Can Nematode Communities Work as an Indicator of Soil Health in a Multiyear Miscanthus × Giganteus Plantation Growing in Lead-Contaminated Soil?
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Site Location and Soil Characteristics
2.2. Plants
2.3. Nematode Sampling, Isolation, and Identification
2.4. Nematode Community Analysis
2.5. Statistical Evaluation
3. Results
3.1. Response of M × g Biomass in a Gradient of Soil Contamination
3.2. Variation in Concentration of Chemical Elements in Soil and Plant Organs
3.3. Nematode Abundance and Community Indexes
3.4. Responses of Nematode Species to Gradient of Environmental Factors
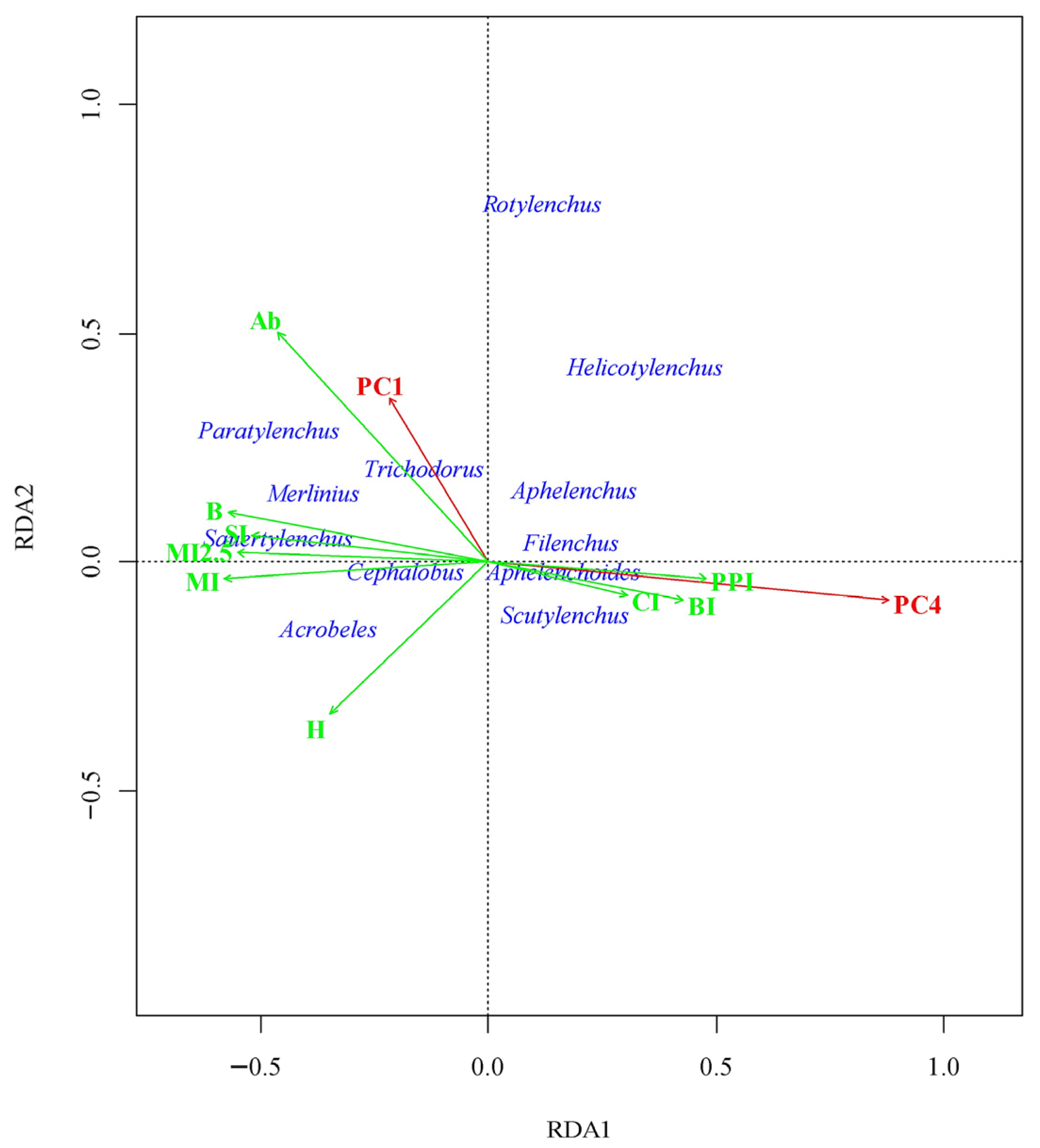
3.5. Nematode Community Ordination
3.6. Nematode Community Maturity and Food Chain Dynamics Induced by Soil Contamination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rather, R.A.; Wani, A.W.; Mumtaz, S.; Padder, S.A.; Khan, A.H.; Almohana, A.I.; Almojil, S.F.; Alam, S.S.; Baba, T.R. Bioenergy: A Foundation to Environmental Sustainability in a Changing Global Climate Scenario. J. King Saud. Univ. Sci. 2022, 34, 101734. [Google Scholar] [CrossRef]
- Erickson, L.E.; Pidlisnyuk, V. Phytotechnology with Biomass Production; Erickson, L.E., Pidlisnyuk, V., Eds.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 9781003082613. [Google Scholar]
- Mamirova, A.; Pidlisnyuk, V.; Amirbekov, A.; Ševců, A.; Nurzhanova, A. Phytoremediation Potential of Miscanthus sinensis And. in Organochlorine Pesticides Contaminated Soil Amended by Tween 20 and Activated Carbon. Environ. Sci. Pollut. Res. 2021, 28, 16092–16106. [Google Scholar] [CrossRef]
- DalCorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy Metal Pollutions: State of the Art and Innovation in Phytoremediation. Int. J. Mol. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef] [Green Version]
- Hedde, M.; van Oort, F.; Boudon, E.; Abonnel, F.; Lamy, I. Responses of Soil Macroinvertebrate Communities to Miscanthus Cropping in Different Trace Metal Contaminated Soils. Biomass Bioenergy 2013, 55, 122–129. [Google Scholar] [CrossRef]
- Skwiercz, A.; Stefanovska, T.; Pidlisnyuk, V.; Zouhar, M.; Kornobis, F.; Obruch, M.; Sozanskyi, M. Nematode Community Composition Associated with Miscanthus × Giganteus Growing at the Polluted Site. Commun. Agric. Appl. Biol. Sci. Ghent Univ. 2017, 82, 281–287. [Google Scholar]
