Abstract
Field research was conducted in Poland in 2019–2021 to determine the effect of microbial products and living mulches on grain yield and grain yield structure elements as well as the biological index of soil fertility (BIF) in spring barley grown in organic agriculture. Two factors were examined: I. microbial products: control (no treatment with microbial products), inoculation with phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis), and co-inoculation (simultaneous inoculation) with phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum); II. living mulch: control (no living mulch—spring barley grown in a pure stand), red clover, red clover and Italian ryegrass, and Italian ryegrass. The study results demonstrated that the highest grain yield at 4.5 t ha−1 with superior structure was produced by spring barley following co-inoculation with phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum). The highest value of the biological index of soil fertility (BIF II) determined at the flowering stage was obtained in plots with spring barley cultivated with the living mulch of red clover mixed with Italian ryegrass or red clover following inoculation with phosphorus-releasing bacteria and nitrogen-fixing bacteria 6.9 and 5.7, respectively.
1. Introduction
In recent years, a steady increase in consumers’ interest in organic food has been observed. As a result, organic agriculture has been gaining importance. In this agricultural system, cereals are the most prominent crops. Spring barley grain produced in organic agriculture is a valuable raw material for the production of groats and flakes, which are natural in character [1].
In the system of organic agriculture, natural and green manure are the nutrient sources for plants [2]. At present, innovative technologies based on the application of bacterial products are increasingly popular [3,4,5,6]. Biofertilizers stand out due to a number of assets. They provide nutrients directly to plants and enhance the process of microbiological increase in the availability of nutrients, which can be easily assimilated by plants [7]. The microorganisms reside in the rhizosphere (rhizosphere bacteria) or inside the plant (endophytic bacteria) and improve host plant growth by making it easier for the host plant to obtain nutrients [8,9]. Biofertilizers enhance plant growth by supplying nutrients through biological nitrogen fixation or increasing the availability of insoluble nutrients in the soil [10,11,12,13]. Microorganisms such as bacteria which fix nitrogen or dissolve phosphates tend to convert atmospheric nitrogen into a plant-available form, produce enzymes, and solubilise insoluble phosphate from organic and inorganic sources [14]. The mechanism of nitrogen supply to a plant is the same in all free-living bacteria. Non-symbiotic bacteria carry out BNF (biological nitrogen fixation) only during growth and assimilate nitrogen for the metabolism of their cells, without releasing the surplus into the environment. Only after cell death is the plant or soil enriched with this element. The main mechanism for the dissolution of P to plant-accessible forms via phosphorus-releasing bacteria is the production of mineral-solubilising compounds, such as organic acids, siderophores, protons, hydroxyl ions, and CO2 [15]. Organic acids, together with their carboxyl and hydroxyl ions, chelate cations or reduce pH to release P [16]. The organic acids are produced in the periplasmic space via the direct oxidation pathway [17]. The excretion of these organic acids is accompanied by a drop in pH that results in the acidification of the microbial cells and the surroundings; thus, P ions are released via the substitution of H+ for Ca2+ [18]. Moreover, in recent years, the application of beneficial microbes in cereals has demonstrated their positive effect on cereal yield quantity and quality under adverse environmental conditions [19,20]. Given the limited amount of research into this issue, such studies should be conducted, in particular in terms of the organic system of cereal cultivation, including living mulches. The development of innovative technologies of cereal cultivation, including living mulches in combination with the application of microbial products, is gaining particular importance in modern agriculture. It contributes to the increased efficiency of nutrient utilisation and the protection of resources, which are more balanced in terms of environmental protection. Additionally, ecosystems should be made more diverse by increasing the number of cultivated species and using more legumes [21] or inclusion of cover crops [22]. Benefits associated with living mulches used as a cultivation system include lower water run-off and soil erosion as well as the stunted sprouting and development of weeds via the competition for limited resources, the production of allelochemicals, and the increased microbial activity of soil [23]. Species grown as catch crops inhabit different niches in time and space using complementary resources [21]. The introduction of leguminous living mulches contributes to increased biological diversity, and these represent a source of biologically fixed nitrogen for cereals, which is of great importance in organic agriculture. Additionally, living mulches are compatible in both organic and conserving agriculture systems [24].
The persistence of a soil ecosystem can be evaluated using biological Indicators, and soil enzymes have been successfully used as soil quality indicators in various agricultural systems [25]. The biological index of soil fertility (BIF) is gaining importance in soil evaluation at different plant development stages [26,27]. As there are a limited amount of studies on the combined application of living mulch and bacterial products in cereal cultivation, we attempted to undertake such research in order to determine the effect of biological products and living mulches on grain yield, elements of yield structure, and the biological index of oil fertility in spring barley grown in organic agriculture.
2. Materials and Methods
2.1. Experimental Design
Field research was conducted in Poland from 2019 to 2021 on an organic farm located at Wyłazy, a locality near Siedlce. The soil on which the field experiment was set up was a Stagnic Luvisol characterised by the following contents of available minerals determined according to the recommendations of the Chemical and Agricultural Station in Warsaw City [28] in the topsoil prior to the experiment set-up: P—8.3; K—12.1; and Mg—4.2 mg per 100 g−1 soil. The soil reaction was neutral (pH in KCl 6.1) and the organic carbon content was 1.05% a.d.m. The granulometric composition of the arable layer of the soil before the establishment of the experiment was as follows: fraction content 2.0–0.05 mm, 79.49%; 0.05–0.02, mm 9.58%; 0.02–0.002 mm, 9.57%; and ˂0.002 mm, 1.37%. The trial was established as a split-block arrangement with three replicates and the following two factors: factor A—microbial products: control (no treatment with microbial products), inoculation with phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis), co-inoculation (simultaneous inoculation) with phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum); factor B—living mulch: control without living mulch (a pure stand spring barley), red clover, red clover and Italian ryegrass, and Italian ryegrass. The area of the plot was 20 m2 (4 × 5 m). Figure 1 shows the scheme of the experiment and the randomization of research factors.
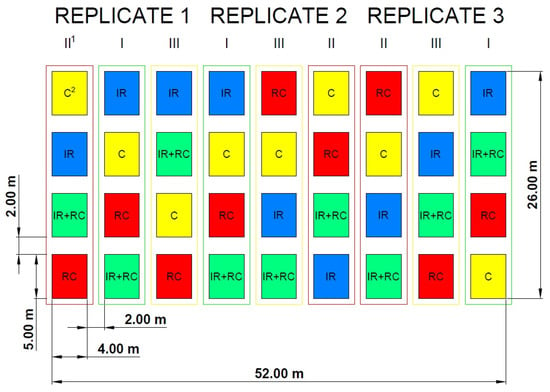
Figure 1.
Scheme of the experimental field. 1 I—control (no treatment with microbial products); II—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis); III—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum); 2 C—control (without living mulch), RC—red clover; IR—Italian ryegrass.
The forecrop for spring barley was winter rye. After harvesting the winter rye, a post-harvest cultivation was carried out. In October, the field was fertilised with 15 t ha of goat manure. The field was then ploughed and left until the following spring. At the beginning of April, pre-sowing cultivation was carried out. Both spring barley and plants grown as a living mulch were sown on the same day in early April. The following respective rates were used for spring barley, red clover, red clover and Italian ryegrass, and Italian ryegrass: 160, 18, 9 and 15, and 30 kg ha−1. The sowing material was organically grown, and for spring barley the Eunova variety was used. The sowing of spring barley and live mulches was carried out using a cereal drill in two passes, and the row spacing was 12.5 cm. The sowing depth of the spring barley was 5–6 cm, while the living mulches were sown at a depth of 1–2 cm. No weed control treatments were applied in plots where spring barley was grown with living mulches. In plots with spring barley cultivation without living mulches, two mechanical treatments were applied. The first treatment with a weeder harrow was carried out after plant emergence, while the second treatment with a medium harrow was carried out after the development of 5–6 leaves. The application of phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) was performed at two dates: on the day of spring barley sowing (date 0) and at the stage of spring barley tillering (BBCH 29-30) at the rate of 1 L of inoculant per 150 L water∙ha−1. The bacteria Azospirillum lipoferum Br17 were applied twice in the growing season. Firstly, barley grain was treated with an inoculant suspension (100 mL 15 kg−1 grain), and later, inoculant spraying (the inoculant rate of 1 L/150 L water∙ha−1) was performed at the emergence stage (BBCH 10–15). The application of the Azotobacter chroococcum inoculant was made twice during the spring barley growing season, that is, on the day of spring barley sowing (day 0) and at the stage of spring barley tillering (BBCH 29-30) at a rate of 1 L of inoculant per 250 L water ha−1.
2.1.1. Trait and Ability of Used Bacterial Strains
The bacterial species used for inoculation came from the collection of the Department of Soil Science and Microbiology of the Poznań University of Life Sciences. They were isolated from under cultivated plants, on selective medium, and then genetically identified on the basis of a fragment of the 16S rRNA gene sequence. Azotobacter chroococcum was isolated on Jensen medium [29], Azospirillum lipoferum Br 17 from the maize rhizosphere on DAS medium [30], Bacillus megaterium var. phosphaticum on calcium phosphate solubilizing Ca3(PO4)2 [31], and Arthrobacter agilis on medium, as described by Hagedorn and Holt [32].
The metabolic properties of Bacillus megaterium var. phosphaticum and Arthrobacter agilis strains for phosphorus solubilisation were initially determined via a qualitative method using MPA medium containing bromocresol purple at pH = 7.2, according to Promwee et al. [33] (Figure 2). The prepared cultures with the isolates discussed above were incubated for 7 days, and the rate of colour change of the medium (from purple to yellow) was assessed according to a five-point scale (“−” no colour change, “+” weak colour change, “++”—moderate colour change, “+++”—intense colour change, and “++++”—very intense colour change).
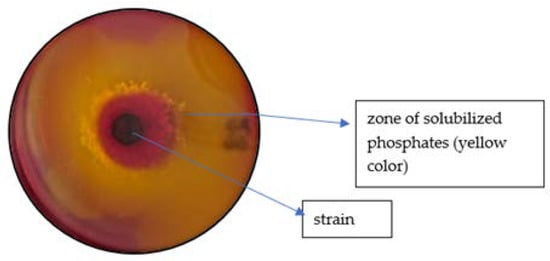
Figure 2.
