Abstract
In response to the new concept of the impact of total climate production factors on plant phenology, this study verifies the feasibility of simulating plant phenology and triggering thresholds based on total climatic production factors by using the phenological and meteorological observation data of S. krylovii plants from 1985 to 2018 at the Xilinhot National Climate Observatory of China Meteorological Administration. The results indicate that the total climate production factors have a significant impact on plant phenological changes and can be effectively utilized for simulating phenology and determining triggering thresholds. The mutation of cumulative climate production potential based on total climate production factors can effectively indicate the green-up date and the wilting date of S. krylovii plants, and their triggering thresholds depend on the parameters of climate resource changes and the characteristics of plant biology, which are (0.085, −5.363) and (0.086, −27.620), respectively. The cumulative climate production potential based on total climate production factors can effectively indicate the heading date of S. krylovii plants, and its triggering thresholds also depend on the parameters of climate resource changes and the characteristics of plant biology, which is (394.632, −38,026.268). Furthermore, the results support the viewpoint that abrupt changes to the climate determine the beginning and ending of plant growth, while the accumulative climate resources determine the other phenological dates. This study provides new ideas for the study of plant phenology.
1. Introduction
Plant phenology is a mutually adaptive growth and development rhythm formed by the long-term adaptation of plants to seasonal changes in environmental conditions such as temperature, precipitation, and light [1], which not only reflects plant growth and development but also indicates climate change [2] and has been widely used to guide agricultural activities and disaster prevention and mitigation [3]. Meanwhile, plant phenology affects water-heat exchange and carbon cycling in ecosystems [4,5], and is an important parameter in land surface process models and plant productivity models [6].
It is found that plant spring phenology tends to be earlier and autumn phenology tends to be later in a warming context [7,8], and this phenomenon is more pronounced in the high latitudes of the Northern Hemisphere [9]. However, there is a clear spatial variability in the response of grassland plant phenology to climate change in Inner Mongolia. In the southern region, there is a trend of earlier green-up and later wilting, while in the central and northern regions, there is a trend of later green-up and earlier wilting [10]. However, the factors causing spatial variabilities in grassland plant phenology are still unclear. The meteorological factors that affect plant phenology include temperature, precipitation, light, air humidity, and carbon dioxide concentration. Among them, temperature is considered to be the most important environmental factor affecting plant phenology [11], and plants can only grow and develop in a certain temperature environment and require a certain cumulative temperature to complete their life cycle [12]. A water deficit limits the utilization of light and heat conditions by plants [13] and is considered a key factor in regulating vegetation activity in arid and semi-arid regions [14]. Light is the source of energy for photosynthesis in plants, and the organic matter produced by photosynthesis is the material basis for plant growth and development [15]. Additionally, the photoperiod has also been shown to be an important indicator of how light affects plant phenology [16]. Meanwhile, there are significant interactions between temperature, precipitation, and light, which collectively influence the changes in plant phenology [17,18]. Phenology models have evolved from statistical to mechanistic models, and have been widely used to simulate phenology and predict the impacts of future climate change in areas where phenology observations are lacking [19]. The original phenological models can be traced back to the thermal time model, which assumes that crop development is proportional to the accumulated thermal time. Considering the effects of dormancy processes and environmental factors during both endodormancy and ecodormancy on plant phenology, a two-phase model has been developed, including a sequential model and parallel model [19]. However, the two-phase model gives an unclear relationship between endodormancy and ecodormancy. Furthermore, there are models that have proposed that plant dormancy includes more than two phases, such as the deepening rest model and the four-phase model [19]. The models mentioned above assume that plant spring phenology is solely induced by air temperature. Some recent studies have also incorporated photoperiod effects into phenological models. The research on autumn phenology models is limited due to the inadequate and inaccurate ground observation data. Currently, the phenological research conducted through different models has been useful and insightful in identifying and demonstrating the causal relationship between various climatic factors and plant phenology [19]. However, the literature lacks a comprehensive model that can determine the combined effects of environmental factors on plant phenology [20]. Therefore, by including the comprehensive response of plant phenology to all environmental factors, the accuracy of the prediction model can be improved.
Research has shown that plant phenology is closely related to the dynamics of photosynthesis [21]. Plant photosynthesis is the result of the interaction between environmental factors and plant biological properties, reflecting the influence of all climate factors affecting plant production, which is referred to as the total climate production factors. Climate is the most important factor affecting plant growth and development, and it also serves as the foundation for the establishment of plant morphology and physiological and biochemical changes [22,23]. Climatic production potential refers to the highest biological or agricultural yield per unit area of land when other conditions (such as soil, nutrients, carbon dioxide) are optimal and the local climatic resources such as light, heat, and water are fully and rationally utilized [24]. Climate production potential not only reflects the influence of all climate factors (such as temperature, humidity, light) and their combined effects on plant production, but also ensures the uniformity of the influencing factors throughout the process of plant growth and the cyclical changes in their interaction with the environment. At the same time, climate production potential also reflects the combined effects of biological factors (such as leaf area), environmental factors, and their interactions, and can reflect the effects of extreme weather and climate events. Therefore, utilizing climate production potential as a driving factor for plant phenology changes can avoid the limitations of existing models and achieve an accurate simulation of phenology [25].
