Abstract
Soil salinity poses a significant challenge to plant growth and agricultural productivity. Research demonstrated the potential of exopolysaccharides (EPS) to enhance plant growth and improve resistance to abiotic stress. Nevertheless, the precise regulatory mechanism by which EPS mediates salt tolerance in alfalfa (Medicago sativa) remains largely unexplored. To investigate the protective effects of EPS from Erwinia persicina Cp2 in enhancing salt stress tolerance in alfalfa, a hydroponic experiment was conducted on the ‘Aohan’ cultivar of alfalfa, and changes in germination rate, biomass, chlorophyll content, electrolyte leakage (EL), hydrogen peroxide (H2O2), malondialdehyde (MDA), proline (Pro), soluble sugar (SS), soluble protein (SP), and activities of superoxide dismutase (SOD) and peroxidase (POD) were analyzed with and without Cp2 EPS under 75 mmol/L NaCl conditions. The results show that the exposure of alfalfa to salt conditions seriously inhibited its germination and growth. However, it is interesting that the application of Cp2 EPS greatly alleviated the damage of salt stress on alfalfa and promoted the germination of alfalfa as well as the root length, seedling length, fresh weight, and dry weight of the plants. In addition, the increases in MDA, H2O2 content, and EL rate caused by salt stress were inhibited after Cp2 EPS treatment, while chlorophyll, Pro, SP, and SS contents as well as SOD and CAT activities were increased. Therefore, Cp2 EPS can significantly alleviate the toxic effect of 75 mmol/L NaCl stress on alfalfa, and comprehensive analysis shows that 1.5 g/L Cp2 EPS had the best alleviating effect on alfalfa at this salt stress concentration. This study lays a practical and theoretical foundation for the development of biogenic agents used to alleviate the growth of alfalfa in saline alkali soil.
1. Introduction
Soil salinity emerged as a significant contributor to resource and environmental ecological challenges, with reports indicating that nearly 20% of arable land and 50% of irrigated land worldwide are affected by salinity [1]. As one of the primary abiotic stresses, salt stress has a severe impact on plant growth and development [2]. Specifically, salt stress impedes plant seed germination, resulting in slower germination rates. Additionally, prolonged exposure to salt stress can lead to the erosion of seeds and seedlings, ultimately reducing the survival rate of seedlings [3]. Furthermore, the imposition of salt stress can result in the occurrence of osmotic stress and ion toxicity, which can trigger the buildup of reactive oxygen species, thereby inducing oxidative stress [4]. Simultaneously, plants employ diverse mechanisms to counteract the detrimental effects of salt stress, such as the accumulation of osmoregulatory substances to mitigate osmotic potential, preservation of intracellular homeostasis, and elimination of reactive oxygen species by augmenting antioxidant enzyme activity [5,6].
Exopolysaccharides (EPS) is a high polymer that is soluble in water and is secreted by some special microorganisms during their growth and metabolic processes [7]. In recent years, there was an increasing interest among scholars in the mechanism of the salt tolerance mechanism of plants mediated by EPS. It was demonstrated that EPS not only facilitates plant seed germination, but also promotes plant growth in adversity [8,9]. When subjected to salt stress, EPS can chelate free Na+ in the soil, restrict the absorption of Na+ by plant roots, and maintain a balance between K+ and Na+. This is attributed to the polyanionic nature of EPS and its ability to bind cations [10,11]. Moreover, it was shown that EPS serves as a remarkably effective antioxidant by scavenging free radicals and safeguarding plants against lipid peroxidation induced by reactive oxygen species during salt stress [12]. More importantly, compared with animal and plant polysaccharides, microbial polysaccharides possess a short culture cycle, low cost, high yield, controllable process, and facile isolation [13]. Therefore, microbial EPS is a potential novel biogenic macromolecule for improving salt tolerance in plants. Erwinia persicina is a typical Gram-negative (G-) bacterium, strain number Cp2, which produces large amounts of mucilaginous exopolysaccharides on King’s B medium and is widely present in the natural environment. However, little is known about the salinity resistance of this bacterium to plants.
Alfalfa (Medicago sativa), a perennial herb, is one of the most widely planted legumes in sustainable animal husbandry due to its high yield, good palatability and wide adaptability [14]. It is grown across more than 80 countries on diverse continents, covering an expansive area of over 35 million hectares [15,16]. In addition, Alfalfa can form a new root organ root nodule with nitrogen fixing rhizobia in the soil, and convert nitrogen in the air into ammonium nitrogen that can be absorbed and utilized by plants. It can not only provide nutrition for its own growth, but also reduce the application of nitrogen fertilizer and promote the efficient use of nitrogen [17]. Therefore, the high quality and high yield of Alfalfa is crucial to the sustainable development of the animal husbandry production system, agricultural production system, and ecosystem health [18,19]. As we all know, despite the constant expansion of alfalfa cultivation in China, its planting sites are often located in arid and semi-arid areas [20]. These areas have dry climate, less rain, strong light, large evaporation, coupled with repeated human cycles of irrigation and fertilization, which further aggravate soil salinization, and salt stress seriously affects its nutrient absorption, photosynthesis, and growth [21,22]. Studies showed that when salt stress concentration reaches 50~200 mmol/L NaCl, it significantly inhibits the growth and yield of alfalfa [23]. How to improve the yield and quality of alfalfa cultivation in such an environment became an important issue facing the current development of animal husbandry.
Aohan alfalfa was first planted in Gansu Province, and then introduced to Aohan Banner, Chifeng City, Inner Mongolia. This variety is drought resistant, cold resistant, sand resistant, and barren resistant, but poor in salt resistance, so enhancing the salt resistance of Aohan alfalfa is crucial for the development of local animal husbandry [24]. Therefore, different concentrations of Cp2 EPS were used in this study to explore the induction mechanism of Cp2 EPS on salt tolerance of alfalfa, providing a certain theoretical basis for the development of EPS of Erwinia persicina Cp2 and the quality and yield improvement of alfalfa, and providing a new strategy for the restoration of saline–alkali land and sustainable agricultural development in China.