The MPA substrate inoculated with inoculants.
In addition, a quantitative analysis was used to establish the ability of the isolates to solubilise phosphorus on liquid NBRIP medium, according to Saravanakumar et al. [34], using a spectrophotometric method at 600 nm. The ability of the strains to make calcium phosphate available was determined from the absorbance readings and the standard curve for KH2PO4 and expressed as phosphate equivalent in mg 1 mL−1.
The nitrogen-fixing activity of Azotobacter chroococum and Azospirillum lipoferum Br17 was assessed by PCR, where polF/polR primers duplicating a specific fragment of the nifH gene were used. The quantitative assessment of nitrogen fixation was validated on liquid media using the acetylene-to-ethylene reduction method. For this purpose, Aztobacter bacteria were cultured on Jensen’s medium with a limited amount of nitrogen and Azospirillum on D-limited medium, respectively, according to Okon et al. [35], and the level of nitrogen fixation was expressed as nMC2H4 mL−1 of the culture.
2.1.2. Determination of Mutual Interactions between the Bacteria Used in the Construction of Inoculates
In order to select bacterial strains for the composed inoculants and to test their compatibility, the mutual interaction between the selected bacterial strains was determined using the ring method.
- Bacillus megaterium var. phosphaticum on Arthrobacter agilis;
- Arthrobacter agilis on Bacillus megaterium var. phosphaticum;
- Azotobacter chroococum on Azospirillum lipoferum Br17;
- Azospirillum lipoferum Br17 on Azotobacter chroococum;
- Bacillus megaterium var. phosphaticum on Azotobacter chroococum;
- Azotobacter chroococum on Bacillus megaterium var. phosphaticum;
- Arthrobacter agilis on Azotobacter chroococum;
- Azotobacter chroococum on Arthrobacter agilis;
- Bacillus megaterium var. phosphaticum on Azospirillum lipoferum Br17;
- Azospirillum lipoferum Br17 on Bacillus megaterium var. phosphaticum;
- Arthrobacter agilis on Azospirillum lipoferum Br17;
- Azospirillum lipoferum Br17 on Arthrobacter agilis.
The analysis of the interactions between bacterial strains showed a lack of antagonistic interactions, as evidenced by the lack of brightening (halo) around the wells for all tested bacteria used to inoculate spring barley.
2.1.3. Preparation of Liquid Modifier and Its Application in the Field
The endophytic bacterial isolates were preserved in test tubes containing agar slants, which were stored in a refrigerator at a temperature of 8 °C. Prior to the field experiment, the isolates underwent two passages onto prepared agar slants with a suitable medium specific to each bacterial species. This process aimed to revive and activate the selected strains for the study. For each date of barley inoculation, the liquid cultures of the chosen inoculates were prepared in 100 mL flasks with five replicates. To suspend the three-day-old starter cultures of bacteria, 5 mL of saline was added diagonally to each tube. The microbial cultures were then scraped using a loop, and 0.5 mL of the resulting bacterial suspension was mixed with 100 mL of liquid NB medium (nutrient broth). The liquid cultures were incubated at the temperature of 28 °C on a shaker set at 70 rpm for a duration 48 h. The concentration of microorganisms in 1 mL of the liquid culture was found to be 1012 cells. Following the incubation, the cultures of each bacterial species were pooled and concentrated through centrifugation at 4000 rpm. The prepared bacterial Bacillus megaterium var. phosphaticum, Arthrobacter agilis, and Azotobacter chroococum were applied with a sprayer twice throughout the growing season of spring barley:
Date 1—spring barley sowing (day 0—grain inoculation);
Date 2—spring barley tillering (BBCH 29-30).
In contrast, the strain Azospirillum lipoferum Br 17 at the emergence stage (BBCH 10-15) was used, due to the fact that these microorganisms are characterised by slower growth and adaptation in the soil–plant system. The bacteria of the genus Azotobacter form a plant–bacteria–soil system and colonise only the rhizosphere. The bacteria of the genus Azospirillum, on the other hand, belong to facultative endophytes that thrive in the soil but are able to colonise both the outer surface of roots and their inner tissues, which requires a longer period of time.
To prepare the concentrated consortium, 300 mL of the obtained inoculum was mixed with 60 L of water, following the recommended ratio for the commercial consortia of 1 L of preparation to 150 L of water per hectare. The density of bacterial cells in the resulting suspension was determined using direct microscopy in a Thoma cell counting chamber, revealing 108 cells in 1 mL of culture. The inoculates were applied on a warm, yet cloudy day, with temperatures ranging from 18 to 25 °C.
2.2. Spring Barley Grain Yield and Yield Structure
Spring barley was harvested in late July. Directly before the harvest, 10 ears were sampled from each plot to determine ear length, grain number per ear, and grain weight per ear. During harvest, grain yield was determined in each plot and was converted into t per 1 ha. Next, samples were collected to determine 1000 grain weight.
2.3. Calculation of the Biological Index of Soil Fertility BIF
During spring barley growing season, soil samples were collected to determine the biological index of soil fertility (BIF). Soil samples were taken at three dates: date I (BIF I) at the stage of spring barley emergence (BBCH 16-17), date II (BIF II) at the stage of spring barley flowering (BBCH 61-65), and date III (BIF III) after spring barley harvest. The biological index of soil fertility (BIF) was determined based on DHA (dehydrogenase activity) and CAT (catalase activity), according to the formula (DHA + kCAT)/2, where k is the coefficient of proportionality and equals 0.01 [36,37].
2.4. Statistical Analysis
The study results were analysed statistically using a three-way ANOVA for the splitblock design, according to the following mathematical model:
where
Yijl = m + ai + gj + ll + alil + eij(1) + bp + blpl + ejp(2) + abip + ablipl + eijpl(3)
yijl—the value of the characteristic; m—average of the population; ai—the effect of the i-th level of factor A (microbial products); gj—the effect of replicates (blocks); ll—the effect of the l-th level of years; alil—the effect of the interaction: factor A × years; eij(1)—error 1 resulting from the interaction: factor A × replicates; bp—the effect of the p-th level of factor B (living mulch); blpl—the effect of the interaction: factor B × years; ejp(2)—error from the interaction: factor B × replicates; abip—effect of the interaction: factor A × factor B; ablipl—effect of the interaction: factor A × factor B × years; and eijl(3)—random error.
The significance of sources of variability was tested using the Fisher–Snedecor F-test (F ≤ 0.05) and the differences between the compared averages were verified using Tukey’s HSD test (p ≤ 0.05). The strength of the relationships between spring barley grain yield, yield structure, and BIF was assessed by calculating Pearson’s correlation coefficients. All the calculations were performed in Statistica, version 13.3 (Hamburg, Germany).
2.5. Weather Conditions
The course of weather conditions varied in the study years (Figure 3).
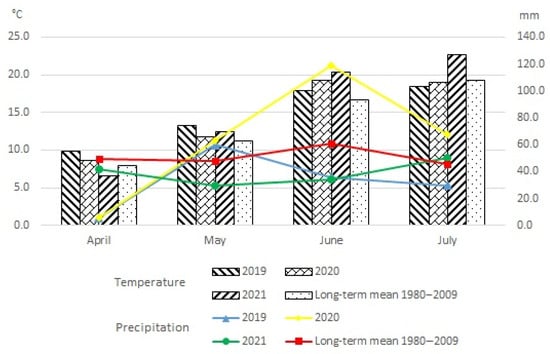
Figure 3.
Weather conditions during the growing season of spring barley, according to the Zawady Meteorological Station.
The lowest precipitation sum during the spring barley crop of 131.3 mm was recorded in 2019. The average temperature that year was 14.9 °C. The year 2020 had the highest precipitation total of 255.7 mm of the years analysed, and the mean air temperature in that year was 14.7 °C. The year 2021 had the highest mean temperature of the analysed years at 15.5 °C, while the precipitation sum in that year was 155.3 mm.
3. Results
Spring barley grain yield was significantly affected by the experimental conditions and their interactions (Figure 4).

Figure 4.
Spring barley grain yield according to microbial products and living mulches (means across 2019–2021), t ha−1—tonnes hectare−1. 1 I—control (no treatment with microbial products); II—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Ar-throbacter agilis); III—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum); 2 Values in living mulches for the interaction (living mulches and microbial products) indicated by the same small letter above the bar (a, b, c) do not differ significantly at p ≤ 0.05; values in bars of the same colour for the interaction (microbial products and living mulches) indicated by the same small letter at bottom of bar (a, b, c) do not differ significantly at p ≤ 0.05; means for the living mulches and microbial products indicated by the same capital letter (A, B, C) do not differ significantly at p ≤ 0.05; ±standard deviation.
The highest grain yield at 4.49 t ha−1 was associated with co-inoculation with phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum), and was significantly lower by 0.59 t ha−1 after the application of phosphorus-releasing bacteria, and lowest at 3.14 t ha−1 for the non-treated control. Living mulches significantly affected spring barley grain yield. The highest grain yield at 4.34 t ha−1 was produced by spring barley grown with the living mulch of either red clover mixed with Italian ryegrass or red clover at 4.22 t ha−1. By contrast, the lowest grain yield at 3.44 t ha−1 was determined for spring barley cultivated with the living mulch of Italian ryegrass or the control spring barley grown in a pure stand at 3.37 t ha−1. The interactions of the experimental factors (living mulches and microbial products) showed that when a live mulch mixture of red clover and Italian ryegrass was used, the highest yield at 5.36 t ha−1 was obtained after the application of nitrogen-fixing bacteria and phosphorus-releasing bacteria. On the other hand, when spring barley was grown on a live mulch of red clover, the highest grain yield at 5.02 t ha−1 was obtained after simultaneous inoculation with phosphorus-releasing bacteria. The cultivation of spring barley with the live mulch of Italian ryegrass and without live mulch produced the highest yields after the application of nitrogen-fixing bacteria, at 4.18 and 4.14 t ha−1, respectively. In addition, no significant differences were found in these cases between the application of phosphorus-releasing bacteria and the control object without microbial products. On the other hand, the interaction of microbial products and living mulches demonstrated that cultivation of spring barley without the application of microbial products yielded the highest grain yield of spring barley with living mulch red clover and mixture of red clover and Italian ryegrass, at 3.36 and 3.53 t ha−1, respectively. When phosphorus-releasing bacteria were used, the highest yields of spring barley were obtained when grown with living mulch red clover, while significantly lower yields were obtained when grown with a living mulch mixture of red clover and Italian ryegrass. The lowest spring barley yields after the application of phosphorus-releasing bacteria were obtained when growing with a living mulches Italian ryegrass and when growing spring barley without a living mulch. When nitrogen-fixing bacteria and phosphorus-releasing bacteria were inoculated simultaneously, the highest yield was obtained when spring barley was grown with a living mulch mixture of red clover and Italian ryegrass. Significantly lower but not significantly different yields were obtained when spring barley was grown with a living mulch of red clover or Italian ryegrass or without a living mulch.