- Alasmary, Z.; Todd, T.; Hettiarachchi, G.M.; Stefanovska, T.; Pidlisnyuk, V.; Roozeboom, K.; Erickson, L.; Davis, L.; Zhukov, O. Effect of Soil Treatments and Amendments on the Nematode Community under Miscanthus Growing in a Lead Contaminated Military Site. Agronomy 2020, 10, 1727. [Google Scholar] [CrossRef]
- Gebremikael, M.T.; Steel, H.; Buchan, D.; Bert, W.; De Neve, S. Nematodes Enhance Plant Growth and Nutrient Uptake under C and N-Rich Conditions. Sci. Rep. 2016, 6, 32862. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Q.; Huang, R.; Wu, K.; Li, Z. Contrasting Impacts of Mobilisation and Immobilisation Amendments on Soil Health and Heavy Metal Transfer to Food Chain. Ecotoxicol. Environ. Saf. 2021, 209, 111836. [Google Scholar] [CrossRef]
- Du Preez, G.; Daneel, M.; De Goede, R.; Du Toit, M.J.; Ferris, H.; Fourie, H.; Geisen, S.; Kakouli-Duarte, T.; Korthals, G.; Sánchez-Moreno, S.; et al. Nematode-Based Indices in Soil Ecology: Application, Utility, and Future Directions. Soil Biol. Biochem. 2022, 169, 108640. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Mamirova, A.; Newton, R.A.; Stefanovska, T.; Zhukov, O.; Tsygankova, V.; Shapoval, P. The Role of Plant Growth Regulators in Miscanthus × Giganteus Growth on Trace Elements-Contaminated Soils. Agronomy 2022, 12, 2999. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Shapoval, P.; Zgorelec, Ž.; Stefanovska, T.; Zhukov, O. Multiyear Phytoremediation and Dynamic of Foliar Metal(Loid)s Concentration during Application of Miscanthus × Giganteus Greef et Deu to Polluted Soil from Bakar, Croatia. Environ. Sci. Pollut. Res. 2020, 27, 31446–31457. [Google Scholar] [CrossRef] [PubMed]
- Krzyżak, J.; Rusinowski, S.; Sitko, K.; Szada-Borzyszkowska, A.; Stec, R.; Jensen, E.; Clifton-Brown, J.; Kiesel, A.; Lewin, E.; Janota, P.; et al. The Effect of Different Agrotechnical Treatments on the Establishment of Miscanthus Hybrids in Soil Contaminated with Trace Metals. Plants 2022, 12, 98. [Google Scholar] [CrossRef]
- Seinhorst, J.W. Killing Nematodes for Taxonomic Study with Hot f.a. 4: 1. Nematologica 1966, 12, 178–178a. [Google Scholar] [CrossRef]
- Brzeski, M.W. Nematodes of Tylenchina in Poland and Temperate Europe; Muzeum i Instytutu Zoologii, Polska Akademia Nauk (MiIZ PAN): Warsaw, Poland, 1998. [Google Scholar]
