Root System Response and Yield of Irrigated Rice in Relation to Irrigation, Potassium and Nitrogen under Subtropical Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Plant Materials
2.2. Experimental Design and Crop Management
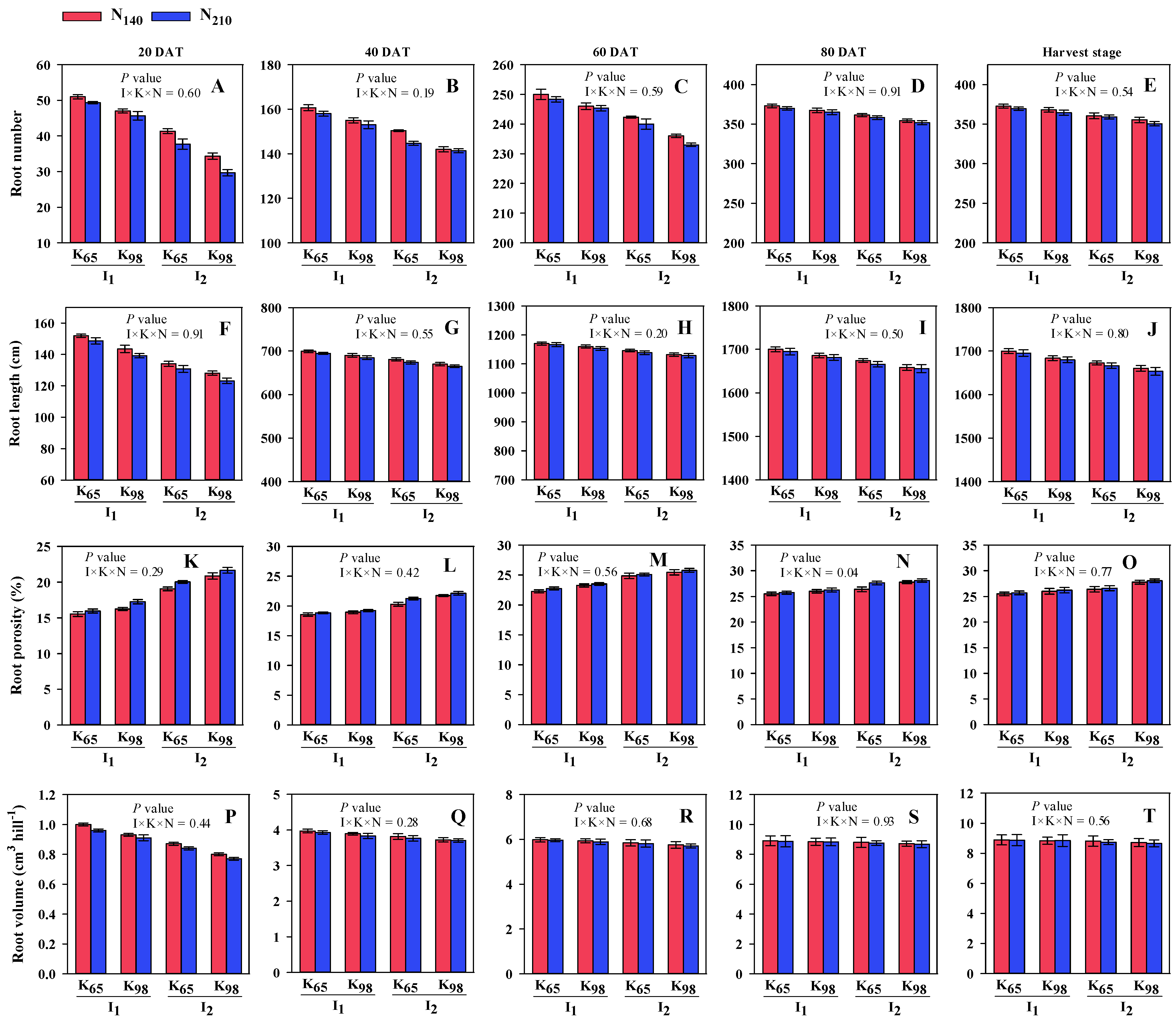
2.3. Determination of Root Morphological and Physiological Traits
2.3.1. Number of Root (RN)
2.3.2. Root Length (RL, cm)
2.3.3. Root Volume (RV, cm3 hill−1)
2.3.4. Root Porosity (RP, %)
2.3.5. Leaf Area Index (LAI)
2.3.6. Total Dry Matter (TDM)
2.3.7. Yield Attributes and Yield
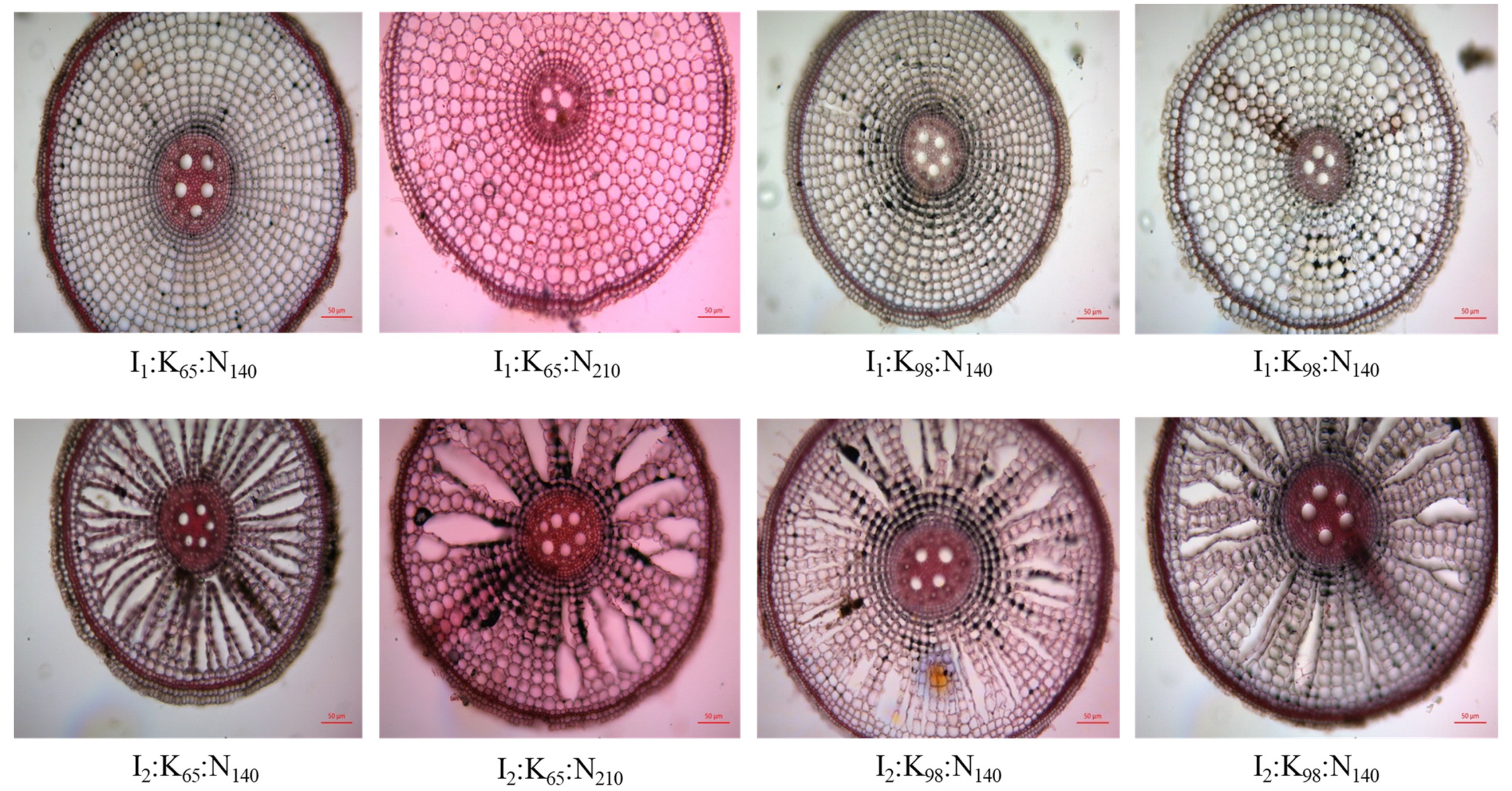
2.4. Root Cross-Section
2.5. Statistical Analysis of Data
3. Results
3.1. Morphological Traits of Root, Total Dry Matter and Leaf Area Index
3.2. Growth Parameters
3.3. Yield Contributing Parameters and Yield
3.4. Relationship among Root Traits, Growth Indices, Yield and Yield Attributes
3.5. Root Cross-Sectional View
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, C.; Chen, M.; Li, X.; Dai, Q.; Xu, K.; Guo, B.; Hu, Y.; Wang, W.; Huo, Z. Effects of soil types and irrigation modes on rice root morphophysiological traits and grain quality. Agronomy 2021, 11, 120. [Google Scholar] [CrossRef]
- Kreszies, T.; Schreiber, L.; Ranathunge, K. Suberized transport barriers in Arabidopsis, barley and rice roots: From the model plant to crop species. J. Plant Physiol. 2018, 227, 5–83. [Google Scholar] [CrossRef]
- Yang, C.M.; Yang, L.Z.; Yang, Y.X.; Ouyang, Z. Rice root growth and nutrient uptake as influenced by organic manure in continuously and alternately flooded paddy soils. Agric. Water Manag. 2004, 70, 67–81. [Google Scholar] [CrossRef]
- Waines, J.G.; Ehdaie, B. Domestication and crop physiology: Roots of green-revolution wheat. Ann. Bot. 2007, 100, 991–998. [Google Scholar] [CrossRef]
