Genome-Wide Identification of R2R3-MYB Transcription Factor Family in Tartary Buckwheat (Fagopyrum tataricum) Identifies a Member Involved in Anthocyanin Biosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, RNA Extraction, and cDNA Synthesis
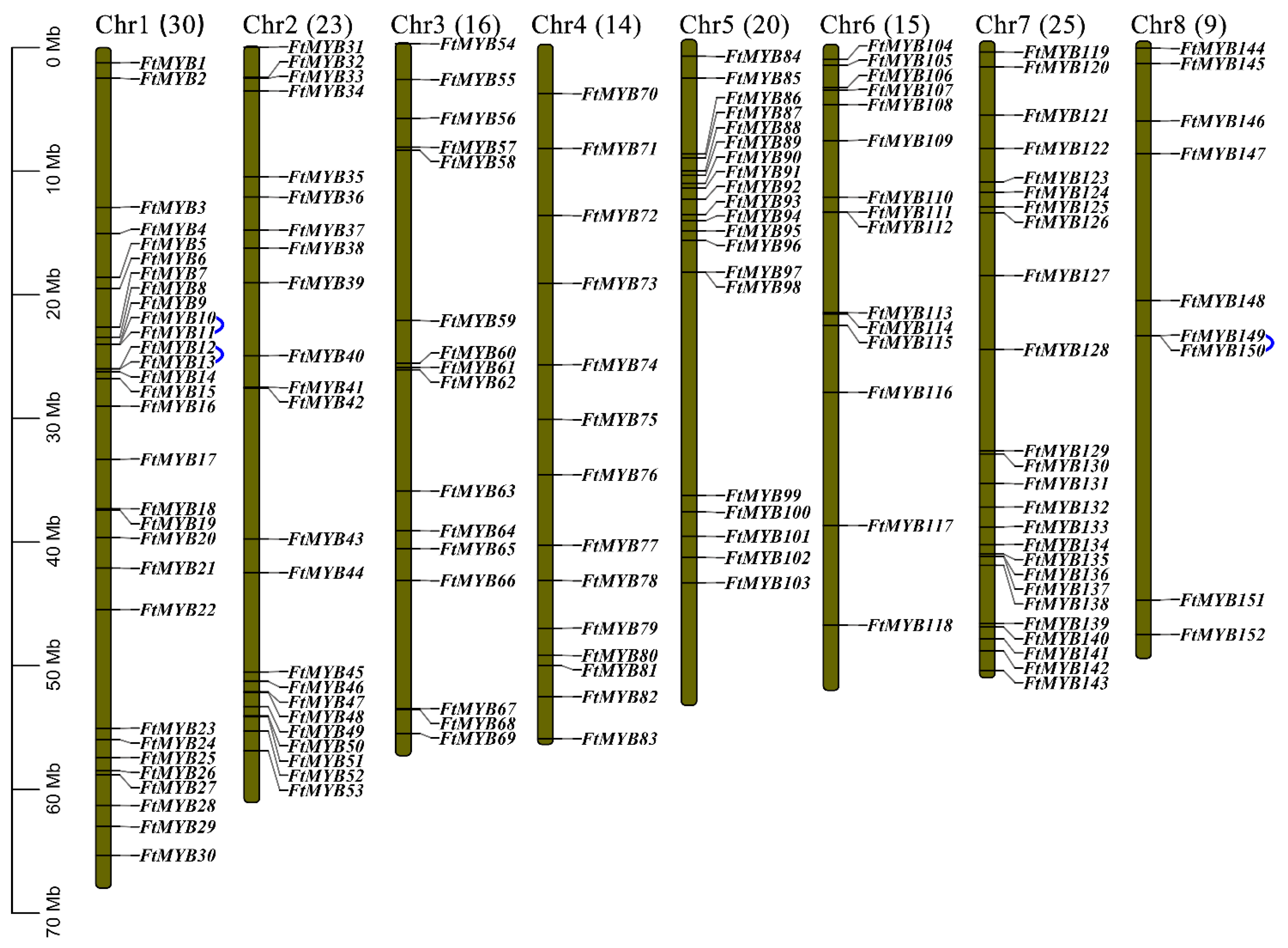
2.2. Identification and Chromosomal Localization Analysis of FtR2R3-MYBs
2.3. Physico-Chemical Analysis of R2R3-MYB Transcription Factors
2.4. Conserved Domains and Phylogenetic Tree Analysis
2.5. Conserved Motif, Gene Structure, Cis-Elements and Synteny Analysis
2.6. Expression Patterns of Ftr2r3-MYB in Different Tissues, under Different Light and Salt Treatments
2.7. Arabidopsis Protoplast Transient Expression Assay
2.8. Yeast-Two-Hybrid Analysis
2.9. Overexpression of FtMYB43 in Arabidopsis
2.10. Determination of Anthocyanin Content
2.11. Real-Time Quantitative PCR (qRT-PCR)
3. Results
3.1. Identification and Chromosome Location of FtR2R3-MYB Genes
3.2. Protein Information Analysis of FtR2R3-MYB TFs
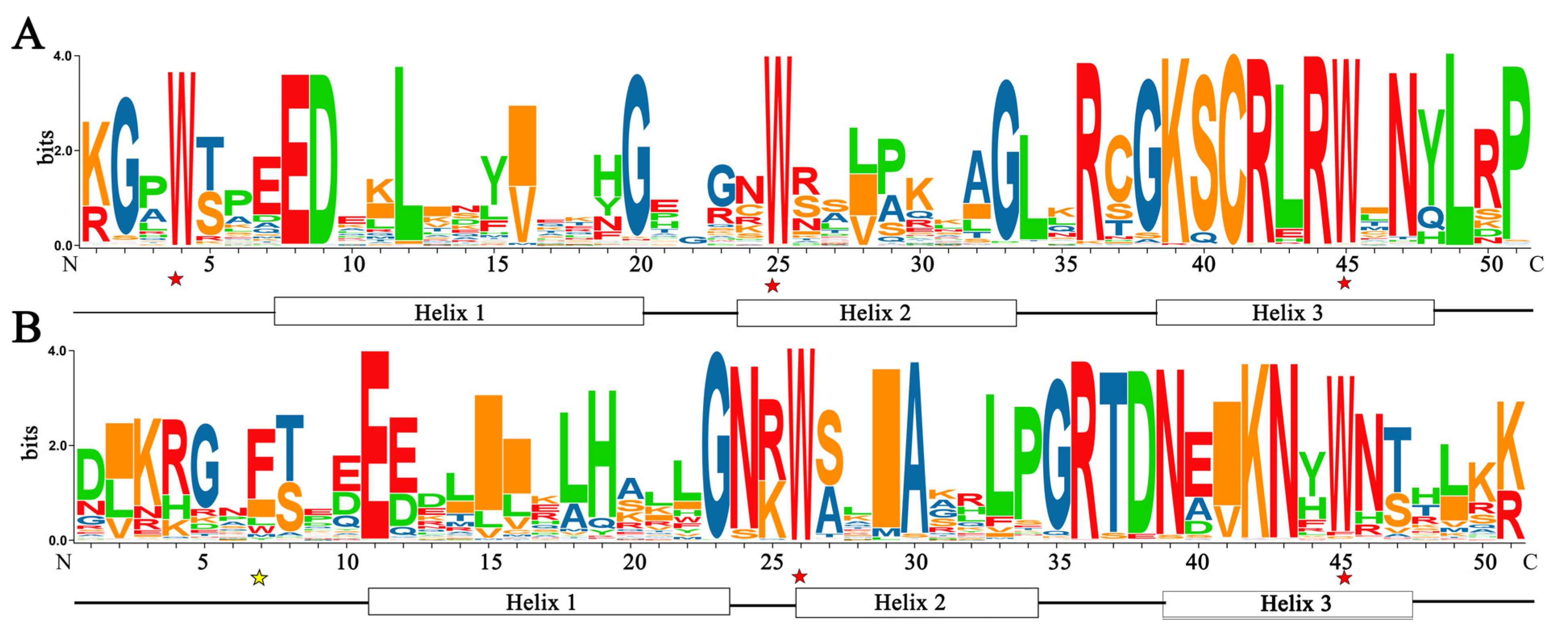
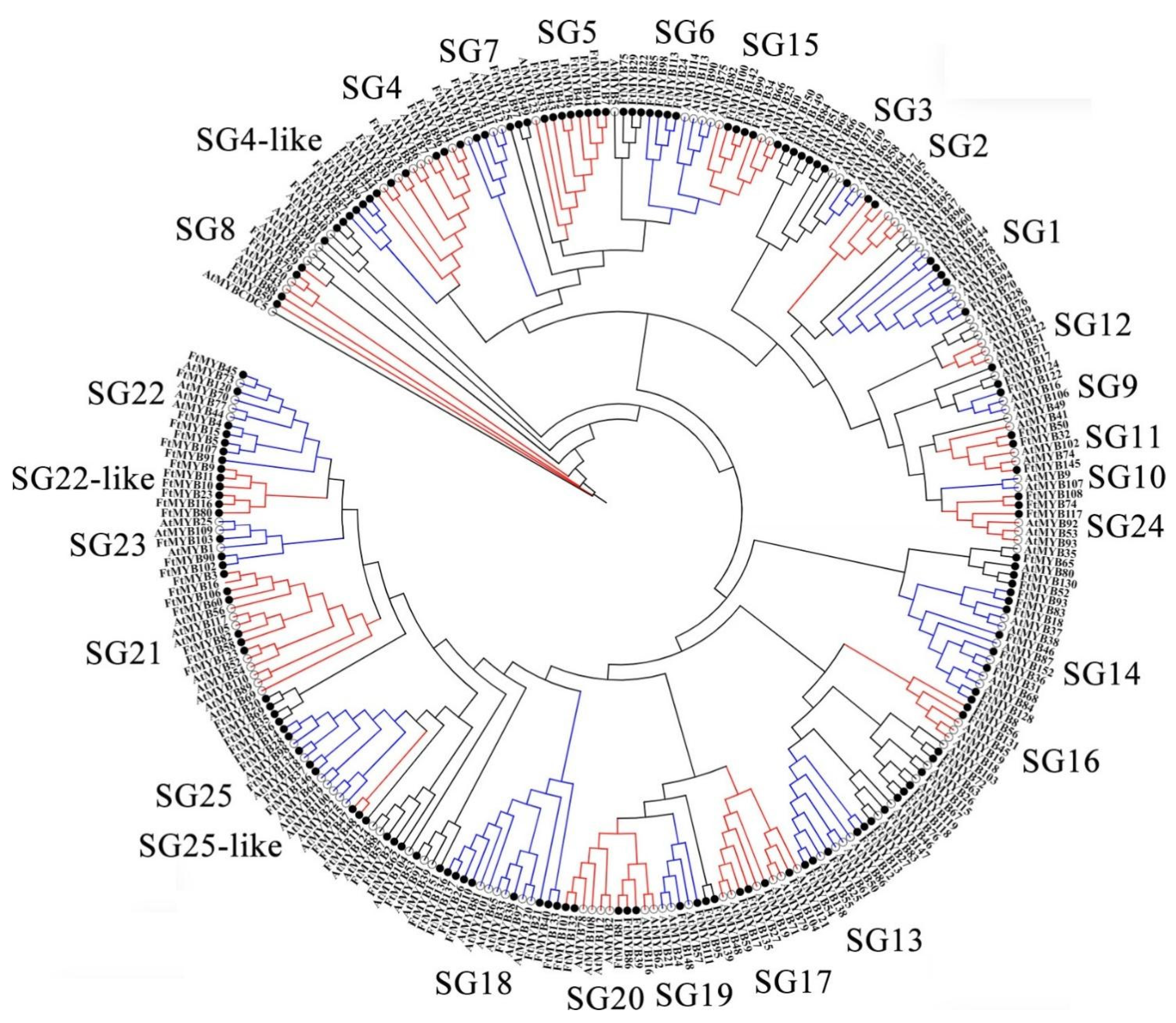
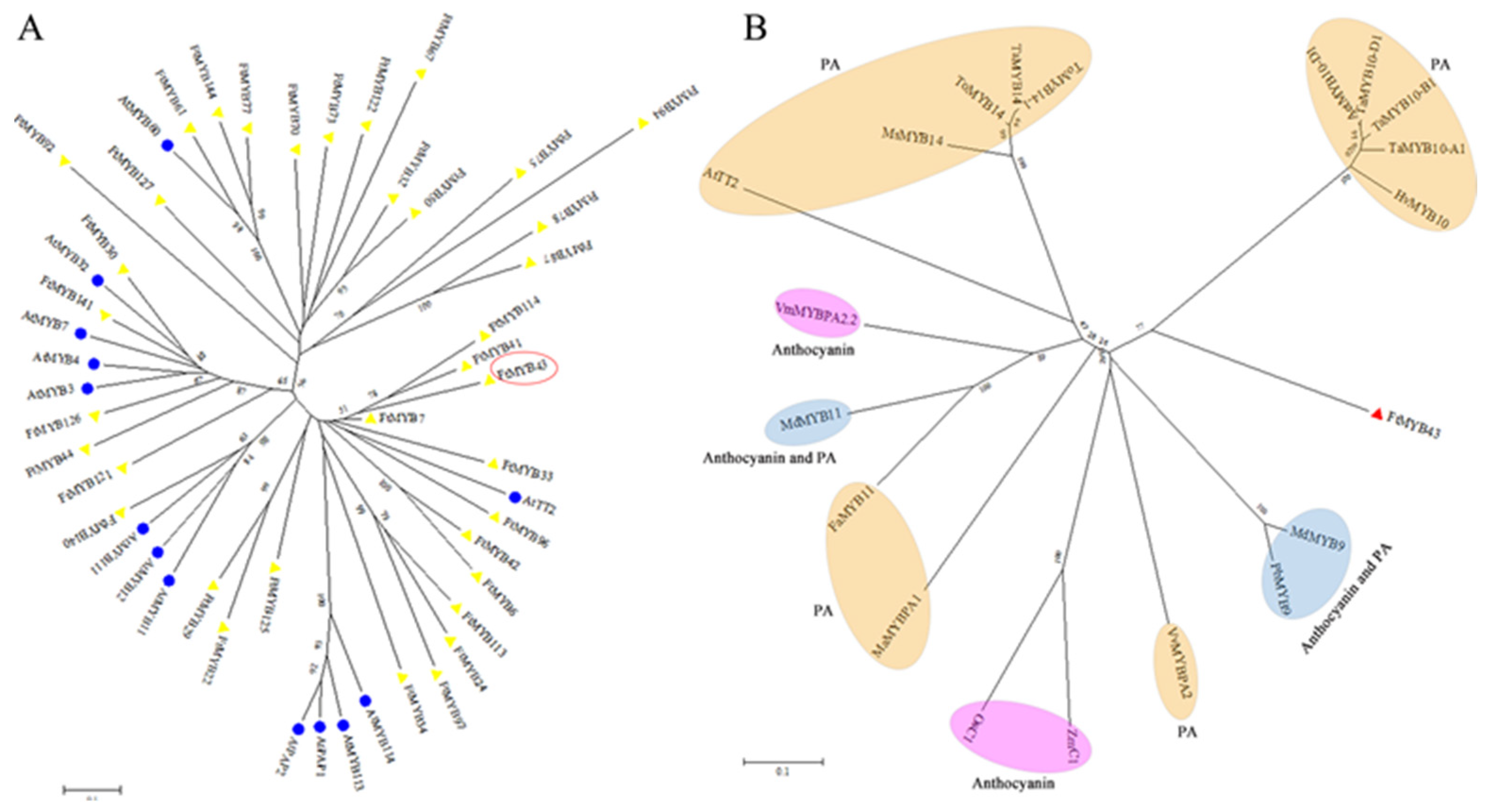
3.3. Conserved Domains and Phylogenetic Relationship Analysis
3.4. Analysis of Motifs, Gene Structure and Cis-Acting Elements of FtR2R3-MYB TFs
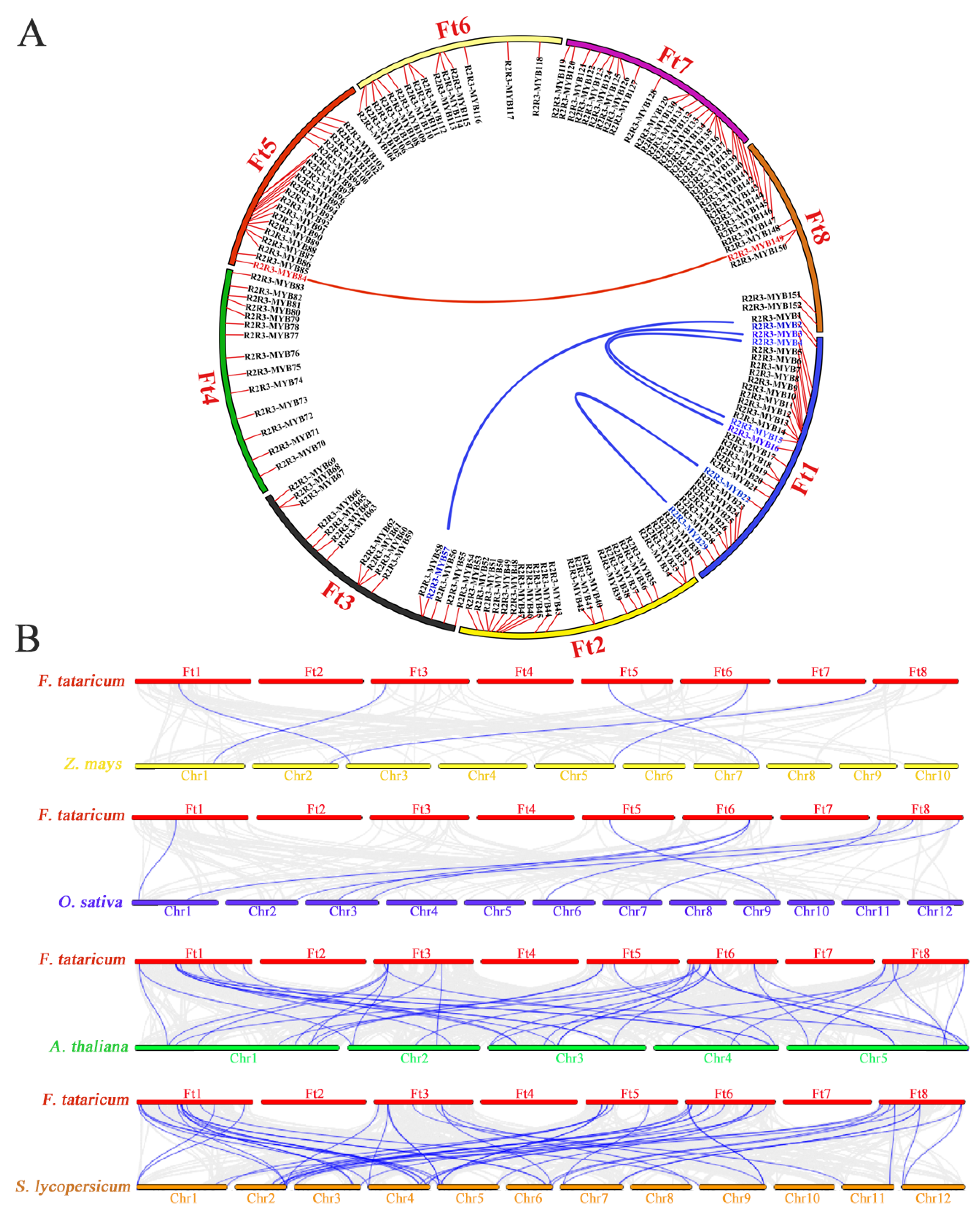
3.5. Synteny Analysis of R2R3-MYB TFs
3.6. Expression Patterns of FtR2R3-MYBs in Different Tissues, and under Salt and Light Treatments
3.7. Potential FtR2R3-MYB Proteins Involved in Anthocyanin Biosynthesis
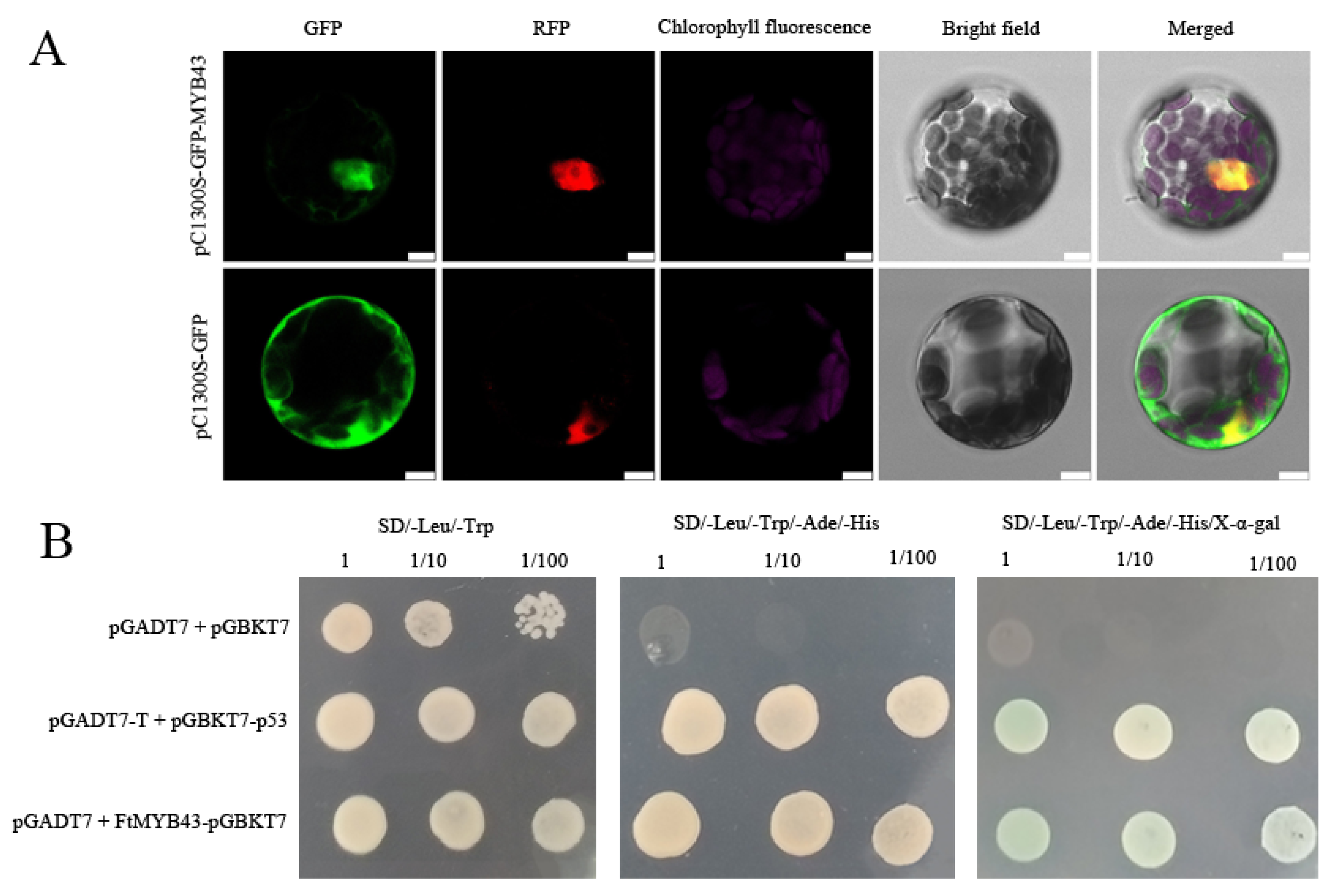
3.8. Characterization of Candidate Gene FtMYB43 That May Promote Anthocyanin Biosynthesis
3.9. Overexpression of FtMYB43 in Arabidopsis
4. Discussion
4.1. Identification and Characteristics of R2R3-MYB Genes in Tartary Buckwheat
4.2. Phylogenesis and Evolution of FtR2R3-MYB Genes
4.3. Expression Profiles of FtR2R3-MYB Gene in Different Tissues, and under Salt and Light Stress
4.4. FtMYB43 Is a Transcript Factor and Participates in Positively Regulating Anthocyanin Biosynthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thakur, S.; Vasudev, P.G. MYB Transcription Factors and their Role in Medicinal Plants. Mol. Biol. Rep. 2022, 49, 10995–11008. [Google Scholar] [CrossRef] [PubMed]
- Stracke, R.; Werber, M.; Weisshaar, B. The R2R3-MYB Gene Family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2001, 4, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Stracke, R.; Grotewold EWeisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; He, K.; Liu, M.; Li, J.; Gao, Z.; Lin, Z.; Zhang, Y.; Wang, X.; Qiu, X.; et al. The MYB Transcription Factor Superfamily of Arabidopsis: Expression Analysis and Phylogenetic Comparison with the Rice MYB Family. Plant Mol. Biol. 2006, 60, 107–124. [Google Scholar]
