A Systematic Review on the Improvement of Cd Stress Tolerance in Ramie Crop, Limitations and Future Prospective
Abstract
1. Introduction
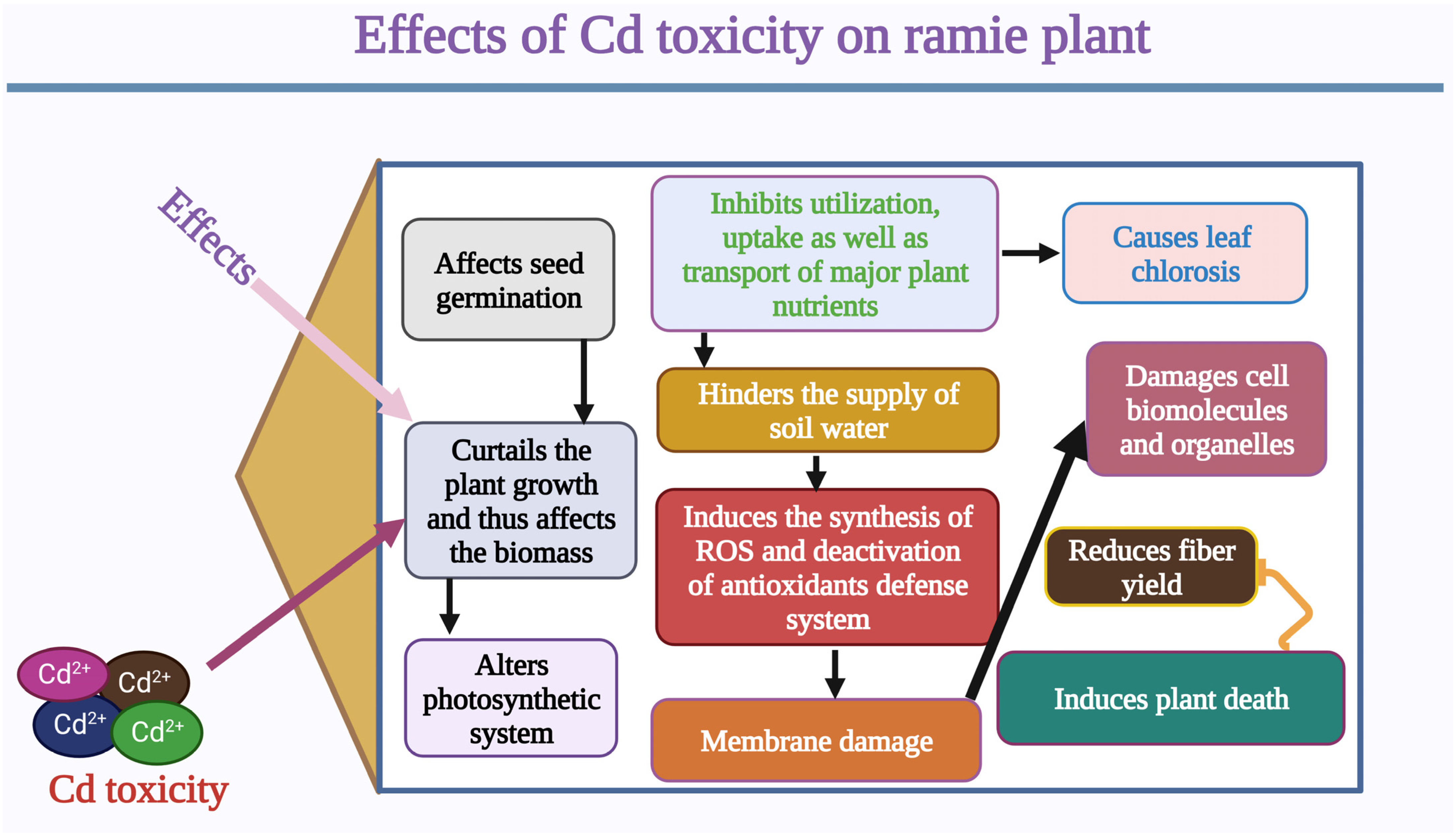
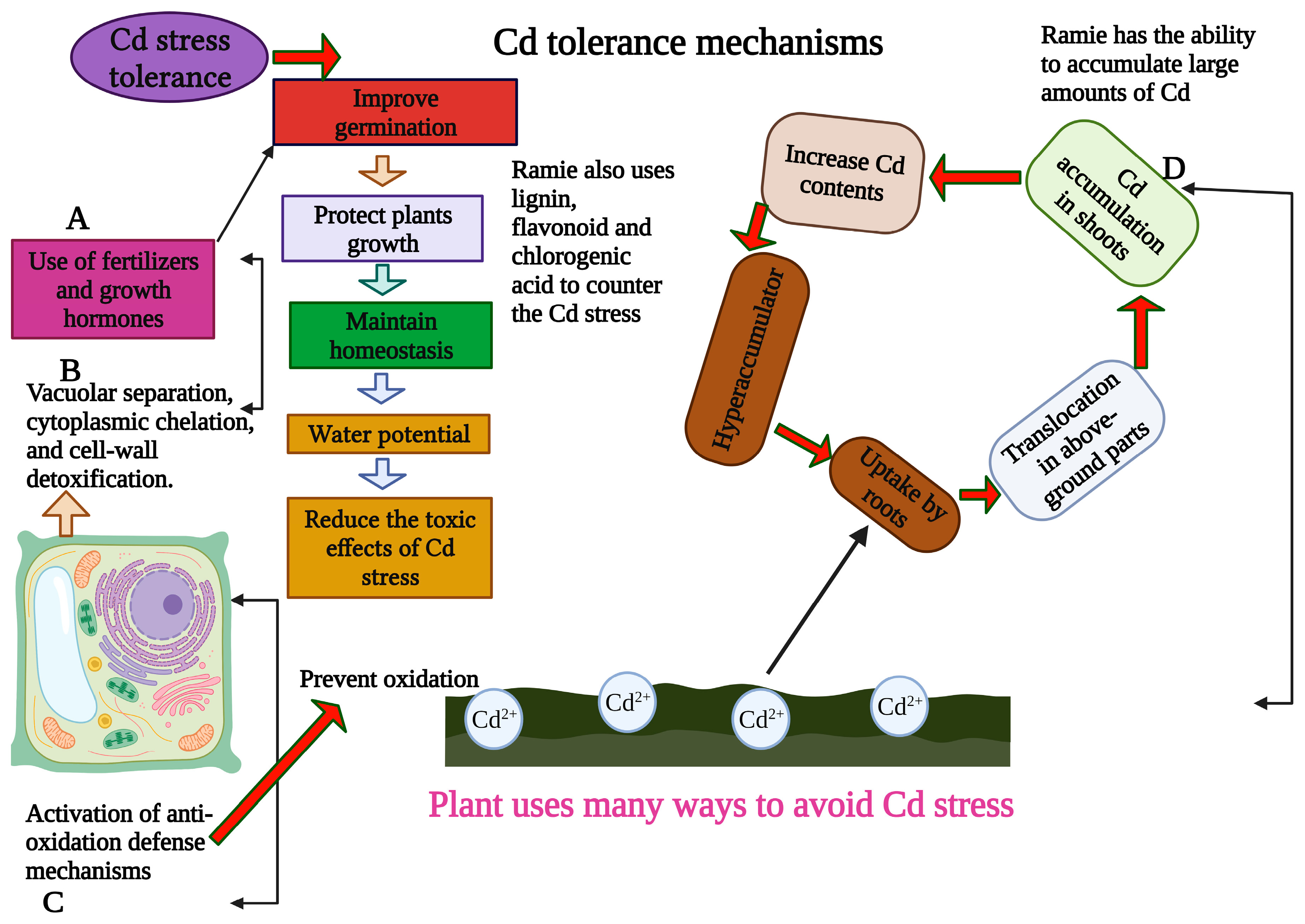
2. Effects of Cd Stress and Genetic Mechanisms
3. Agronomic Approaches to Enhance Cd Tolerance in Ramie
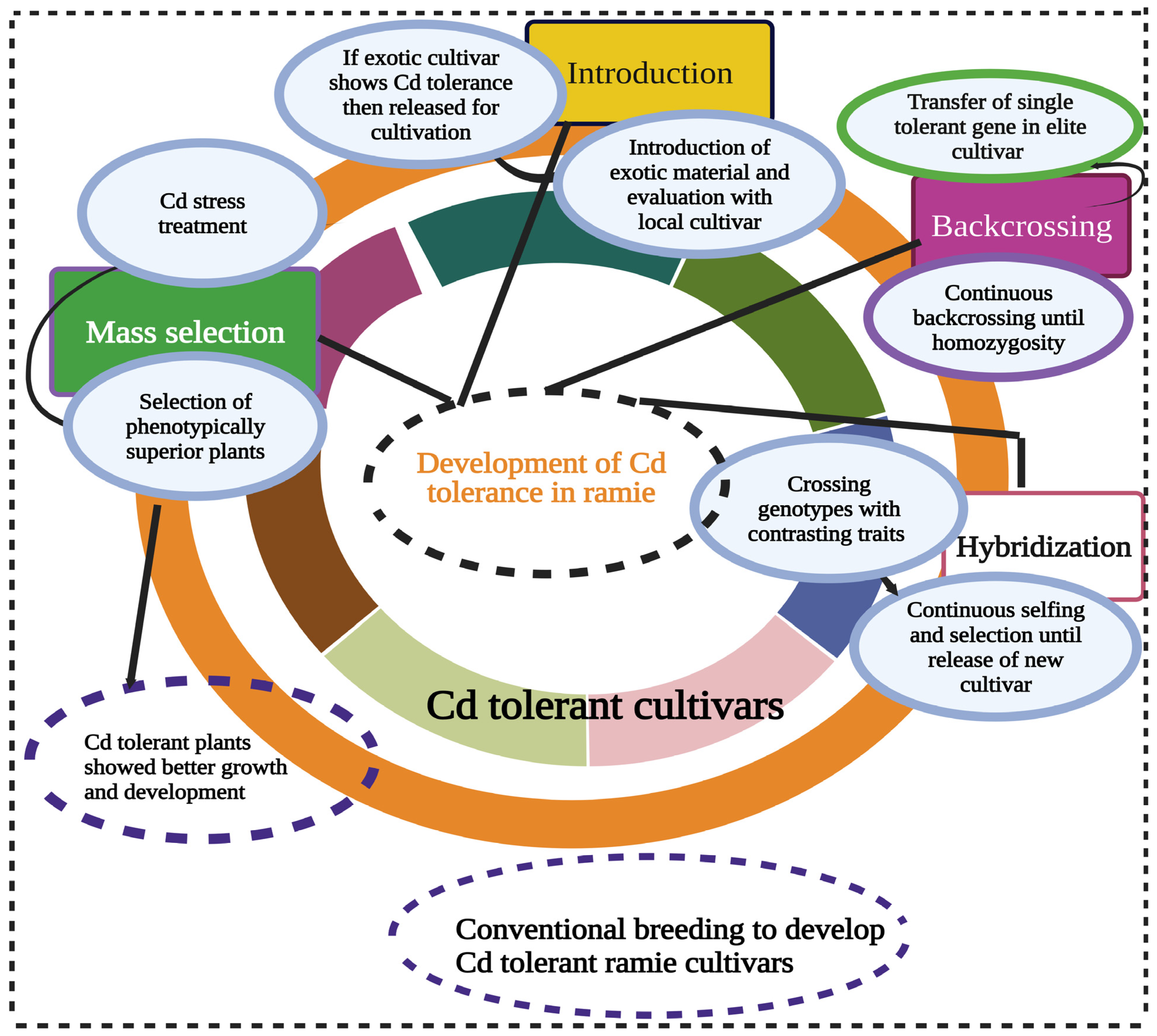
4. Genetic Diversity for Cd Tolerance in Ramie
5. QTL Mapping for Cd Tolerance in Ramie
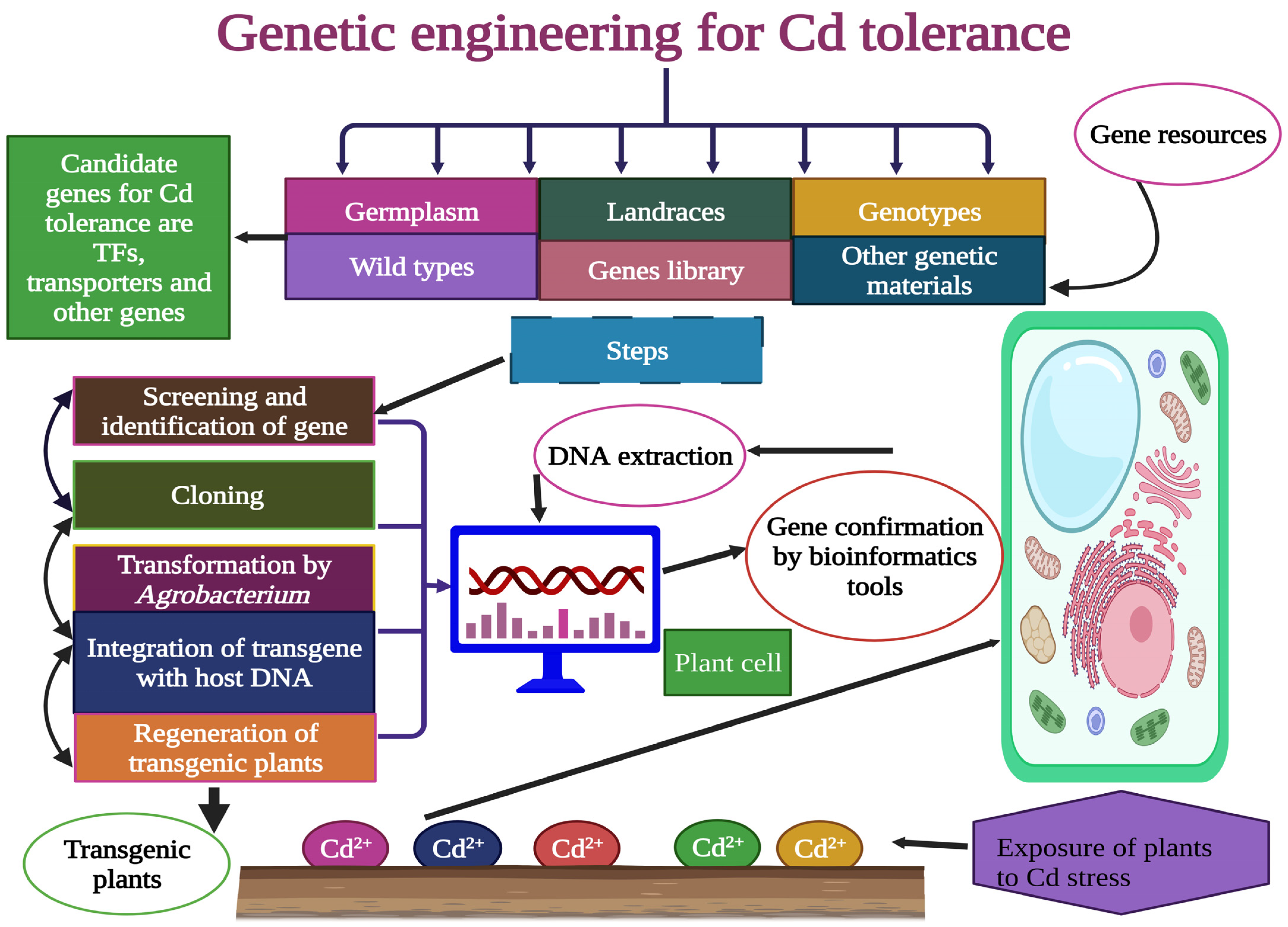
6. Genetically Engineered Ramie for Cd Tolerance
7. Transcription Factors (TFs) Analysis for Cd Tolerance in Ramie
8. CRISPR/Cas9-Mediated Gene Editing for Cd Tolerance
9. Conclusions and Future Perspectives
- The screening of ramie germplasm will lead to the identification of several tolerant genotypes, which can serve as a source of Cd-tolerant genes for developing Cd-tolerant ramie.
- Investigation of physiological- and biochemical-based Cd tolerance mechanisms.
- Selection of wild relatives of ramie and their screening for Cd-tolerant genes.
- Exposure of ramie cultivars to Cd stress levels and increase the expression of TFs families and the selection of potential targets for CRISPR/Cas9-mediated gene editing to develop Cd-tolerant mutants.
- Conservation of the genetic diversity of ramie for future breeding programs.