- Gewin, V. Food: An underground revolution. Nature 2010, 466, 552–553. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, L.; Fabrice, M.R.; Tian, Y.; Qi, K.; Chen, Q.; Cao, P.; Wang, P.; Zhang, S.; Wu, J.; et al. Physiological and nutritional responses of pear seedlings to nitrate concentrations. Front. Plant Sci. 2018, 9, 1679. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, A.C.M.; Mcclymont, S.A.; Holmes, B.M.; Lyons, E.; Raizada, M.N. Novel temporal, fine-scale and growth variation phenotypes in roots of adult-stage maize (Zea mays L.) in response to low nitrogen stress. Plant Cell Environ. 2011, 34, 2122–2137. [Google Scholar] [CrossRef]
- Fan, J.B.; Zhang, Y.L.; Turner, D.; Duan, Y.H.; Wang, D.S.; Shen, Q.R. Root physiological and morphological characteristics of two rice cultivars with different nitrogen-use efficiency. Pedosphere 2010, 20, 446–455. [Google Scholar] [CrossRef]
- Yang, G.Z.; Chu, K.Y.; Tang, H.Y.; Nie, Y.C.; Zhang, X.L. Fertilizer 15N accumulation, recovery and distribution in cotton plant as affected by N rate and split. J. Integr. Agric. 2013, 12, 999–1007. [Google Scholar] [CrossRef]
- White, P.J.; Karley, A.J. Potassium Cell Biology of Metals and Nutrients; Springer: Berlin, Germany, 2010; pp. 199–224. [Google Scholar]
- Oosterhuis, D.; Loka, D.; Kawakami, E.; Pettigrew, W. The physiology of potassium in crop production. Adv. Agron. 2014, 126, 203–234. [Google Scholar] [CrossRef]
- Sano, T.; Becker, D.; Ivashikina, N.; Wegner, L.H.; Zimmermann, U.; Roelfsema, M.R.; Nagata, T.; Hedrich, R. Plant cells must pass a K+ threshold to re-enter the cell cycle. Plant J. 2007, 50, 401–413. [Google Scholar] [CrossRef]
- Peters, J.; Chin, C.K. Potassium loss is involved in tobacco cell death induced by palmitoleic acid and ceramide. Arch. Biochem. Biophys. 2007, 465, 180–186. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, L.; Qu, H.; Lian, J.; Liu, W.; Hu, Y.; Xu, G. Alteration of nutrient allocation and transporter genes expression in rice under N, P, K, and Mg deficiencies. Acta Physiol. Plant 2012, 34, 939–946. [Google Scholar] [CrossRef]