Chinese temperate grasslands are the third largest in the world [26], sensitive to climate change, and play an important role in the global carbon cycle [27]. The grassland of S. krylovii is one of the representative types of typical grasslands [28], which occupies an important position in livestock production [29] and has been significantly affected by the warm and dry climate [30]. The climatic mechanism of delaying the start and advancing the end of the growing season of S. krylovii and the effects of phenological changes on plant production of S. krylovii in a semi-arid region were revealed [31,32,33]. Using the long-term phenological and corresponding meteorological observation data from 1985 to 2018 in the grassland of S. krylovii, this study proposes new concepts of climate production potential, cumulative climate production potential (reflecting resource accumulation), the first-order derivative of cumulative climate production potential (reflecting the rate of resource change), and the second-order derivative of cumulative climate production potential (reflecting sudden resource change) based on the total climate production factors influencing plant phenological changes [25]. On this basis, this study intends to (1) verify the feasibility of simulating plant phenology and triggering thresholds based on the total climatic production factors, and (2) clarify the relationship between the main phenological periods of S. krylovii and total climatic production factors, as well as their triggering thresholds, to improve the understanding of the response of phenology to the combined effect of meteorological conditions and provide a basis for the development of phenological models.
2. Materials and Methods
2.1. Study Area and Data
The study data are from the Xilinhot National Climate Observatory in Xilinhot, Inner Mongolia, China (44°08′ 03′ N, 116°19′ 43′ E, 990 m a.s.l.). It is located in the middle of the Inner Mongolian Autonomous Region, which is a typical temperate semi-arid continental climate zone. The average annual temperature and average annual precipitation are 2.0 °C and 260 mm, respectively. Winter is cold and dry, summer is warm and humid, and solar radiation is strong. The soil type is chestnut soil. The dominant species of the ecosystem is S. krylovii, and the accompanying species include Leymus chinensis (Trin.) Tzvel.), Allium tenuissimum, Cleistogenes squarrosa (Trin.) Keng, Artemisia frigida Willd., Allium amsopodium Ledeb., Kochia prostrata (Linn.) Schrad., Artemisia scoparia Waldst. Et Kit., Heteropappus altaicus (Willd.) Novopokr. The phenology and meteorological data have been observed at the Xilinhot National Climate Observatory, Inner Mongolia, China Meteorological Administration since 1985. All meteorological data were examined and verified by the National Meteorological Information Center of the China Meteorological Administration. Plant phenology was obtained at the natural pasture observation site from the Xilinhot National Climate Observatory, Inner Mongolia, China Meteorological Administration. The fence area of the observation field is 100 × 100 m, which is divided into 4 plots of 50 × 50 m, and each plot is divided into 4 replicates for observation (Figure 1). The multi-spectral phenological observation camera (made in China) is located in the center of the phenology observation site. Phenological periods, which were recorded by local professional observers in accordance with standard observations [34], were observed every 2 d. And 10 plants with respectable growth and complete life history for 3 consecutive years were selected for observation. In this study, the phenological dataset of dominant species S. krylovii in typical grassland include the green-up date, the heading date, and the wilting date from 1985 to 2018. The green-up date is defined as the date when 50% of the individual plants in the plots have their elasticity restored and turned from yellow to green. The heading date is defined as the date when the aristae of 50% of plants are exposed from the top or side of the leaf sheath. The wilting date is defined as the date when two-thirds of the aboveground part of the first plant in the plot is withered and yellow. Each phenological stage is recorded. The phenological observation records are converted into the daily sequence from January 1st by using the method of Julian day. The meteorological dataset includes daily mean air temperature (°C), precipitation (mm), sunshine duration (h), average pressure (hpa), mean wind speed (m/s) at the height of 10 m, and relative humidity (%) from 1985 to 2018.

Figure 1.
Phenology observation site at the natural pasture from the Xilinhot National Climate Observatory, Inner Mongolia, China Meteorological Administration.
2.2. Method
2.2.1. Climate Tendency Rate
The climate tendency rate reflects the changing trend of meteorological elements in a region. Xi is a meteorological element value at the Xilinhot National Climate Observatory from 1985 to 2018, Ti is the corresponding chronological order, and the linear regression equation is as follows:
where a is the regression constant, b is the regression coefficient, and b × 10 is the climate tendency rate. If the climate tendency rate is positive, it indicates that the element is showing an increasing trend; if it is negative, it indicates a decreasing trend.
2.2.2. Climate Production Potential
Climate production potential (LNPP) refers to the climate production per unit leaf area [25], which is obtained by dividing the climate production (YW) by the leaf area correction function f(L), thus making the climate production potential comparable among different phenological periods.