- Increase the Cd accumulation in ramie to increase its phytoremediation capability.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Notes
- Liu, J.; Gai, L.; Zong, H. Foliage application of chitosan alleviates the adverse effects of cadmium stress in wheat seedlings (Triticum aestivum L.). Plant Physiol. Biochem. 2021, 164, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Guedes, F.R.C.M.; Maia, C.F.; da Silva, B.R.S.; Batista, B.L.; Alyemeni, M.N.; Ahmad, P.; da Silva Lobato, A.K. Exogenous 24-Epibrassinolide stimulates root protection, and leaf antioxidant enzymes in lead stressed rice plants: Central roles to minimize Pb content and oxidative stress. Environ. Pollut. 2021, 280, 116992. [Google Scholar] [CrossRef]
- Neidhardt, H. Arbuscular mycorrhizal fungi alleviate negative effects of arsenic-induced stress on crop plants: A meta-analysis. Plants People Planet 2021, 3, 523–535. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Huang, W.; Zhang, D.; Wu, J.; Li, B.; Li, M.; Liu, L.; Yan, M. Abscisic-Acid-Regulated Responses to Alleviate Cadmium Toxicity in Plants. Plants 2023, 12, 1023. [Google Scholar] [CrossRef] [PubMed]
- Griffith, A.; Wise, P.; Gill, R.; Paukett, M.; Donofrio, N.; Seyfferth, A.L. Combined effects of arsenic and Magnaporthe oryzae on rice and alleviation by silicon. Sci. Total Environ. 2021, 750, 142209. [Google Scholar] [CrossRef]
- Bhat, J.A.; Faizan, M.; Bhat, M.A.; Huang, F.; Yu, D.; Ahmad, A.; Bajguz, A.; Ahmad, P. Defense interplay of the zinc-oxide nanoparticles and melatonin in alleviating the arsenic stress in soybean (Glycine max L.). Chemosphere 2022, 288, 132471. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation—A review. Earth-Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Geng, N.; Wu, Y.; Zhang, M.; Tsang, D.C.; Rinklebe, J.; Xia, Y.; Lu, D.; Zhu, L.; Palansooriya, K.N.; Kim, K.-H. Bioaccumulation of potentially toxic elements by submerged plants and biofilms: A critical review. Environ. Int. 2019, 131, 105015. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Kučerová, D.; Vivodová, Z.; Kollárová, K. Silicon alleviates the negative effects of arsenic in poplar callus in relation to its nutrient concentrations. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 145, 275–289. [Google Scholar] [CrossRef]
- El Rasafi, T.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Tack, F.M.; Sebastian, A.; Prasad, M.; Rinklebe, J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2020, 52, 675–726. [Google Scholar] [CrossRef]
- Zhao, H.; Guan, J.; Liang, Q.; Zhang, X.; Hu, H.; Zhang, J. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci. Rep. 2021, 11, 9913. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, X.; Hou, L.; Ji, S.; Zhang, Y.; Fan, W.; Li, T.; Zhang, L.; Liu, P.; Yang, L. Effects of cadmium on transcription, physiology, and ultrastructure of two tobacco cultivars. Sci. Total Environ. 2023, 869, 161751. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.; Gul, I.; Kallerhoff, J.; Arshad, M. Fungi-assisted phytoextraction of lead: Tolerance, plant growth–promoting activities and phytoavailability. Environ. Sci. Pollut. Res. 2019, 26, 23788–23797. [Google Scholar] [CrossRef] [PubMed]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. App. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, U.; Ayub, A.; Hussain, S.; Waraich, E.A.; El-Esawi, M.A.; Ishfaq, M.; Ahmad, M.; Ali, N.; Maqsood, M.F. Cadmium toxicity in plants: Recent progress on morpho-physiological effects and remediation strategies. J. Soil Sci. Plant Nutrit. 2021, 22, 212–269. [Google Scholar] [CrossRef]
- Smolders, E.; Brans, K.; Földi, A.; Merckx, R. Cadmium fixation in soils measured by isotopic dilution. Soil Sci. Soc. Amer. J. 1999, 63, 78–85. [Google Scholar] [CrossRef]
- Astolfi, S.; Ortolani, M.R.; Catarcione, G.; Paolacci, A.R.; Cesco, S.; Pinton, R.; Ciaffi, M. Cadmium exposure affects iron acquisition in barley (Hordeum vulgare) seedlings. Physiol. Plant. 2014, 152, 646–659. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Abbas, T.; Zia-ur-Rehman, M.; Hannan, F.; Keller, C.; Al-Wabel, M.I.; Ok, Y.S. Cadmium minimization in wheat: A critical review. Ecotoxicol. Environ. Saf. 2016, 130, 43–53. [Google Scholar] [CrossRef]
- Hussain, B.; Ashraf, M.N.; Abbas, A.; Li, J.; Farooq, M. Cadmium stress in paddy fields: Effects of soil conditions and remediation strategies. Sci. Total Environ. 2021, 754, 142188. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, X.; Guo, X.; Pan, Y.; Yu, B.; Tang, Z.; Guo, Q. Differential responses to Cd stress induced by exogenous application of Cu, Zn or Ca in the medicinal plant Catharanthus roseus. Ecotoxicol. Environ. Saf. 2018, 157, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, J.; Li, K.; Zhang, Z.; Yu, B.; Lu, X.; Yang, J.; Zhu, Q. Correlations between cadmium and mineral nutrients in absorption and accumulation in various genotypes of rice under cadmium stress. Chemosphere 2003, 52, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhou, M.; Shu, W.; Lan, C.; Ye, Z.; Qiu, R.; Jie, Y.; Cui, G.; Wong, M.H. Constitutional tolerance to heavy metals of a fiber crop, ramie (Boehmeria nivea), and its potential usage. Environ. Pollut. 2010, 158, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Zeng, G.; Qu, D.; Gu, J.; Zhou, M.; Chai, L. Cadmium-induced oxidative stress and response of the ascorbate–glutathione cycle in Bechmeria nivea (L.) Gaud. Chemosphere 2007, 69, 99–107. [Google Scholar] [CrossRef]
- Wang, C.-L.; Liu, Y.-G.; Zeng, G.-M.; Hu, X.-J.; Ying, Y.-C.; Xi, H.; Lu, Z.; Wang, Y.-Q.; Li, H.-Y. Mechanism of exogenous selenium alleviates cadmium induced toxicity in Bechmeria nivea (L.) Gaud (Ramie). Transact. Nonferrous Met. Soc. China 2014, 24, 3964–3970. [Google Scholar] [CrossRef]
- Du, Y.; Hu, X.F.; Wu, X.H.; Shu, Y.; Jiang, Y.; Yan, X.J. Affects of mining activities on Cd pollution to the paddy soils and rice grain in Hunan province, Central South China. Environ. Monit. Assess. 2013, 185, 9843–9856. [Google Scholar] [CrossRef]
- An, X.; Wei, J.; Liu, Q.; Ying, J.; Zhou, H.; Luo, X.; Li, W.; Liu, T.; Zou, L.; Zhu, G. Progress in the Study of the absorption, accumulation, and tolerance of ramie to the heavy metal cadmium. Mol. Plant Breed. 2023, 14, 3. [Google Scholar]
- Lei, M.; Yue, Q.; Chen, T.; Huang, Z.; Liao, X.; Liu, Y.; Zheng, G.; Chang, Q. Heavy metal concentrations in soils and plants around Shizhuyuan mining area of Hunan Province. Acta Ecol. Sin. 2005, 25, 1146–1151. [Google Scholar]
- She, W.; Jie, Y.-C.; Xing, H.-C.; Lu, Y.-W.; Kang, W.-L.; Wang, D. Heavy metal concentrations and bioaccumulation of ramie (Boehmeria nivea) growing on 3 mining areas in Shimen, Lengshuijiang and Liuyang of Hunan Province. Shengtai Xuebao/Acta Ecol. Sin. 2010, 31, 874–881. [Google Scholar]
- Feng, X.; Abubakar, A.; Chen, K.; Yu, C.; Zhu, A.; Chen, J.; Gao, G.; Wang, X.; Mou, P.; Chen, P. Genome-wide analysis of R2R3-MYB transcription factors in Boehmeria nivea (L.) Gaudich revealed potential cadmium tolerance and anthocyanin biosynthesis genes. Front. Genet. 2023, 14, 1080909. [Google Scholar] [CrossRef]
- Satya, P.; Mitra, S.; Ray, D.P. Ramie (Boehmeria nivea L. Gaud) genetic improvement. In Advances in Plant Breeding Strategies: Industrial and Food Crops; Springer: Berlin/Heidelberg, Germany, 2019; pp. 115–150. [Google Scholar]
- Zhu, Q.; Huang, D.; Liu, S.; Luo, Z.; Rao, Z.; Cao, X.; Ren, X. Accumulation and subcellular distribution of cadmium in ramie (Boehmeria nivea L. Gaud.) planted on elevated soil cadmium contents. Plant Soil Environ. 2013, 59, 57–61. [Google Scholar] [CrossRef]
- Ma, Y.; Jie, H.; Tang, Y.; Xing, H.; Jie, Y. The Role of hemicellulose in cadmium tolerance in ramie (Boehmeria nivea (L.) Gaud.). Plants 2022, 11, 1941. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Y.; Huang, Y.; Zeng, G.; Wang, Y.; Hu, X.; Zhou, L. Effects of indole-3-acetic, kinetin and spermidine assisted with EDDS on metal accumulation and tolerance mechanisms in ramie (Boehmeria nivea (L.) Gaud.). Ecol. Engin. 2014, 71, 108–112. [Google Scholar] [CrossRef]
- Tang, H.; Liu, Y.; Gong, X.; Zeng, G.; Zheng, B.; Wang, D.; Sun, Z.; Zhou, L.; Zeng, X. Effects of selenium and silicon on enhancing antioxidative capacity in ramie (Boehmeria nivea (L.) Gaud.) under cadmium stress. Environ. Sci. Pollut. Res. 2015, 22, 9999–10008. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Wang, Y.; Liu, C.; Yang, X.; Wang, H.; Li, F.; Liu, T. Linkage mapping of quantitative trait loci for fiber yield and its related traits in the population derived from cultivated ramie and wild B. nivea var. tenacissima. Sci. Rep. 2019, 9, 16855. [Google Scholar] [CrossRef]
- Zhu, S.; Shi, W.; Jie, Y.; Zhou, Q.; Song, C. A MYB transcription factor, BnMYB2, cloned from ramie (Boehmeria nivea) is involved in cadmium tolerance and accumulation. PLoS ONE 2020, 15, e0233375. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhou, J.; Jie, Y.; Xing, H.; Zhong, Y.; She, W.; Wei, G.; Yu, W.; Ma, Y. A ramie (Boehmeria nivea) bZIP transcription factor BnbZIP3 positively regulates drought, salinity and heavy metal tolerance. Mol. Breed. 2016, 36, 120. [Google Scholar] [CrossRef]