- Moriconi, J.I.; Buet, A.; Simontacchi, M.; Santa-Marıa, G.E. Nearisogenic wheat lines carrying altered function alleles of the Rht-1 genes exhibit differential responses to potassium deprivation. Plant Sci. 2012, 185, 199–207. [Google Scholar] [CrossRef]
- Huang, Y.D.; Zhang, Z.L.; Wei, F.Z.; Li, J.C. Ecophysiological effect of dry cultivated and plastic film-mulched rice planting. Chin. J. Appl. Ecol. 1999, 10, 305–308. [Google Scholar]
- Geng, D.; Chen, P.; Shen, X.; Zhang, Y.; Li, X.; Jiang, L.; Xie, Y.; Niu, C.; Zhang, J.; Huang, X.; et al. Mdmyb88 and Mdmyb124 enhance drought tolerance by modulating root vessels and cell walls in apple. Plant Physiol. 2018, 178, 1296–1309. [Google Scholar] [CrossRef]
- Bengough, A.G.; McKenzie, B.M.; Hallet, P.D.; Valentine, T.A. Root elongation, water stress, and mechanical impedance: A review of limiting stresses and beneficial root tip traits. J. Exp. Bot. 2011, 62, 59–68. [Google Scholar] [CrossRef]
- Ali, M.H.; Khatun, M.M.; Mateo, L.G. Influences of various level of water depth on rice growth in rice-fish culture under wetland rice ecosystems. J. Geo. Environ. 2000, 4, 23–30. [Google Scholar]
- Li, S.; Wang, Z.; Malhi, S.S.; Li, S.; Gao, Y.; Tian, X. Nutrient and Water Management Effects on Crop Production, and Nutrient and Water Use Efficiency in Dryland Areas of China. Adv. Agron. 2009, 102, 223–265. [Google Scholar] [CrossRef]
- Lower, S.S.; Orians, C.M. Soil nutrients and water availability interact to influence willow growth and chemistry but not leaf beetle performance. Entomol. Exp. Appl. 2003, 107, 69–79. [Google Scholar] [CrossRef]
