Evaluation of the Intra- and Interspecific Development of Different Accessions of Silphium perfoliatum L. and Silphium integrifolium Michx.
Abstract
1. Introduction
2. Materials and Methods
2.1. Growing and Climate Conditions
2.2. Trial Setup
2.3. Assistance for the Chronology of Incidents
2.4. Description of the Germination
2.5. Data Collection
2.6. Data Processing
3. Results
3.1. Development Description of the Establishment Year
3.1.1. Germination
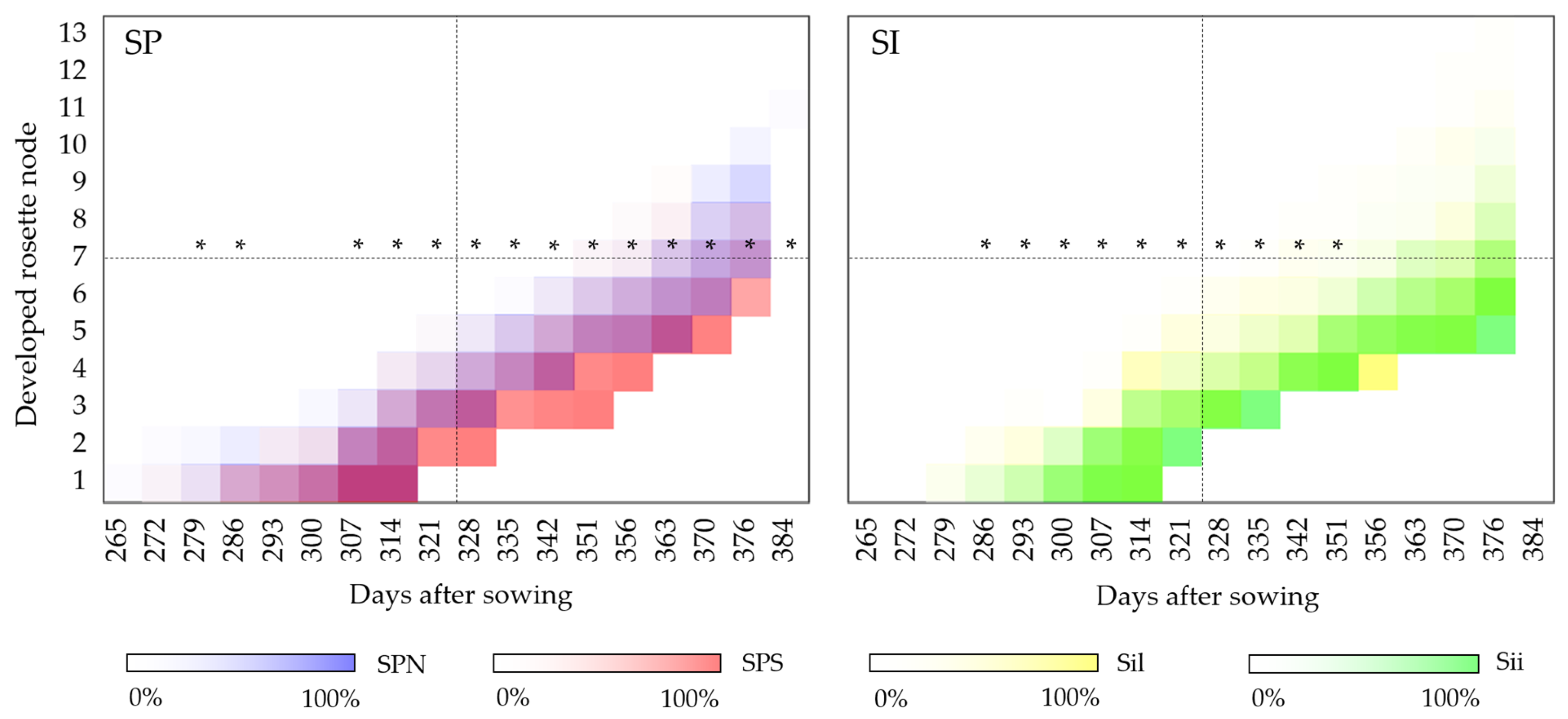
3.1.2. Rosette Development
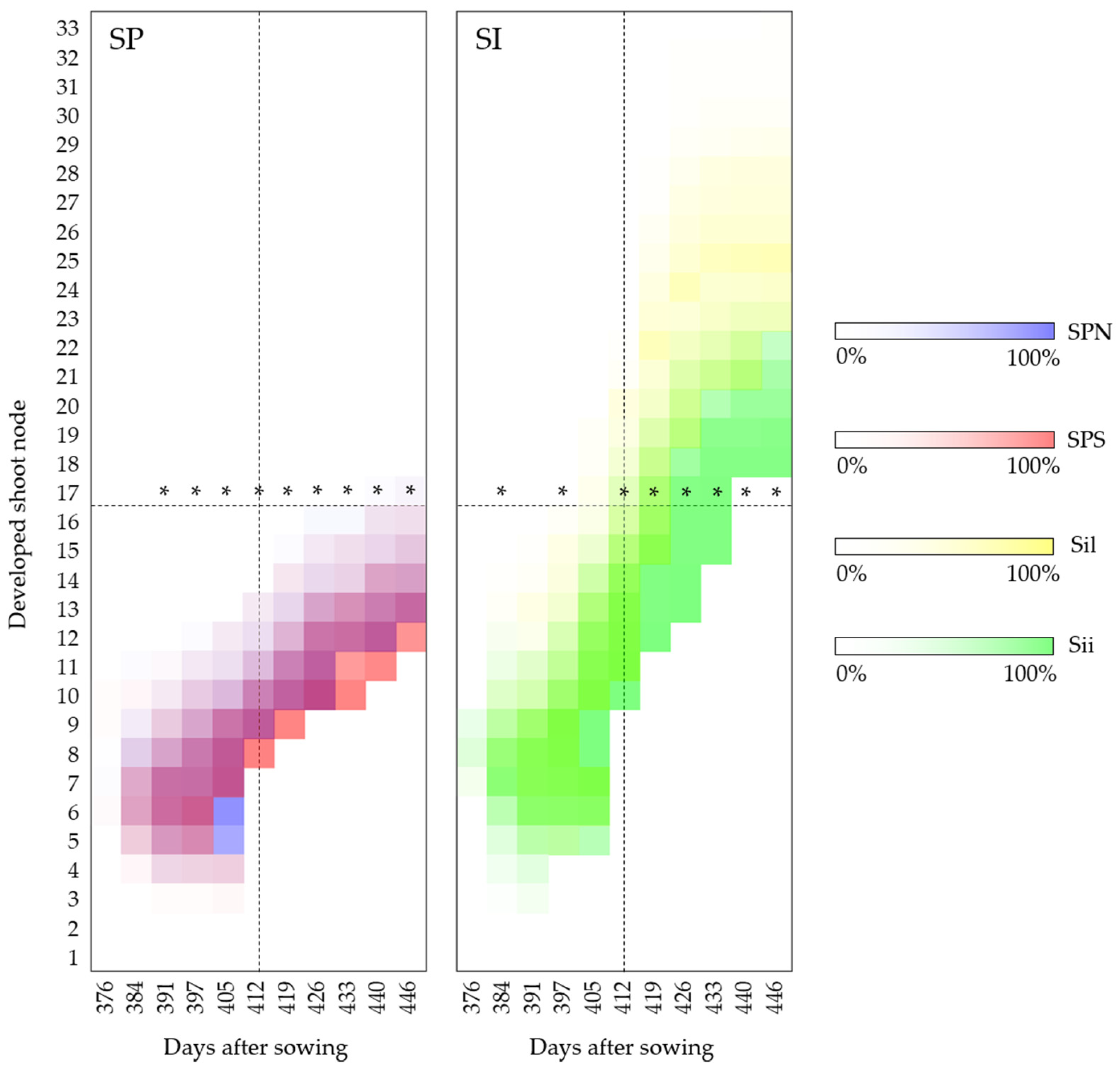
3.1.3. Shoot Development
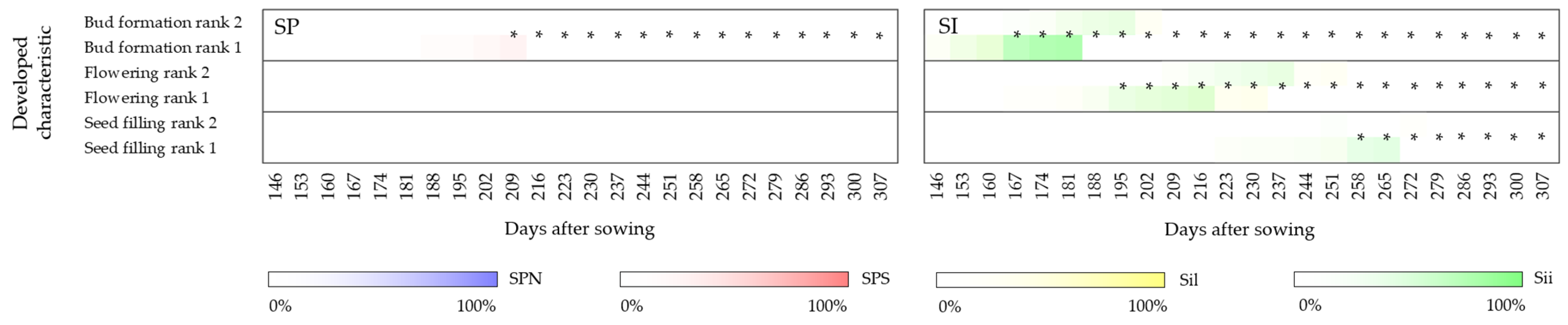
3.1.4. Generative Stage
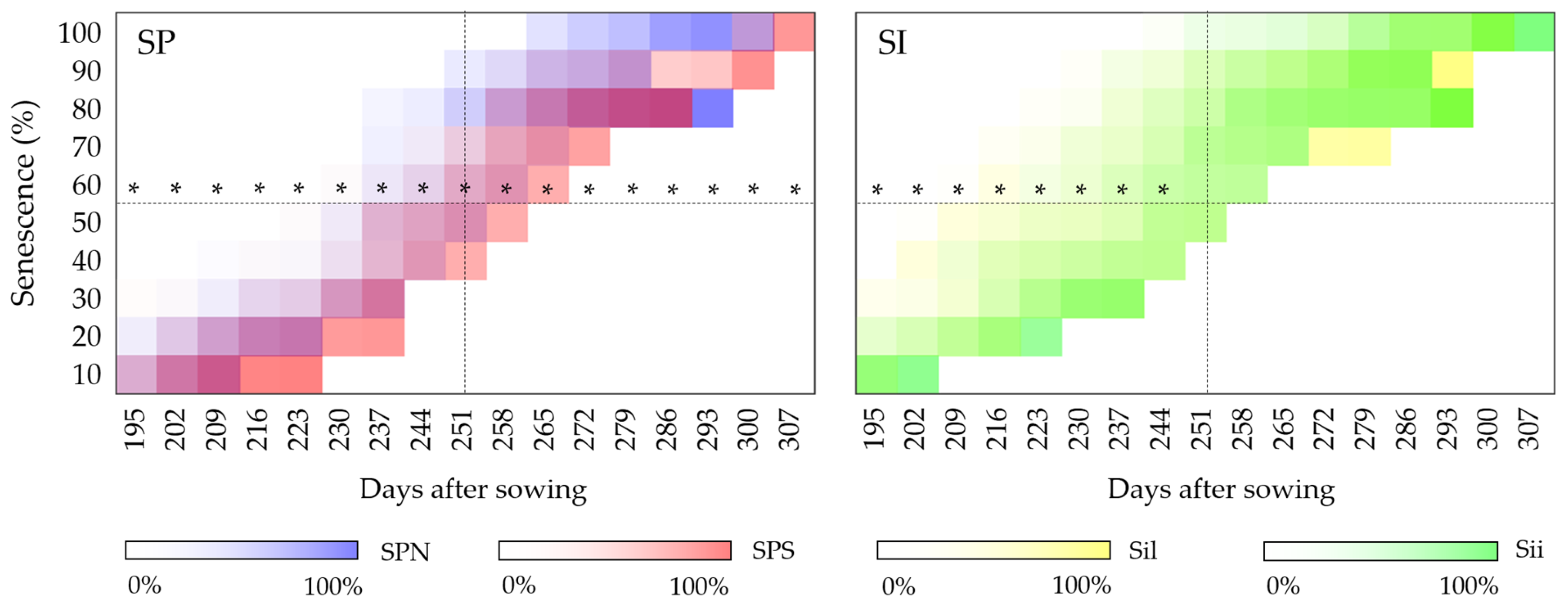
3.1.5. Senescence
3.2. Development Description of the Second Year of Development
3.2.1. Rosette Development