- Baumann, K.; Perez-Rodriguez, M.; Bradley, D.; Venail, J.; Bailey, P.; Jin, H.; Koes, R.; Roberts, K.; Martin, C. Control of Cell and Petal Morphogenesis by R2R3 MYB Transcription Factors. Development 2007, 134, 1691–1701. [Google Scholar] [CrossRef]
- Ambawat, S.; Sharma, P.; Yadav, N.R.; Yadav, R.C. MYB Transcription Factor Genes as Regulators for Plant Responses: An Overview. Physiol. Mol. Biol. Plants 2013, 19, 307–321. [Google Scholar] [CrossRef]
- Oshima, Y.; Mitsuda, N. The MIXTA-Like Transcription Factor MYB16 is a Major Regulator of Cuticle Formation in Vegetative Organs. Plant Signal. Behav. 2013, 8, e26826. [Google Scholar] [CrossRef]
- Rosany, C.R.; Beatriz, V.T.; Blanca, S.S. MiR858-Mediated Regulation of Flavonoid-Specific MYB Transcription Factor Genes Controls Resistance to Pathogen Infection in Arabidopsis. Plant Cell Physiol. 2018, 1, 190–204. [Google Scholar]
- Yan, H.; Pei, X.; Zhang, H.; Li, X.; Zhang, X.; Zhao, M.; Chiang, V.L.; Sederoff, R.R.; Zhao, X. MYB-Mediated Regulation of Anthocyanin Biosynthesis. Int. J. Mol. Sci. 2021, 22, 3103. [Google Scholar] [CrossRef]
- He, Y.; Li, M.; Wang, Y.; Shen, S. The R2R3-MYB transcription factor MYB44 modulates carotenoid biosynthesis in Ulva prolifera. Algal Res. 2022, 62, 102578. [Google Scholar] [CrossRef]
- Jia, N.; Liu, J.; Tan, P.; Sun, Y.; Lv, Y.; Liu, J.; Sun, J.; Huang, Y.; Lu, J.; Jin, N.; et al. Citrus Sinensis MYB Transcription Factor CsMYB85 Induce Fruit Juice Sac Lignification through Interaction with other CsMYB Transcription Factors. Front. Plant Sci. 2019, 25, 213. [Google Scholar] [CrossRef]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription Factors Associated with Abiotic and Biotic Stress Tolerance and Their Potential for Crops Improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef]
- Jin, J.P.; Tian, F.; Yang, D.C.; Meng, Y.Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a Central Hub for Transcription Factors and Regulatory Interactions in Plants. Nucleic Acids Res. 2017, 45, D1040–D1045. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, Q.; Sun, Y.; Yang, L.; Wang, Z. Genome-Wide Identification and Characterization of R2R3-MYB Family in Hypericum perforatum under Diverse Abiotic Stresses. Int. J. Biol. Macromol. 2020, 145, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, P.; Gu, M.; Lin, X.; Hou, B.; Zheng, Y.; Sun, Y.; Jin, S.; Ye, N. R2R3-MYB Transcription Factor Family in Tea Plant (Camellia sinensis): Genome-Wide Characterization, Phylogeny, Chromosome Location, Structure and Expression Patterns. Genomics 2021, 11, 1565–1578. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; He, W.; Guo, X.; Pan, J. Genome-Wide Identification, Classification and Expression Analysis of the MYB Transcription Factor Family in Petunia. Int. J. Mol. Sci. 2021, 22, 4838. [Google Scholar] [CrossRef] [PubMed]
- Naik, J.; Rajput, R.; Pucker, B.; Stracke, R.; Pandey, A. The R2R3-MYB Transcription Factor MtMYB134 Orchestrates Flavonol Biosynthesis in Medicago truncatula. Plant Mol. Biol. 2021, 106, 157–172. [Google Scholar] [CrossRef]
- Zhou, X.L.; Chen, Z.D.; Zhou, Y.M.; Shi, R.H.; Li, Z.J. The Effect of Tartary Buckwheat Flavonoids in inhibiting the Proliferation of MGC80-3 Cells during Seed Germination. Molecules 2019, 24, 3092. [Google Scholar] [CrossRef]
- Cao, P.; Wu, Y.; Li, Y.; Xiang, L.; Cheng, B.; Hu, Y.; Jiang, X.; Wang, Z.; Wu, S.; Si, L.; et al. The Important Role of Glycerophospholipid Metabolism in the Protective Effects of Polyphenol-Enriched Tartary Buckwheat Extract against Alcoholic Liver Disease. Food Funct. 2022, 13, 10415–10425. [Google Scholar] [CrossRef]
- Li, H.; Lv, Q.; Liu, A.; Wang, J.; Sun, X.; Deng, J.; Chen, Q.; Wu, Q. Comparative Metabolomics Study of Tartary (Fagopyrum tataricum (L.) Gaertn) and Common (Fagopyrum esculentum Moench) Buckwheat Seeds. Food Chem. 2021, 371, 131125. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Y.; Qin, L.K. The Cause of Germination Increases the Phenolic Compound Contents of Tartary Buckwheat (Fagopyrum tataricum). J. Future Foods 2022, 2, 372–379. [Google Scholar] [CrossRef]
- Kubatka, P.; Mazurakova, A.; Samec, M.; Koklesova, L.; Zhai, K.; Al-Ishaq, R.; Kajo, K.; Biringer, K.; Vybohova, D.; Brockmueller, A.; et al. Flavonoids Against Non-Physiologic Inflammation Attributed to Cancer Initiation, Development, and Progression-3PM Pathways. EPMA J. 2021, 12, 559–587. [Google Scholar] [CrossRef]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant Flavonoids in Cancer Chemoprevention: Role in Genome Stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.C.; Zhang, K.; Wang, C.; Chandora, R.; Khurshid, M.; Li, J.; He, M.; Georgiev, M.I.; Zhou, M. Strategic Enhancement of Genetic Gain for nutraceutical development in buckwheat: A Genomics-Driven Perspective. Biotechnol. Adv. 2020, 39, 107479. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hou, Z.; Liu, D.; Yang, X. Tartary buckwheat Flavonoids Protect Hepatic Cells against High Glucose-Induced Oxidative Stress and Insulin Resistance via MAPK Signaling Pathways. Food Funct. 2016, 7, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Jang, I.S.; Yang, K.E.; Yoon, S.J.; Baek, S.; Lee, J.Y.; Suzuki, T.; Chung, K.Y.; Woo, S.H.; Choi, J.S. Effect of Rutin from Tartary Buckwheat Sprout on serum Glucose-Lowering in Animal Model of Type 2 Diabetes. Acta Pharm. 2016, 66, 297–302. [Google Scholar] [CrossRef]