Climate production (YW) is calculated based on the step-by-step correction method recommended by the Food and Agriculture Organization of the United Nations (FAO) [25]. Firstly, photosynthetic production (YQ) is estimated based on plant physiological mechanisms and energy conversion. Secondly, light temperature production (YT) is evaluated by temperature corrections on YQ. Finally, climate production (YW) is obtained by moisture corrections on YT. YW can be calculated based on daily scale meteorological data during plant growth:
where YW is the climate production per unit area (kg·hm−2); Q is the total solar radiation during the plant growth period (MJ·m−2); f(Q) is photosynthetic efficiency coefficient (g·kJ−1), f(t) is the temperature correction coefficient, f(w) is the moisture correction coefficient; YQ is the photosynthetic production potential per unit area (kg·hm−2); YT is the production potential of light and temperature per unit area (kg·hm−2).
YQ is the plant yield determined solely by solar radiation under assumed optimal conditions such as temperature, moisture, soil conditions, CO2 concentration, and agricultural facilities, calculated as follows:
where Q is the total solar radiation during the plant growth season (∑Qi) (MJ·m−2) and f(L) is the leaf area correction function. The other parameters listed in the equation are constants related to the biological characteristics of the plant itself (Table 1).

Table 1.
Explanation of each parameter in the equation.
Daily solar radiation () can be expressed as [39]
where is astronomical radiation (MJ·m–2); n and N are the actual daily sunshine hours (h) and the maximum possible sunshine hours (h), respectively; and a and b are empirical coefficients, taken as 0.29 and 0.557, respectively [40].
where T is the time in days (=24 × 60 min), I0 is the solar constant (0.082 MJ·m−2·min−1), ω is the time angle (rad), φ is the geographic latitude (rad), δ is the solar declination (rad), ρ is the distance between the Sun and the Earth, and ρ2 is the correction coefficient of eccentricity of Earth’s orbit.
where θ is the solar angle (rad) and dn is the chronological order, with January 1st as 1, January 2nd as 2, and so on.
Light temperature production (YT) is the plant yield determined solely by solar radiation and air temperature under assumed optimal conditions such as moisture, soil conditions, CO2 concentration, and agricultural facilities, calculated as follows:
where is the temperature correction coefficient, 0 ≤ f(t) ≤ 1; t is the daily average temperature (°C); and tmin, ts, tmax are the lower-limit temperature, optimal temperature, and upper-limit temperature (°C) of the growth period, where 0 °C, 20 °C, and 35 °C are taken in this study, respectively [35].
Climate production (YW) is the plant yield determined solely by solar radiation, air temperature, and moisture under assumed optimal conditions such as soil conditions, CO2 concentration, and agricultural facilities, calculated as follows:
where VPD is the water vapor pressure deficit (Pa); t the daily average temperature (°C); RH is the average relative humidity (%); and VPDmax and VPDmin are constants determined by vegetation type, where VPDmax and VPDmin are taken as 3100 Pa and 650 Pa, respectively [41].
To achieve the prediction of plant phenology, it is necessary to calculate LNPP for each day starting from January 1st and calculate the cumulative LNPP to reflect the climate resource status, i.e., the cumulative climate production potential. The cumulative climate production potential is a function of time and conforms to the logistic curve. The first derivative and second derivative can be obtained according to the cumulative climate production potential, which, respectively, reflects the change speed and acceleration (abrupt change) of climate resources. The mutation (second derivative) of the cumulative climate production potential can be used to indicate the beginning and the ending of plant growth, while the cumulative climate production potential or its change speed (first derivative) can be used to indicate the other phenological dates between the beginning and end of plant growth [25].
2.2.3. Logistic Curve
The accumulation of daily climate production potential can reflect the degree to which plants can utilize climate resources. Thus, the relationship between plant phenology and climate production potential can be analyzed. To quantitatively describe the accumulation of climate production potential over time, the logistic curve is used to simulate it. The trend equation of accumulative climate production potential based on the logistic curves is calculated as follows:
The integral equation can be expressed as
where dY/dt is the instantaneous growth rate, is the theoretical upper limit of climate production potential, is time (d), Y is the cumulative value of climate production potential from January 1st, a is the coefficient, and r is the intrinsic growth rate and is the maximum instantaneous growth rate under specific conditions.
2.3. Data Processing
Microsoft Excel 2019 (Redmond, WA, USA) is used to collate and summarize the data, Matlab 2018a software (Mathworks, Natick, MA, USA) is used to fit the logistic curve and calculate the change rate (the first derivative of the cumulative climate production potential) and mutation (the second derivative of the cumulative climate production potential) of the cumulative climate production potential, and SPSS 21.0 (IBM, Armonk, NY, USA) is used to analyze the change trend of the main phenological dates of S. krylovii and their relationships with the cumulative climate production potential, its change rate, and mutation [21]. Origin 2018a is used to plot the figures.