- Wang, X.B.; Zhou, B.Y.; Sun, X.F.; Yue, Y.; Ma, W.; Zhao, M. Soil tillage management affects maize grain yield by regulating spatial distribution coordination of roots, soil moisture and nitrogen status. PLoS ONE 2015, 10, e0129231. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, C.S.; Dell, B.; Zhao, Z.G.; Guo, J.J.; Xu, D.P.; Zeng, J. Growth and nutrient dynamics of Betulaalnoides seedlings under exponential fertilization. J. For. Res. 2018, 29, 111–119. [Google Scholar] [CrossRef]
- Gregory, P.J.; Simmonds, L.P.; Warren, G.P. Interactions between plant nutrients, water and carbon dioxide as factors limiting crop yields. Philos. Trans. R. Soc. B Biol. Sci. 1997, 352, 987–996. [Google Scholar] [CrossRef]
- UNDP; FAO (United Nations Development Program and Food and Agriculture Organization). Land Resources Appraisal of Bangladesh for Agricultural Development. Report 2; Agro-Ecological Region of Bangladesh; United Nations Development Program and Food and Agricultural Organization: Dhaka, Bangladesh, 1988; pp. 212–221. [Google Scholar]
- FRG. Fertilizer Recommendation Guide; Bangladesh Agricultural Research Council (BARC): Dhaka, Bangladesh, 2018; p. 274. [Google Scholar]
- Bridgit, A.J.; Potty, N.N. Influence of root characters on rice productivity in iron soils of Kerala. Int. Rice Res. News 2002, 27, 45–46. [Google Scholar]
- Jensen, C.R.; Luxmoore, R.J.; Van Gundy, S.D.; Stolzy, L.H. Root air space measurements by a pycnometer method. Agron. J. 1969, 61, 474–475. [Google Scholar] [CrossRef]
- Radford, P.J. Growth analysis formulae-their use and abuse. Crop Sci. 1967, 7, 171–175. [Google Scholar] [CrossRef]
- Hunt, R. Plant Growth Analysis. Studies in Biology; Edward Arnold: London, UK, 1978. [Google Scholar]
- Yoshida, S.; Foron, D.A.; Cock, J.H. Laboratory Manual for Physiological Studies of Rice; International Rice Research Institute: Los Baños, Philippines, 1971; p. 70. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation Statistical Computation: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 20 March 2023).