Statistical analysis revealed the significant effect of growing season and its interaction with bacterial products (Figure 5).
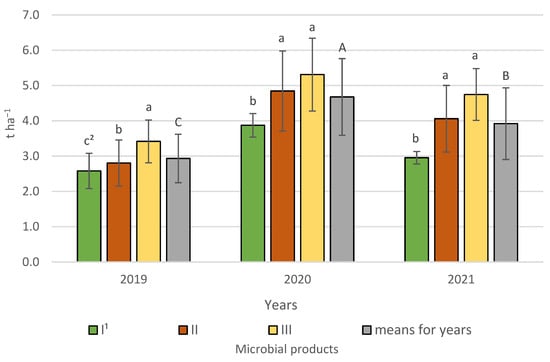
Figure 5.
Spring barley grain yield according to microbial products in 2019−2021, t ha−1—tonnes hectare−1. 1 I—control (no treatment with microbial products); II—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Ar-throbacter agilis); III—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum); 2 values in years for interactions (years and microbial products) represented by the same small letter above the bar (a, b, c) do not differ significantly at p ≤ 0.05; means for the years represented by the same capital letter (A, B, C) do not differ significantly at p ≤ 0.05; ±standard deviation.
The highest spring barley grain yield at 4.68 t ha−1 was obtained in the favourable year 2020 and was significantly lower by 0.76 t ha−1 in 2021 and the lowest by 1.75 t ha−1 compared to 2020 in the dry year of 2019. The interaction indicated that in 2020–2021 the highest grain yield was recorded for spring barley following either simultaneous inoculation with phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum) 5.31 and 4.75 t ha−1, respectively, or phosphorus-releasing bacteria only 5.31 t ha−1 in 2020 and 4.06 t ha−1 in 2021, respectively, and is lowest in the control. In 2019, the highest grain yield was produced by spring barley after inoculation with phosphorus-releasing bacteria and nitrogen-fixing bacteria at 3.42 t ha−1, which is significantly the lowest for amendment with phosphorus-releasing bacteria and the non-inoculated control.
An Interaction between years and living mulches was confirmed (Figure 6).
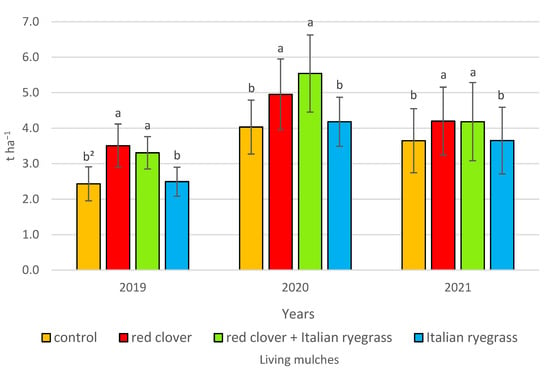
Figure 6.
Spring barley grain yield according to living mulches in 2019–2021, t ha−1—tonnes hectare−1. 2 values in years for the interactions (years and living mulches) represented by the same small letters above the bar (a, b) do not differ significantly at p ≤ 0.05; ±standard deviation.
In 2019–2021, the highest grain yield was determined for spring barley cultivated with the living mulch of red clover mixed with Italian ryegrass or red clover alone, which is lowest for the living mulch of Italian ryegrass and the control without a living mulch.
Spring barley ear length was significantly affected by the experimental factors and their interaction (Figure 7).
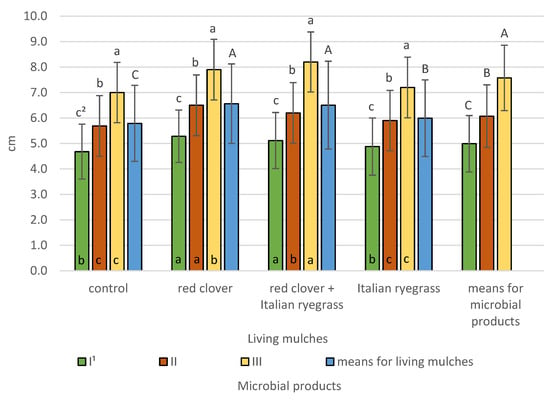
Figure 7.
Spring barley ear length according to microbial products and living mulches (means across 2019–2021), cm—centimetre. 1 I—control (no treatment with microbial products); II—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Ar-throbacter agilis); III—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum); 2 values in living mulches for the interaction (living mulches and microbial products) represented by the same small letter above the bar (a, b, c) do not differ significantly at p ≤ 0.05; values in bars of the same colour for the interaction (microbial products and living mulches) represented by the same small letter at bottom of bar (a, b, c) do not differ significantly at p ≤ 0.05; means for the living mulches and microbial products represented by the same capital letter (A, B, C) do not differ significantly at p ≤ 0.05; ±standard deviation.
The longest spring barley ears were found following co-inoculation with phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum) at 7.6 cm, which are significantly shorter by 1.5 cm for inoculation with phosphorus-releasing bacteria and shorter by 2.6 cm with respect to simultaneous bacterial inoculation for the control without the application of microbial products. Living mulches significantly influenced spring barley ear length. The longest ears at 6.6 cm were determined for spring barley grown with either red clover alone or mixed with Italian ryegrass (6.5 cm) and are significantly shorter at 6.0 cm for spring barley cultivated with the living mulch of Italian ryegrass and the shortest at 5.8 cm for the control without living mulch. The interaction between living mulches and microbial products was confirmed and it revealed that in all the plots with living mulches and in the control, the longest spring barley ears were found following co-inoculation with phosphorus-releasing bacteria and nitrogen-fixing bacteria, and these were significantly shorter for treatment with phosphorus-releasing bacteria and the shortest for the non-treated control. Simultaneous inoculation with phosphorus-releasing bacteria and nitrogen-fixing bacteria resulted in ear lengths of 8.2 cm when barley was grown with living mulches mixed of red clover and Italian ryegrass, 7.9 cm with living mulches red clover, 7.2 cm when barley was grown with Italian ryegrass and 7.0 cm when barley was grown without living mulches. The study also demonstrated a significant interaction between microbial products and living mulches. It demonstrated that the longest spring barley ears in cultivation without the use of microbial products were obtained when the living mulch was red clover and a mixture of red clover and Italian ryegrass. On the other hand, significantly lower ear length was obtained when spring barley was grown with living mulch Italian ryegrass and without living mulch. On the other hand, the application of phosphorus-releasing bacteria resulted in the longest spring barley ears at 6.5 cm when grown with a living mulch red clover and were significantly shorter at 6.2 cm, when grown with a living mulch mixture of red clover and Italian ryegrass. The significantly shortest spring barley ears were observed with living mulch Italian ryegrass and without a living mulch. The application of the simultaneous inoculation of phosphorus-releasing bacteria and nitrogen-fixing bacteria resulted in the longest ears of all cultivations with a living mulch mixture of red clover and Italian ryegrass; these were significantly shorter in the cultivation with living mulch of red clover and the shortest in the cultivation with living mulch Italian ryegrass and spring barley without any living mulch.
Statistical analysis confirmed a significant impact of experimental factors and their interaction on grain number per ear in spring barley (Figure 8).

Figure 8.
Spring barley grain number per ear according to microbial products and living mulches (means across 2019–2021), pcs.—pieces. 1 I—control (no treatment with microbial products); II—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Ar-throbacter agilis); III—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum); 2 values in living mulches for the interactions (living mulches and microbial products) represented by the same small letter above the bar (a, b, c) do not differ significantly at p ≤ 0.05; values in bars of the same colour for the interaction (microbial products and living mulches) represented by the same small letter at bottom of bar (a, b, c) do not differ significantly at p ≤ 0.05; means for the living mulches and microbial products represented by the same capital letter (A, B, C, D) do not differ significantly at p ≤ 0.05; ±standard deviation.
The highest grain number at 26 pcs. was recorded for spring barley following simultaneous inoculation with phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum) and was significantly lower by 7 pcs. for inoculation with phosphorus-releasing bacteria and the lower by 10 pcs. for the non-treated control. Living mulches significantly affected grain number per ear of spring barley. The highest grain number at 22 pcs. was recorded for spring barley ears sampled in plots with the living mulch of red clover but was significantly lower at 21 pcs. for living mulch of red clover mixed with Italian ryegrass, and even lower at 19 pcs. for Italian ryegrass and lowest at 18 pcs. for the control spring barley grown in a pure stand. The interaction of living mulches and microbial products was confirmed, indicating that the highest grain number per ear was associated with the introduction of a living mulch combined with co-inoculation with phosphorus-releasing bacteria and nitrogen-fixing bacteria, and was significantly lower for treatment with phosphorus-releasing bacteria and lowest for plots without the application of microbial inoculants. Accordingly, simultaneous inoculation with phosphorus-releasing bacteria and nitrogen-fixing bacteria in the plots of a living mulch mixture of red clover and Italian ryegrass resulted in a grain number per ear at 29 pcs., in the plots of a living mulch of red clover at 27 pcs., and in the plots of cultivation spring barley with a living mulch of Italian ryegrass at 25 pcs. and 24 pcs. for spring barley cultivation without living mulch. The interactions of microbial products and living mulches in the experiment conducted were also significant. With no application of microbial products and with the application of phosphorus-releasing bacteria, the highest number of grains per ear was obtained when spring barley was grown with red clover living mulch, 18 pcs. and 22 pcs., respectively. A significantly lower number of grains in both cases was obtained when spring barley was grown with a living mulch mixture of red clover and Italian ryegrass, and the lowest number with a living mulch of Italian ryegrass and without a living mulch. The application of the simultaneous inoculation of phosphorus-releasing bacteria and nitrogen-fixing bacteria induced the highest number of grains when spring barley was grown with a living mulch mixture of red clover and Italian ryegrass. A significantly lower number of grains was observed with a living mulch of red clover and Italian ryegrass, while the lowest number of grains was achieved without the use of a living mulch.
