, C.H.; Sermarini, R.A.; Otto, R. Urease Inhibitor NBPT on Ammonia Volatilization and Crop Productivity: A Meta-Analysis. Agron. J. 2017, 109, 1–13. [Google Scholar] [CrossRef]
- Guo, B.; Zheng, X.; Yu, J.; Ding, H.; Luo, S.; Carswell, A.; Misselbrook, T.; Zhang, J.; Mueller, C.; Shen, J.; et al. Liming and nitrification inhibitor affects crop N uptake efficiency and N loss through changing soil N processes. Biol. Fertil. Soils 2022, 58, 949–959. [Google Scholar] [CrossRef]
- Wang, J.; Kang, J.; Sha, Z.; Qu, Z.; Niu, X.; Xu, W.; Zhang, H.; Goulding, K.; Liu, X. Mitigation of ammonia volatilization on farm using an N stabilizer—A demonstration in Quzhou, North China Plain. Agric. Ecosyst. Environ. 2022, 336, 108011. [Google Scholar] [CrossRef]
- Jariwala, H.; Santos, R.M.; Lauzon, J.D.; Dutta, A.; Wai Chiang, Y. Controlled release fertilizers (CRFs) for climate-smart agriculture practices: A comprehensive review on release mechanism, materials, methods of preparation, and effect on environmental parameters. Environ. Sci. Pollut. Res. 2022, 29, 53967–53995. [Google Scholar] [CrossRef]
- Irfan, S.A.; Razali, R.; KuShaari, K.; Mansor, N.; Azeem, B.; Versypt, A.N.F. A review of mathematical modeling and simulation of controlled-release fertilizers. J. Control. Release 2018, 271, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.-T.; Du, X.; Zhou, J.-C.; Peng, Y.; Li, X.-L.; Tao, P.-J.; Zhang, Y.-C. Network Analysis Reveals the Combination of Controlled-Release and Regular Urea Enhances Microbial Interactions and Improves Maize Yields. Front. Microbiol. 2022, 13, 825787. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Gao, Y.; Chen, Q.; Li, Z.; Gao, F.; Meng, Q.; Li, T.; Liu, A.; Wang, Q.; Wu, L.; et al. Blended controlled-release nitrogen fertilizer with straw returning improved soil nitrogen availability, soil microbial community, and root morphology of wheat. Soil Tillage Res. 2021, 212, 105045. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.; Geisseler, D.; Wu, Z.; Gong, P.; Xue, Y.; Yu, C.; Juan, Y.; Horwath, W.R. Available C and N affect the utilization of glycine by soil microorganisms. Geoderma 2016, 283, 32–38. [Google Scholar] [CrossRef]
- Turan, M.A.; Taban, S.; Katkat, A.V.; Kucukyumuk, Z. The evaluation of the elemental sulfur and gypsum effect on soil pH, EC, SO4-S and available Mn content. J. Food Agric. Environ. 2013, 11, 572–575. [Google Scholar]
- Glaser, K.; Hackl, E.; Inselsbacher, E.; Strauss, J.; Wanek, W.; Zechmeister-Boltenstern, S.; Sessitsch, A. Dynamics of ammonia-oxidizing communities in barley-planted bulk soil and rhizosphere following nitrate and ammonium fertilizer amendment. FEMS Microbiol. Ecol. 2010, 74, 575–591. [Google Scholar] [CrossRef]
- Shi, X.; Hu, H.-W.; Kelly, K.; Chen, D.; He, J.-Z.; Suter, H. Response of ammonia oxidizers and denitrifiers to repeated applications of a nitrification inhibitor and a urease inhibitor in two pasture soils. J. Soils Sediments 2017, 17, 974–984. [Google Scholar] [CrossRef]
- Zhang, K.; Li, D.; Du, Y.; Xue, Y.; Song, Y.; Zhang, Y.; Li, Y.; Zheng, Y.; Zhang, J.; Cui, Y. Effects of coated and stabilized nitrogen fertilizer on improving physicochemical and biological fertility and delaying acidification of brown soil. J. Plant Nutr. Fertil. 2023, 29, 472–482. [Google Scholar]
- Shen, X.-Y.; Zhang, L.-M.; Shen, J.-P.; Li, L.-H.; Yuan, C.-L.; He, J.-Z. Nitrogen loading levels affect abundance and composition of soil ammonia oxidizing prokaryotes in semiarid temperate grassland. J. Soils Sediments 2011, 11, 1243–1252. [Google Scholar] [CrossRef]
- Erguder, T.H.; Boon, N.; Wittebolle, L.; Marzorati, M.; Verstraete, W. Environmental factors shaping the ecological niches of ammonia-oxidizing archaea. FEMS Microbiol. Rev. 2009, 33, 855–869. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C.; Shen, J.-P.; Winefield, C.S.; O’Callaghan, M.; Bowatte, S.; He, J.-Z. Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol. Ecol. 2010, 72, 386–394. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, L.; Chen, H.; Chen, G.; Wang, S.; Zhao, X.; Wang, Y. Responses of soil phosphorus pools accompanied with carbon composition and microorganism changes to phosphorus-input reduction in paddy soils. Pedosphere 2021, 31, 83–93. [Google Scholar] [CrossRef]
- Li, Y.N.; Wang, T.Y.; Wang, C.Y.; Li, M.S.; Wang, Y.; Liu, S.X. Responses of Soil Rhizosphere Fungi to N Application Levels in Different Types of Soil. Appl. Ecol. Environ. Res. 2021, 19, 1645–1659. [Google Scholar] [CrossRef]