- Fageria, N.K.; Baligar, V.C.; Clark, R.B. Physiology of Crop Production; Haworth Press Inc.: Philadelphia, PA, USA, 2006; pp. 1–356. [Google Scholar]
- Elmi, A.A.; Madramootoo, C.; Egeh, M.; Hamel, C. Water and fertilizer nitrogen management to minimize nitrate pollution from a cropped soil in southwestern Quebec, Canada. Water Air Soil Pollut. 2004, 151, 117–134. [Google Scholar] [CrossRef]
- Guttieri, M.J.; McLean, R.; Stark, J.C.; Souza, E. Managing irrigation and nitrogen fertility of hard spring wheats for optimum and noodle quality. Crop Sci. 2005, 45, 2049–2059. [Google Scholar] [CrossRef]
- Islam, S.M.M.; Gaihre, Y.K.; Biswas, J.C.; Jahan, M.S.; Singh, U.; Adhikary, S.K.; Satter, M.A.; Saleque, M.A. Different nitrogen rates and methods of application for dry season rice cultivation with alternate wetting and drying irrigation: Fate of nitrogen and grain yield. Agric. Water Manag. 2018, 196, 144–153. [Google Scholar] [CrossRef]
- Fageria, N.K.; Moreira, A.; Moraes, L.A.C.; Moraes, M.F. Root Growth, Nutrient Uptake, and Nutrient-Use Efficiency by Roots of Tropical Legume Cover Crops as Influenced by Phosphorus Fertilization. Commun. Soil Sci. Plant Anal. 2014, 45, 555–569. [Google Scholar] [CrossRef]
- Blevins, D.G.; Barnett, N.M.; Frost, W.B. Role of Potassium and Malate in Nitrate Uptake and Translocation by Wheat Seedlings. Plant Physiol. 1978, 62, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Cramer, M.D. Root Nitrogen Acquisition and Assimilation. In Root Physiology: From Gene to Function; Plant Ecophysiology; Lambers, H., Colmer, T.D., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 1–36. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Kronzucker, H.J. The nitrogen–potassium intersection: Membranes, metabolism, and mechanism. Plant Cell Environ. 2016, 10, 2029–2041. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Romero, L. Relationship between potassium fertilisation and nitrate assimilation in leaves and fruits of cucumber (Cucumissativus) plants. Ann. Appl. Biol. 2002, 140, 241–245. [Google Scholar] [CrossRef]
- Alou, I.N.; Steyn, J.M.; Annandale, J.G.; van der Laan, M. Growth, phenological, and yield response of upland rice (Oryza sativa L. cv. Nerica 4®) to water stress during different growth stages. Agric. Water Manag. 2018, 198, 39–52. [Google Scholar] [CrossRef]