- Andrássy, I. Free-Living Nematodes of Hungary (Nematoda Errantia). In Pedozoologica Hungarica No. 4.; Csuzdi, C., Mahunka, S., Eds.; Hungarian Natural History Museum: Budapest, Hungary, 2007; p. 496. ISBN 9637093982. [Google Scholar]
- Bongers, T.; Bongers, M. Functional Diversity of Nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Hua, E.; Zhu, Y.; Huang, D.; Liu, X. Are Free-Living Nematodes Effective Environmental Quality Indicators? Insights from Bohai Bay, China. Ecol. Indic. 2021, 127, 107756. [Google Scholar] [CrossRef]
- Bongers, T. The Maturity Index: An Ecological Measure of Environmental Disturbance Based on Nematode Species Composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ettema, C.H.; Bongers, T. Characterization of Nematode Colonization and Succession in Disturbed Soil Using the Maturity Index. Biol. Fertil. Soils 1993, 16, 79–85. [Google Scholar] [CrossRef]
- Bongers, T.; Korthals, G. The Behaviour of Maturity Index and Plant Parasite Index under Enriched Conditions. In Proceedings of the 22nd International Symposium of the European Society of Nematologists, Ghent, Belgium, 7–12 August 1994; p. 39. [Google Scholar]
- Sieriebriennikov, B.; Ferris, H.; de Goede, R.G.M. NINJA: An Automated Calculation System for Nematode-Based Biological Monitoring. Eur. J. Soil Biol. 2014, 61, 90–93. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.B. The Ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef] [Green Version]
- StatSoft Inc. STATISTICA Data Analysis Software System, Version 12.0, 1984–2014; StatSoft Inc.: Tulsa, OK, USA, 2014.
- Jansen, F. Hierarchical Species Response Curves in Package EHOF. 2013. Volume 1, pp. 1–9. Available online: ftp://mirror.hmdc.harvard.edu/mirrors/cran.r-project.org/web/packages/eHOF/vignettes/eHOF.pdf (accessed on 23 April 2023).
- Jansen, F.; Oksanen, J. How to Model Species Responses along Ecological Gradients—Huisman-Olff-Fresco Models Revisited. J. Veg. Sci. 2013, 24, 1108–1117. [Google Scholar] [CrossRef]
- Michaelis, J.; Diekmann, M.R. Biased Niches—Species Response Curves and Niche Attributes from Huisman-Olff-Fresco Models Change with Differing Species Prevalence and Frequency. PLoS ONE 2017, 12, e0183152. [Google Scholar] [CrossRef] [Green Version]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Community Ecology Package; R Package Version 2.5-2. 2018. Available online: http://CRAN.Rproject.org/package=vegan (accessed on 6 May 2023).