- Tatsuro Suzuki, T.; Morishita, T.; Takigawa, S.; Noda, T.; Ishiguro, K. Development of Rutin-Rich Noodles using Trace-Rutinosidase Variety of Tartary buckwheat (Fagopyrum tataricum. Gaertn.) ‘Manten-Kirari’. Food Sci. Technol. Res. 2019, 25, 915–920. [Google Scholar] [CrossRef]
- Schaart, J.G.; Dubos, C.; Romero De La Fuente, I.; van Houwelingen, A.M.M.L.; de Vos, R.C.H.; Jonker, H.H.; Xu, W.; Routaboul, J.M.; Lepiniec, L.; Bovy, A.G. Identification and Characterization of MYB-bHLH-WD40 Regulatory Complexes Controlling Proanthocyanidin Biosynthesis in Strawberry (Fragaria × ananassa) Fruits. New Phytol. 2013, 197, 454–467. [Google Scholar] [CrossRef]
- Liu, H.; Chen, H.X.; Chen, X.X.; Lu, J.; Chen, D.L.; Luo, C.; Cheng, X.; Huang, C.L. Transcriptomic and Metabolomic Analyses Reveal that MYB transcription Factors Regulate Anthocyanin Synthesis and Accumulation in the Disc Florets of the Anemone form of Chrysanthemum Morifolium. Sci. Hortic. 2023, 307, 110847. [Google Scholar] [CrossRef]
- Subban, P.; Prakash, S.; Bootbool Mann, A.; Kutsher, Y.; Evenor, D.; Levin, I.; Reuveni, M. Functional Analysis of MYB Alleles from Solanum chilense and Solanum lycopersicum in Controlling Anthocyanin Levels in Heterologous Tobacco Plants. Physiol. Plant. 2021, 172, 1630–1640. [Google Scholar] [CrossRef]
- Wang, N.; Xu, H.; Jiang, S.; Zhang, Z.; Lu, N.; Qiu, H.; Qu, C.; Wang, Y.; Wu, S.; Chen, X. MYB12 and MYB22 Play Essential Roles in Proanthocyanidin and Flavonol Synthesis in Red-Fleshed Apple (Malus sieversii f. niedzwetzkyana). Plant J. 2017, 90, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, D.J.; Espley, R.V.; Deng, C.H.; Dare, A.P.; Günther, C.S.; Jaakola, L.; Karppinen, K.; Boase, M.R.; Wang, L.; Luo, H.; et al. The Coordinated Action of MYB Activators and Repressors Controls Proanthocyanidin and Anthocyanin Biosynthesis in Vaccinium. Front. Plant Sci. 2022, 13, 910155. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Du, W.; Hao, Y.; Han, Y.; Li, H.; Liu, L.; Zhang, K.; Zhou, M.; Sun, Z. Elucidation of the Regulatory Network of Flavonoid Biosynthesis by Profiling the Metabolome and Transcriptome in Tartary Buckwheat. J. Agric. Food Chem. 2021, 69, 7218–7229. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lv, Q.; Ma, C.; Qu, J.; Cai, F.; Deng, J.; Huang, J.; Ran, P.; Shi, T.; Chen, Q. Metabolite Profiling and transcriptome Analyses Provide Insights into the Flavonoid Biosynthesis in the Developing Seed of Tartary Buckwheat (Fagopyrum tataricum). J. Agric. Food Chem. 2019, 67, 11262–11276. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Ma, B.; Gao, Q.; Du, H.; Han, Y.; Li, Y.; Cao, Y.; Qi, M.; Zhu, Y.; et al. The Tartary Buckwheat Genome Provides Insights into Rutin Biosynthesis and Abiotic Stress Tolerance. Mol. Plant. 2017, 10, 1224–1237. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Nesi, N.; Jond, C.; Debeaujon, I.; Caboche, M.; Lepiniec, L. The Arabidopsis TT2 Gene Encodes an R2R3 MYB Domain Protein that Acts as a Key Determinant for Proanthocyanidin Accumulation in Developing Seed. Plant Cell. 2001, 13, 2099–2114. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, C.; Huang, C.; Wen, D.; Lu, J.; Chen, S.; Zhang, T.; Shi, Y.; Xue, J.; Ma, W.; et al. The light-Induced Transcription Factor FtMYB116 Promotes Accumulation of Rutin in Fagopyrum tataricum. Plant Cell Environ. 2019, 42, 1340–1351. [Google Scholar] [CrossRef]
- Wu, Q.; Bai, X.; Zhao, W.; Xiang, D.; Wan, Y.; Yan, J.; Zou, L.; Zhao, G. De Novo Assembly and Analysis of Tartary Buckwheat (Fagopyrum tataricum Garetn.) Transcriptome Discloses Key Regulators Involved in Salt-Stress Response. Genes 2017, 8, 255. [Google Scholar] [CrossRef]
- Xiong, L.; Li, C.; Li, H.; Lyu, X.; Zhao, T.; Liu, J.; Zuo, Z.; Liu, B. A transient Expression System in Soybean Mesophyll Protoplasts Reveals the Formation of Cytoplasmic GmCRY1 Photobody-Like Structures. Sci. China Life Sci. 2019, 2, 1070–1077. [Google Scholar] [CrossRef]
- Zale, J.M.; Agarwal, S.; Loar, S.; Steber, C.M. Evidence for stable transformation of wheat by floral dip in Agrobacterium tumefaciens. Plant Cell Rep. 2009, 28, 903–913. [Google Scholar] [CrossRef]
- Labra, M.; Vannini, C.; Grassi, F.; Bracale, M.; Balsemin, M.; Basso, B.; Sala, F. Genomic Stability in Arabidopsis thaliana Transgenic Plants Obtained by Floral Dip. Theor. Appl. Genet. 2004, 109, 1512–1518. [Google Scholar] [CrossRef]
- Park, N.I.; Li, X.; Suzuki, T.; Kim, S.J.; Woo, S.H.; Park, C.H.; Park, S.U. Differential Expression of Anthocyanin Biosynthetic Genes and Anthocyanin Accumulation in Tartary Buckwheat Cultivars ‘Hokkai t8’ and ‘Hokkai t10’. J. Agric. Food Chem. 2011, 59, 2356–2361. [Google Scholar] [CrossRef]