Grain weight per spring barley ear was significantly influenced by experimental factors and their interaction (Figure 9).
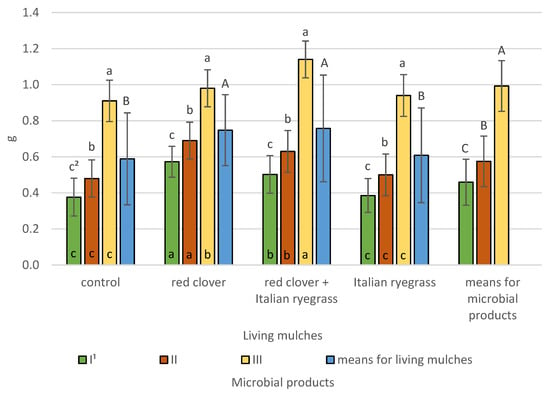
Figure 9.
Spring barley grain weight per ear according to microbial products and living mulches (means across 2019–2021), g—gram. 1 I—control (no treatment with microbial products); II—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Ar-throbacter agilis); III—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum); 2 values in living mulches for the interactions (living mulches and microbial products) represented by the same small letter above the bar (a, b, c) do not differ significantly at p ≤ 0.05; values in bars of the same colour for the interactions (microbial products and living mulches) represented by the same small letter at bottom of bar (a, b, c) do not differ significantly at p ≤ 0.05; means for the living mulches and microbial products represented by the same capital letter (A, B, C) do not differ significantly at p ≤ 0.05; ±standard deviation.
The highest grain weight per ear at 1.0 g was determined for co-inoculation with phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum) and is significantly lower at 0.6 g for phosphorus-releasing bacteria and lowest at 0.5 g for the non-inoculated control. Living mulches had a significant influence on grain weight per ear in spring barley. The highest weight was found for spring barley grown with the living mulch of either red clover mixed with Italian ryegrass or red clover alone at 0.8 and 0.7 g, respectively. The significantly lowest weight for Italian ryegrass living mulch and the control spring barley, both cultivated in a pure stand, was 0.6 g. The interactions between living mulches and microbial products were confirmed, and it was indicated that, regardless of living mulch, the greatest grain weight per ear was found for co-inoculation with phosphorus-releasing bacteria and nitrogen-fixing bacteria and was significantly lower for phosphorus-releasing bacteria inoculant and lowest for the untreated control. The use of co-inoculation with phosphorus-releasing bacteria and nitrogen-fixing bacteria with the living mulch mixture of red clover and Italian ryegrass resulted in a grain weight per ear of 1.1 g, in the plots of living mulch of red clover the weight was 1.0 g, and in the plots of living mulch of Italian ryegrass and spring barley cultivation without living mulch the weights were both 0.9 g. The experiment also demonstrated the significant interactions of microbial products and living mulches. The cultivation of spring barley without microbial products and with phosphorus-releasing bacteria demonstrated the highest grain weight per ear with living mulch red clover of 0.6 g and 0.7 g, respectively. Significantly lower grain weights per ear were obtained when grown with a living mulch mixture of red clover and Italian ryegrass and lowest when growing spring barley with a living mulch of Italian ryegrass and without a living mulch. In the case of the application of the simultaneous inoculation of phosphorus-releasing bacteria and nitrogen-fixing bacteria, the highest grain weight per ear was obtained when spring barley was grown with a living mulch mixture of red clover and Italian ryegrass, a lower weight was obtained with a living mulch of red clover, and the significantly lowest weight was obtained with a living mulch of Italian ryegrass and without a living mulch on the control plots.
Statistical analysis confirmed a significant impact of experimental factors and their interaction on the 1000 grain weight of spring barley (Figure 10).
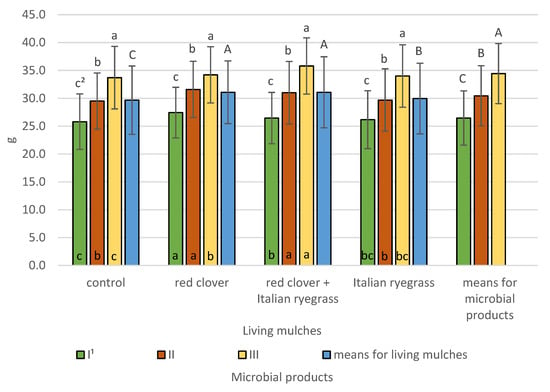
Figure 10.
Spring barley 1000 grain yield according to microbial products and living mulches (means across 2019–2021), g—gram. 1 I—control (no treatment with microbial products); II—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Ar-throbacter agilis); III—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum); 2 values in living mulches for the interactions (living mulches and microbial products) represented by the same small letter above the bar (a, b, c) do not differ significantly at p ≤ 0.05; values in bars of the same colour for the interactions (microbial products and living mulches) represented by the same small letter at bottom of bar (a, b, c) do not differ significantly at p ≤ 0.05; means for the living mulches and microbial products represented by the same capital letter (A, B, C) do not differ significantly at p ≤ 0.05; ±standard deviation.
The highest 1000 grain weight at 34.4 g was recorded for simultaneous inoculation with phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum), and was significantly lower at 30.5 g for phosphorus-releasing bacteria and lowest at 26.5 g for the control spring barley without the application of microbial products. Living mulches significantly affected the 1000 grain weight of spring barley. The greatest 1000 grain weight was recorded for spring barley grown with a mixture of red clover and Italian ryegrass or red clover only, namely 31.1 g for both. Significantly lower values were obtained at 29.9 g for the living mulch comprising Italian ryegrass and the lowest value was for the control without living mulch at 27.7 g. The interactions of living mulches and microbial products were identified and revealed that, regardless of the test living mulches, the highest 1000 grain weight was found for spring barley grown following inoculation with phosphorus-releasing bacteria and nitrogen-fixing bacteria, and was significantly lower for phosphorus-releasing bacteria and lowest for the non-treated control without living mulch. The application of phosphorus-releasing bacteria and nitrogen-fixing bacteria with living mulch mixture of red clover and Italian ryegrass resulted in a 1000 grain weight of 35.8 g; in the plots of living mulch of red clover, it resulted in a weight of 34.2 g; in the plots of living mulch of Italian ryegrass, it resulted in a weight of 34.0 g; and for spring barley cultivation without living mulch, it resulted in a weight of 33.7 g. The interaction of microbial products and living mulches demonstrated that in cases of a lack of microbial products, the highest 1000 grain weight was exhibited by spring barley grown with a living mulch red clover. In addition, when spring barley was grown without microbial products, no significant differences were demonstrated between the cultivation with a living mulch of mixtures of red clover and Italian ryegrass and living mulch Italian ryegrass and between the control without a living mulch and living mulch Italian ryegrass. The cultivation of spring barley with the application of phosphorus-releasing bacteria demonstrated the highest 1000 grain weight with living mulch red clover and a mixture of red clover and Italian ryegrass, and significantly lower values were obtained with living mulch Italian ryegrass and cultivation spring barley without living mulch. The application of a simultaneous inoculation of phosphorus-releasing bacteria and nitrogen-fixing bacteria also significantly differentiated the weight of 1000 grains in the used living mulches. The highest 1000 grain weight for simultaneous inoculation was obtained with the living mulch of mixtures of red clover and Italian ryegrass. A lower value was obtained when spring barley was grown with a living mulch of mixtures of red clover and Italian ryegrass. In addition, no significant difference was revealed between growing spring barley with living mulch Italian ryegrass and without living mulch.
The biological Index of soil fertility determined at the stage of spring barley emergence (BIF I) was significantly affected by the experimental factors and their interactions (Figure 11).
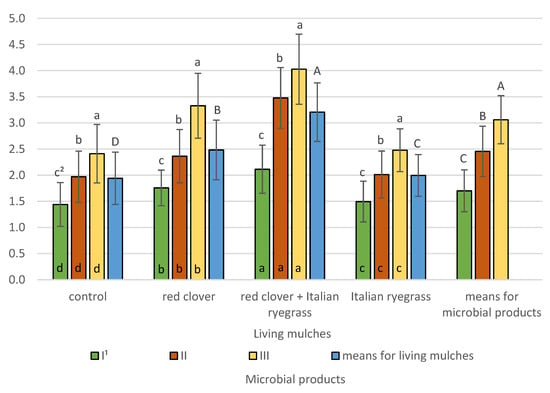
Figure 11.
Biological index of soil fertility BIF I determined after spring barley emergence (means across 2019–2021). 1 I—control (no treatment with microbial products); II—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Ar-throbacter agilis); III—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum); 2 values in living mulches for the interaction (living mulches and microbial products) represented by the same small letter above the bar (a, b, c) do not differ significantly at p ≤ 0.05; values in bars of the same colour for the interaction (microbial products and living mulches) represented by the same small letter at bottom of bar (a, b, c, d) do not differ significantly at p ≤ 0.05; means for the living mulches and microbial products represented by the same capital letter (A, B, C, D) do not differ significantly at p ≤ 0.05; ±standard deviation.