3. Results
3.1. Climate Change Trends
From 1985 to 2018, the annual average temperature at the Xilinhot National Climate Observatory in Inner Mongolia showed an increasing trend, while the annual precipitation and annual sunshine duration showed a decreasing trend (Figure 2). From 1985 to 2018, the annual average temperature in the region ranged from 1.3 to 4.8 °C, with an average of 3.3 °C, showing a significant upward trend (0.40 °C/10a, p < 0.01). During this period, there was a slight decrease in the average temperature from December to January of the following year, while there was an increasing trend in the average temperature from February to November. Specifically, the average temperature changes in March, July, August, and September were significant, with rates of 1.17 °C/10a (p < 0.01), 0.89 °C/10a (p < 0.05), 0.53 °C/10a (p < 0.05), and 0.55 °C/10a (p < 0.05), respectively. The annual precipitation showed a large interannual variation, ranging from 121.1 to 511.7 mm. The annual average precipitation was 272.4 mm, showing an insignificant decreasing trend (−8.24 mm/10a, p > 0.05). The main decrease in precipitation occurred in July and August (−9.73 mm/10a (p > 0.05) and −10.76 mm/10a (p > 0.05), respectively). The average annual sunshine duration ranged from 2720.8 to 3215.8 h, with an average of 2991.9 h, showing an insignificant downward trend (−32.10 h/10a, p > 0.05). Except for January, February, July, and August, the average monthly sunshine duration in the Xilinhot region showed a decreasing trend, especially in May and June with significant changes (−9.06 h/10a (p < 0.05) and −10.76 h/10a (p < 0.05), respectively).
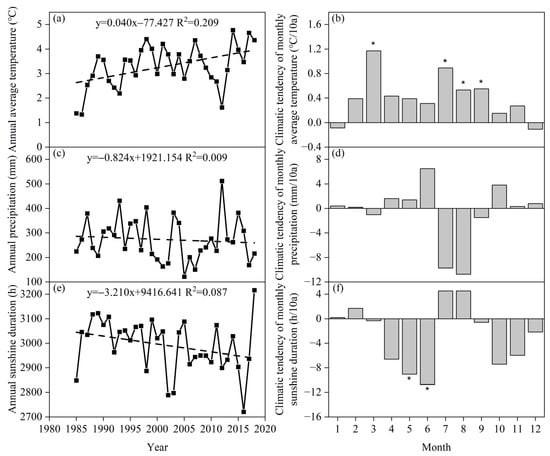
Figure 2.
The changing trends of meteorological factors from 1985 to 2018 at the Xilinhot National Climate Observatory, Inner Mongolia, China Meteorological Administration. (a) Annual average temperature; (b) climatic tendency of monthly average temperature; (c) annual precipitation; (d) climatic tendency of monthly precipitation; (e) annual sunshine duration; and (f) climatic tendency of monthly sunshine duration. * indicates that the changing trend of this factor is significant from 1985 to 2018 (p < 0.05).
3.2. Phenological Change Trends of S. krylovii Plant
From 1985 to 2018, the green-up of S. krylovii plants in the Xilinhot region of Inner Mongolia mainly occurred in mid- and late April. The earliest green-up date was on 2 April 1993, and the latest green-up was on 7 May 2009. The green-up date showed a significant delay trend, with an average delay of 5.4 d/10a (p < 0.05) (Figure 3). There were significant interannual differences in the heading dates, occurring from early July to the middle of September, with a difference of nearly three months between the earliest and latest heading dates. The heading date showed an insignificant trend of advancing, with an average of 5.4 d/10a (p > 0.05). After September, S. krylovii plants gradually entered the wilting period, mainly concentrated in late September and early October, with the latest wilting date in the middle of October (1987, 1998, and 2000). In contrast, the wilting date showed a slight advance trend, with an average advance of 1.2 d/10a (p > 0.05). Overall, the significant delay in the green-up date and the advance in the wilting date have led to a significant reduction in the length of the growth season of S. krylovii plants in the Xlinhot region, with an average reduction of 6.3 d/10a (p < 0.05).
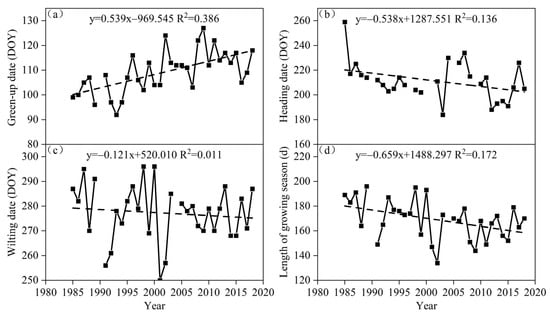
Figure 3.
The phenological change trends of S. krylovii from 1985 to 2018 at the Xilinhot National Climate Observatory, Inner Mongolia, China Meteorological Administration. (a) Green-up date trend; (b) heading date trend; (c) wilting date trend; and (d) trend of the length of the growing season.
3.3. Relationship between the Main Phenology and Climate Production Potential
The cumulative climate production potential of S. krylovii plants followed a logistic curve over time (Table 2). The determination coefficients of the cumulative climate production potential of S. krylovii plants from 1985 to 2018 were all greater than 0.996.

Table 2.
Results of logistic curve fitting the accumulative climate productivity potential.