- An, X.H.; Tian, Y.; Chen, K.Q.; Liu, X.J.; Liu, D.D.; Xie, X.B.; Cheng, C.G.; Cong, P.H.; Hao, Y.J. MdMYB9 and MdMYB11 are Involved In the regulation of the JA-Induced Biosynthesis of Anthocyanin and Proanthocyanidin in Apples. Plant Cell Physiol. 2015, 56, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Liang, L.X.; Li, L.B.; Wang, T. Genome-Wide Analysis of R2R3-MYB Transcription Factors in Phalaenopsis equestris. For. Res. 2018, 31, 104–113. [Google Scholar]
- Zhang, M.; Liu, W.; Bi, Y.P. Dehydration-Responsive Element-Binding (DREB) Transcription Factor in Plants and its Role during Abiotic Stresses. Yi Chuan 2009, 31, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Suzuki, A.; Washida, H.; Takaiwa, F. The GCN4 Motif in a Rice Glutelin Gene is Essential for endosperm-Specific Gene Expression and is Activated by Opaque-2 in Transgenic Rice Plants. Plant J. 1998, 14, 673–683. [Google Scholar] [CrossRef]
- Himi, E.; Maekawa, M.; Miura, H.; Noda, K. Development of PCR Markers for Tamyb10 Related to R-1, Red grain Color Gene in Wheat. Theor. Appl. Genet. 2011, 122, 1561–1576. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, Y.; Zheng, D.; Pang, J.; Liu, Y.; Luo, S.; Meng, S.; Qian, L.; Wei, D.; Dai, S.; et al. Chromosomal-Level Genome Assembly of the Orchid Tree Bauhinia variegata (Leguminosae; Cercidoideae) Supports the Allotetraploid Origin Hypothesis of Bauhinia. DNA Res. 2022, 29, dsac012. [Google Scholar] [CrossRef]
- Terrier, N.; Torregrosa, L.; Ageorges, A.; Vialet, S.; Verriès, C.; Cheynier, V.; Romieu, C. Ectopic Expression of VvMybPA2 Promotes Proanthocyanidin Biosynthesis in Grapevine and Suggests Additional Targets in the Pathway. Plant Physiol. 2009, 149, 1028–1041. [Google Scholar] [CrossRef]
- Hancock, K.R.; Collette, V.; Fraser, K.; Greig, M.; Xue, H.; Richardson, K.; Jones, C.; Rasmussen, S. Expression of the R2R3-MYB Transcription Factor TaMYB14 from Trifolium Arvense Activates Proanthocyanidin Biosynthesis in the Legumes Trifolium repens and Medicago sativa. Plant Physiol. 2012, 159, 1204–1220. [Google Scholar] [CrossRef]
- Rajput, R.; Naik, J.; Stracke, R.; Pandey, A. Interplay between R2R3 MYB-Type Activators and Repressors Regulates Proanthocyanidin Biosynthesis in Banana (Musa acuminata). New Phytol. 2022, 236, 1108–1127. [Google Scholar] [CrossRef]
- Dong, Z.D.; Chen, J.; Li, T.; Chen, F.; Cui, D.Q. Molecular Survey of Tamyb10-1 Genes and their Association with Grain Colour and Germinability in Chinese Wheat and Aegilops tauschii. J. Genet. 2015, 94, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Pu, X.; Gao, R.; Demurtas, O.C.; Fleck, S.J.; Richter, M.; He, C.; Ji, A.; Sun, W.; Kong, J.; et al. Tandem Gene Duplications Drive Divergent Evolution of Caffeine and Crocin Biosynthetic Pathways in Plants. BMC Biol. 2020, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin-Wang, K.; Liu, Z.; Allan, A.C.; Qin, S.; Zhang, J.; Liu, Y. Genome-Wide Analysis and Expression Profiles of the StR2R3-MYB transcription Factor Superfamily in Potato (Solanum tuberosum L.). Int. J. Biol. Macromol. 