- Song, T.; Xu, F.; Yuan, W.; Zhang, Y.; Liu, T.; Chen, M.; Hu, Q.; Tian, Y.; Xu, W.; Zhanh, J. Comparison on physiological adaptation and phosphorus use efficiency of upland rice and lowland rice under alternate wetting and drying irrigation. Plant Growth Regul. 2018, 86, 195–210. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Costa, C.; Dwyer, L.M.; Zhou, X.; Dutilleul, P.; Hamel, C.; Reid, L.M.; Smith, D.L. Root morphology of contrasting maize genotypes. Agron. J. 2002, 94, 96–101. [Google Scholar] [CrossRef]
- Mahajan, G.; Chauhan, B.S.; Timsina, J.; Singh, P.P.; Singh, K. Crop Performance and Water and Nitrogen-Use Efficiencies in Dry-Seeded Rice in Response to Irrigation and Fertilizer Amounts in Northwest India. Field Crops Res. 2012, 134, 59–70. [Google Scholar] [CrossRef]
- Reis, A. Aerobic rice system improves water productivity, nitrogen recovery and crop performance in brazilian weathered lowland soil. Field Crops Res. 2018, 218, 59–68. [Google Scholar] [CrossRef]
- McMurtrie, R.E.; Iversen, C.M.; Dewar, R.C.; Medlyn, B.E.; Näsholm, T.; Pepper, D.A.; Norby, R.J. Plant Root Distributions and Nitrogen Uptake Predicted by a Hypothesis of Optimal Root Foraging. Ecol. Evol. 2012, 2, 1235–1250. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.; Deng, Y.; Chen, X.; Xu, X.; Chen, R.; Lv, Y.; Zhao, Y.; Zhao, X.; He, X.; Li, B.; et al. Characterization of root response to phosphorus supply from morphology to gene analysis in field-grown wheat. J. Exp. Bot. 2013, 64, 1403–1411. [Google Scholar] [CrossRef]
- Fageria, N.K.; Oliveira, J.P. Nitrogen, Phosphorus and Potassium Interactions in Upland Rice. J. Plant Nutr. 2014, 37, 1586–1600. [Google Scholar] [CrossRef]
- Deng, Y.; Teng, W.; Tong, Y.; Chen, X.; Zou, C. Phosphorus Efficiency Mechanisms of Two Wheat Cultivars as Affected by a Range of Phosphorus Levels in the Field. Front. Plant Sci. 2018, 9, 1614. [Google Scholar] [CrossRef]
- Li, X.; Lu, J.; Wu, L.; Chen, F. The difference of potassium dynamics between yellowish red soil and yellow cinnamon soil under rapeseed (Brassica napus L.)–rice (Oryza sativa L.) rotation. Plant Soil 2009, 320, 141–151. [Google Scholar] [CrossRef]
- Duan, Y.; Shi, X.; Li, S.; Sun, X.; He, X. Nitrogen Use Efficiency as Affected by Phosphorus and Potassium in Long-Term Rice and Wheat Experiments. J. Integr. Agric. 2014, 13, 588–596. [Google Scholar] [CrossRef]
- Duncan, E.G.; O’Sullivan, C.A.; Roper, M.M.; Biggs, J.S.; Peoples, M.B. Influence of Co-Application of Nitrogen with Phosphorus, Potassium and Sulphur on the Apparent Efficiency of Nitrogen Fertiliser Use, Grain Yield and Protein Content of Wheat: Review. Field Crops Res. 2018, 226, 56–65. [Google Scholar] [CrossRef]