3.3.1. Relationship between the Green-Up Date and Climate Production Potential
The relationship between the green-up date of S. krylovii plants and the cumulative climate production potential showed a significant positive correlation (Figure 4). The correlation coefficients (R2) were 0.667, 0.822, and 0.862 for the cumulative climate production potential and its first and second derivative, respectively. Among them, the relationship between the green-up date and the second derivative of cumulative climate production potential was best, indicating that the drastic changes in meteorological conditions were important reasons for triggering the green-up date of S. krylovii plants. Based on this relationship, it can be concluded that the triggering threshold of the green-up date depended on both resource change parameters and biological characteristic parameters, namely the slope (0.085) and intercept (−5.363) of the second derivative of cumulative climate production potential with diurnal variation.
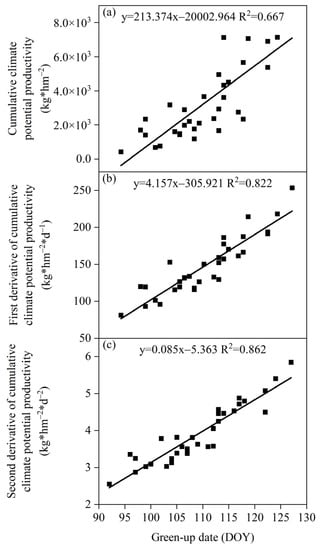
Figure 4.
Relationship between green-up date of S. krylovii plant and climatic productivity potential accumulation. (a) Cumulative climate potential productivity; (b) first derivative of cumulative climate potential productivity; and (c) second derivative of cumulative climate potential productivity.
3.3.2. Relationship between the Heading Date and Climate Production Potential
The heading date of S. krylovii plants in the Xilinhot region showed a significant positive correlation with the cumulative climate production potential (Figure 5). However, there was a significant negative correlation between the heading date and the first and second derivative of the cumulative climate production potential. The correlation between the heading date and the cumulative climate production potential (0.840) was significantly better than the first and second derivative of the cumulative climate production potential (0.754 and 0.607, respectively), reflecting that the heading date of S. krylovii plants mainly depended on the cumulative degree of climate resources. Based on the relationship between the heading date and the cumulative climate production potential, it can be concluded that the triggering threshold of the heading date depended on both resource change parameters and biological characteristic parameters, namely the slope (394.632) and intercept (−38,026.268) of the cumulative climate production potential with diurnal changes.
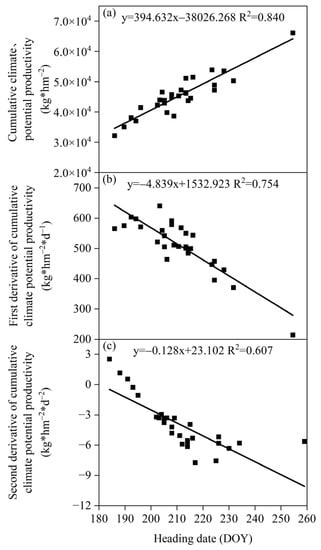
Figure 5.
Relationship between the heading date of S. krylovii plant and climatic potential productivity potential. (a) Cumulative climate potential productivity; (b) first derivative of cumulative climate potential productivity; and (c) second derivative of cumulative climate potential productivity.
3.3.3. Relationship between the Wilting Date and Climate Production Potential
There was a significant positive correlation between the wilting date of S. krylovii plants and the cumulative climate production potential and its second derivative, but a significant negative correlation with the first derivative of cumulative climate production potential (Figure 6). The correlation coefficients between the wilting date and the cumulative climate production potential and its first and second derivative were 0.315, 0.917, and 0.915, respectively. The wilting date of S. krylovii plants showed a strong correlation with the first and second derivative of cumulative climate production potential. However, there was a significant negative correlation between the wilting date and the first derivative of the cumulative climate production potential, indicating a decrease in the utilization of climate resources rather than a complete halt. Considering the variability between the green-up phenology and the wilting phenology [25], the abrupt changes in cumulative climate production potential can better reflect the changes in plant withering and yellowing periods. Based on the relationship between the wilting date of S. krylovii plants and the second derivative of the cumulative climate production potential, it can be concluded that the triggering threshold of the wilting date also depended on both resource change parameters and biological characteristic parameters, namely the slope (0.086) and intercept (−27.620) of the second derivative of cumulative climate production potential with diurnal variation.
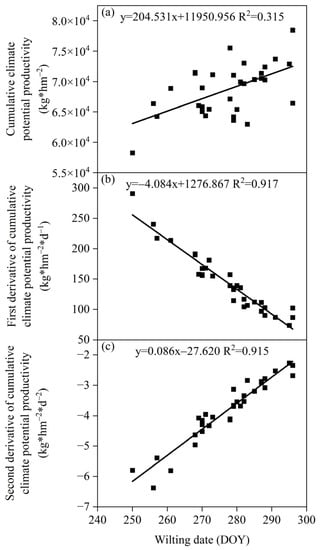
Figure 6.
Relationship between the wilting date of S. krylovii plant and climatic potential productivity potential. (a) Cumulative climate potential productivity; (b) first derivative of cumulative climate potential productivity; and (c) second derivative of cumulative climate potential productivity.