2. Materials and Methods
2.1. Experimental Materials
The variety of alfalfa tested was ‘Aohan’, which was provided by Forage Germplasm Resources Laboratory of Gansu Agricultural University.
EPS was extracted from Erwinia persicina Cp2 and provided by Pasture Pathology Laboratory of Gansu Agricultural University.
2.2. Experimental Design
Alfalfa seeds with healthy and full seeds and basically the same size were selected and soaked in 75% alcohol for 1 min and then transferred to 1% sodium hypochlorite NaClO solution for 5 min, and were then repeatedly rinsed with sterile water 4 to 5 times and set aside.
Firstly, we conducted a NaCl concentration screening test and added different concentrations of sodium chloride (0, 75, 100, 150, and 200 mmol/L) solutions to the growth bottle and autoclaved at 121 °C and 0.11 Mpa for 21 min. After cooling, alfalfa seeds were placed equidistantly (seed spacing = 1 cm) on the germination bed of the growth bottle with tweezers in an ultra-clean bench, with 8 replicates per treatment and 25 seeds per replicate. After inoculation, the growth bottles were placed in a growth chamber at a constant temperature of 20 °C with 12 h of light and 12 h of darkness for incubation. During the incubation period, the germination of seeds was counted every 24 h, and the germination standard was defined as the embryonic root breaking through the seed coat by 2 mm.
Then, based on the preliminary NaCl concentration screening test results, subsequent experiments were conducted. The experimental treatments included (i) control (CK1) with distilled water only; (ii) 75 mmol/L NaCl (CK2); (iii) 75 mmol/L NaCl and 0.5 g/L Cp2 EPS (T1); (iv) 75 mmol/L NaCl and 1.0 g/L Cp2 EPS (T2); (v) 75 mmol/L NaCl and 1.5 g/L Cp2 EPS (T3); (vi) 75 mmol/L NaCl and 2.0 g/L Cp2 EPS (T4); and (vii) 75 mmol/L NaCl and 2.5 g/L Cp2 EPS (T5). A total of 200 mL of each treatment solution was added to the growth bottle. The subsequent operation is the same as the salt stress screening test mentioned above.
2.3. Measurement of Relevant Indicators
2.3.1. Germination Index Determination
The calculation formulae were as follows: germinating potential (GP) = the germination number on the 4th day/25 ×100%; germination rate (GR) = the germination number on the 10th day/25 ×100%; germination index (GI) = ∑(Gt/Dt) (where, Gt is germination number at different times (10 days); and Dt is the number of days of germination).
2.3.2. Measurement of Growth Indicators
Seedling length (SL) and root length (RL): were determined by taking alfalfa seedlings grown in growth bottles for 14 d. The root length and seedling length of seedlings were measured with a straightedge, and four plants were randomly selected from each growth bottle.
Fresh weight (FW) and dry weight (DW) were determined by randomly selecting 10 seedlings from each growth bottle, absorbing the water with filter paper, dividing them into seedling and root parts, weighing seedling fresh weight (SFW) and root fresh weight (RFW), respectively, using an analytical balance, and then drying them to constant weight in an oven at 80 °C. The seedling fresh weight (SFW) and root fresh weight (RFW) were weighed on an analytical balance and then dried to constant weight in an oven at 80 °C. The seedling dry weight (SDW) and root dry weight (RDW) were weighed separately.
Root morphological index: the root systems of seedlings were scanned by a root scanner (Seiko Epson Corporation, Suwa, Nagano, Japan) and then analyzed by Win-RHIZO root analysis software (Regent Istruments Canada Ine) to obtain root surface area (RSA), root volume (RV), and average root diameter (ARD).
2.3.3. Determination of Chlorophyll Content
We referred to the method of Yao et al. [25] to determine the chlorophyll content of the samples with slight modifications. Briefly, fresh leaves (0.1 g) from the control and treatment groups were placed in 10 mL centrifuge tubes and 5 mL of 95% ethanol was added. Then, each tube was wrapped in tin foil and left at room temperature for 24 h until the leaves were completely discolored. The absorbance of the supernatant was read at 665 and 649 nm and used to estimate the content of chlorophyll a and chlorophyll b, respectively.
2.3.4. Determination of Oxidative Damage
The determination of the electrolyte leakage (EL) was referred to the method of Lutts et al. [26] with minor modifications. In short, fresh leaves were washed thoroughly with deionized water, dried with filter paper, and 0.1 g of the fresh sample was quickly weighed in a graduated test tube of 10 mL deionized water and soaked at room temperature for 24 h. The conductivity EC1 of the extracts was measured with an electrical conductivity meter, then the samples were heated in a boiling water bath for 30 min and the conductivity EC2 was measured while the conductivity EC0 of the deionized water was measured. The EL was calculated as follows: EL = (EC1 − EC0)/(EC2 − EC0) × 100%.
Hydrogen peroxide (H2O2) and malondialdehyde (MDA) were measured using a microplate reader (Spectra Max iD3, Molecular Devices, San Jose, CA, USA) at 415 and 532 nm according to the H2O2 (M0107A) and MDA (M0103A) kits (Suzhou Michy Biomedical Technology Co., Ltd., Suzhou, China), respectively. The content of H2O2 was expressed as μmol/g FW; the content of MDA was expressed as nmol/g FW.
2.3.5. Determination of Osmoregulatory Substances
The proline (Pro) content was measured at 520 nm using a microplate reader (Spectra Max iD3, Molecular Devices, San Jose, CA, USA) according to the Pro (M0108A) kit (Suzhou Mickey Biopharmaceutical Technology Co., Ltd., Suzhou, China).