- Gillani, S.F.; Rasheed, A.; Yuhong, G.; Wei, J.; Tariq, H.; Ilyas, M.; Yunling, P. Assessment of cold stress tolerance in maize through quantitative trait locus, genome-wide association study and transcriptome analysis. Not. Bot. Horti Agrobot. Cluj-Nap. 2021, 49, 12525. [Google Scholar] [CrossRef]
- Shanying, H.; Xiaoe, Y.; Zhenli, H.; Baligar, V.C. Morphological and physiological responses of plants to cadmium toxicity: A review. Pedosphere 2017, 27, 421–438. [Google Scholar]
- Baliardini, C.; Meyer, C.-L.; Salis, P.; Saumitou-Laprade, P.; Verbruggen, N. CATION EXCHANGER1 cosegregates with cadmium tolerance in the metal hyperaccumulator Arabidopsis halleri and plays a role in limiting oxidative stress in Arabidopsis spp. Plant Physiol. 2015, 169, 549–559. [Google Scholar] [CrossRef]
- She, W.; Jie, Y.-C.; Xing, H.-C.; Lu, Y.-W.; Huang, M.; Kang, W.-L.; Wang, D. Tolerance to cadmium in ramie (Boehmeria nivea) genotypes and its evaluation indicators. Acta Agron. Sinica 2011, 37, 348–353. [Google Scholar] [CrossRef]
- Zhu, S.; Shi, W.; Zhang, J. Effect of different ramie (Boehmeria nivea L. Gaud) cultivars on the adsorption of heavy metal ions cadmium and lead in the remediation of contaminated farmland soils. Open Chem. 2022, 20, 444–454. [Google Scholar] [CrossRef]
- Quan, R.; Chen, J.; Zhang, L.; Xu, M.; Yang, R.; She, W.; Cui, G. Responses of ramie to antioxidant enzymes and plant chelating peptides to Cd stress. Chin. J. Trop. Crops 2022, 43, 1023. [Google Scholar]
- She, W.; Cui, G.; Li, X.; Su, X.; Jie, Y.; Yang, R. Characterization of cadmium concentration and translocation among ramie cultivars as affected by zinc and iron deficiency. Acta Physiol. Plant. 2018, 40, 104. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y.; Hu, X.; Zeng, G.; Wang, Y.; Hu, X.; Zhou, Y.; Tan, X.; Jiang, L.; Zeng, X. Time-dependent antioxidative responses of ramie (Boehmeria nivea (L.) Gaudich) to moderate cadmium stress and its up-regulation mechanism by spermidine antioxidant. RSC Adv. 2015, 5, 76141–76149. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zeng, G.; Chai, L.; Song, X.; Min, Z.; Xiao, X. Subcellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Gaud. Environ. Expe. Bot. 2008, 62, 389–395. [Google Scholar] [CrossRef]
- She, W.; Jie, Y.-C.; Xing, H.-C.; Luo, Z.-Q.; Kang, W.-L.; Huang, M.; Zhu, S.-J. Absorption and accumulation of cadmium by ramie (Boehmeria nivea) cultivars: A field study. Acta Agricul. Scand. Section B-Soil Plant Sci. 2011, 61, 641–647. [Google Scholar] [CrossRef]
- Zhao, X.; Luan, M.; Qiu, C.; Guo, Y.; Long, S.; Wang, Y.; Qiu, H. Analysis of the potential of 165 ramie germplasms to be used for cadmium-contamination remediation. Indust. Crops Prod. 2021, 171, 113841. [Google Scholar] [CrossRef]
- Djemal, R.; Khoudi, H. The ethylene-responsive transcription factor of durum wheat, TdSHN1, confers cadmium, copper, and zinc tolerance to yeast and transgenic tobacco plants. Protoplasma 2022, 259, 19–31. [Google Scholar] [CrossRef]
- Rono, J.K.; Le Wang, L.; Wu, X.C.; Cao, H.W.; Zhao, Y.N.; Khan, I.U.; Yang, Z.M. Identification of a new function of metallothionein-like gene OsMT1e for cadmium detoxification and potential phytoremediation. Chemosphere 2021, 265, 129136. [Google Scholar] [CrossRef]
- Sterckeman, T.; Thomine, S. Mechanisms of cadmium accumulation in plants. Crit. Rev. Plant Sci. 2020, 39, 322–359. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Jiang, W.; Xiukang, W.; Hussain, S.; Ahmad, M.; Maqsood, M.F.; Ali, N.; Ishfaq, M.; Kaleem, M.; Haider, F.U. Cadmium phytotoxicity, tolerance, and advanced remediation approaches in agricultural soils; a comprehensive review. Front. Plant Sci. 2022, 13, 773815. [Google Scholar] [CrossRef]
- Ying, Z.; Yushen, M.; Yamei, W.; Zehang, L.; Yuejun, X.; Hucheng, X.; Yucheng, J. Genotype differences in cadmium accumulation ability of ramie on cadmium-polluted Field. J. Agricul. Sci. Tech. 2021, 23, 54. [Google Scholar]
- Hasan, M.K.; Ahammed, G.J.; Sun, S.; Li, M.; Yin, H.; Zhou, J. Melatonin inhibits cadmium translocation and enhances plant tolerance by regulating sulfur uptake and assimilation in Solanum lycopersicum L. J. Agric. Food Chem. 2019, 67, 10563–10576. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Xu, W.; Liu, A.; Chen, S. Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ. Exp. Bot. 2019, 161, 303–311. [Google Scholar] [CrossRef]
- Rehman, S.; Chattha, M.U.; Khan, I.; Mahmood, A.; Hassan, M.U.; Al-Huqail, A.A.; Salem, M.Z.; Ali, H.M.; Hano, C.; El-Esawi, M.A. Exogenously applied trehalose augments cadmium stress tolerance and yield of mung bean (Vigna radiata L.) grown in soil and hydroponic systems through reducing cd uptake and enhancing photosynthetic efficiency and antioxidant defense systems. Plants 2022, 11, 822. [Google Scholar] [CrossRef]
- Yeboah, A.; Lu, J.; Gu, S.; Liu, H.; Shi, Y.; Amoanimaa-Dede, H.; Agyenim-Boateng, K.G.; Payne, J.; Yin, X. Evaluation of two wild castor (Ricinus communis L.) accessions for cadmium tolerance in relation to antioxidant systems and lipid peroxidation. Environ. Sci. Pollut. Res. 2021, 28, 55634–55642. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, S.; Tang, Q.; Tang, S. Genome-wide transcriptomic profiling of ramie (Boehmeria nivea L. Gaud) in response to cadmium stress. Gene 2015, 558, 131–137. [Google Scholar] [CrossRef]
- Yeboah, A.; Lu, J.; Ting, Y.; Karikari, B.; Gu, S.; Xie, Y.; Liu, H.; Yin, X. Genome-wide association study identifies loci, beneficial alleles, and candidate genes for cadmium tolerance in castor (Ricinus communis L.). Indust. Crops Prod. 2021, 171, 113842. [Google Scholar] [CrossRef]
- Hou, F.; Zhou, X.; Liu, P.; Yuan, G.; Zou, C.; Lübberstedt, T.; Pan, G.; Ma, L.; Shen, Y. Genetic dissection of maize seedling traits in an IBM Syn10 DH population under the combined stress of lead and cadmium. Mol. Genet. Gen. 2021, 296, 1057–1070. [Google Scholar] [CrossRef]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone priming: Regulator for heavy metal stress in plants. J. Plant Growth Reg. 2019, 38, 739–752. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Gautam, S.; Rattan, A.; Bhardwaj, R.; Sharma, A. Brassinosteroids in plant nutrition and heavy metal tolerance. In Brassinosteroids in Plant Developmental Biology and Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2022; pp. 217–235. [Google Scholar]
- Rehman, R.S.; Ali, M.; Zafar, S.A.; Hussain, M.; Pasha, A.; Naveed, M.S.; Ahmad, M.; Waseem, M. Abscisic Acid Mediated Abiotic Stress Tolerance in Plants. Asian J. Res. Crop. Sci. 2022, 7, 1–17. [Google Scholar] [CrossRef]
- Gray, W.M. Hormonal regulation of plant growth and development. PLoS Biol. 2004, 2, e311. [Google Scholar] [CrossRef] [PubMed]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between plant hormones and heavy metals responses. Genet. Mol. Biol. 2017, 40, 373–386. [Google Scholar] [CrossRef]
- Xu, Y.-X.; Mao, J.; Chen, W.; Qian, T.-T.; Liu, S.-C.; Hao, W.-J.; Li, C.-F.; Chen, L. Identification and expression profiling of the auxin response factors (ARFs) in the tea plant (Camellia sinensis (L.) O. Kuntze) under various abiotic stresses. Plant Physiol. Biochem. 2016, 98, 46–56. [Google Scholar] [CrossRef]
- Yaashikaa, P.; Kumar, P.S.; Jeevanantham, S.; Saravanan, R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ. Pollut. 2022, 301, 119035. [Google Scholar] [CrossRef]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop. J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Shaukat, K.; Zahra, N.; Hafeez, M.B.; Naseer, R.; Batool, A.; Batool, H.; Raza, A.; Wahid, A. Role of salicylic acid–induced abiotic stress tolerance and underlying mechanisms in plants. In Emerging Plant Growth Regulators in Agriculture; Elsevier: Amsterdam, The Netherlands, 2022; pp. 73–98. [Google Scholar]
- Wang, H.; Zhong, G.; Shi, G.; Pan, F. Toxicity of Cu, Pb, and Zn on seed germination and young seedlings of wheat (Triticum aestivum L.). In Proceedings of the International Conference on Computer and Computing Technologies in Agriculture, Nanchang, China, 22–25 October 2010; pp. 231–240. [Google Scholar]
- Bulak, P.; Walkiewicz, A.; Brzezińska, M. Plant growth regulators-assisted phytoextraction. Biol. Plant. 2014, 58, 1–8. [Google Scholar] [CrossRef]
- Chen, K.; Chen, P.; Qiu, X.; Chen, J.; Gao, G.; Wang, X.; Zhu, A.; Yu, C. Regulating role of abscisic acid on cadmium enrichment in ramie (Boehmeria nivea L.). Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- He, Y.; He, S.; Luo, J.; Zeng, Y.; Zhang, X.; Huo, Y.; Jie, Y.; Xing, H. Exogenous Plant growth Regulator and Foliar Fertilizers for Phytoextraction of Cadmium with Boehmeria nivea [L.] Gaudich from Contaminated Field Soil. 2022. Available online: https://www.researchsquare.com/article/rs-1197456/latest.pdf (accessed on 20 May 2023).