The highest BIF I was obtained in the topsoil at 3.1 following co-inoculation with phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum), and was significantly lower at 2.5 for treatment with the microbial product containing phosphorus-releasing bacteria and lowest at 1.7 for the untreated control. Living mulches significantly affected BIF I, its highest value of 3.2 being confirmed for the living mulch of red clover mixed with Italian ryegrass. Significantly lower values at 2.5 were determined for the red clover living mulch, even lower at 2.0 for Italian ryegrass, and lowest at 1.9 for the control where spring barley was grown in a pure stand. The interactions of living mulches and microbial products were confirmed, indicating that, regardless of the type of test living mulch, the highest BIF I was determined for plots treated with phosphorus-releasing bacteria and nitrogen-fixing bacteria, and BIF I was significantly lower for treatment with phosphorus-releasing bacteria and lowest for the untreated control. The experiment demonstrated the interaction of microbial products and living mulches, which determined that regardless of whether a combination of microbial products was used or not, the highest BIF I value was obtained when spring barley was grown with a living mulch mixture of red clover and Italian ryegrass. Indeed, the lowest values were seen in plots without living mulch across all microbial products combinations used.
Statistical analysis confirmed a significant impact of experimental factors and their interactions on the biological index of soil fertility determined in the topsoil at the stage of spring barley flowering (BIF II) (Figure 12).
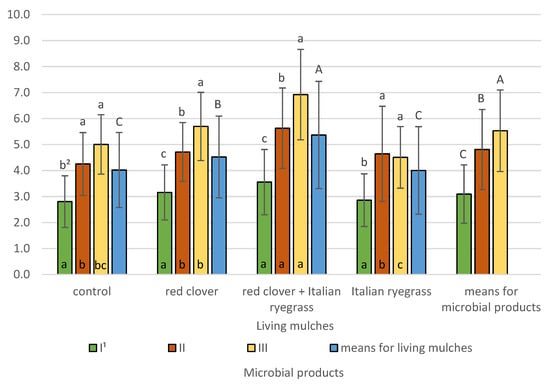
Figure 12.
Biological index of soil fertility BIF II determined at the stage of spring barley flowering (means across 2019–2021). 1 I—control (no treatment with microbial products); II—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Ar-throbacter agilis); III—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum); 2 values in living mulches for the interactions (living mulches and microbial products) represented by the same small letter above the bar (a, b, c) do not differ significantly at p ≤ 0.05; values in bars of the same colour for the interactions (microbial products and living mulches) represented by the same small letter at bottom of bar (a, b, c) do not differ significantly at p ≤ 0.05; means for the living mulches and microbial products represented by the same capital letter (A, B, C) do not differ significantly at p ≤ 0.05; ±standard deviation.
The highest values of this index, at 5.5, were determined for units treated with phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum), and were lower at 4.8 for inoculation with phosphorus-releasing bacteria and lowest at 3.1 for the untreated control. Living mulches significantly influenced BIF II. The highest value at 5.4 of this index was recorded for soil planted with spring barley grown using the living mulch of red clover mixed with Italian ryegrass and was significantly lower, at 4.5, for red clover living mulch and lowest for Italian ryegrass or the control spring barley grown in a pure stand, both 4.0. The interactions of living mulches and microbial products was confirmed, and it demonstrated that in units with the living mulch of red clover mixed with Italian ryegrass or red clover only, the highest BIF II values were recorded following co-inoculation with phosphorus-releasing bacteria and nitrogen-fixing bacteria and were significantly lower for phosphorus-releasing bacteria and lowest for the control without microbial inoculation. In turn, for plots with the living mulch of Italian ryegrass and for the control, the highest BIF II values were determined following simultaneous inoculation with phosphorus-releasing bacteria and nitrogen-fixing bacteria, as well as inoculation with phosphorus-releasing bacteria. The lowest BIF II was found for units without the application of microbial products. The interaction of microbial products and living mulches demonstrated that in plots where no microbial products were applied, no significant differences were found between the living mulches used. The highest BIF II value using phosphorus-releasing bacteria was obtained when spring barley was grown using a living mulch mixture of red clover and Italian ryegrass. In other living mulches and in cases without living mulch, no significant difference was observed in the experiment. With the simultaneous inoculation of phosphorus-releasing bacteria and nitrogen-fixing bacteria, the highest value of BIF II was revealed in plots where spring barley was grown with a living mulch mixture of red clover and Italian ryegrass, significantly lower when spring barley was grown with a living mulch red clover and in plots without a living mulch. In addition, no significant differences were found in the control plots (without living mulch) and plots with living mulch Italian ryegrass.
The biological index of soil fertility determined in the topsoil after spring barley harvest (BIF III) was significantly affected by experimental factors and their interactions (Figure 13).
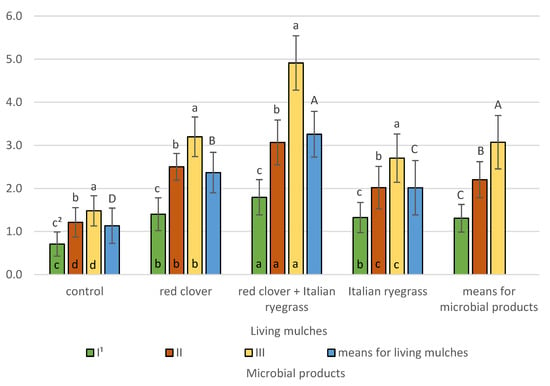
Figure 13.
Biological index of soil fertility BIF III determined after spring barley harvest (means across 2019–2021). 1 I—control (no treatment with microbial products); II—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Ar-throbacter agilis); III—phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum); 2 values in living mulches for the interactions (living mulches and microbial products) represented by the same small letter above the bar (a, b, c) do not differ significantly at p ≤ 0.05; values in bars of the same colour for the interactions (microbial products and living mulches) represented by the same small letter at bottom of bar (a, b, c, d) do not differ significantly at p ≤ 0.05; means for the living mulches and microbial products represented by the same capital letter (A, B, C, D) do not differ significantly at p ≤ 0.05; ±standard deviation.
The highest BIF III values at 3.1 were found for plots co-inoculated with phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum) and were significantly lower at 2.2 for inoculation with phosphorus-releasing bacteria and lowest at 1.3 for the control without microbial treatment. Also, living mulches significantly affected BIF III. The highest values of this index at 3.3 were determined for soil after harvesting spring barley grown with the living mulch of red clover mixed with Italian ryegrass and were significantly lower at 2.4 for red clover living mulch, lower still at 2.0 for Italian ryegrass, and lowest at 1.1 for the control where spring barley had been grown in a pure stand. The interactions between living mulches and microbial products was confirmed, and it was revealed that, regardless of the test living mulches, the highest BIF III values were recorded for units with co-inoculation with phosphorus-releasing bacteria and nitrogen-fixing bacteria and were significantly lower for phosphorus-releasing bacteria and for non-treated units. The experiment also revealed an interaction between microbial products and living mulches. The application of phosphorus-releasing bacteria and simultaneous inoculation with phosphorus-releasing bacteria and nitrogen-fixing bacteria resulted in the highest value of BIF III in the cultivation of spring barley with a living mulch mixture of red clover and Italian ryegrass; in the remaining plots, significantly lower values were obtained. The highest BIF III value, in the plots without microbial products, was observed for the cultivation of spring barley with a living mulch mixture of red clover and Italian ryegrass, and these values were significantly lower in the other plots where living mulches were used and in those without living mulches.
The analysis of the biological index of soil fertility determined on three dates revealed that the highest values were reached at date II (BIF II), and these were lower at date III (BIF III) and at date I (BIF I). The highest BIF values obtained at date II (BIF II), which is the stage of spring barley flowering, are associated with the highest nutrient availability for plants, which positively affects spring barley grain yield.
Correlation analysis showed a significant relationship between spring barley grain yield, yield parameters, and BIF (Table 1 and Table 2). The relationship between the analysed traits showed a significance level of p < 0.01. A positive correlation was obtained between grain yield and the number and weight of grains per ear, ear length and 1000 grain weight. In the present study, individual yield structure parameters were also highly correlated with each other. In addition, a significant positive correlation of spring barley grain yield and yield structure parameters with BIF was also shown for all three analysis dates. The highest correlation coefficient was shown between yield and yield structure parameters and BIF was analysed at the flowering stage of spring barley.

Table 1.
Correlation coefficients (n = 108) between spring barley grain yield and yield structure.

Table 2.
Correlation coefficients (n = 108) between spring barley grain yield, yield structure and BIF.
4. Discussion
At present, the development of organic agriculture is very much visible, with cereals being the dominant crop in this system. Worldwide, biofertilizers are gaining importance in agriculture. Microorganisms are capable of mobilising important nutrients in soil, which is transforming them from substances which cannot be used to those that plants can utilise by means of biological processes [38]. Research by Game et al. [39] and Gayatri et al. [38] demonstrated that the application of microbial fertilisers based on Azotobacter, Azospirillum, P-releasing bacteria and other biofertilizers has a positive impact on plant production. This is supported by the results of the authors’ own research, in which simultaneous inoculation with phosphorus-releasing bacteria and nitrogen-fixing bacteria had a positive effect on spring barley yields. According to Gayatri et al. [38], the positive effect of microbial products on crop yield should be attributed primarily to the provision of additional plant-available nitrogen and phosphorus, which largely determine crop yield. Also, results obtained by Jain et al. [40] showed a positive effect of Azotobacter inoculation on shoot length, root length, root number and total chlorophyll content in maize seedlings. According to a study by Volkogon et al. [41], the application of microbial preparations can result in an increase in barley yields equivalent to the application of N60P60K60 mineral fertiliser. Thus, the use of microbial preparations may bring about positive effects not only on organic farms but also on conventional ones, as a result of the possibility of reducing mineral fertilisation without any yield loss effects.