Soluble sugar (SS) and soluble protein (SP) were measured at 620 and 562 nm, respectively, using a microplate reader (Spectra Max iD3, Molecular Devices, San Jose, CA, USA) according to the SS (M1503A) and BCA protein (M1806A) kits (Suzhou Mickey Biomedical Technology Co., Ltd., Suzhou, China). The content of SP was determined by the bicinchoninic acid (BCA) method, and the content of SS was determined according to the anthrone method, both at mg/g FW.
2.3.6. Determination of Antioxidant Enzyme Activity
Superoxide dismutase (SOD) and catalase (CAT) activities were measured using a microplate reader (Spectra Max iD3, Molecular Devices, San Jose, CA, USA) at 450 and 240 nm according to the SOD (M0102A) and CAT (M0103A) kits (Suzhou Michy Biomedical Technology Co., Ltd., Suzhou, China), respectively. SOD activity was determined by the WST-8 method at U/g FW, and CAT activity was determined by the UV colorimetric method at μmol/min/g FW.
2.4. Statistical Analysis
The mean of three replications was used to represent per parameter. Data were evaluated with variance analysis using SPSS 26.0. Correlation analysis was performed using Spearman’s correlation. Principal component analyses (PCA) were performed using Origin 2022, and Topsis comprehensive analysis was performed using the online software Chiplot (https://www.chiplot.online/#Pie-plot (accessed on 11 May 2023).
3. Results
3.1. NaCl Concentration Screening Test
As the concentration of NaCl gradually increases, the germination potential and germination rate of alfalfa seeds show a gradually decreasing trend (Figure 1C,D). Compared with the control, 75~200 mmol/L NaCl significantly inhibited the germination potential and germination rate of seeds (p < 0.05). The germination of seeds was inhibited under 75 mmol/L NaCl treatment, but the survival rate of seedlings was relatively high; the germination rate of seeds under 100 mmol/L NaCl treatment reached 50% of the control, but the survival rate of seedlings was extremely low, and the seeds basically did not germinate under the treatment of 150 and 200 mmol/L NaCl; although some seeds germinated, with the increase in stress time, the seeds basically rotted (Figure 1A,B). Therefore, 75 mmol/L NaCl was used as the salt stress treatment in this experiment.
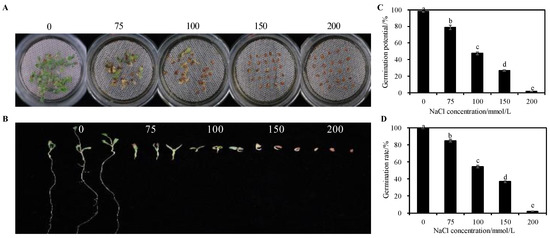
Figure 1.
Effects of different concentrations of NaCl stress on seed germination of alfalfa. (A) Germination state. (B) Growth state. (C) Germination potential. (D) Germination rate. Bars superscripted by different lowercase letters indicate significant differences (p < 0.05), mean ± SE.
3.2. Effects of Different Concentrations of Cp2 EPS on Seed Germination of Alfalfa under Salt Stress
The CK2 group exhibited a significant inhibition of alfalfa seed germination in comparison to the CK1 group (p < 0.05). Compared with the CK2 group, the germination potential, germination rate, and germination index of seeds showed a trend of first increasing and then decreasing with the increase in Cp2 EPS concentration from T1 to T5 treatment groups, and all reached their maximum under the T3 treatment group, which increased by 19.88%, 15.88%, and 25.81%, respectively (p < 0.05). The results show that the germination of alfalfa seeds was inhibited under 75 mmol/L NaCl stress, while the addition of 0.5~2.5 g/L Cp2 EPS could promote the germination of alfalfa seeds in alfalfa under this salt stress (Table 1).

Table 1.
Effects of different concentrations of Cp2 EPS on germination rate, germination potential, germination index and vitality index of salt-stressed alfalfa seeds.
3.3. Effects of Different Concentrations of Cp2 EPS on Growth of Alfalfa Seedlings under Salt Stress
In comparison to the CK1 group, the CK2 group significantly inhibited the growth of alfalfa seedlings (p < 0.05), resulting in a decrease of 69.05% in seedling length and 94.19% in root length, respectively. The seedling length of alfalfa under the T3 treatment group was the longest with an increase of 91.11%, followed by the T2 treatment group with an increase of 64.42%; the root length of alfalfa under the T2 treatment group was the longest with an increase of 552.30%, followed by the T3 treatment group with an increase of 532.57% (Figure 2). The results show that the growth of alfalfa seedlings was inhibited under 75 mmol/L NaCl stress, while the addition of 0.5~2.5 g/L Cp2 EPS promoted the growth of alfalfa under this salt stress.
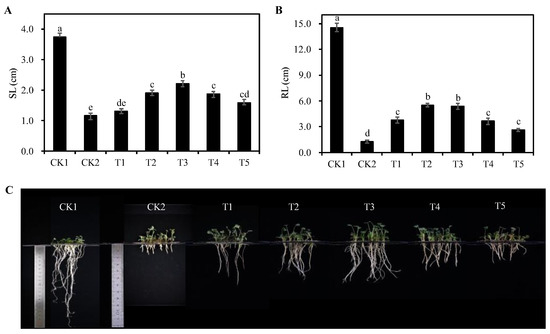
Figure 2.
Effects of different concentrations of Cp2 EPS on the seeding length (A) and root length (B) and apparent morphology (C) of salt-stressed alfalfa seedlings. Bars superscripted by different lowercase letters indicate significant differences (p < 0.05), and the error bars were expressed in the form of ± standard error (SE).