4. Discussion and Conclusions
Meteorological factors are important factors that affect plant growth and development, as well as the basis for plant morphogenesis and physiological and biochemical changes. The meteorological factors that affect the green-up date of grassland plants include temperature, sunshine duration [42], accumulated temperature [19,43], and moisture [44,45]. When the air temperature is below the threshold, the autumn phenology occurs [46,47]; when the photoperiod is shortened to the threshold that limits plant growth and development, it induces plant leaf senescence and enters a dormant state. An in situ simulation experiment of S. krylovii grassland also indicates that it is environmental factors, rather than plant productivity, that drive leaf senescence [48]. In this study, the pronounced relationship between the green-up date and wilting date of S. krylovii plants and the second derivative of cumulative climate production potential was found to be best and significant. This finding reconfirmed the strong relationship existing between meteorological conditions and the green-up to wilting cycles. However, as a novelty, it also highlighted that the plant phenology simulation and trigger threshold could be determined by the total climatic production factors. The heading date of S. krylovii plants had the best relationship with the cumulative climate production potential, indirectly proving the impact of rising summer temperature as a driver of climate production potential on the phenological periods between the beginning and ending phenology, reflecting the cumulative climate resource effects.
In this study, the long-term phenology and corresponding meteorological observation data of S. krylovii grassland from 1985 to 2018 were used to verify the indication of climate production potential based on total climate production factors on plant phenology, and the relationship between the main phenological stages of S. krylovii plants and climate production potential was clarified. The climate production potential not only reflects the comprehensive effect of environmental factors on plant production and their consistency throughout the process of plant growth, but also reflects the effects of biological factors, environmental factors, and their interactions, as well as extreme weather and climate events, which will be helpful for achieving accurate simulations of plant phenology. The main conclusions are as follows:
(1) The feasibility of simulating plant phenology and triggering thresholds based on total climatic production factors is verified. The climate production potential, which is based on these factors, serves as a good indicator for predicting the phenology of S. krylovii plants. The abrupt change in cumulative climate production potential reflects the drastic change in climate conditions, effectively indicating the green-up and wilting periods of S. krylovii plants. On the other hand, the cumulative climate production potential reflects the rate of change in climate resources and, to some extent, reflects resource utilization, which can effectively indicate the heading period of S. krylovii plants.
(2) The relationship between the main phenological periods of S. krylovii and total climatic production factors and their triggering thresholds is clarified. The mutation of cumulative climate production potential based on total climate production factors can effectively indicate the green-up date and the wilting date of S. krylovii plants, and their triggering thresholds depend on the parameters of climate resource changes and plant biology, which are (0.085, −5.363) and (0.086, −27.620), respectively. The cumulative climate production potential based on total climate production factors can effectively indicate the heading date of S. krylovii plants, and its triggering thresholds also depend on the parameters of climate resource changes and plant biological parameters, which is (394.632, −38,026.268), respectively.
Author Contributions
Conceptualization, G.Z. and W.G.; methodology, G.Z. and W.G.; validation, G.Z., W.G. and L.Z.; formal analysis, W.G.; investigation, W.G., X.S. and X.L.; writing—original draft preparation, G.Z. and W.G.; writing—review and editing, G.Z., X.L., E.L. and Y.J.; funding acquisition, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported by the National Key Research and Development Program of China (No. 2018YFA0606103) and the National Natural Science Foundation of China (42130514).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Acknowledgments
Sincere thanks go to the editor and anonymous reviewers for their thoughtful comments that improved this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, L.X.; Chen, H.L.; Li, Q. Research advances in plant phenology and climate. Acta Eco. Sin. 2010, 30, 447–454. (In Chinese) [Google Scholar]