- Weng, J.H.; Takeda, T.; Agata, W.; Hakoyama, S. Studies on dry matter and grainproduction of rice plants: I. Influence of the reserved carbohydrate until heading stage and the assimilation products during the ripening period on grain production. Jpn. J. Crop Sci. 1982, 51, 500–509. [Google Scholar] [CrossRef]
- Bindra, A.D.; Kalia, B.D.; Kumar, S. Effect of nitrogen levels and dates of transplanting on growth, yield and yield attributes of scented rice. Adv. Agric. Res. 2000, 10, 45–48. [Google Scholar]
- Laroo, N.; Shivay, Y.S. Effect of nitrogen and sulphur levels on growth and productivity of scented rice. Curr. Adv. Agric. Sci. 2011, 3, 45–48. [Google Scholar]
- Lynch, J.; Marschner, P.; Rengel, Z. Effect of internal and external factors on root growth and development. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; pp. 331–346. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Colmer, T.D.; Pedersen, O.; Nakazono, M. Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. J. Plant Physiol. 2018, 176, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Root phenotypes for improved nutrient capture: An underexploited opportunity for global agriculture. New Phytol. 2019, 223, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, T.; Wang, Z.; Zhang, H.; Yang, J.; Zhang, J. Combination of site-specific nitrogen management and alternate wetting and drying irrigation increases grain yield and nitrogen and water use efficiency in super rice. Field Crops Res. 2013, 154, 226–235. [Google Scholar] [CrossRef]
- Yang, J.C. Approaches to achieve high grain yield and high resource use efficiency in rice. Front. Agric. Sci. Eng. 2015, 2, 115–123. [Google Scholar] [CrossRef]
- Mandal, K.G.; Kundu, D.K.; Thakur, A.K.; Kannan, K.; Brahmanand, P.S.; Kumar, A. Aerobic Rice Response to Irrigation Regimes and Fertilizer Nitrogen Rates. J. Food Agric. Environ. 2013, 11, 1148–1153. [Google Scholar]
- Rajeev, K.; Sandeep, K.; Anil, K.; Harnam, S.; Sanjeev, K.K. Effect of top dressing nitrogen and potassium on yield and yield component of rice (Oryza sativa L). Agriways 2013, 1, 90–94. [Google Scholar]
- Metho, L.A.; Hammes, P.S.; de Beer, J.M.; Groeneveld, H.T. Interaction between Cultivar and Soil Fertility on Grain Yield, Yield Components and Grain Nitrogen Content of Wheat. South Afr. J. Plant Soil 1997, 14, 158–164. [Google Scholar] [CrossRef]
- Epie, K.E.; Maral, E. Shoot and Root Biomass, Phosphorus and Nitrogen Uptake of Spring Wheat Grown in Low Phosphorus and Moisture Content Conditions in a Pot Experiment. J. Plant Nutr. 2018, 41, 2273–2280. [Google Scholar] [CrossRef]


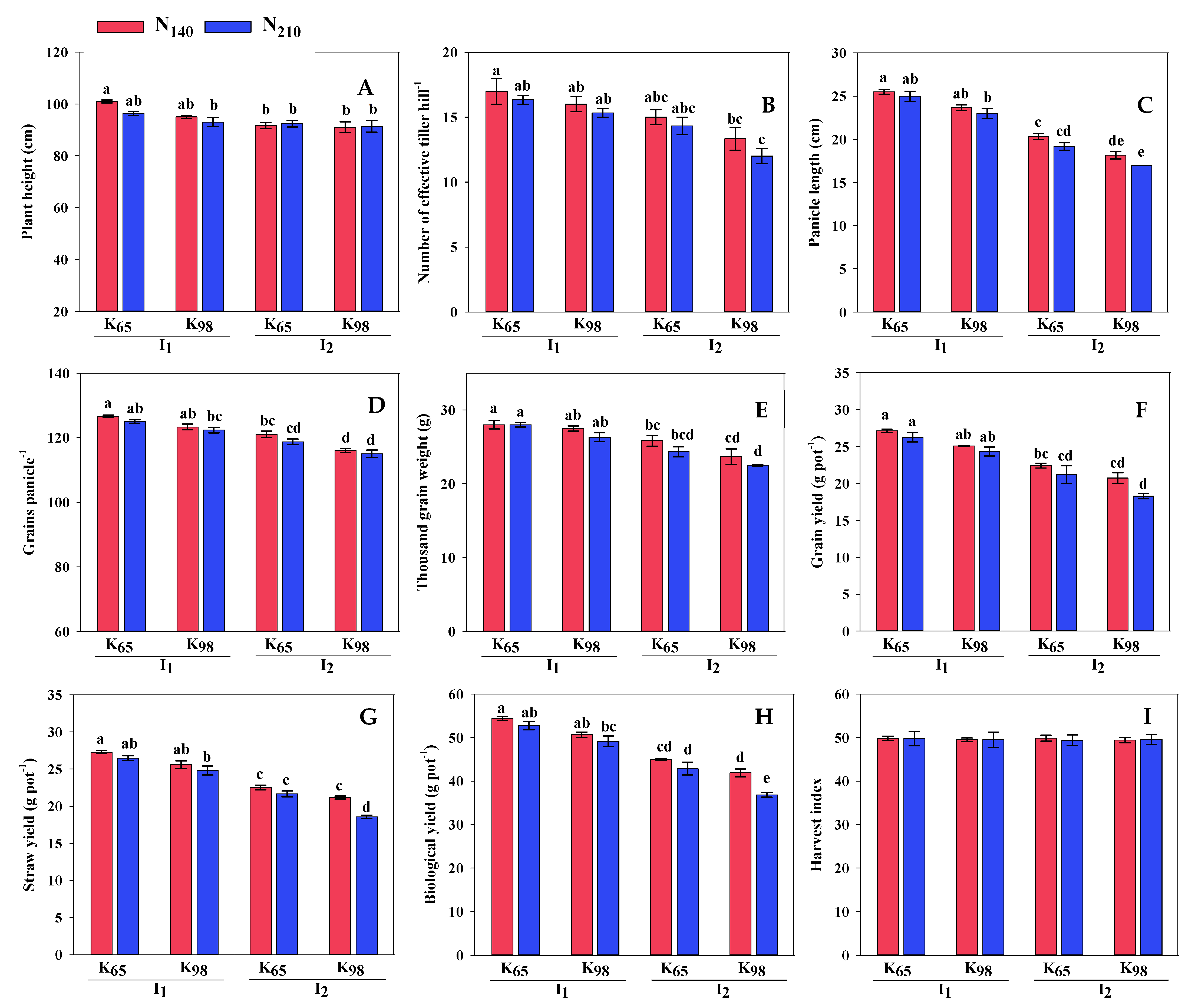