- Mroue, S.; Simeunovic, A.; Robert, H.S. Auxin production as an integrator of environmental cues for developmental growth regulation. J. Exp. Bot. 2018, 69, 201–212. [Google Scholar] [CrossRef]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yang, H.; Shang, C.; Ma, S.; Liu, L.; Cheng, J. The roles of auxin biosynthesis YUCCA gene family in plants. Int. J. Mol. Sci. 2019, 20, 6343. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.P.; Zhu, J.; Wang, P.; Zeng, J.; Tan, R.; Yang, Y.Z.; Liu, Z.M. Effect of Cd on growth, physiological response, Cd subcellular distribution and chemical forms of Koelreuteria paniculata. Ecotoxicol. Environ. Saf. 2018, 160, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Chaca, P.; Vigliocco, A.; Reinoso, H.; Molina, A.; Abdala, G.; Zirulnik, F.; Pedranzani, H. Effects of cadmium stress on growth, anatomy and hormone contents in Glycine max (L.) Merr. Acta Physiol. Plant. 2014, 36, 2815–2826. [Google Scholar] [CrossRef]
- Ahmad, P.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Ahanger, M.A.; Alamri, S.A. Jasmonic acid alleviates negative impacts of cadmium stress by modifying osmolytes and antioxidants in faba bean (Vicia faba L.). Arch. Agron. Soil Sci. 2017, 63, 1889–1899. [Google Scholar] [CrossRef]
- Shahid, M.; Javed, M.T.; Mushtaq, A.; Akram, M.S.; Mahmood, F.; Ahmed, T.; Noman, M.; Azeem, M. Microbe-mediated mitigation of cadmium toxicity in plants. In Cadmium Toxicity and Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 427–449. [Google Scholar]
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Manganese-induced cadmium stress tolerance in rice seedlings: Coordinated action of antioxidant defense, glyoxalase system and nutrient homeostasis. Comptes Rendus Biol. 2016, 339, 462–474. [Google Scholar] [CrossRef]
- Erguuml, N. Effects of some heavy metals and heavy metal hormone interactions on wheat (Triticum aestivum L. cv. Gun 91) seedlings. Afri. J. Agricul. Res. 2012, 7, 1518–1523. [Google Scholar]
- Villiers, F.; Jourdain, A.; Bastien, O.; Leonhardt, N.; Fujioka, S.; Tichtincky, G.; Parcy, F.; Bourguignon, J.; Hugouvieux, V. Evidence for functional interaction between brassinosteroids and cadmium response in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 1185–1200. [Google Scholar] [CrossRef]
- Munzuroglu, O.; Zengin, F.K. Effect of cadmium on germination, coleoptile and root growth of barley seeds in the presence of gibberellic acid and kinetin. J. Environ. Biol. 2006, 27, 671–677. [Google Scholar]
- Jie, H.; Zhao, L.; Ma, Y.; Rasheed, A.; Jie, Y. Integrated transcriptome and metabolome analysis reveal that exogenous gibberellin application regulates lignin synthesis in ramie. Agronomy 2023, 13, 1450. [Google Scholar] [CrossRef]
- Jie, H.; Ma, Y.; Xie, D.-Y.; Jie, Y. Transcriptional and metabolic characterization of feeding ramie growth enhanced by a combined application of gibberellin and ethrel. Int. J. Mol. Sci. 2022, 23, 12025. [Google Scholar] [CrossRef] [PubMed]
- Karcz, W.; Kurtyka, R. Effect of cadmium on growth, proton extrusion and membrane potential in maize coleoptile segments. Biol. Plant. 2007, 51, 713–719. [Google Scholar] [CrossRef]
- Krantev, A.; Yordanova, R.; Popova, L. Salicylic acid decreases Cd toxicity in maize plants. Gen. App. Plant Physiol. 2006, 3, 45–52. [Google Scholar]
- Krantev, A.; Yordanova, R.; Janda, T.; Szalai, G.; Popova, L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 2008, 165, 920–931. [Google Scholar] [CrossRef]
- Gondor, O.K.; Pál, M.; Darkó, É.; Janda, T.; Szalai, G. Salicylic acid and sodium salicylate alleviate cadmium toxicity to different extents in maize (Zea mays L.). PLoS ONE 2016, 11, e0160157. [Google Scholar] [CrossRef]
- Kalai, T.; Bouthour, D.; Manai, J.; Kaab, L.B.B.; Gouia, H. Salicylic acid alleviates the toxicity of cadmium on seedling growth, amylases and phosphatases activity in germinating barley seeds. Arc. Agro. Soil Sci. 2016, 62, 892–904. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, R.; Hu, X.; Song, J.; Li, B.; Ou, D.; Hu, X.; Zhao, Y. Exogenous spermidine elevating cadmium tolerance in Salix matsudana involves cadmium detoxification and antioxidant defense. Int. J. Phytoremed. 2019, 21, 305–315. [Google Scholar] [CrossRef]
- Nahar, K.; Rahman, M.; Hasanuzzaman, M.; Alam, M.; Rahman, A.; Suzuki, T.; Fujita, M. Physiological and biochemical mechanisms of spermine-induced cadmium stress tolerance in mung bean (Vigna radiata L.) seedlings. Environ. Sci. Pollut. Res. 2016, 23, 21206–21218. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shi, G.; Li, W.; Wu, W. Exogenous spermidine enhances Hydrocharis dubia cadmium tolerance. Russian J. Plant Physiol. 2013, 60, 770–775. [Google Scholar] [CrossRef]
- Rady, M.M.; Hemida, K.A. Modulation of cadmium toxicity and enhancing cadmium-tolerance in wheat seedlings by exogenous application of polyamines. Ecotoxicol. Environ. Saf. 2015, 119, 178–185. [Google Scholar] [CrossRef]
- Gong, X.; Liu, Y.; Huang, D.; Zeng, G.; Liu, S.; Tang, H.; Zhou, L.; Hu, X.; Zhou, Y.; Tan, X. Effects of exogenous calcium and spermidine on cadmium stress moderation and metal accumulation in Boehmeria nivea (L.) Gaudich. Environ. Sci. Pollut. Res. 2016, 23, 8699–8708. [Google Scholar] [CrossRef] [PubMed]
- She, W.; Cui, G.-X.; Jie, Y.-C.; Bai, Y.-C.; Cao, Y.; Xiao, C.-X. Comparative effects of chelants on plant growth, cadmium uptake and accumulation in nine cultivars of Ramie (Boehmeria nivea). Acta Agricul. Scand. Sect. B-Soil Plant Sci. 2014, 64, 71–78. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, Y.; Liu, Y.; Zeng, G.; Hu, X.; Hu, X.; Zhou, L.; Guo, Y.; Li, J. Cadmium accumulation and apoplastic and symplastic transport in Boehmeria nivea (L.) Gaudich on cadmium-contaminated soil with the addition of EDTA or NTA. RSC Adv. 2015, 5, 47584–47591. [Google Scholar] [CrossRef]
- Wu, S.; Xue, S.; Iqbal, Y.; Xing, H.; Jie, Y. Seasonal nutrient cycling and enrichment of nutrient-related soil microbes aid in the adaptation of ramie (Boehmeria nivea L.) to nutrient-deficient conditions. Front. Plant Sci. 2021, 12, 644904. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, T.; Zheng, Q.; Li, J.; Qian, Y.; Li, J.; Zhan, Q. Identification of cold tolerance and analysis of genetic diversity for major wheat varieties in Jianghuai region of China. Pak. J. Bot. 2019, 52, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Pandit, E.; Guru, M.; Nayak, D.; Tasleem, S.; Barik, S.; Mohanty, D.; Mohanty, S.; Patra, B.; Pradhan, S. Genetic diversity, population structure, marker validation and kinship analysis for seedling stage cold tolerance in indica rice. ORYZA-An Int. J. Rice 2018, 55, 396–405. [Google Scholar] [CrossRef]
- Swarup, S.; Cargill, E.J.; Crosby, K.; Flagel, L.; Kniskern, J.; Glenn, K.C. Genetic diversity is indispensable for plant breeding to improve crops. Crop. Sci. 2021, 61, 839–852. [Google Scholar] [CrossRef]
- Negisho, K.; Shibru, S.; Pillen, K.; Ordon, F.; Wehner, G. Genetic diversity of Ethiopian durum wheat landraces. PLoS ONE 2021, 16, e0247016. [Google Scholar] [CrossRef]
- Govindaraj, M.; Vetriventhan, M.; Srinivasan, M. Importance of genetic diversity assessment in crop plants and its recent advances: An overview of its analytical perspectives. Genet. Res. Int. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Esquinas-Alcázar, J. Protecting crop genetic diversity for food security: Political, ethical and technical challenges. Nat. Rev. Gen. 2005, 6, 946–953. [Google Scholar] [CrossRef]