2020, 148, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, G.; Sun, C.; Sui, N. Research advances of MYB transcription factors in plant stress resistance and breeding. Plant Signal. Behav. 2019, 14, 1613131. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.P.; Zhao, H.X.; Yao, P.F.; Li, Q.Q.; Huang, Y.J.; Li, C.L.; Chen, H.; Wu, Q. An R2R3-MYB Transcription Factor FtMYB15 Involved in the Synthesis of Anthocyanin and Proanthocyanidins from Tartary Buckwheat. J. Plant Growth Regul. 2018, 37, 76–84. [Google Scholar] [CrossRef]
- Mu, R.L.; Cao, Y.R.; Liu, Y.F.; Lei, G.; Zou, H.F.; Liao, Y.; Wang, H.W.; Zhang, W.K.; Ma, B.; Du, J.Z.; et al. An R2R3-Type Transcription Factor Gene AtMYB59 Regulates Root Growth and Cell Cycle Progression in Arabidopsis. Cell Res. 2009, 19, 1291–1304. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, H.X.; Yao, H.P.; Li, C.L.; Chen, H.; Wang, A.H.; Park, S.U.; Wu, Q. Identification, Isolation and Expression Analysis of Eight Stress-Related R2R3-MYB Genes in Tartary Buckwheat (Fagopyrum tataricum). Plant Cell Rep. 2016, 35, 1385–1396. [Google Scholar] [CrossRef]
- Lin, Z.; Cao, D.; Damaris, R.N.; Yang, P. Genome-Wide Identification of MADS-box Gene Family in Sacred Lotus (Nelumbo nucifera) Identifies a SEPALLATA Homolog Gene Involved in Floral Development. BMC Plant Biol. 2020, 20, 497. [Google Scholar] [CrossRef]
- Kumar, M.; Kherawat, B.S.; Dey, P.; Saha, D.; Singh, A.; Bhatia, S.K.; Ghodake, G.S.; Kadam, A.A.; Kim, H.U.; Manorama Chung, S.M. Genome-Wide Identification and Characterization of PIN-FORMED (PIN) Gene Family Reveals Role in Developmental and Various Stress Conditions in Triticum aestivum L. Int. J. Mol. Sci. 2021, 22, 7396. [Google Scholar] [CrossRef] [PubMed]
- Kesawat, M.S.; Kherawat, B.S.; Singh, A.; Dey, P.; Kabi, M.; Debnath, D.; Saha, D.; Khandual, A.; Rout, S.; Manorama; et al. Genome-Wide Identification and Characterization of the Brassinazole-resistant (BZR) Gene Family and Its Expression in the Various Developmental Stage and Stress Conditions in Wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2021, 22, 8743. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Chen, W.; Zhu, X.; Zhou, X.; Jin, Y.; Zhan, C.; Liu, G.; Liu, X.; Ma, D.; Qiao, Y. Genome-Wide Identification and Characterization of Wheat 14-3-3 Genes Unravels the Role of TaGRF6-A in Salt Stress Tolerance by Binding MYB Transcription Factor. Int. J. Mol. Sci. 2021, 22, 1904. [Google Scholar] [CrossRef]