- Loecher, M.; Ropkins, K. RgoogleMaps and Loa: Unleashing R Graphics Power on Map Tiles. J. Stat. Softw. 2015, 63, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy Metals in Agricultural Soils of the European Union with Implications for Food Safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Etim, E.U.; Onianwa, P.C. Lead Contamination of Soil in the Vicinity of a Military Shooting Range in Ibadan, Nigeria. Toxicol. Environ. Chem. 2012, 94, 895–905. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Q.; Su, W.; Xing, C.; Liu, C. Satellite Spectroscopy Reveals the Atmospheric Consequences of the 2022 Russia-Ukraine War. Sci. Total Environ. 2023, 869, 161759. [Google Scholar] [CrossRef] [PubMed]
- Schaffartzik, A.; Plank, C.; Brad, A. Ukraine and the Great Biofuel Potential? A Political Material Flow Analysis. Ecol. Econ. 2014, 104, 12–21. [Google Scholar] [CrossRef]
- Shen, S.; Chen, J.; Chang, J.; Xia, B. Using Bioenergy Crop Cassava (Manihot esculenta) for Reclamation of Heavily Metal-Contaminated Land. Int. J. Phytoremedia. 2020, 22, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Canqui, H. Energy Crops and Their Implications on Soil and Environment. Agron. J. 2010, 102, 403–419. [Google Scholar] [CrossRef]
- Ussiri, D.A.N.; Guzman, J.G.; Lal, R.; Somireddy, U. Bioenergy Crop Production on Reclaimed Mine Land in the North Appalachian Region, USA. Biomass Bioenergy 2019, 125, 188–195. [Google Scholar] [CrossRef]
- Silveira, M.L.; Comerford, N.B.; Reddy, K.R.; Prenger, J.; DeBusk, W.F. Influence of Military Land Uses on Soil Carbon Dynamics in Forest Ecosystems of Georgia, USA. Ecol. Indic. 2010, 10, 905–909. [Google Scholar] [CrossRef]
- Bastia, G.; Al Souki, K.S.; Pourrut, B. Evaluation of Miscanthus × Giganteus Tolerance to Trace Element Stress: Field Experiment with Soils Possessing Gradient Cd, Pb, and Zn Concentrations. Plants 2023, 12, 1560. [Google Scholar] [CrossRef] [PubMed]
- Luque-Espinar, J.A.; Pardo-Igúzquiza, E.; Grima-Olmedo, J.; Grima-Olmedo, C. Multiscale Analysis of the Spatial Variability of Heavy Metals and Organic Matter in Soils and Groundwater across Spain. J. Hydrol. 2018, 561, 348–371. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Kapusta, P.; Zubek, S.; Stanek, M.; Woch, M.W. Soil Organic Matter Prevails over Heavy Metal Pollution and Vegetation as a Factor Shaping Soil Microbial Communities at Historical Zn–Pb Mining Sites. Chemosphere 2020, 240, 124922. [Google Scholar] [CrossRef] [PubMed]
- Falko, N.; Zhukov, O. Transition from Hierarchy to Adhocratic Organizational Culture in a Ukrainian University: From Survival to Successful Development in the Conditions of War. Probl. Perspect. Manag. 2023, 21, 15–22. [Google Scholar] [CrossRef]
- Shelford, V.E. Some Concepts of Bioecology. Ecology 1931, 12, 455–467. [Google Scholar] [CrossRef]