3.4. Effects of Different Concentrations of Cp2 EPS on Fresh and Dry Weight of Alfalfa under Salt Stress
The CK2 group significantly reduced the fresh weight and dry weight of alfalfa seedlings compared with the CK1 group (p < 0.05) (Figure 3). The addition of different concentrations of Cp2 EPS all significantly increased seedling fresh weight (Figure 3A), root fresh weight (Figure 3B), seedling dry weight (Figure 3C), and root dry weight (Figure 3D) of alfalfa under salt stress (p < 0.05). Compared with the CK2 group, seedling fresh weight and root dry weight of alfalfa under the T3 treatment group were the largest, increasing by 189.60% and 187.06% (p < 0.05), respectively; root fresh weight of alfalfa under the T2 treatment group was the largest, increasing by 220.90% (p < 0.05); seedling dry weight of alfalfa under T5 treatment group was the largest, increasing by 209.27% (p < 0.05).
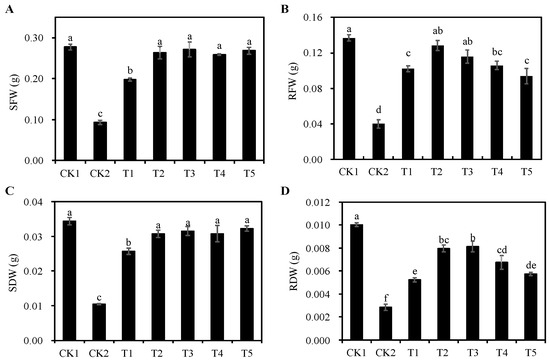
Figure 3.
Effects of different concentrations of Cp2 EPS on fresh and dry weights of salt-damaged alfalfa seedlings. (A) Seedling fresh weight (SFW). (B) Root fresh weight (RFW). (C) Seedling dry weight (SDW). (D) Root dry weight (RDW). Bars superscripted by different lowercase letters indicate significant differences (p < 0.05), mean ± SE.
3.5. Effects of Different Concentrations of Cp2 EPS on Root Morphology of Alfalfa under Salt Stress
In comparison to the CK1 group, the CK2 group significantly reduced root surface area and root volume of alfalfa by 82.34% and 50.94% (p < 0.05), respectively, and increased the average root diameter by 229.41% (Table 2). Under NaCl stress, the root surface area and root volume of alfalfa tended to increase and then decrease as the concentration of Cp2 EPS increased, and the average root diameter tended to decrease and then increase. Compared with the CK2 group, the average root diameter of alfalfa under the T2 treatment group was the smallest, decreasing by 48.31%; the root surface area and root volume of alfalfa under T3 treatment group were the largest, increasing by 331.29% and 111.54%, respectively (p < 0.05).

Table 2.
Effects of different concentrations of Cp2 EPS on average root diameter, root surface area, and root volume of the salt-stressed Alfalfa seedlings.
3.6. Effects of Different Concentrations of Cp2 EPS on Chlorophyll Content of Alfalfa under Salt Stress
In comparison to the CK1 group, chlorophyll a, chlorophyll b, and total chlorophyll content (Figure 4A–C) of seedlings under the CK2 group were significantly reduced by 57.92%, 65.99%, and 61.72% (p < 0.05), respectively. Compared with CK2 group, all treatment groups from T1 to T5 significantly alleviated the inhibitory effect of salt stress on the chlorophyll content of alfalfa leaves (p < 0.05), among which, the T2 treatment group had the best effect with 122.58%, 65.67%, and 98.13% increase in chlorophyll a, chlorophyll b, and total chlorophyll content, respectively, followed by the T3 treatment group, which was not significantly different from the T2 treatment group (p > 0.05).
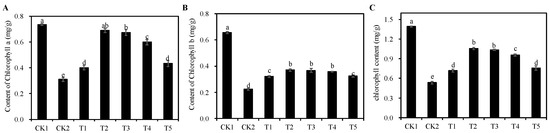
Figure 4.
Effect of different concentrations of Cp2 EPS on chlorophyll content of salt-damaged alfalfa seedlings. (A) Chlorophyll a. (B) Chlorophyll b. (C) Total chlorophyll content. Bars superscripted by different lowercase letters indicate significant differences (p < 0.05), mean ± SE.
3.7. Oxidative Damage of Different Concentrations of Cp2 EPS on Alfalfa Seedlings under Salt Stress
In comparison to the CK1 group, the CK2 group exhibited a significant increase in the EL, MDA, and H2O2 content of alfalfa seedlings (p < 0.05), with respective increases of 194.81%, 58.70%, and 229.27% (Figure 5). Conversely, the EL, MDA, and H2O2 content of alfalfa seedlings under T1 to T5 treatment groups were significantly reduced in comparison to the CK2 group (p < 0.05). Notably, the T3 treatment group was the most effective, with the EL, MDA content, and H2O2 content reduced by 63.58%, 33.99%, and 40.25%, respectively (p < 0.05). These results show that an appropriate concentration of Cp2 EPS could effectively reduce cell membrane oxidative damage and protect cell structure stability under 75 mmol/L NaCl stress.
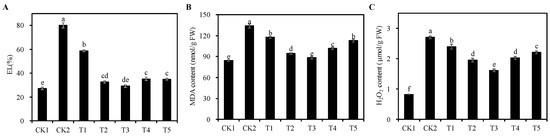
Figure 5.
Effects of different concentrations of Cp2 EPS on EL, MDA content and H2O2 content of the salt-stressed Alfalfa seedlings. (A) Electrolyte leakage (EL). (B) Malondialdehyde (MDA) content. (C) Hydrogen peroxide (H2O2) content. Bars superscripted by different lowercase letters indicate significant differences (p < 0.05), mean ± SE.
3.8. Effects of Different Concentrations of Cp2 EPS on Osmoregulatory Substances in Alfalfa Seedlings under Salt Stress
Pro, SS, and SP are important osmoregulatory substances in plants. Salt stress dramatically induced the accumulation of Pro and SS in alfalfa seedlings, with further augmentation of Pro, SS, and SP content observed upon treatment with Cp2 EPS (Figure 6). Further analysis showed that the content of Pro, SS, and SP in all mitigation groups was significantly different from that of the CK2 group (p < 0.05), except for the Pro content in T1 treatment group. Notably, the T3 treatment group exhibited the highest accumulation of osmoregulatory substances, with a respective increase of 122.23%, 135.67%, and 122.23% compared to the CK2 group, respectively (p < 0.05). In summary, Cp2 EPS can alleviate the damage of salt stress on alfalfa seedlings by accumulating osmoregulatory substances.