Although it is essential for plant growth and development, phosphorus is an element which is often a limiting nutrient. Thus, the possibility of obtaining soil P through plant roots is of great interest in agriculture. An organic option would be to use bacteria solubilising P, which is released to plants by means of various mechanisms [42,43,44]. In their research, Zaballa et al. [45] inoculated plants with the bacteria Enterobacter ludwigii and Azospirillum brasilense and found that these had a beneficial effect on barley yield. The use of phosphorus-releasing bacteria in agriculture could lead to a reduction in soil P content as a result of its constant removal from crop yields. It is therefore advisable to constantly replenish it through fertilisation, which, in organic farming, can be achieved through fertilisation with animal manure. In the present study, the application of only phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) was followed by a decline in spring barley grain yield compared with instances of their use as a co-inoculant with nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum). However, even if applied alone, they contributed to the positive effects of spring barley compared with the control without the application of microbial products. The use of phosphorus-releasing bacteria can achieve similar effects in crop yields to the application of 50 kg P2O5 ha−1 as superphosphate [45]. Therefore, the increase in yield with the application of phosphorus-releasing bacteria can be attributed, as with other microbial products, to the increased availability of bioavailable nutrients to plants.
In organic farming systems, it is recommended to grow cereals with a living mulch. In the present study, growing spring barley with live mulch showed positive effects on both yield and structure. Also, research conducted in Germany by Gerhards [46] demonstrated the validity of growing cereals with legumes and grasses as a living mulch. It has been confirmed that cereal grain yields were higher when compared with cereal crop yields grown in a pure stand. Living mulches, even when sown simultaneously with cereals, did not reduce grain yield, which agrees with the findings of Hartwig and Ammon [47] and Brust et al. [48]. Only Bhaskar et al. [49] found a 14% decline in yields of cereals grown with living mulches. By contrast, living mulches in the Norwegian production of spring cereals substantially increased grain yield by as much as 16–22% [50]. The clover living mulch was able to biologically fix elemental nitrogen from the air. This nitrogen is also able to benefit mainstream crops grown with living mulch, thus enabling higher yields to be achieved. In turn, grasses effectively take up nutrients from the soil and prevent their leaching [22,51,52,53]. Moreover, living mulches enhance soil microbial activity, preserve nutrients in the topsoil, increase biodiversity and control weeds, which is of great importance in organic agriculture [48]. Species grown as living mulches occupy different niches in time and space using complementary resources [21,54].
Bearing in mind the numerous benefits of the application of microbial products and living mulches, in the experiment discussed here, their combined use for the organic management of spring barley was evaluated, as there is a distinct lack of research into this issue. The obtained results are very promising. To improve spring barley yields on organic or conventional farms with reduced mineral fertilisation, the use of following simultaneous inoculation with phosphorus-releasing bacteria (Bacillus mega-terium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum) with the living mulch of red clover and red clover mixed with Italian ryegrass is recommended.
World agriculture constantly faces multiple environmental challenges connected with climate change, including water shortages and temperature increases [55,56]. Also, in the present study, adverse weather conditions recorded in 2019 were followed by a significant decline in spring barley grain yield compared with 2020–2021, when the amount of precipitation was higher. This being the case, innovative technologies of cereal cultivation should be sought to relieve the effects of drought. Azotobacter-based biofertilizers have unique properties, such as the formation of nodules, which make plants more resistant to environmental stresses [57]. In the present study, both in the dry year 2019 and the more favourable years of 2020 and 2021, characterised by a higher amount of precipitation, simultaneous inoculation with phosphorus-releasing bacteria and nitrogen-fixing bacteria contributed to superior spring barley grain yields. This has also been confirmed by Alharbi et al. [56], who demonstrated that seed inoculation with multiple bacterial inoculants produces better yields compared with those inoculated with bacterial strain. Also, Ikan et al. [58] demonstrated improved wheat growth following inoculation with bacterial preparations under drought conditions. Under drought conditions, the use of bacterial preparations activated the photosynthetic mechanism and increased the activity of antioxidants such as polyphenoloxidase, thereby improving the plants coping with drought [58]. It is also of importance that plants grown as living mulch assist spring barley plants in mitigating the effects of drought. In the research reported here, it was found that, regardless of weather conditions were favourable during the spring barley growing season, the highest grain yield was harvested from plots planted with the living mulch of red clover or red clover mixed with Italian ryegrass. Thus, it seems necessary to develop innovative cultivation systems to enhance the efficiency of nutrient use and preserve resources, which are much more diverse due to more species being grown and the utilisation of a greater number of legumes or their mixes with grasses [21,22].
Yield structure elements, such as ear length, grain number per ear, grain weight per ear and 1000 grain weight, are yield determinants. Research by Gayatri et al. [38] showed that the application of various liquid bioproducts (Azotobacter and phosphorus-releasing bacteria) increased ear length, spikelet number per ear and 1000 grain weight in wheat. Pan et al. [43] and Zaballa et al. [45] reported that treatment with phosphorus-releasing bacteria Enterobacter ludwigii positively affected barley grain structure elements, which agrees with the findings of this study in which different phosphorus-releasing bacteria were used (Bacillus megaterium var. phosphaticum, Arthrobacter agilis). In addition to biological nitrogen fixation and phosphorus solubilisation, bacterial preparations are able to influence plants by synthesising plant growth hormones, such as indole acetic acid, gibberellins and cytokinins [38,57]. Among other things, these hormones increase the root mass of plants and thus improve nutrient uptake, which presumably affects structure elements related to grain yield [45,57].
Living mulches, in particular legumes, favourably affect the elements of cereal grain yield structure [22]. Also, in the present work, spring barley cultivated with the living mulch of red clover or red clover mixed with Italian ryegrass resulted in an improvement in the yield structure compared that which grew without a living mulch. However, there are no studies on a combined application of living mulches and microbial products and their effects on grain yield structure elements in organically managed spring barley. In the present study, their effect was beneficial.
Soil biology is believed to be a significant and a key element of organic agriculture. The persistence of the soil ecosystem can be evaluated by means of biological indicators, and soil enzymes have been effectively used as indicators of soil quality in various agricultural systems [25,26]. Thus, in order to accurately determine soil quality, a number of enzymatic activities have to be assessed. Catalases and dehydrogenases are present in soil as basic components of complete microbial living cells. They can be used as a measure of overall activity of microbes in soil; therefore, they can also be utilised to calculate the biological index of soil fertility (BIF) [26,59]. In the present work, the BIF determined at three dates corresponding to three developmental stages of spring barley was significantly affected by bacterial products. The highest BIF values at the three dates were obtained following the simultaneous application of phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum), which was a result of the highest activity of soil microorganisms. Biostimulants, which act in a similar manner, increase the microbial activity in the rhizosphere and soil enzymes and stimulate hormone production and photosynthesis [36,60]. The present study demonstrated that the cultivation of spring barley with a living mulch increased BIF values corresponding to three development stages, in particular the spring barley flowering stage, when the cereal was grown with the living mulch of red clover mixed with Italian ryegrass or red clover only. However, the best effects were obtained for spring barley with a living mulch of red clover mixed with Italian ryegrass or red clover following the application of phosphorus-releasing bacteria and nitrogen-fixing bacteria. Also, Wolna-Maruwka et al. [27], who applied the bacteria Bacillus spp. as a biofertilizer, reported that the increased BIF values determined at three dates during the maize growing season, the highest BIF being recorded at the stage of maize flowering, which concurs with the results of the present work pertaining to the stage of spring barley flowering. Hupe et al. [61] noticed that the plant development stage affected nutrient dynamics in the rhizosphere and thus the soil enzymatic activity. The researchers observed carbon and nitrogen deposition in the rhizosphere in the period from plant emergence to flowering. They stressed the fact that after flowering, nitrogen deposition in the rhizosphere was markedly reduced, in their opinion, due to plants’ removal of nitrogen in order to produce yield. The authors believed that after flowering, the quantity of organic nitrogen substances relative to carbon is reduced in the rhizosphere as a result of the lower metabolic activity of soil enzymes, with this inference confirmed in the present study, which showed that, after spring barley harvest, BIF III was much lower than at the flowering stage.
Grain yield is simultaneously determined by a number of plant and grain traits [62]. The most important yield traits affecting yield are 1000 seed weight, the number of grains per ear and number of ears per area [63]. Farmers tend to sow crops at the fixed density recommended for the crop [64]. Thus, in practical terms, the 1000 seed weight and the number of grains per ear have the greatest influence on the yields obtained [64]. This statement is confirmed by the results of the authors’ research, in which the highest correlation coefficient was obtained between the yield and 1000 seed weight and the number of grains per ear. Also, other authors obtained significant correlation coefficients between grain yield and yield structure traits [65,66,67]. However, when analysing the correlation values obtained by these other authors, the yield structure trait that is most important for the yield obtained cannot be clearly identified. In the research results quoted by other authors, the highest correlation coefficient between yield and yield structure traits concerned a different trait each time. This could have been caused by the different climatic zones in which the research was conducted, i.e., different air temperatures and precipitation sums during the growing season of the plants. These factors may have influenced the intensive development of one yield trait at the expense of another. These suppositions are confirmed by Levakova [68] who, on the basis of her research, found the interactions of individual yield traits. According to the aforementioned author, the formation of one of these elements can be compensated by a more significant development of another under different growing season conditions. The present study also revealed the significant correlation between the individual yield structure traits of spring barley. The authors’ research also showed a highly significant correlation between spring barley yield, structure and BIF. Analogous correlations between yield and its structure and soil DHA and CAT were also shown in studies conducted by other authors [69]. The highest correlation coefficient in the authors’ own study was shown between the yield and its structure and the BIF analysed at the flowering stage of cereals. A possible reason for the results obtained is that the plants at the flowering and grain formation stages have the highest nutrient requirements. Therefore, a high BIF during this period is crucial for the yield.
The research reported here has allowed the development of an innovative technology of organically managed spring barley cultivation. Spring barley grown with the living mulch of red clover or red clover mixed with Italian ryegrass following co-inoculation with phosphorus-releasing bacteria (Bacillus megaterium var. phosphaticum, Arthrobacter agilis) and nitrogen-fixing bacteria (Azospirillum lipoferum Br17, Azotobacter chroococcum) produces superior grain yield characterised by the best structure and contributes to the highest values of biological index of soil fertility (BIF), which is so important in organic agriculture. The above variants of the innovative spring barley cultivation technology contributed to a stable grain yield even in the dry year of 2019, which is a promising factor in terms of changing climatic conditions.