- Renčo, M.; Čerevková, A.; Hlava, J. Life in a Contaminated Environment: How Soil Nematodes Can Indicate Long-Term Heavy-Metal Pollution. J. Nematol. 2022, 54, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P. Effect of an Artificial Metal Pollution on Nematode Assemblage of a Calcareous Loamy Chernozem Soil. Plant Soil 1999, 212, 35–43. [Google Scholar] [CrossRef]
- Okada, H.; Harada, H.; Kadota, I. Fungal-Feeding Habits of Six Nematode Isolates in the Genus Filenchus. Soil Biol. Biochem. 2005, 37, 1113–1120. [Google Scholar] [CrossRef]
- Rodríguez Martín, J.A.; Gutiérrez, C.; Escuer, M.; García-González, M.T.; Campos-Herrera, R.; Águila, N. Effect of Mine Tailing on the Spatial Variability of Soil Nematodes from Lead Pollution in La Union (Spain). Sci. Total Environ. 2014, 473–474, 518–529. [Google Scholar] [CrossRef] [Green Version]
- Chauvin, C.; Trambolho, M.; Hedde, M.; Makowski, D.; Cérémonie, H.; Jimenez, A.; Villenave, C. Soil Nematodes as Sndicators of Heavy Metal Pollution: A Meta-Analysis. Open J. Soil Sci. 2020, 10, 579–601. [Google Scholar] [CrossRef]
- El Baz, S.; Baz, M.; Barakate, M.; Hassani, L.; El Gharmali, A.; Imziln, B. Resistance to and Accumulation of Heavy Metals by Actinobacteria Isolated from Abandoned Mining Areas. Sci. World J. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Boshoff, M.; De Jonge, M.; Dardenne, F.; Blust, R.; Bervoets, L. The Impact of Metal Pollution on Soil Faunal and Microbial Activity in Two Grassland Ecosystems. Environ. Res. 2014, 134, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, C.; Fernández, C.; Escuer, M.; Campos-Herrera, R.; Beltrán Rodríguez, M.E.; Carbonell, G.; Rodríguez Martín, J.A. Effect of Soil Properties, Heavy Metals and Emerging Contaminants in the Soil Nematodes Diversity. Environ. Pollut. 2016, 213, 184–194. [Google Scholar] [CrossRef]
- Hol, W.H.G.; de Boer, W.; Termorshuizen, A.J.; Meyer, K.M.; Schneider, J.H.M.; van Dam, N.M.; van Veen, J.A.; van der Putten, W.H. Reduction of Rare Soil Microbes Modifies Plant-Herbivore Interactions. Ecol. Lett. 2010, 13, 292–301. [Google Scholar] [CrossRef]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of Heavy Metals through Terrestrial Food Webs: A Review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef] [Green Version]
- Ruess, L.; Ferris, H. Decomposition Pathways and Successional Changes. In Proceedings of the Fourth International Congress of Nematology, Tenerife, Spain, 8–13 June 2002; Cook, R., Hunt, D., Eds.; BRILL: Tenerife, Spain, 2004; pp. 547–556. [Google Scholar]
- Ferris, H.; Bongers, T.; De Goede, R.G.M. A Framework for Soil Food Web Diagnostics: Extension of the Nematode Faunal Analysis Concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Bhusal, D.R.; Sgardelis, S.P. Nematode Community Indices for Microhabitat Type and Large Scale Landscape Properties. Ecol. Indic. 2017, 73, 472–479. [Google Scholar] [CrossRef]
- Wardle, D.A.; Yeates, G.W.; Watson, R.N.; Nicholson, K.S. The Detritus Food-Web and the Diversity of Soil Fauna as Indicators of Disturbance Regimes in Agro-Ecosystems. Plant Soil 1995, 170, 35–43. [Google Scholar] [CrossRef]