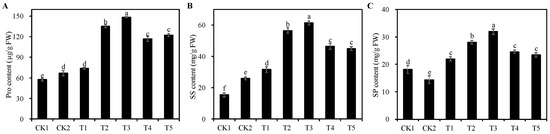
Figure 6.
Effects of different concentrations of Cp2 EPS on osmoregulatory substances of the salt-stressed Alfalfa seedlings. (A) Proline (Pro) content. (B) Soluble sugar (SS) content. (C) Soluble protein (SP) content. Bars superscripted by different lowercase letters indicate significant differences (p < 0.05), mean ± SE.
3.9. Effects of Different Concentrations of Cp2 EPS on Antioxidant Enzyme Activities of Alfalfa Seedlings under Salt Stress
SOD and CAT are important antioxidant enzymes that protect cells from oxidative damage by eliminating excessive reactive oxygen species from plants. The SOD and CAT activities of alfalfa seedlings were significantly decreased in the CK2 group as compared to the CK1 group (p < 0.05). Compared with the CK2 group, the SOD and CAT activities of seedlings treated with T1 to T5 were significantly increased (p < 0.05), and reached their maximum values under the T2 and T3 treatment groups, with an increase of 71.63% and 105.25%, respectively (p < 0.05) (Figure 7). The results show that the application of certain concentrations of Cp2 EPS could improve the antioxidant enzyme activity to alleviate the salt stress on alfalfa seedlings.
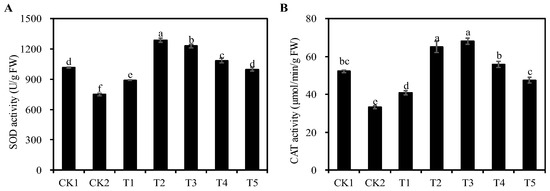
Figure 7.
Effects of different concentrations of Cp2 EPS on antioxidant enzyme activities of the salt-stressed Alfalfa seedlings. (A) Superoxide dismutase (SOD) activity. (B) Catalase (CAT) activity. Bars superscripted by different lowercase letters indicate significant differences (p < 0.05), mean ± SE.
3.10. Correlation Analysis and Principal Component Analysis
Spearman correlation analysis was performed on 24 parameters, including plant germination index, growth index, and physiological index (Figure 8A). The results show that ARD, EL, MDA, and H2O2 were negatively correlated with other indicators.
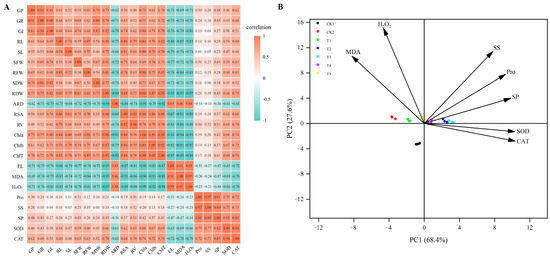
Figure 8.
Heatmap of correlation between germination, growth, and physiological indicators (A). The image show Spearman correlation and the values in the grid represent correlation coefficients (r values). The red color indicates a positive (0 < r < 1.0) correlation and the green color indicates a negative (−1.0 < r < 0) correlation. GP, germination potential; GR, germination rate; GI, germination index; SL, seeding length; RL, root length; SFW, seedling fresh weight; RFW, root fresh weight; SDW, seedling dry weight; RDW, root dry weight; ARD, average root diameter; RSA, root surface area; RV, root volume; Chla, chlorophyll a; Chlb, chlorophyll b; ChlT, total chlorophyll; EL, electrolyte leakage; MDA, malondialdehyde; H2O2, hydrogen peroxide; Pro, proline; SS, soluble sugar; SP, soluble protein; SOD, superoxide dismutase; and CAT, catalase activity. Principal component analysis of physiological indicators (B). The percent total variance is shown for PC1 and PC2 in parenthesis in the axis. PCA, principal component analysis; PC1, principal component 1; and PC2, principal component 2.
Principal component analysis was further performed on the physiological indicators of alfalfa response after Cp2 EPS treatment (Figure 8B). The results show that these seven parameters can be divided into PC1 (68.5%) and PC2 (21.7%), both of which have a cumulative contribution rate of 90.2%, thus explaining the distribution of each sample well. The MDA and H2O2 were distributed in the second quadrant, and they almost showed the opposite relationships to the others distributed in the first and fourth quadrants.
3.11. Comprehensive Analysis
The indicators used for each treatment were normalized to obtain the contribution values of all indicators for each treatment (Figure 9A). Subsequently, a comprehensive analysis was conducted based on the contribution values (Figure 9B). The results show that the addition of different concentrations of Cp2 EPS could alleviate salt stress in alfalfa to different degrees. Notably, the T3 (1.5 g/L Cp2 EPS) treatment group had the best effect on the germination of alfalfa seeds and seedling growth under 75 mmol/L NaCl stress. The comprehensive analysis yielded the following ranking: CK1 > T3 > T2 > T4 > T5 > T1 > CK2.
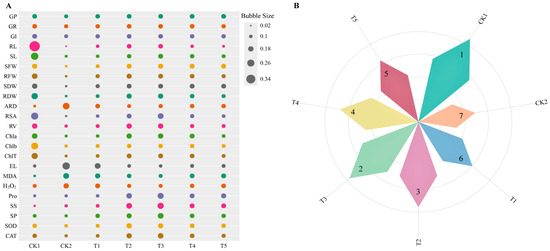
Figure 9.