- Kesawat, M.S.; Kherawat, B.S.; Singh, A.; Dey, P.; Routray, S.; Mohapatra, C.; Saha, D.; Ram, C.; Siddique, K.H.M.; Kumar, A.; et al. Genome-Wide Analysis and Characterization of the Proline-Rich Extensin-like Receptor Kinases (PERKs) Gene Family Reveals Their Role in Different Developmental Stages and Stress Conditions in Wheat (Triticum aestivum L.). Plants 2022, 11, 496. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Xu, N.; Rui, C.; Fan, Y.; Wang, J.; Han, M.; Wang, Q.; Sun, L.; Chen, X.; et al. Genome-Wide Expression Analysis of Phospholipase A1 (PLA1) Gene Family Suggests Phospholipase A1-32 Gene Responding to Abiotic Stresses in Cotton. Int. J. Biol. Macromol. 2021, 192, 1058–1074. [Google Scholar] [CrossRef]
- Bai, Y.C.; Li, C.L.; Zhang, J.W.; Li, S.J.; Luo, X.P.; Yao, H.P.; Chen, H.; Zhao, H.X.; Park, S.U.; Wu, Q. Characterization of Two Tartary Buckwheat R2R3-MYB Transcription Factors and their Regulation of Proanthocyanidin Biosynthesis. Physiol. Plant. 2014, 152, 431–440. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Wang, L.; Damaris, R.N.; Zhao, J.; Zhang, L.; Wang, T.; Yang, C.; Huang, J.; Shi, T.; Zhu, L.; et al. Genome-Wide Identification of R2R3-MYB Transcription Factor Family in Tartary Buckwheat (Fagopyrum tataricum) Identifies a Member Involved in Anthocyanin Biosynthesis. Agronomy 2023, 13, 2117. https://doi.org/10.3390/agronomy13082117
Deng J, Wang L, Damaris RN, Zhao J, Zhang L, Wang T, Yang C, Huang J, Shi T, Zhu L, et al. Genome-Wide Identification of R2R3-MYB Transcription Factor Family in Tartary Buckwheat (Fagopyrum tataricum) Identifies a Member Involved in Anthocyanin Biosynthesis. Agronomy. 2023; 13(8):2117. https://doi.org/10.3390/agronomy13082117
Chicago/Turabian StyleDeng, Jiao, Lijuan Wang, Rebecca Njeri Damaris, Jiali Zhao, Lan Zhang, Tingting Wang, Chaojie Yang, Juan Huang, Taoxiong Shi, Liwei Zhu, and et al. 2023. "Genome-Wide Identification of R2R3-MYB Transcription Factor Family in Tartary Buckwheat (Fagopyrum tataricum) Identifies a Member Involved in Anthocyanin Biosynthesis" Agronomy 13, no. 8: 2117. https://doi.org/10.3390/agronomy13082117
APA StyleDeng, J., Wang, L., Damaris, R. N., Zhao, J., Zhang, L., Wang, T., Yang, C., Huang, J., Shi, T., Zhu, L., Meng, Z., Cai, F., & Chen, Q. (2023). Genome-Wide Identification of R2R3-MYB Transcription Factor Family in Tartary Buckwheat (Fagopyrum tataricum) Identifies a Member Involved in Anthocyanin Biosynthesis. Agronomy, 13(8), 2117. https://doi.org/10.3390/agronomy13082117