- Choudhury, B.I.; Khan, M.L.; Dayanandan, S. Patterns of nucleotide diversity and phenotypes of two domestication related genes (OsC1 and Wx) in indigenous rice varieties in Northeast India. BMC Genet. 2014, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Haun, W.J.; Hyten, D.L.; Xu, W.W.; Gerhardt, D.J.; Albert, T.J.; Richmond, T.; Jeddeloh, J.A.; Jia, G.; Springer, N.M.; Vance, C.P. The composition and origins of genomic variation among individuals of the soybean reference cultivar Williams 82. Plant Physiol. 2011, 155, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Van de Wouw, M.; van Hintum, T.; Kik, C.; van Treuren, R.; Visser, B. Genetic diversity trends in twentieth century crop cultivars: A meta analysis. Theoret. App. Genet. 2010, 120, 1241–1252. [Google Scholar] [CrossRef]
- Fukuoka, S.; Suu, T.D.; Ebana, K.; Trinh, L.N.; Nagamine, T.; Okuno, K. Diversity in phenotypic profiles in landrace populations of Vietnamese rice: A case study of agronomic characters for conserving crop genetic diversity on farm. Genet. Res. Crop Evolut. 2006, 53, 753–761. [Google Scholar] [CrossRef]
- Wendel, J.F.; Greilhuber, J.; Dolezel, J.; Leitch, I.J. Plant Genome Diversity Volume 1: Plant Genomes, Their Residents, and Their Evolutionary Dynamics; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Murat, F.; Peer, Y.V.D.; Salse, J. Decoding plant and animal genome plasticity from differential paleo-evolutionary patterns and processes. Gen. Biol. Evol. 2012, 4, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cai, K.; Zhang, G.; Zeng, F. Metabolite profiling of barley grains subjected to water stress: To explain the genotypic difference in drought-induced impacts on malting quality. Fronti. Plant Sci. 2017, 8, 1547. [Google Scholar] [CrossRef]
- Belete, Y.; Shimelis, H.; Laing, M.; Mathew, I. Genetic diversity and population structure of bread wheat genotypes determined via phenotypic and SSR marker analyses under drought-stress conditions. J. Crop Imp. 2021, 35, 303–325. [Google Scholar] [CrossRef]
- Pandey, J.; Scheuring, D.C.; Koym, J.W.; Coombs, J.; Novy, R.G.; Thompson, A.L.; Holm, D.G.; Douches, D.S.; Miller, J.C.; Vales, M.I. Genetic diversity and population structure of advanced clones selected over forty years by a potato breeding program in the USA. Sci. Rep. 2021, 11, 1–18. [Google Scholar] [CrossRef]
- Vaughan, D.; Balazs, E.; Heslop-Harrison, J. From crop domestication to super-domestication. Ann. Bot. 2007, 100, 893–901. [Google Scholar] [CrossRef]
- Andersen, J.R.; Lübberstedt, T. Functional markers in plants. Trend. Plant Sci. 2003, 8, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wei, Q.; Lin, L.; Shi, L.; Cui, Z.; Li, Y.; Huang, C.; Wei, C. Physicochemical properties of a new starch from ramie (Boehmeria nivea) root. Int. J. Biol. Macromol. 2021, 174, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Glaszmann, J.-C.; Kilian, B.; Upadhyaya, H.D.; Varshney, R.K. Accessing genetic diversity for crop improvement. Curr. Opin. Plant Biol. 2010, 13, 167–173. [Google Scholar] [CrossRef]
- Zhu, G.; Huang, D.; Zhu, Q.; Liu, S.; Liu, G.; Jia, L. Tolerance and phytoremediation potential of ramie for cadmium contaminated soil. Res. Agricul. Modern. 2009, 30, 752–755. [Google Scholar]
- She, W.; Jie, Y.; Xing, H.; Lu, Y.; Huang, M.; Kang, W.; Wang, D. Comparison and screening indicators for ramie (Boehmeria nivea) genotypes tolerant to cadmium. Acta Agro. Sin. 2011, 37, 348–354. [Google Scholar] [CrossRef]
- She, W.; Jie, Y.; Xing, H.; Huang, M.; Kang, W.; Lu, Y.; Wang, D. Uptake and accumulation of heavy metal by ramie growing on antimony mining area in Lengshuijiang City of Hunan Province. J. Agro-Environ. Sci. 2010, 29, 91–96. [Google Scholar]
- Wu, Z.; Tang, Q.; Wang, Y.; Qiu, C.; Long, S.; Zhao, X.; Hu, Z.; Guo, Y. Ramie (Boehmeria nivea) as Phytoremediation Crop for heavy metal-contaminated paddy soil in Southern China: Variety comparison, cd accumulation, and assessment of fiber recycling. J. Nat. Fib. 2021, 19, 11078–11091. [Google Scholar] [CrossRef]
- Derakhshani, B.; Jafary, H.; Zanjani, B.M.; Hasanpur, K.; Mishina, K.; Tanaka, T.; Kawahara, Y.; Oono, Y. Combined QTL mapping and RNA-Seq profiling reveals candidate genes associated with cadmium tolerance in barley. PLoS ONE 2020, 15, e0230820. [Google Scholar] [CrossRef]
- Liu, T.; Tang, S.; Zhu, S.; Tang, Q. QTL mapping for fiber yield-related traits by constructing the first genetic linkage map in ramie (Boehmeria nivea L. Gaud). Mol. Breed. 2014, 34, 883–892. [Google Scholar] [CrossRef]
- Chen, J.; Rao, J.; Wang, Y.; Zeng, Z.; Liu, F.; Tang, Y.; Chen, X.; Liu, C.; Liu, T. Integration of quantitative trait loci mapping and expression profiling analysis to identify genes potentially involved in ramie fiber lignin biosynthesis. Genes 2019, 10, 842. [Google Scholar] [CrossRef]
- Zhu, S.; Zheng, X.; Dai, Q.; Tang, S.; Liu, T. Identification of quantitative trait loci for flowering time traits in ramie (Boehmeria nivea L. Gaud). Euphytica 2016, 210, 367–374. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, K.; Niu, J.; Zhong, Y.; Sun, Z.; Luan, M.; Chen, J. Association analysis and validation of simple sequence repeat markers for fiber fineness in ramie (Boehmeria Nivea L. Gaudich). J. Nat. Fib. 2020, 19, 3615–3623. [Google Scholar] [CrossRef]
- Ranjbar, S.; Malcata, F.X. Is Genetic Engineering a Route to Enhance Microalgae-Mediated Bioremediation of Heavy Metal-Containing Effluents? Molecules 2022, 27, 1473. [Google Scholar] [CrossRef]
- Bansal, M.; Wani, S.H. Recent advancement in plant genetic engineering for efficient phytoremediation. In Phytoremediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–202. [Google Scholar]
- Raza, A.; Habib, M.; Charagh, S.; Kakavand, S.N. Genetic engineering of plants to tolerate toxic metals and metalloids. In Handbook of Bioremediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 411–436. [Google Scholar]
- Sonawane, H.; Arya, S.; Bedi, A.; Jaiswar, A. Targeted genetic modification technologies: Potential benefits of their future use in Phytoremediation. In Phytoremediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 203–226. [Google Scholar]
- She, W.; Zhu, S.; Jie, Y.; Xing, H.; Cui, G. Expression profiling of cadmium response genes in ramie (Boehmeria nivea L.) root. Bullet. Environ. Contaminat. Toxicol. 2015, 94, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Kumar, P.; Parmar, N.; Shandil, R.K.; Aggarwal, G.; Gaur, A.; Srivastava, D.K. Achievements and prospects of genetic engineering in poplar: A review. New For. 2021, 52, 889–920. [Google Scholar] [CrossRef]
- Cai, X.; Wang, M.; Jiang, Y.; Wang, C.; Ow, D.W. Overexpression of OsABCG48 lowers cadmium in rice (Oryza sativa L.). Agronomy 2021, 11, 918. [Google Scholar] [CrossRef]
- Mehta, V.; Kansara, R.; Srivashtav, V.; Savaliya, P. A novel insight into phytoremediation of heavy metals through genetic engineering and phytohor-mones. J. Nanosci. Nanomed. Nanobiol. 2021, 4, 10. [Google Scholar]
- Yue, E.; Rong, F.; Liu, Z.; Ruan, S.; Qian, H. A Great Potential genetic editing target, the non-Coding RNA Microrna535, knockout through CRISPR/Cas9 technology to reduce the accumulation of cadmium in rice. Knockout through Crispr/cas9 Technology to Reduce the Accumulation of Cadmium in Rice.