Comparison of the classification of germination, growth, and physiological indicators of alfalfa under different treatments (A). Comprehensive evaluation of the growth characteristics of alfalfa under salt stress with different concentrations of Cp2 EPS treatments (B). CK1 means distilled water; CK2 means 75 mmol/L NaCl; T1, T2, T3, T4, and T5 mean at 75 mmol/L NaCl stress solution added 0.5, 1.0, 1.5, 2.0, and 2.5 g/L Cp2 EPS treatment.
4. Discussion
The germination of seeds is a critical stage in the life cycle of plants, which is closely related to the proper development of seedlings [27]. Salt stress is a prominent factor that hinders seed germination and the normal growth of seedlings [28]. In this study, we found that salt stress significantly reduced the index related to alfalfa seed germination and severely inhibited the phenotypic growth of seedlings, mainly characterized by stunted and thickened root systems, yellowing of leaves, and a marked reduction in plant height and biomass [29]. It was demonstrated that EPS not only serves as a regulator of nutrient transport, hypocotyl elongation, cotyledon greening, and shoot development, but also plays a direct role in plant salt tolerance [30]. In the current study, we found that the germination ability of alfalfa seeds and seedling growth ability were significantly enhanced by the addition of Cp2 EPS under salt stress conditions, consistent with the findings of Sun [31]. In addition, this study revealed that salt stress had a more pronounced effect on root morphology than on aboveground structures. The impact of salt stress on root morphology was observed through a significant reduction in total root length, total root volume, and total root surface area. This phenomenon may be attributed to the root system’s initial perception of salt stress signals, which triggers physiological responses that ultimately affect root structure [32]. Conversely, the application of Cp2 EPS resulted in a notable improvement in seedling root growth. This effect could be attributed to the formation of a biofilm by EPS, which adhered to the root surface and provided protection against salt damage [33]. Similar findings were reported by Liu et al. [34].
Photosynthesis is an important component of plant growth and development, and chlorophyll content can serve as an indicator of a plant’s ability to absorb, convert, and transfer light energy [35]. This study showed that exposure to 75 mmol/L NaCl stress resulted in a significant reduction in the levels of chlorophyll a, chlorophyll b, and total chlorophyll in alfalfa. Conversely, different concentrations of Cp2 EPS significantly increased chlorophyll content of alfalfa. This indicated that Cp2 EPS has a protective effect on the degradation of chlorophyll in alfalfa leaves under salt stress, providing a guarantee for photosynthesis under salt stress environments and providing an energy basis for the normal physiological and biochemical activities of plants. The reason may be that EPS inhibits the absorption of Na+ by the root system and reduces the transportation of Na+ to the aboveground, thus protecting photosynthetic pigments in alfalfa from salt damage and maintaining the normal operation of plant photosynthesis, and subsequently alleviating the toxic effects of salt stress on alfalfa leaves [10]. Additionally, previous studies demonstrated that the inoculation of EPS-producing bacteria under salt stress has a beneficial effect on the photosynthetic pigment content of maize [34] and wheat [36] seedlings, which is in agreement with the findings of the present investigation.
Under salt stress conditions, plants can accumulate osmoregulatory substances, including proline, soluble sugar, and soluble protein, to counteract the osmotic imbalance caused by salt stress [37]. It was shown that these osmoregulatory substances, specifically amino acids and soluble sugars play a crucial role in maintaining cell expansion pressure, safeguarding cell structure, and eliminating reactive oxygen species (ROS). Of particular note is the protective function of proline, which can prevent damage to protein conversion mechanisms and upregulate stress protective proteins. This is the main mechanism by which plants enhance their resistance to salt stress [37,38]. In this study, we found that salt stress induced the synthesis of proline in alfalfa seedlings, which greatly resisted salt stress injury. This phenomenon may be attributed to the plant’s stress response mechanism under salt stress. Additionally, the addition of EPS further increased the proline content of alfalfa seedlings, indicating its potential in mitigating the osmotic stress imbalance caused by salt stress. Furthermore, the results of this study also demonstrate a significant elevation in the levels of soluble sugar and soluble protein in alfalfa seedlings upon the addition of EPS. This observation is consistent with the report by Liu et al. [34], who suggested that EPS produced by salt-tolerant rhizobium can enhance osmotic stress tolerance and promote growth in maize. Similarly, Sun et al. [31] demonstrated that EPS produced by Pantoea alhagi NX-11 protected rice seedlings from salt stress by increasing the content of osmoregulatory substances. These results provide further evidence that EPS can alleviate osmotic stress induced by salt stress.
ROS are products of aerobic metabolic processes within biological cells. Under normal circumstances, the production and clearance of ROS in plants are typically maintained in a dynamic balance. Nevertheless, exposure to salt stress conditions can disturb this balance, resulting in an excessive accumulation of ROS. Excessive ROS levels can inflict harm upon cell membranes, elevate membrane permeability, cause significant electrolyte loss, and disrupt regular cellular function [39,40]. EL and MDA are important physiological indicators to identify the strength of plant resistance, while H2O2 is one of the main components of ROS, and its content can directly reflect the degree of cell membrane damage [41]. In this study, the levels of EL, MDA, and H2O2 were extremely increased in alfalfa seedlings under salt stress, indicating a heightened degree of oxidative damage. However, treatment with Cp2 EPS resulted in a significant reduction in the levels of EL, MDA, and H2O2, indicating that Cp2 EPS can significantly reduce the degree of cell membrane lipid peroxidation and increase the salt tolerance of alfalfa. The reason may be that microbial EPS, as a natural antioxidant, can eliminate free radicals to reduce ROS levels, thus protecting plants from ROS-induced lipid peroxidation [42].