- Zhu, K.Z.; Wan, M.W. Phenology; Science Press: Beijing, China, 1973. (In Chinese) [Google Scholar]
- Zhu, K.Z. A preliminary research of climatic change in China about five thousand years past. Sci. Sin. 1973, 2, 168–189. (In Chinese) [Google Scholar]
- Penuelas, J.; Rutishauser, T.; Filella, I. Ecology. Phenology feedbacks on climate change. Science 2009, 324, 887–888. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.F.; Gray, J.; Friedl, M.A.; Toomey, M.; Bohrer, G.; Hollinger, D.Y.; Munger, J.W.; O’Keefe, J.; Schmid, H.P.; Wing, I.S.; et al. Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nat. Clim. Chang. 2014, 4, 598–604. [Google Scholar] [CrossRef]
- Li, R.P.; Zhou, G.S.; Zhang, H.L. Research advances in plant phenology. Chin. J. Appl. Ecol. 2006, 17, 541–544. (In Chinese) [Google Scholar]
- Piao, S.L.; Friedlingstein, P.; Ciais, P.; Viovy, N.; Demarty, J. Growing season extension and its impact on terrestrial carbon cycle in the Northern Hemisphere over the past 2 decades. Glob. Biogeochem. Cy. 2007, 21, GB3018. [Google Scholar] [CrossRef]
- Jeong, S.J.; Ho, C.H.; Gim, H.J.; Brown, M. Phenology shifts at start vs. end of growing season in temperate vegetation over the Northern Hemisphere for the period 1982–2008. Glob. Chang. Biol. 2011, 17, 2385–2399. [Google Scholar] [CrossRef]
- Root, T.; MacMynowski, D.; Mastrandrea, M. Human-modified temperatures induce species changes: Joint attribution. Proc. Natl. Acad. Sci. USA 2005, 102, 7465–7469. [Google Scholar] [CrossRef]
- Shi, G.H. Phenological variation of main herbages during the last 20 years in the typical steppe of Inner Mongolia Plateau China. Chin. J. Grassl. 2019, 41, 80–88. (In Chinese) [Google Scholar]
- Lesica, P.; Kittelson, P.M. Precipitation and temperature are associated with advanced flowering phenology in a semi-arid grassland. J. Arid Environ. 2020, 74, 1013–1017. [Google Scholar] [CrossRef]
- Zhang, X.B.; Ren, J.R.; Zhang, D.R. Phenological observations on Larix principis-rupprechtii Mayr. in primary seed orchard. J. For. Res. 2001, 12, 201–204. [Google Scholar]
- Tao, Z.X.; Wang, H.J.; Liu, Y.C.; Xu, Y.J.; Dai, J.H. Phenological response of different vegetation types to temperature and precipitation variations in northern China during 1982–2012. Int. J. Remote Sens. 2017, 38, 3236–3252. [Google Scholar] [CrossRef]
- Pennington, D.D.; Collins, S.L. Response of an aridland ecosystem to interannual climate variability and prolonged drought. Landsc. Ecol. 2007, 22, 897–910. [Google Scholar] [CrossRef]
- Hu, Y.; Li, P.; Yang, J.G. Applied Meteorology, 2nd ed; China Meteorological Press: Beijing, China, 2005. [Google Scholar]
- Way, D.A.; Montgomery, R.A. Photoperiod constraints on tree phenology, performance and migration in a warming world. Plant Cell Environ. 2015, 38, 1725–1736. [Google Scholar] [CrossRef]
- Tao, F.L.; Yokozawa, M.; Zhang, Z.Z.; Hayashi, Y.; Ishigooka, Y. Land surface phenology dynamics and climate variations in the North East China Transect (NECT), 1982–2000. Int. J. Remote Sens. 2008, 29, 5461–5478. [Google Scholar] [CrossRef]
- Fu, Y.H.; Zhao, H.F.; Piao, S.L.; Peaucelle, M.a.r.c.; Peng, S.S.; Zhou, G.Y.; Ciais, P.; Huang, M.T.; Menzel, A.; Peñuelas, J.; et al. Declining global warming effects on the phenology of spring leaf unfolding. Nature 2015, 526, 104–107. [Google Scholar] [CrossRef]
- Fu, Y.H.; Li, X.X.; Zhou, X.C.; Geng, X.J.; Guo, Y.H.; Zhang, Y.R. Progress in plant phenology modeling under global climate change. Sci. Chin. Earth Sci. 2020, 63, 1237–1247. [Google Scholar] [CrossRef]
- Hänninen, H.; Kramer, K.; Tanino, K.; Zhang, R.; Wu, J.S.; Fu, Y.H. Experiments are necessary in process–based tree phenology modelling. Trends Plant Sci. 2019, 24, 199–209. [Google Scholar] [CrossRef]
- Hu, M.X.; Zhou, G.S. Phenological change and its ecophysiological mechanism of spring maize responding to drought at jointing stage and rewatering. Acta Eco. Sin. 2020, 40, 274–283. (In Chinese) [Google Scholar]
- Dai, W.J.; Jin, H.Y.; Zhang, Y.H. Advances in plant phenology. Acta Ecol. Sin. 2020, 40, 6705–6719. (In Chinese) [Google Scholar]
- Hossain, A.; Teixeira da Silva, J.A.; Lozovskaya, M.V.; Zvolinsky, V.P. High temperature combined with drought affect rainfed spring wheat and barley in South-Eastern Russia: I. Phenology and growth. Saudi. J. Biol. Sci. 2012, 19, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Yang, X.G.; Sun, S. Comparison of potential yield and resource utilization efficiency of main food crops in three provinces of Northeast China under climate change. Chin. J. Appl. Ecol. 2015, 26, 3091–3102. (In Chinese) [Google Scholar]