- Wojas, S.; Hennig, J.; Plaza, S.; Geisler, M.; Siemianowski, O.; Skłodowska, A.; Ruszczyńska, A.; Bulska, E.; Antosiewicz, D.M. Ectopic expression of Arabidopsis ABC transporter MRP7 modifies cadmium root-to-shoot transport and accumulation. Environ. Pollut. 2009, 157, 2781–2789. [Google Scholar] [CrossRef]
- Zhang, Y.; Sa, G.; Zhang, Y.; Hou, S.; Wu, X.; Zhao, N.; Zhang, Y.; Deng, S.; Deng, C.; Deng, J. Populus euphratica annexin1 facilitates cadmium enrichment in transgenic Arabidopsis. J. Hazard. Mat. 2021, 405, 124063. [Google Scholar] [CrossRef]
- He, L.; Ma, X.; Li, Z.; Jiao, Z.; Li, Y.; Ow, D.W. Maize OXIDATIVE STRESS2 homologs enhance cadmium tolerance in Arabidopsis through activation of a putative SAM-dependent methyltransferase gene. Plant Physiol. 2016, 171, 1675–1685. [Google Scholar] [CrossRef]
- Wu, L.; Yu, Y.; Hu, H.; Tao, Y.; Song, P.; Li, D.; Guan, Y.; Gao, H.; Sui, X.; Volodymyr, T. New SFT2-like Vesicle Transport Protein (SFT2L) enhances cadmium tolerance and reduces cadmium accumulation in common wheat grains. J. Agricul. Food Chem. 2022, 70, 5526–5540. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Wang, M.; Zhang, T.; Liu, X.; Jiang, H.; Sun, Y.; Cheng, X.; Yan, Q. Enhanced cadmium accumulation and tolerance in transgenic hairy roots of Solanum nigrum L. Expressing Iron-Regulated Transporter Gene IRT1. Life 2020, 10, 324. [Google Scholar] [CrossRef]
- Sahoo, K.K.; Tripathi, A.K.; Pareek, A.; Singla-Pareek, S.L. Taming drought stress in rice through genetic engineering of transcription factors and protein kinases. Plant Stress 2013, 7, 60–72. [Google Scholar]
- Nester, E.W. Agrobacterium: Nature’s genetic engineer. Front. Plant Sci. 2015, 5, 730. [Google Scholar] [CrossRef]
- Tang, W.; Charles, T.M.; Newton, R.J. Overexpression of the pepper transcription factor CaPF1 in transgenic Virginia pine (Pinus virginiana Mill.) confers multiple stress tolerance and enhances organ growth. Plant Mol. Biol. 2005, 59, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, D.; Ramasamy, K.; Sellamuthu, G.; Jayabalan, S.; Venkataraman, G. CRISPR for crop improvement: An update review. Front. Plant Sci. 2018, 9, 985. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, R.; Javed, T.; Afzal, I.; Sabagh, A.E.; Ali, A.; Vicente, O.; Chen, P. Modern biotechnologies: Innovative and sustainable approaches for the improvement of sugarcane tolerance to environmental stresses. Agronomy 2021, 11, 1042. [Google Scholar] [CrossRef]
- Sandler, R. The ethics of genetic engineering and gene drives in conservation. Conservat. Biol. 2020, 34, 378–385. [Google Scholar] [CrossRef]
- Zhu, B.; Gan, C.; Gu, L.; Du, X.; Wang, H. Identification of NRAMP4 from Arabis paniculata enhance cadmium tolerance in transgenic Arabidopsis. J. Genet. 2021, 100, 89. [Google Scholar] [CrossRef]
- Huo, J.; Du, B.; Sun, S.; He, S.; Zhao, N.; Liu, Q.; Zhai, H. A novel aldo-keto reductase gene, IbAKR, from sweet potato confers higher tolerance to cadmium stress in tobacco. Front. Agricul. Sci. Engi. 2018, 5, 206–213. [Google Scholar]
- Bhuiyan, M.S.U.; Min, S.R.; Jeong, W.J.; Sultana, S.; Choi, K.S.; Song, W.Y.; Lee, Y.; Lim, Y.P.; Liu, J.R. Overexpression of a yeast cadmium factor 1 (YCF1) enhances heavy metal tolerance and accumulation in Brassica juncea. Plant Cell Tissue Organ Cult. (PCTOC) 2011, 105, 85–91. [Google Scholar] [CrossRef]
- Shi, J.; Du, P.; Luo, H.; Wu, H.; Zhang, Y.; Chen, J.; Wu, M.; Xu, G.; Gao, H. Soil contamination with cadmium and potential risk around various mines in China during 2000–2020. J. Environ. Manag. 2022, 310, 114509. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, T.; Li, Y.; Wang, M.; Chen, W.; Dai, Y. Mitigating cadmium contamination of rice soils supporting tobacco–rice rotation in southern China: Win–win or lose–lose? J. Hazard. Mat. 2022, 425, 128052. [Google Scholar] [CrossRef] [PubMed]
- Liptáková, Ľ.; Demecsová, L.; Valentovičová, K.; Zelinová, V.; Tamás, L. Early gene expression response of barley root tip to toxic concentrations of cadmium. Plant Mol. Biol. 2022, 108, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhou, J.; Jie, Y.; Xing, H.; Zhong, Y.; Yu, W.; She, W.; Ma, Y.; Liu, Z.; Zhang, Y. A ramie bZIP transcription factor BnbZIP2 is involved in drought, salt, and heavy metal stress response. DNA Cell Biol. 2016, 35, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Shi, W. Cloning and expression pattern analysis of BnMYB3 transcription factor gene in ramie. Acta Bot. Boreali-Occident. Sin. 2019, 39, 422–429. [Google Scholar]
- Zhu, S.; Shi, W.; Jie, Y.; Zhou, Q. Cloning and expression analysis of cadmium-responsive transcription factor gene BnMYB1 from ramie (Boehmeria nivea). J. Agricul. Biotech. 2018, 26, 774–783. [Google Scholar]
- Liu, T.; Zhu, S.; Tang, Q.; Tang, S. Identification of 32 full-length NAC transcription factors in ramie (Boehmeria nivea L. Gaud) and characterization of the expression pattern of these genes. Mol. Genet. Gen. 2014, 289, 675–684. [Google Scholar] [CrossRef]
- Sternberg, S.H.; Richter, H.; Charpentier, E.; Qimron, U. Adaptation in CRISPR-Cas systems. Mol. Cell 2016, 61, 797–808. [Google Scholar] [CrossRef]
- Amitai, G.; Sorek, R. CRISPR–Cas adaptation: Insights into the mechanism of action. Nat. Rev. Microbiol. 2016, 14, 67–76. [Google Scholar] [CrossRef]
- Ahmad, S.; Wei, X.; Sheng, Z.; Hu, P.; Tang, S. CRISPR/Cas9 for development of disease resistance in plants: Recent progress, limitations and future prospects. Brief. Funct. Gen. 2020, 19, 26–39. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P. Evolutionary classification of CRISPR–Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef]
- Tang, Y.; Fu, Y. Class 2 CRISPR/Cas: An expanding biotechnology toolbox for and beyond genome editing. Cell Biosci. 2018, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef]
- Kantor, A.; McClements, M.E.; MacLaren, R.E. CRISPR-Cas9 DNA base-editing and prime-editing. Int. J. Mol. Sci. 2020, 21, 6240. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, W.; Li, J. The CRISPR/Cas9 revolution continues: From base editing to prime editing in plant science. J. Genet. Gen. 2021, 48, 661–670. [Google Scholar] [CrossRef]
- Huang, C.-J.; Wu, T.-L.; Zheng, P.-X.; Ou, J.-Y.; Ni, H.-F.; Lin, Y.-C. Comparative genomic analysis uncovered evolution of pathogenicity factors, horizontal gene transfer events, and heavy metal resistance traits in citrus canker Bacterium Xanthomonas citri subsp. citri. Front. Microbiol. 2021, 12, 2605. [Google Scholar] [CrossRef]