In response to salt stress, plants also protect themselves from oxidative damage by activating antioxidant defense systems to scavenge excess ROS, including SOD, CAT, and POD, etc. [2]. Among these enzymes, SOD is the most effective scavenger of reactive oxygen species and serves as the primary defense mechanism against ROS damage [43]. SOD converts O2− into O2 and H2O2, and H2O2 is further broken down into H2O by CAT and POD, etc., thus preventing the production of ROS [44]. Multiple studies demonstrated the efficacy of EPS as an antioxidant. In the present study, the activities of SOD and CAT in alfalfa seedlings were observed to decrease under salt stress, but were significantly increased following treatment with EPS, an antioxidant enzyme. Yang et al. [42] reported that EPS secreted by Bacillus amyloliquefaciens exhibited extremely strong free radical scavenging effects. Additionally, Arroussi et al. [45], reported that EPS from Dunaliella salina can enhance the activity of antioxidant enzymes in tomato plants under salt stress. The results of the above studies were basically the same as the results of this experiment, further suggesting that EPS can eliminate excess ROS by upregulating antioxidant enzyme activity, thus protecting plants from salt stress damage.
In summary, EPS can improve the salt tolerance of alfalfa seedlings. Moreover, EPS secreted by Erwinia persicina Cp2 has great application prospects in plant adaptation or improvement of saline–alkali land. However, the current understanding of EPS from this bacterium remains limited, and the possibility of other resistance needs to be further explored.
5. Conclusions
A total of 75 mmol/L NaCl stress severely inhibited the germination of alfalfa seeds and the normal growth of seedlings. However, the application of Cp2 EPS resulted in increased antioxidant enzyme activity, elevated the content of osmoregulatory substance, and reduced membrane permeability, thereby alleviating the damage of salt stress on alfalfa seedlings and promoting their growth. Furthermore, EPS demonstrated a dose-dependent function in improving alfalfa seed germination and seedling growth under salt stress. The comprehensive analysis revealed that 1.5 g/L Cp2 EPS had the most effective alleviating effect on alfalfa under this salt stress concentration. This study provides guidance for effectively utilizing bacterial EPS to improve plant salt tolerance. However, the mechanism of its induction of plant salt resistance is not entirely clear, and especially on the molecular level, research needs to be further explored.
Author Contributions
Conceptualization: Z.Z. and H.C. data curation: Z.J., L.H. and H.C. formal analysis: R.H., W.T. and H.C. methodology: Z.Z., L.Z. and H.C. writing—original draft: H.C. writing—review and editing: Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32060396), Natural Science Foundation of Gansu Province, China (20JR10RA562), Youth Tutor Fund of Gansu Agricultural University (GAU-QDFC-2019-08), and Key Laboratory of Grassland Ecosystem of the Ministry of Education (KLGE202203).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Yao Bo for his help in data analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shu, K.; Qi, Y.; Chen, F.; Meng, Y.J.; Luo, X.F.; Shuai, H.W.; Zhou, W.G.; Ding, J.; Du, J.B.; Liu, J.; et al. Salt stress represses soybean seed germination by negatively regulating GA biosynthesis while positively mediating ABA biosynthesis. Front. Plant Sci. 2017, 8, 1372. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef]
- Lombardi, T.; Lupi, B. Effect of salinity on the germination and growth of Hordeum secalinum Schreber (Poaceae) in relation to the seeds after-ripening time. Biology 2006, 113, 37–42. [Google Scholar]
- Qureshi, M.I.; Abgin, M.Z.; Ahmad, J.; Muhammad, I. Effect of long-term salinity on cellular antioxidants, compatible solute and fatty acid profile of Sweet Annie (Artemisia annua L.). Phytochemistry 2013, 95, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Bai, Z.; et al. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Zannini, E.; Waters, D.M.; Coffey, A.; Arendt, E.K. Production, properties and industrial food application of lactic acid bacteria-derived exopolysaccharides. Appl. Microbiol. Biotechnol. 2016, 100, 1121–1135. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, J.S.; Singh, D.P. Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 2011, 21, 214–222. [Google Scholar] [CrossRef]
- Alami, Y.; Achouak, W.; Marol, C.; Heulin, T. Rhizosphere soil aggregation and plant growth promotion of sunflowers by an exopolysaccharide-producing Rhizobium sp. strain isolated from sunflower roots. Appl. Environ. Microbiol. 2000, 66, 3393–3398. [Google Scholar] [CrossRef]
- Qurashi, A.W.; Sabri, A.N. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz. J. Microbiol. 2012, 43, 1183–1191. [Google Scholar] [CrossRef]
- Ashraf, M.; Hasnain, S.; Berge, O.; Mahmood, T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fertil. Soils. 2004, 40, 157–162. [Google Scholar] [CrossRef]
- Nunkaew, T.; Kantachote, D.; Nitoda, T.; Kanzaki, H.; Ritchie, R.J. Characterization of exopolymeric substances from selected Rhodopseudomonas palustris strains and their ability to adsorb sodium ions. Carbohydr. Polym. 2015, 115, 334–341. [Google Scholar] [CrossRef]
- Yang, F.R.; Chen, J.L.; Ye, S.H.; Liu, Z.F. Characterization of antioxidant activity of exopolysaccharides from endophytic Lysinibacillus sphaericus Ya6 under osmotic stress conditions. Process Biochem. 2022, 113, 87–96. [Google Scholar] [CrossRef]