- Park, B.Y.; Lee, J.K.; Ro, H.M.; Kim, Y.H. Short-Term Effects of Low-Level Heavy Metal Contamination on Soil Health Analyzed by Nematode Community Structure. Plant Pathol. J. 2016, 32, 329–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, J.G.; Torres, M.A.; dos Santos, G.; Moens, T. Influence of Heavy Metals on Nematode Community Structure in Deteriorated Soil by Gold Mining Activities in Sibutad, Southern Philippines. Ecol. Indic. 2018, 91, 712–721. [Google Scholar] [CrossRef] [Green Version]



| Parameters | Unit of Measuring | Value | Measuring Standard |
|---|---|---|---|
| Macroelements | |||
| Nitrogen N | mg kg−1 | 4.6 | DSTU 4729:200 |
| Hydrolyzed N | mg kg−1 | 95 | DSTU:7863-2015 |
| Available P | mg kg−1 | 496 | DSTU:4115:2002 |
| Available K | mg kg−1 | 550 | DSTU:4115:2002 |
| General indicators | |||
| Organic matter | % | 4.9 | DSTU 7632:2014 |
| pH (H2O) | pH unit | 7.9 | DSTU 8346:2015 |
| pH (KCl) | pH unit | 6.9 | DSTU:ISO:10390:2001 |
| Soil salinity | mg 100 g–1 | 80 | DSTU 7827:2015 |
| Hydrolytic acidity | meq100 g−1 | 0.16 | DSTU 7537:2014 |
| Microelements | |||
| Available S | mg/kg | 26 | DSTU:8347-2015 |
| Exchangeable Ca | meq100 g−1 | 0.3 | DSTU:7861:2015 Soil Quality |
| Exchangeable Mn | meq100 g−1 | 8.8 | DSTU:7861:2015 Soil Quality |
| Element | PC 1, λ = 6.6, 38.7% | PC 2, λ = 2.6, 15.2% | PC 3, λ = 1.8, 10.9% | PC 4, λ = 1.4, 8.5% |
|---|---|---|---|---|
| Mg | 0.24 | – | 0.62 | 0.26 |
| Al | 0.42 | 0.60 | 0.26 | –0.15 |
| Si | 0.35 | 0.42 | 0.60 | – |
| P | 0.61 | –0.67 | – | – |
| S | 0.67 | –0.47 | – | –0.17 |
| K | 0.63 | –0.26 | –0.30 | –0.27 |
| Ca | 0.51 | –0.25 | 0.55 | – |
| Ti | 0.73 | 0.36 | – | – |
| Mn | 0.76 | – | – | 0.15 |
| Fe | 0.78 | 0.27 | – | –0.18 |
| Cu | 0.79 | –0.24 | – | – |
| Zn | 0.80 | –0.24 | – | –0.25 |
| Rb | 0.82 | – | –0.24 | – |
| Sr | 0.76 | – | 0.25 | 0.25 |
| Y | 0.32 | 0.72 | –0.29 | – |
| Zr | 0.63 | 0.59 | –0.30 | – |
| Pb | – | –0.17 | –0.54 | 0.73 |
| Index | Variant of Contamination by Lead | PC1 | PC2 | PC3 | PC4 | |||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||
| Total abundance, ind. | 462.42 ± 95.50 | 477.42 ± 81.15 | 478.25 ± 76.07 | 360.58 ± 48.08 | 0.46 | – | – | –0.31 |
| Total biomass, mg | 1.55 ± 0.12 | 1.19 ± 0.18 | 1.10 ± 0.13 | 0.78 ± 0.22 | – | – | – | –0.90 |
| Shannon diversity, bit/genera | 4.11 ± 0.10 | 3.92 ± 0.24 | 4.01 ± 0.13 | 4.03 ± 0.20 | –0.32 | – | – | – |
| MI | 2.20 ± 0.07 | 2.01 ± 0.04 | 2.01 ± 0.04 | 2.01 ± 0.03 | – | – | 0.32 | –0.72 |
| MI 2–5 | 2.63 ± 0.11 | 2.41 ± 0.08 | 2.38 ± 0.05 | 2.26 ± 0.09 | – | – | 0.32 | –0.87 |
| PPI | 2.83 ± 0.10 | 2.90 ± 0.08 | 2.93 ± 0.05 | 2.91 ± 0.05 | – | – | – | 0.41 |
| CI | 21.78 ± 5.03 | 27.52 ± 4.59 | 29.48 ± 2.00 | 42.96 ± 11.90 | – | – | – | 0.84 |
| BI | 19.12 ± 2.16 | 21.83 ± 2.35 | 23.10 ± 1.54 | 28.70 ± 4.92 | – | – | – | 0.86 |
| EI | 71.43 ± 2.08 | 72.14 ± 2.53 | 70.84 ± 1.65 | 65.79 ± 3.90 | – | – | – | –0.68 |
| SI | 63.21 ± 5.68 | 49.87 ± 5.41 | 47.24 ± 4.75 | 36.60 ± 10.36 | – | – | – | –0.92 |