- Kesari, N. Role of CRISPR-Cas9 and amiRNA in conferring abiotic and biotic stress tolerance to plants. 2021. [Google Scholar]
- Ahmed, T.; Noman, M.; Shahid, M.; Muhammad, S.; Tahir ul Qamar, M.; Ali, M.A.; Maqsood, A.; Hafeez, R.; Ogunyemi, S.O.; Li, B. Potential application of CRISPR/Cas9 system to engineer abiotic stress tolerance in plants. Protein Pept. Lett. 2021, 28, 861–877. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, B.; Ding, W.; Liu, X.; Yang, D.-L.; Wei, P.; Cao, F.; Zhu, S.; Zhang, F.; Mao, Y. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 2013, 23, 1229–1232. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J.-L. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotech. 2013, 31, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Nekrasov, V.; Staskawicz, B.; Weigel, D.; Jones, J.D.; Kamoun, S. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat. Biotech. 2013, 31, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.D.; Scott, D.A.; Weinstein, J.A.; Ran, F.A.; Konermann, S.; Agarwala, V.; Li, Y.; Fine, E.J.; Wu, X.; Shalem, O. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotech. 2013, 31, 827–832. [Google Scholar] [CrossRef]
- Montague, T.G.; Cruz, J.M.; Gagnon, J.A.; Church, G.M.; Valen, E. CHOPCHOP: A CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 2014, 42, W401–W407. [Google Scholar] [CrossRef]
- Lei, Y.; Lu, L.; Liu, H.Y.; Li, S.; Xing, F.; Chen, L.L. CRISPR-P: A web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol. Plant 2014, 7, 1494–1496. [Google Scholar] [CrossRef]
- Belhaj, K.; Chaparro-Garcia, A.; Kamoun, S.; Nekrasov, V. Plant genome editing made easy: Targeted mutagenesis in model and crop plants using the CRISPR/Cas system. Plant Methods 2013, 9, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Wang, Y.; Li, J.; Gao, C. Genome editing in rice and wheat using the CRISPR/Cas system. Nat. Prot. 2014, 9, 2395–2410. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.-L.; Dong, L.; Wang, Z.-P.; Zhang, H.-Y.; Han, C.-Y.; Liu, B.; Wang, X.-C.; Chen, Q.-J. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014, 14, 327. [Google Scholar] [CrossRef]
- Kumar, V.; Jain, M. The CRISPR–Cas system for plant genome editing: Advances and opportunities. J. Exp. Bot. 2015, 66, 47–57. [Google Scholar] [CrossRef]
- Jain, M. Function genomics of abiotic stress tolerance in plants: A CRISPR approach. Front. Plant Sci. 2015, 6, 375. [Google Scholar] [CrossRef]
- Sarma, H.; Islam, N.; Prasad, R.; Prasad, M.; Ma, L.Q.; Rinklebe, J. Enhancing phytoremediation of hazardous metal (loid) s using genome engineering CRISPR–Cas9 technology. J. Hazard. Mat. 2021, 414, 125493. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Sheng, Z.; Jalal, R.S.; Tabassum, J.; Ahmed, F.K.; Hu, S.; Shao, G.; Wei, X.; Abd-Elsalam, K.A.; Hu, P. CRISPR–Cas technology towards improvement of abiotic stress tolerance in plants. In CRISPR and RNAi Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 755–772. [Google Scholar]
- Liu, C.-X.; Yang, T.; Zhou, H.; Ahammed, G.J.; Qi, Z.-Y.; Zhou, J. The E3 Ubiquitin Ligase Gene Sl1 Is critical for cadmium tolerance in Solanum lycopersicum L. Antioxidants 2022, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Lu, Y.; Zhang, X.; Yang, G.; Chao, D.; Wang, Z.; Shi, M.; Chen, J.; Chao, D.-Y.; Li, R. The ABC transporter ABCG36 is required for cadmium tolerance in rice. J. Exp. Bot. 2019, 70, 5909–5918. [Google Scholar] [CrossRef] [PubMed]
| Cd Stress | Hormones, Polyamines, Metalloids, and Nutrients | Effects | References |
|---|---|---|---|
| 30 mg kg−1 | Spd and IAA 100 μm | Increased the activity of antioxidant enzymes | [34] |
| 6 and 9 mg/L | Se, 1.2 μmol/L | Improve the action of POD and SOD and control methylation of DNA in ramie leaves | [25] |
| 1 mM | Ca 5-mM, and Spd 1 mM | Reduced MDA contents, enhancing plants’ dry weight, and chlorophyll contents | [97] |
| Cd 30 μM | Spd 0.1 mM | Decreased Cd content, stabilized cellular macromolecules like protein and sugar | [46] |
| Crops/Plants | Gene | Role | References |
|---|---|---|---|
| Wheat | TaSFT2L | Enhanced root and shoot growth, chlorophyll contents, and root and shoot dry weight | [142] |
| Rice | OsMT1e | Enhanced shoot and dry root weight and chlorophyll contents | [51] |
| Arabidopsis | ApNRAMP4 | Reduced Cd contents | [150] |
| Tobacco | IbAKR | Increased ability for scavenging MDA, and enhanced proline contents | [151] |
| Arabidopsis | ZmOXS2b and ZmO2L1 | Enhanced Cd tolerance by activating the targeted gene | [141] |
| Brassica | YCF1 | Increased plant fresh weight and biomass | [152] |
| TFs Families/Genes | Gene | Function | Reference |
|---|---|---|---|
| MYB | BnGMYB10/12/41 | Interact with genes regulating the biosynthesis of flavonoids and enhanced Cd tolerance | [30] |
| MYB | BnMYB2 | Enhanced Cd tolerance in Arabidopsis | [37] |
| MYB | BnMYB3 | Higher expression levels with an increase in stress time and intensity | [157] |
| MYB | BnMYB1 | Up-regulated and higher expression in leaves | [158] |
| bZIP | BnbZIP3 | Enhanced Cd tolerance | [38] |
| bZIP | BnbZIP2 | Enhanced sensitivity to Cd stress | [156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasheed, A.; Jie, H.; He, P.; Lv, X.; Ali, B.; Ma, Y.; Xing, H.; Almari, S.; Elnour, R.O.; Hassan, M.U.; et al. A Systematic Review on the Improvement of Cd Stress Tolerance in Ramie Crop, Limitations and Future Prospective. Agronomy 2023, 13, 1793. https://doi.org/10.3390/agronomy13071793
Rasheed A, Jie H, He P, Lv X, Ali B, Ma Y, Xing H, Almari S, Elnour RO, Hassan MU, et al. A Systematic Review on the Improvement of Cd Stress Tolerance in Ramie Crop, Limitations and Future Prospective. Agronomy. 2023; 13(7):1793. https://doi.org/10.3390/agronomy13071793
Chicago/Turabian StyleRasheed, Adnan, Hongdong Jie, Pengliang He, Xueying Lv, Basharat Ali, Yushen Ma, Hucheng Xing, Saad Almari, Rehab O. Elnour, Muhammad Umair Hassan, and et al. 2023. "A Systematic Review on the Improvement of Cd Stress Tolerance in Ramie Crop, Limitations and Future Prospective" Agronomy 13, no. 7: 1793. https://doi.org/10.3390/agronomy13071793
APA StyleRasheed, A., Jie, H., He, P., Lv, X., Ali, B., Ma, Y., Xing, H., Almari, S., Elnour, R. O., Hassan, M. U., Gillani, S. F. A., & Jie, Y. (2023). A Systematic Review on the Improvement of Cd Stress Tolerance in Ramie Crop, Limitations and Future Prospective. Agronomy, 13(7), 1793. https://doi.org/10.3390/agronomy13071793