- Guo, S.; Ma, X.; Cai, W.; Wang, Y.; Gao, X.; Fu, B.; Li, S. Exogenous proline improves salt tolerance of alfalfa through modulation of antioxidant capacity, ion homeostasis, and proline metabolism. Plants 2022, 11, 2994. [Google Scholar] [CrossRef] [PubMed]
- Radović, J.; Sokolović, D.; Marković, J. Alfalfa-most important perennial forage legume in animal husbandry. Biotechnol. Anim. Husb. 2009, 25, 465–475. [Google Scholar] [CrossRef]
- Sun, T.; Li, Z.Z. Alfalfa-corn rotation and row placement affects yield, water use, and economic returns in Northeast China. Field Crops Res. 2019, 241, 07558. [Google Scholar] [CrossRef]
- Lúa, J.; Roda, C.; Zanetti, M.E.; Blanco, F.A. Compatibility between legumes and Rhizobia for the establishment of a successful nitrogen-fixing symbiosis. Genes 2018, 9, 125. [Google Scholar] [CrossRef]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Kulkarni, K.P.; Tayade, R.; Asekova, S.; Song, T.G.; Shannon, G.J.; Lee, D.J. Harnessing the potential of forage legumes, alfalfa, soybean, and cowpea for sustainable agriculture and global food security. Front. Plant Sci. 2018, 9, 1314. [Google Scholar] [CrossRef]
- Song, Y.G.; Lv, J.; Ma, Z.Q.; Dong, W. The mechanism of alfalfa (Medicago sativa L.) response to abiotic stress. Plant Growth Regul. 2019, 89, 239–249. [Google Scholar] [CrossRef]
- Singer, S.D.; Hannoufa, A.; Acharya, S. Molecular improvement of alfalfa for enhanced productivity and adaptability in a changing environment. Plant Cell Env. 2018, 41, 1955–1971. [Google Scholar] [CrossRef] [PubMed]
- Van, Z.E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Xiong, X. Salt Tolerance Mechanism of Alfalfa under Non-Uniform Salt Stress; Nanjing Agricultural University: Nanjing, China, 2019; p. 13. [Google Scholar] [CrossRef]
- Mao, P.S.; Zhang, Y.; Huang, Q.; Mao, C.L.; Tang, J.; Sun, M. Physiological responses of Aohan alfalfa seeds to Melatonin priming under alkaline salt stress. Chin. J. Grassl. 2020, 42, 30–36. [Google Scholar] [CrossRef]
- Yao, B.; Huang, R.; Zhang, Z.F.; Shi, S.L. Seed-borne Erwinia persicina affects the growth and physiology of alfalfa (Medicago sativa L.). Front. Microbiol. 2022, 13, 891188. [Google Scholar] [CrossRef] [PubMed]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Song, J.; Shi, W.W.; Liu, R.R.; Xu, Y.G.; Sui, N.; Zhou, J.C.; Feng, G. The role of the seed coat in adaptation of dimorphic seeds of the euhalophyte Suaeda salsa to salinity. Plant Species Biol. 2017, 32, 107–114. [Google Scholar] [CrossRef]
- Ehab, A.I. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef]
- Lastdrager, J.; Hanson, J.; Smeekens, S. Sugar signals and the control of plant growth and development. J. Exp. Bot. 2014, 65, 799–807. [Google Scholar] [CrossRef]
- Shobhit, R.V.; Vikas, K.P.; Jay, S.S. Plant growth promoting Curtobacterium albidum strain SRV4: An agriculturally important microbe to alleviate salinity stress in paddy plants. Ecol. Indic. 2019, 105, 553–562. [Google Scholar] [CrossRef]
- Sun, L.; Cheng, L.; Ma, Y.; Lei, P.; Wang, R.; Gu, Y.; Li, S.; Zhang, F.; Xu, H. Exopolysaccharides from Pantoea alhagi NX-11 specifically improve its root colonization and rice salt resistance. Int. J. Biol. Macromol. 2022, 209, 396–404. [Google Scholar] [CrossRef]
- Cramer, G.R.; Läuchli, A.; Epstein, E. Effects of NaCl and CaCl2 on ion activities in complex nutrient solutions and root growth of cotton. Plant Physiol. 1986, 81, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Luo, Y.T.; Li, Z.F.; Wang, J.M.; Wei, G.H. Role of exopolysaccharide in salt stress resistance and cell motility of Mesorhizobium alhagi CCNWXJ12-2T. Appl. Microbiol. Biotechnol. 2017, 101, 2967–2978. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.T.; Chai, J.L.; Zhang, Y.C.; Zhang, C.; Lei, Y.; Li, Q.P.; Yao, T. Halotolerant rhizobacteria mitigate the effects of salinity stress on maize growth by secreting exopolysaccharides. Environ. Exp. Bot. 2022, 204, 105098. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Pawelzik, E. Contribution of amino acids to strawberry fruit quality and their relevance as stress indicators under NaCl salinity. Food Chem. 2008, 111, 642–647. [Google Scholar] [CrossRef]
- Din, B.U.; Sarfraz, S.; Xia, Y.; Kamran, M.A.; Javed, M.T.; Sultan, T.; Munis, M.F.H.; Chaudhary, H.J. Mechanistic elucidation of germination potential and growth of wheat inoculated with exopolysaccharide and ACC-deaminase producing Bacillus strains under induced salinity stress. Ecotoxicol. Environ. Saf. 2019, 183, 109466. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2019, 251, 3. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef]
- Soltabayeva, A.; Ongaltay, A.; Omondi, J.O.; Srivastava, S. Morphological, physiological and molecular markers for salt-stressed plants. Plants 2021, 10, 243. [Google Scholar] [CrossRef]
- Mira, M.M.; Katarzyna, C.; Robert, D.H.; Claudio, S. In vitro differentiation of tracheary elements is induced by suppression of Arabidopsis phytoglobins. Plant Physiol. Biochem. 2019, 135, 141–148. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Yang, H.; Deng, J.; Yuan, Y.; Fan, D.; Zhang, Y.; Zhang, R.; Han, B. Two novel exopolysaccharides from Bacillus amyloliquefaciens C-1: Antioxidation and effect on oxidative stress. Curr. Microbiol. 2015, 70, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Arroussi, H.E.; Benhima, R.; Elbaouchi, A.; Sijilmassi, B.; Mernissi, N.E.; Aafsar, A.; Meftah-Kadmiri, I.; Bendaou, N.; Smouni, A. Dunaliella salina exopolysaccharides: A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J. Appl. Phycol. 2018, 30, 2929–2941. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).