| Irrigation (I) | PH (cm) | ET (no.) | PL (cm) | GP (no.) | TGW (g) | GY (g pot−1) | SY (g pot−1) | HI (%) |
|---|---|---|---|---|---|---|---|---|
| I1 | 96.33 a | 16.17 a | 24.29 a | 124.33 a | 26.90 a | 25.70 a | 26.05 a | 49.66 |
| I2 | 91.58 b | 13.67 b | 18.67 b | 117.67 b | 22.81 b | 20.65 b | 20.98 b | 49.57 |
| CV (%) | 3.24 | 9.07 | 6.15 | 2.01 | 6.02 | 7.07 | 5.94 | 1.75 |
| Potassium (K) | ||||||||
| K65 | 95.33 a | 15.67 a | 22.50 a | 122.83 a | 25.87 a | 24.25 a | 24.49 a | 49.73 |
| K98 | 92.58 b | 14.17 b | 20.46 b | 119.17 b | 23.85 b | 22.10 b | 22.54 b | 49.51 |
| CV (%) | 3.89 | 11.46 | 14.15 | 3.14 | 9.59 | 12.42 | 11.95 | 1.74 |
| Nitrogen (N) | ||||||||
| N140 | 94.67 a | 15.33 a | 21.92 a | 121.75 a | 121.75 a | 23.83 a | 24.14 a | 49.67 |
| N210 | 93.25 b | 14.50 b | 21.04 b | 120.25 b | 120.25 b | 22.52 b | 22.89 b | 49.56 |
| CV (%) | 4.10 | 12.27 | 14.84 | 3.46 | 10.33 | 13.05 | 12.41 | 1.75 |
| ANOVA | ||||||||
| I | ** | ** | ** | ** | ** | ** | ** | NS |
| K | * | ** | ** | ** | ** | ** | ** | NS |
| N | * | * | ** | * | * | ** | ** | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaysar, M.S.; Sarker, U.K.; Kheya, S.A.; Hasan, A.K.; Hossain, M.A.; Somaddar, U.; Saha, G.; Chaki, A.K.; Hashem, A.; Abd_Allah, E.F.; et al. Root System Response and Yield of Irrigated Rice in Relation to Irrigation, Potassium and Nitrogen under Subtropical Conditions. Agronomy 2023, 13, 1626. https://doi.org/10.3390/agronomy13061626
Kaysar MS, Sarker UK, Kheya SA, Hasan AK, Hossain MA, Somaddar U, Saha G, Chaki AK, Hashem A, Abd_Allah EF, et al. Root System Response and Yield of Irrigated Rice in Relation to Irrigation, Potassium and Nitrogen under Subtropical Conditions. Agronomy. 2023; 13(6):1626. https://doi.org/10.3390/agronomy13061626
Chicago/Turabian StyleKaysar, Md. Salahuddin, Uttam Kumer Sarker, Sinthia Afsana Kheya, Ahmed Khairul Hasan, Md. Alamgir Hossain, Uzzal Somaddar, Gopal Saha, Apurbo Kumar Chaki, Abeer Hashem, Elsayed Fathi Abd_Allah, and et al. 2023. "Root System Response and Yield of Irrigated Rice in Relation to Irrigation, Potassium and Nitrogen under Subtropical Conditions" Agronomy 13, no. 6: 1626. https://doi.org/10.3390/agronomy13061626
APA StyleKaysar, M. S., Sarker, U. K., Kheya, S. A., Hasan, A. K., Hossain, M. A., Somaddar, U., Saha, G., Chaki, A. K., Hashem, A., Abd_Allah, E. F., & Uddin, M. R. (2023). Root System Response and Yield of Irrigated Rice in Relation to Irrigation, Potassium and Nitrogen under Subtropical Conditions. Agronomy, 13(6), 1626. https://doi.org/10.3390/agronomy13061626