5. Conclusions
In organic farming systems, under a Stagnic Luvisol soil with an average abundance of macroelements, a neutral pH and temperate climatic conditions, the use of the innovative technology of growing spring barley with a living mulch of red clover or a mixture of red clover and Italian ryegrass along with the application of simultaneous inoculation with phosphorus-releasing bacteria and nitrogen-fixing bacteria should be recommended. However, this type of research needs to be further explored under different soil and climate conditions around the world, taking into account bacterial strains and living mulch adapted to the climate. It is therefore necessary to take into account site-specific factors and to conduct field trials to assess the effectiveness and feasibility of the approach presented in the experiment in different agricultural systems and with other cereal species.
Author Contributions
Conceptualization, A.P. and A.N.; methodology, R.G. and R.R.; software, R.G.; validation, A.N. and A.W.-M.; formal analysis, A.P. and A.W.-M.; investigation, R.R.; resources, R.G and A.N.; data curation, A.W.-M.; writing—original draft preparation, R.G. and A.P.; writing—review and editing, R.G. and R.R.; visualization, A.N. and A.W.-M.; supervision, R.G. and A.W.-M.; project administration, A.P. and A.N.; funding acquisition, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education, grant number 29/20/B.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lukinac, J.; Jukić, M. Barley in the production of cereal-based products. Plants 2022, 11, 3519. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Wang, P.Y.; Xiong, X.B.; Wang, Y.B.; Zhou, R.; Tao, H.Y.; Grace, U.A.; Wang, N.; Xiong, Y.C. Environmental risk of multi-year polythene film mulching and its green solution in arid irrigation region. J. Hazard. Mater. 2022, 435, 128981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhao, Z.; Yin, X.A.; Zhu, Y. Impacts of biochars on bacterial community shifts and biodegradation of antibiotics in an agricultural soil during short-term incubation. Sci. Total Environ. 2021, 771, 144751. [Google Scholar] [CrossRef] [PubMed]
- Naseri, R.; Azadi, S.; Rahimi, M.J.; Maleki, A.; Mirzaei, A. Effects of inoculation with Azotobacter Chroococcum and Pseudomonas putida on yield and some of the important agronomic traits in barley (Hordeum vulgar L.). Int. J. Agron. Plant Prod. 2013, 4, 1602–1610. [Google Scholar]
- Koryagin, Y.V.; Kulikova, E.G.; Koryagina, N.V.; Trishina, V.A. Application of microbiological fertilizers in barley cultivation technology. IOP Conf. Ser. Earth Environ. Sci. 2022, 953, 012005. [Google Scholar] [CrossRef]
- Mirskaya, G.V.; Khomyakov, Y.V.; Rushina, N.A.; Vertebny, V.E.; Chizhevskaya, E.P.; Chebotar, V.K.; Chesnokov, Y.V.; Pishchik, V.N. Plant development of early-maturing spring wheat (Triticum aestivum L.) under inoculation with Bacillus sp. V2026. Plants 2022, 11, 1817. [Google Scholar] [CrossRef]
- Kumar, A.; Patel, J.S.; Meena, V.S.; Srivastava, R. Recent advances of PGPR based approaches for stress tolerance in plants for sustainable agriculture. Biocatal. Agric. Biotechnol. 2019, 20, 101271. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, S.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere bacteria in plant growth promotion, biocontrol, and bioremediation of contaminated sites: A Comprehensive review of effects and mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef]
- Sunera, A.; Saqib, S.; Uddin, S.; Zaman, W.; Ullah, F.; Ayaz, A.; Asghar, M.; ur Rehman, S.; Munis, M.F.H.; Chaudhary, H. Characterization and phytostimulatory activity of bacteria isolated from tomato (Lycopersicon esculentum Mill.) rhizosphere. Microb. Pathog. 2020, 140, 103966. [Google Scholar] [CrossRef]
- Ali, M.A.; Naveed, M.; Mustafa, A.; Abbas, A. The Good, the Bad, and the Ugly of Rhizosphere Microbiome: Probiotics and Plant Health; Springer: Singapore, 2017; pp. 253–290. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, I.; Hilger, T.H.; Nadeem, S.M.; Akhtar, M.F.Z.; Jamil, M.; Hussain, A.; Zahir, Z.A. Preliminary study on phosphate solubilizing Bacillus subtilis strain Q3 and Paenibacillus sp. strain Q6 for improving cotton growth under alkaline conditions. PeerJ 2018, 6, e5122. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Bushra; Hussain, A.; Dar, A.; Ahmad, M.; Wang, X.; Brtnicky, M.; Mustafa, A. Combined use of novel endophytic and rhizobacterial strains upregulates antioxidant enzyme systems and mineral accumulation in wheat. Agronomy 2022, 12, 551. [Google Scholar] [CrossRef]
- Ahmad, M.; Zahir, Z.A.; Jamil, M.; Nazli, F.; Iqbal, Z. Field application of ACC-deaminase biotechnology for improving chickpea productivity in Bahawalpur. Soil Environ. 2017, 36, 93–102. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2013, 2, 587. [Google Scholar] [CrossRef]
- Seshachala, U.; Tallapragada, P. Phosphate solubilizers from the rhizosphere of Piper nigrum L. in Karnataka, India. Chil. J. Agric. Res. 2012, 72, 397–403. [Google Scholar] [CrossRef]
- Zhao, K.; Penttinen, P.; Zhang, X.; Ao, X.; Liu, M.; Yu, X.; Chen, Q. Maize rhizosphere in Sichuan, China, hosts plant growth promoting Burkholderia cepacia with phosphate solubilizing and antifungal abilities. Microbiol. Res. 2014, 169, 76–82. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Mumtaz, M.Z.; Barry, K.M.; Baker, A.L.; Nichols, D.S.; Ahmad, M.; Zahir, Z.A.; Britz, M.L. Production of lactic and acetic acids by Bacillus sp. ZM20 and Bacillus cereus following exposure to zinc oxide: A possible mechanism for Zn solubilization. Rhizosphere 2019, 12, 100170. [Google Scholar] [CrossRef]
- Hussain, A.; Zahir, Z.A.; Ditta, A.; Tahir, M.U.; Ahmad, M.; Mumtaz, M.Z.; Hayat, K.; Hussain, S. Production and implication of bio-activated organic fertilizer enriched with zinc-solubilizing bacteria to boost up maize (Zea mays L.) production and biofortification under two cropping seasons. Agronomy 2020, 10, 39. [Google Scholar] [CrossRef]
- Bedoussac, L.; Justes, E. A comparison of commonly used indices for evaluating species interactions and intercrop efficiency: Application to durum wheat–winter pea intercrops. Field Crops Res. 2011, 124, 25–36. [Google Scholar] [CrossRef]
- Wittwer, R.A.; Dorn, B.; Jossi, W.; van der Heijden, M.G. Cover crops support ecological intensification of arable cropping systems. Sci. Rep. 2017, 7, 41911. [Google Scholar] [CrossRef]
- Costanzo, A.; Bàrberi, P. Functional agrobiodiversity and agroecosystem services in sustainable wheat production. A review. Agron. Sustain. Dev. 2014, 34, 327–348. [Google Scholar] [CrossRef]
- Canali, S.; Ortolani, L.; Campanelli, G.; Robačer, M.; von Fragstein, P.; D’Oppido, D.; Kristensen, H.L. Yield, product quality and energy use in organic vegetable living mulch cropping systems: Research evidence and farmers’ perception. Renew. Agric. Food Syst. 2017, 32, 200–213. [Google Scholar] [CrossRef]
- Piotrowska-Długosz, A.; Charzynski, P. The impact of the soil sealing degree on microbial biomass, enzymatic activity, and physicochemical properties in the ekranic technosols of Toruń (Poland). J. Soils Sediments 2015, 15, 47–59. [Google Scholar] [CrossRef]
- Sulewska, H.; Niewiadomska, A.; Ratajczak, K.; Budka, A.; Panasiewicz, K.; Faligowska, A.; Wolna-Maruwka, A.; Dryjański, L. Changes in Pisum sativum L. plants and in soil as a result of application of selected foliar fertilizers and biostimulators. Agronomy 2020, 10, 1558. [Google Scholar] [CrossRef]
- Wolna-Maruwka, A.; Piechota, T.; Niewiadomska, A.; Kamiński, A.; Kayzer, D.; Grzyb, A.; Pilarska, A.A. The effect of biochar-based organic amendments on the structure of soil bacterial community and yield of maize (Zea mays L.). Agronomy 2021, 11, 1286. [Google Scholar] [CrossRef]
- Available online: http://www.oschr-warszawa.pl/ (accessed on 14 July 2023).