- Zhou, G.S.; Song, X.Y.; Zhou, M.Z.; Zhou, L.; Ji, Y.H. Advances in influencing mechanism and model of total climatic production factors of plant phenology change. Sci. Sin. Vitae. 2023, 53, 380–389. (In Chinese) [Google Scholar] [CrossRef]
- Lee, R.; Yu, F.; Price, K.P. Evaluating vegetation phenological patterns in Inner Mongolia using NDVI time-series analysis. Int. J. Remote Sens. 2002, 23, 2505–2512. [Google Scholar] [CrossRef]
- Sui, X.H.; Zhou, G.S.; Zhuang, Q.L. Sensitivity of carbon budget to historical climate variability and atmospheric CO2 concentration in temperate grassland ecosystems in China. Clim. Chang. 2013, 117, 259–272. [Google Scholar] [CrossRef]
- Inner Mongolia Ningxia Comprehensive Expedition, Chinese Academy of Sciences. Vegetation of Inner Mongolia; Science Press: Beijing, China, 1985. [Google Scholar]
- Yuan, W.P.; Zhou, G.S.; Wang, Y.H.; Wang, Y.S. Simulating phenological characteristics of two dominant grass species in a semi-arid steppe ecosystem. Ecol. Res. 2007, 22, 784–791. [Google Scholar] [CrossRef]
- Parry, M.; Canziani, O.; Palutikof, J. Intergovernmental Panel on Climate Change Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Liu, E.H.; Zhou, G.S.; He, Q.J.; Wu, B.Y.; Zhou, H.L.; Gu, W.J. Climatic Mechanism of Delaying the Start and Advancing the End of the Growing Season of Stipa krylovii in a Semi-Arid Region from 1985–2018. Agronomy 2022, 12, 1906. [Google Scholar] [CrossRef]
- Liu, E.H.; Zhou, G.S.; He, Q.J.; Wu, B.Y.; Lv, X.M. Predrought and Its Persistence Determined the Phenological Changes of Stipa krylovii in Inner Mongolia. Agronomy 2023, 13, 1345. [Google Scholar] [CrossRef]
- Yu, H.Y.; Zhou, G.S.; Lv, X.M.; He, Q.J.; Zhou, M.Z. Effects of Phenological Changes on Plant Production—From the View of Stipa krylovii. Agronomy 2022, 12, 3208. [Google Scholar] [CrossRef]
- China Meteorological Administration. Specification for Agrometeorological Observation; China Meteorological Press: Beijing, China, 1993. (In Chinese) [Google Scholar]
- Guo, J.; Gao, S.; Liu, L. Climatic productivity of forage grass and its restricting factors in north region of China. Chin. J. Eco-Agricul. 2002, 3, 48–50. (In Chinese) [Google Scholar]
- Monteith, J.L.; Moss, C.J. Climate and the Efficiency of Crop Production in Britain [and Discussion]. Philos. Trans. R. Soc. B Biol. Sci. 1977, 281, 277–294. [Google Scholar]
- Yu, H.N.; Li, W.G. Analysis and Use of Agricultural Climate Resources; Meteorology Press: Beijing, China, 1985. (In Chinese) [Google Scholar]
- Yu, H.N.; Zhao, F.S. On the light and thermal resources and the crop potential productivity—taking Luancheng County of Hebei province as an example. Acta Meteorol. Sin. 1982, 3, 327–334. (In Chinese) [Google Scholar]
- Weng, D. Climatological calculation methods for total radiation. Acta Meteorol. Sin. 1964, 3, 304–315. (In Chinese) [Google Scholar]
- Wang, B.Z.; Zhang, F.G.; Li, L.X. Solar energy Resources in China. Acta Energ. Sol. Sin. 1980, 1, 1–9. (In Chinese) [Google Scholar]
- Kanniah, K.D.; Beringer, J.; Hutley, L.B.; Tapper, N.J.; Zhu, X. Evaluation of Collections 4 and 5 of the MODIS Gross Primary Productivity product and algorithm improvement at a tropical savanna site in northern Australia. Remote Sens. Environ. 2009, 113, 1808–1822. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, G.S.; Wang, Y. Phenological calendar of stipa krylovii steppe in Inner Mongolia, China and its correlation with climatic variables. Acta Phytoecol. Sin. 2008, 32, 1312–1322. (In Chinese) [Google Scholar]
- Wang, H.J.; Wu, C.Y.; Ciais, P.; Peñuelas, J.; Dai, J.H.; Fu, Y.H.; Ge, Q.S. Overestimation of the effect of climatic warming on spring phenology due to misrepresentation of chilling. Nat. Commun. 2020, 11, 4945. [Google Scholar] [CrossRef]
- Xu, L.L. Non-linear response of dominant plant species regreening to precipitation in mid-west Inner Mongolia in spring. Acta Eco. Sin. 2020, 40, 9120–9128. (In Chinese) [Google Scholar]
- Zhang, X.Y.; Friedl, M.A.; Schaaf, C.B.; Strnhler, A.H. Monitoring the response of vegetation phenology to precipitation in Africa by coupling MODIS and TRMM instruments. J. Geo. Res. Atmos. 2005, 110, D12103. [Google Scholar] [CrossRef]
- Zhang, F. Effects of global warming on plant phenological events in China. Acta Geo. Sin. 1995, 50, 402–410. (In Chinese) [Google Scholar]
- Jeong, S.J.; Medvigy, D. Macroscale prediction of autumn leaf coloration throughout the continental United States. Glob. Ecol. Biogeogr. 2014, 23, 1245–1254. [Google Scholar] [CrossRef]
- Yu, H.Y.; Zhou, G.S.; Lv, X.M.; He, Q.J.; Zhou, M.Z. Environmental factors rather than productivity drive autumn leaf senescence: Evidence from a grassland in situ simulation experiment. Agr. For. Meteorol. 2022, 37, 109221. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).