| Genus | PC 1 | PC 2 | PC 3 | PC 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Model * | IQV ** | Model | IQV | Model | IQV | Model | IQV | |
| Acrobeles | VI | 0.73 | VI | 0.75 | I | 0.68 | VII | 0.41 |
| Allodorylaimus | I | 0.79 | I | 0.54 | I | 0.38 | II | 0.50 |
| Aphelenchoides | VII | 0.80 | III | 0.92 | VII | 0.83 | II | 0.69 |
| Aphelenchus | VII | 0.85 | I | 0.89 | I | 0.93 | II | 0.20 |
| Aporcelaimus | VII | 0.77 | VI | 0.67 | I | 0.37 | II | 0.00 |
| Bitylenchus | IV | 0.94 | VI | 0.74 | I | 0.84 | IV | 0.69 |
| Cephalobus | VII | 0.58 | VII | 0.65 | I | 0.65 | VII | 0.57 |
| Clarkus | I | 0.00 | I | 0.00 | I | 0.00 | I | 0.00 |
| Cuticularia | I | 0.60 | I | 0.56 | I | 0.09 | II | 0.00 |
| Diplogaster | VII | 0.72 | I | 0.44 | I | 0.50 | III | 0.72 |
| Ditylenchus | III | 0.85 | I | 0.47 | I | 0.52 | II | 0.22 |
| Eudorylaimus | VII | 0.62 | VII | 0.62 | VI | 0.79 | II | 0.13 |
| Filenchus | IV | 0.93 | I | 0.92 | I | 0.83 | II | 0.37 |
| Helicotylenchus | VII | 0.53 | VII | 0.62 | VII | 0.55 | VII | 0.69 |
| Merlinius | VII | 0.55 | VII | 0.38 | VII | 0.70 | VII | 0.84 |
| Mesodorylaimus | I | 0.67 | I | 0.76 | I | 0.19 | II | 0.00 |
| Mylonchulus | I | 0.69 | I | 0.73 | IV | 0.51 | IV | 0.73 |
| Paratylenchus | VII | 0.33 | VII | 0.34 | VII | 0.49 | VII | 0.75 |
| Pratylenchus | VII | 0.92 | VII | 0.57 | VII | 0.88 | VI | 0.69 |
| Rhabditis | II | 0.30 | I | 0.49 | I | 0.58 | IV | 0.33 |
| Rotylenchus | VII | 0.38 | VII | 0.65 | VII | 0.59 | VII | 0.17 |
| Sauertylenchus | VII | 0.56 | VII | 0.10 | VII | 0.67 | II | 0.80 |
| Scutylenchus | VII | 0.82 | VII | 0.78 | VI | 0.64 | II | 0.72 |
| Trichodorus | VII | 0.32 | VII | 0.38 | VII | 0.22 | V | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanovska, T.; Skwiercz, A.; Pidlisnyuk, V.; Zhukov, O.; Shapoval, P. Can Nematode Communities Work as an Indicator of Soil Health in a Multiyear Miscanthus × Giganteus Plantation Growing in Lead-Contaminated Soil? Agronomy 2023, 13, 1620. https://doi.org/10.3390/agronomy13061620
Stefanovska T, Skwiercz A, Pidlisnyuk V, Zhukov O, Shapoval P. Can Nematode Communities Work as an Indicator of Soil Health in a Multiyear Miscanthus × Giganteus Plantation Growing in Lead-Contaminated Soil? Agronomy. 2023; 13(6):1620. https://doi.org/10.3390/agronomy13061620
Chicago/Turabian StyleStefanovska, Tatyana, Andrzej Skwiercz, Valentina Pidlisnyuk, Oleksandr Zhukov, and Pavlo Shapoval. 2023. "Can Nematode Communities Work as an Indicator of Soil Health in a Multiyear Miscanthus × Giganteus Plantation Growing in Lead-Contaminated Soil?" Agronomy 13, no. 6: 1620. https://doi.org/10.3390/agronomy13061620
APA StyleStefanovska, T., Skwiercz, A., Pidlisnyuk, V., Zhukov, O., & Shapoval, P. (2023). Can Nematode Communities Work as an Indicator of Soil Health in a Multiyear Miscanthus × Giganteus Plantation Growing in Lead-Contaminated Soil? Agronomy, 13(6), 1620. https://doi.org/10.3390/agronomy13061620