- Jiang, Y.; Qian, H.; Wang, X.; Chen, L.; Liu, M.; Li, H.; Sun, B. Nematodes and microbial community affect the sizes and turnover rates of organic carbon pools in soil aggregates. Soil Biol. Biochem. 2018, 119, 22–31. [Google Scholar] [CrossRef]
- Kou, Y.P.; Wei, K.; Chen, G.X.; Wang, Z.Y.; Xu, H. Effects of 3,4-dimethylpyrazole phosphate and dicyandiamide on nitrous oxide emission in a greenhouse vegetable soil. Plant Soil Environ. 2015, 61, 29–35. [Google Scholar] [CrossRef]
- Dong, D.; Kou, Y.; Yang, W.; Chen, G.; Xu, H. Effects of urease and nitrification inhibitors on nitrous oxide emissions and nitrifying/denitrifying microbial communities in a rainfed maize soil: A 6-year field observation. Soil Tillage Res. 2018, 180, 82–90. [Google Scholar] [CrossRef]
- Liu, G.; Yang, Z.; Du, J.; He, A.; Yang, H.; Xue, G.; Yu, C.; Zhang, Y. Adding NBPT to urea increases N use efficiency of maize and decreases the abundance of N-cycling soil microbes under reduced fertilizer-N rate on the North China Plain. PLoS ONE 2020, 15, e0240925. [Google Scholar] [CrossRef]
- Seo, J.; Jang, I.; Gebauer, G.; Kang, H. Abundance of Methanogens, Methanotrophic Bacteria, and Denitrifiers in Rice Paddy Soils. Wetlands 2014, 34, 213–223. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, H.; Wang, R.; Guo, S. Responses of nitrification and denitrification to nitrogen and phosphorus fertilization: Does the intrinsic soil fertility matter? Plant Soil 2019, 440, 443–456. [Google Scholar] [CrossRef]
- Wei, X.; Hu, Y.; Peng, P.; Zhu, Z.; Atere, C.T.; O’Donnell, A.G.; Wu, J.; Ge, T. Effect of P stoichiometry on the abundance of nitrogen-cycle genes in phosphorus-limited paddy soil. Biol. Fertil. Soils 2017, 53, 767–776. [Google Scholar] [CrossRef]
- Ma, Q.; Qian, Y.; Yu, Q.; Cao, Y.; Tao, R.; Zhu, M.; Ding, J.; Li, C.; Guo, W.; Zhu, X. Controlled-release nitrogen fertilizer application mitigated N losses and modified microbial community while improving wheat yield and N use efficiency. Agric. Ecosyst. Environ. 2023, 349, 108445. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, L.; Deng, Y.; Zhi, X.; Jiang, Y.H.; Tu, Q.; Xie, J.; Van Nostrand, J.D.; He, Z.; Yang, Y. Reproducibility and Quantitation of Amplicon Sequencing-Based Detection. Abstr. Gen. Meet. Am. Soc. Microbiol. 2011, 111, 793. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric-Estimation of the Number of Classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Enguita, F.J.; Leitao, A.L. Hydroquinone: Environmental Pollution, Toxicity, and Microbial Answers. Biomed Res. Int. 2013, 2013, 542168. [Google Scholar] [CrossRef]
- Li, W.X.; Wang, C.; Zheng, M.M.; Cai, Z.J.; Wang, B.R.; Shen, R.F. Fertilization strategies affect soil properties and abundance of N-cycling functional genes in an acidic agricultural soil. Appl. Soil Ecol. 2020, 156, 103704. [Google Scholar] [CrossRef]





| Organic Matter (g/kg) | Total N (g/kg) | Total Phosphorus (g/kg) | Total Potassium (g/kg) | Available N (mg/kg) | Available Phosphorus (mg/kg) | Available Potassium (mg/kg) | pH |
|---|---|---|---|---|---|---|---|
| 23.76 | 1.25 | 0.58 | 25.36 | 112.75 | 19.31 | 70.28 | 6.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, D.; Zhang, K.; Xiao, F.; Li, Y.; Du, Y.; Xue, Y.; Zhang, L.; Gong, P.; Song, Y.; et al. The Effects of Long-Term Application of Stabilized and Coated Urea on Soil Chemical Properties, Microbial Community Structure, and Functional Genes in Paddy Fields. Agronomy 2023, 13, 2190. https://doi.org/10.3390/agronomy13092190
Zhang Y, Li D, Zhang K, Xiao F, Li Y, Du Y, Xue Y, Zhang L, Gong P, Song Y, et al. The Effects of Long-Term Application of Stabilized and Coated Urea on Soil Chemical Properties, Microbial Community Structure, and Functional Genes in Paddy Fields. Agronomy. 2023; 13(9):2190. https://doi.org/10.3390/agronomy13092190
Chicago/Turabian StyleZhang, Yiji, Dongpo Li, Ke Zhang, Furong Xiao, Yonghua Li, Yandi Du, Yan Xue, Lili Zhang, Ping Gong, Yuchao Song, and et al. 2023. "The Effects of Long-Term Application of Stabilized and Coated Urea on Soil Chemical Properties, Microbial Community Structure, and Functional Genes in Paddy Fields" Agronomy 13, no. 9: 2190. https://doi.org/10.3390/agronomy13092190
APA StyleZhang, Y., Li, D., Zhang, K., Xiao, F., Li, Y., Du, Y., Xue, Y., Zhang, L., Gong, P., Song, Y., & Wu, K. (2023). The Effects of Long-Term Application of Stabilized and Coated Urea on Soil Chemical Properties, Microbial Community Structure, and Functional Genes in Paddy Fields. Agronomy, 13(9), 2190. https://doi.org/10.3390/agronomy13092190