- Fenglerowa, W. Simple method for counting azotobacter in soil samples. Acta Microbiol. Pol. 1965, 14, 203–206. [Google Scholar]
- Dőbereiner, J. Forage grasses and grain crops. In Methods for Evaluating Biological Nitrogen Fixation; Bergsen, F.J., Ed.; Wiley and Sons.: New York, NY, USA, 1980; pp. 535–555. [Google Scholar]
- Rodina, A. Mikrobiologiczne Metody Badania Wód; Wydawnictwo PWRiL: Warszawa, Poland, 1968; p. 254. [Google Scholar]
- Hagedorn, C.; Holt, J. Ecology of soil arthrobacters in clation-webster toposequences of Iowa. Appl. Microbiol. 1975, 2, 211–218. [Google Scholar] [CrossRef]
- Promwee, A.; Issarakraisila, M.; Intana, W.; Chamswarng, C.; Yenjit, P. Phosphate solubilization and growth promotion of rubber tree (Hevea brasiliensis Muell. Arg.) by Trichoderma strains. J. Agric. Sci. 2014, 6, 8. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Shanmuga Arasu, V.; Kathiresan, K. Effect of Trichoderma on soil phosphate solubilization and growth improvement of Avicennia marina. Aquat. Bot. 2013, 104, 101–105. [Google Scholar] [CrossRef]
- Okon, Y.; Albrecht, S.L.; Burris, R.H. Factors affecting growth and nitrogen fixation of Spirillum lipoferum. J. Bacteriol. 1976, 127, 3. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomska, A.; Sulewska, H.; Wolna-Maruwka, A.; Ratajczak, K.; Waraczewska, Z.; Budka, A. The influence of bio-stimulants and foliar fertilizers on yield, plant features, and the level of soil biochemical activity in white lupine (Lupinus albus L.) cultivation. Agronomy 2020, 10, 150. [Google Scholar] [CrossRef]
- Stefanic, F.; Ellade, G.; Chirnageanu, J. Researches concerning a biological index of soil fertility. In Proceedings of the 5th Symposium of Soil Biology, Njoro, Kenya, 30 November–2 December 1981; Nemes, M.P., Kiss, S., Papacostea, P., Stefanic, C., Rusan, M., Eds.; Romanian National Society of Soil Science: Bucharest, Romania, 1984; pp. 35–45. [Google Scholar]
- Gayatri, A.K.S.; Verma, S.O.N.; Sahu, U. Effect of Azotobacter and phosphate solubilizing bacteria on the yield of different wheat (Triticum aestivum L.) Cultivar. Pharma Innov. J. 2022, 11, 1629–1633. [Google Scholar]
- Game, B.C.; Ilhe, B.M.; Pawar, V.S.; Khandagale, P.P. Effect of Azotobacter, phosphate solubilising bacteria and potash mobilising bacteria inoculants on productivity of wheat (Triticum aestivum L.). Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2800–2807. [Google Scholar] [CrossRef]
- Jain, D.; Sharma, J.; Kaur, G.; Bhojiya, A.A.; Chauhan, S.; Sharma, V.; Mohanty, S.R.; Maharjan, E. Phenetic and molecular diversity of nitrogen fixating plant growth promoting Azotobacter isolated from semiarid regions of India. BioMed Res. Int. 2021, 2021, 6686283. [Google Scholar] [CrossRef]
- Volkogon, V.; Moskalenko, A.; Dimova, S.; Volkogon, K.; Potapienko, L. The effect of inoculation with Azospirillum brasilense strain 410 on spring barley cv. nosivsky development and yield. Agric. Sci. Pract. 2023, 9, 64–75. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsiebb, G.; Orozco-Mosqueda, M.C.; Glick, B. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Pan, Q.; Shikano, I.; Hoover, K.; Liu, T.X.; Felton, G.W. Enterobacter ludwigii, isolated from the gut microbiota of Helicoverpa zea, promotes tomato plant growth and yield without compromising anti-herbivore defenses. Arthropod-Plant Interact. 2019, 13, 271–278. [Google Scholar] [CrossRef]
- Psakia, O.; Mainaa, S.; Vlysidisb, A.; Papanikolaoua, S.; Machado de Castro, A.; Freired, D.M.G.; Dheskalie, E.; Kookose, I.; Koutinasa, A. Optimisation of 2,3-butanediol production by Enterobacter ludwigii using sugarcane molasses. Biochem. Eng. J. 2019, 152, 107370. [Google Scholar] [CrossRef]
- Zaballa, J.I.; Golluscio, R.; Ribaudo, C.M. Effect of the phosphorus-solubilizing bacterium Enterobacter ludwigii on barley growth promotion. Am. Sci. Res. J. Eng. Technol. Sci. 2020, 63, 144–157. [Google Scholar]
- Gerhards, R. Weed suppression ability and yield impact of living mulch in cereal crops. Agriculture 2018, 8, 39. [Google Scholar] [CrossRef]
- Hartwig, N.L.; Ammon, H.U. Cover crops and living mulches. Weed Sci. 2002, 50, 688–699. [Google Scholar] [CrossRef]
- Brust, J.; Gerhards, R.; Karanisa, T.; Ruff, L.; Kipp, A. Why undersown and cover crops become important again for weed suppression in European cropping systems. Gesunde Pflanz. 2011, 63, 191–198. [Google Scholar] [CrossRef]
- Bhaskar, A.; Vijaya, V.; Davies, W.P.; Cannon, N.D.; Conway, J.S. Weed manifestation under different tillage and legume under-sowing in organic wheat. Biol. Agric. Hortic. 2014, 30, 253–263. [Google Scholar] [CrossRef]
- Brandsæter, L.O.; Goul Thomsen, M.; Wærnhus, K.; Fykse, H. Effects of repeated clover undersowing in spring cereals and stubble treatments in autumn on Elymus repens, Sonchus arvensis and Cirsium arvense. Crop Prot. 2012, 32, 104–110. [Google Scholar] [CrossRef]
- Van Diggelen, R.; Bobbink, R.; Frouz, J.; Harris, J.; Verbruggen, E. Converting agricultural lands into heathlands: The relevance of soil processes. In Soils Landscape Restor; Stanturf, J.A., Callaham, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 357–372. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Zhuang, Q.; Jin, X.; Bian, Z.; Zhou, M.; Meng, Z.; Han, C.; Guo, X.; Jin, W.; et al. A Review on carbon source and sink in arable land ecosystems. Land 2022, 11, 580. [Google Scholar] [CrossRef]
- Sedri, M.H.; Niedbała, G.; Roohi, E.; Niazian, M.; Szulc, P.; Rahmani, H.A.; Feiziasl, V. Comparative analysis of plant growth-promoting rhizobacteria (PGPR) and chemical fertilizers on quantitative and qualitative characteristics of rainfed wheat. Agronomy 2022, 12, 1524. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Shaver, T.M.; Lindquist, J.L.; Shapiro, C.A.; Elmore, R.W.; Francis, C.A.; Hergert, G.W. Cover crops and ecosystem services: Insights from studies in temperate soils. Agron. J. 2015, 107, 2449–2474. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Khan, N. Climate change and salinity effects on crops and chemical communication between plants and plant growth-promoting microorganisms under stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Alharbi, K.; Rashwan, E.; Mohamed, H.H.; Awadalla, A.; Omara, A.E.-D.; Hafez, E.M.; Alshaal, T. Application of silica nanoparticles in combination with two bacterial strains improves the growth, antioxidant capacity and production of barley irrigated with saline water in salt-affected soil. Plants 2022, 11, 2026. [Google Scholar] [CrossRef]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah Kadmiri, I. Nitrogen fixing Azotobacter species as potential soil biological enhancers for crop nutrition and yield stability. Front. Microbiol. 2021, 12, 628379. [Google Scholar] [CrossRef]
- Ikan, C.; Ben-Laouane, R.; Ouhaddou, R.; Ghoulam, C.; Meddich, A. Co-Inoculation of arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria can mitigate the effects of drought in wheat plants (Triticum durum). Plant Biosyst. -Int. J. Deal. All Asp. Plant Biol. 2023, 1–20. [Google Scholar] [CrossRef]
- Ahemad, M.; Khan, S.M. Functional aspects of plant growth promoting Rhizobacteria: Recent Advancements. Microbiol. Insights 2011, 1, 39–54. [Google Scholar] [CrossRef]
- Fageria, N.K.; Filho, M.B.; Moreira, A.; Guimarães, C.M. Foliar fertilization of crop plants. J. Plant Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]
- Hupe, A.; Schulz, H.; Bruns, C.; Haase, T.; Heß, J.; Joergensen, R.G.; Wichern, F. Even Flow? Changes of carbon and nitrogen release from pea roots over time. Plant Soil 2018, 431, 143–157. [Google Scholar] [CrossRef]
- Marza, F.; Bai, G.H.; Carver, B.F.; Zhou, W.C. Quantitative trait loci for yield and related traits in the wheat population Ning7840 × Clark. Theor. Appl. Genet. 2006, 112, 688–698. [Google Scholar] [CrossRef]
- Wang, X.; Guan, P.; Xin, M.; Wang, Y.; Chen, X.; Zhao, A.; Liu, M.; Li, H.; Zhang, M.; Lu, L.; et al. Genome-wide association study identifies QTL for thousand grain weight in winter wheat under normal-and late-sown stressed environments. Theor. Appl. Genet. 2021, 134, 143–157. [Google Scholar] [CrossRef]
- Xu, X.; Sharma, R.; Tondelli, A.; Russell, J.; Comadran, J.; Schnaithmann, F.; Pillen, K.; Kilian, B.; Cattivelli, L.; Thomas, W.T.B.; et al. Genome-wide association analysis of grain yield-associated traits in a Pan-European barley cultivar collection. Plant Genom. 2018, 11, 170073. [Google Scholar] [CrossRef]
- Al-Tabbal, J.A.; Al-Fraihat, A.H. Genetic variation, heritability, phenotypic and genotypic correlation studies for yield and yield components in promising barley genotypes. J. Agric. Sci. 2012, 4, 193. [Google Scholar] [CrossRef]
- Singh, S.; Madakemohekar, A.H.; Prasad, L.C.; Prasad, R. Genetic variability and correlation analysis of yield and its contributing traits in barley (Hordeum vulgare L.) for drought tolerance. Indian Res. J. Genet. Biotechnol. 2015, 7, 103–108. [Google Scholar]
- Wiegmann, M.; Maurer, A.; Pham, A.; March, T.J.; Al-Abdallat, A.; Thomas, W.T.B.; Bull, H.J.; Shahid, M.; Eglinton, J.; Baum, M.; et al. Barley yield formation under abiotic stress depends on the interplay between flowering time genes and environmental cues. Sci. Rep. 2019, 9, 6397. [Google Scholar] [CrossRef] [PubMed]
- Levakova, O.V. Variability of the elements of spring barley yield structure depending on the hydrothermal conditions of vegetation. Agric. Sci. Euro-Northeast 2022, 23, 327–333. [Google Scholar] [CrossRef]
- Taheri, M.; Astaraei, A.; Lakzian, A.; Emami, H. Application of biochar and sulfur-modified biochar in a saline-sodic and calcareous soil: Effects on soil water content, soil biochemical properties and millet (Panicum miliaceum) yield. Res. Sq. 2022, 1–27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).