3.2.2. Shoot Development
3.2.3. Generative Stage
3.2.4. Senescence
3.3. BBCH-Coding of the Phenological Development Stages of Silphium perfoliatum and Silphium integrifolium
- BBCH Macro Stage 0—Germination
| Code | Description | SP 1. Year | SI 1. Year | SP 2. Year | SI 2. Year | |
|---|---|---|---|---|---|---|
| 0 | 0 | Dry seed | 1.00 | 1.00 | - | - |
| 0 | 3 | End of seed swelling | 1.00 | 1.00 | - | - |
| 0 | 5 | Radicle visible | 0.84 | 0.90 | - | - |
| 0 | 6 | Radicle elongated | 0.82 | 0.82 | - | - |
| 0 | 7 | Seed coat breakthrough of the cotyledons | 0.64 | 0.82 | - | - |
| 0 | 8 | Surface breakthrough | 0.52 | 0.76 | - | - |
| 0 | 9 | Cotyledons fully developed | 0.52 | 0.70 | - | - |

- BBCH Macro Stage 1—Rosette Development
| Code | Description | SP 1. Year | SI 1. Year | SP 2. Year | SI 2. Year | |
|---|---|---|---|---|---|---|
| 1 | 1 | 1st rosette node/1st pair of leaves fully developed | 1.00 | 1.00 | 1.00 | 1.00 |
| 1 | 2 | 2nd rosette node/2nd pair of leaves fully developed | 1.00 | 1.00 | 1.00 | 1.00 |
| 1 | 3 | 3rd rosette node/3rd pair of leaves fully developed | 0.97 | 1.00 | 1.00 | 1.00 |
| 1 | 4 | 4th rosette node/4th pair of leaves fully developed | 0.97 | 1.00 | 1.00 | 1.00 |
| 1 | 5 | 5th rosette node/5th pair of leaves fully developed | 0.94 | 1.00 | 1.00 | 1.00 |
| 1 | 6 | 6th rosette node/6th pair of leaves fully developed | 0.83 | 1.00 | 1.00 | 1.00 |
| 1 | 7 | 7th rosette node/7th pair of leaves fully developed | 0.63 | 1.00 | 0.92 | 0.83 |
| 1 | 8 | 8th rosette node/8th pair of leaves fully developed | - | 1.00 | 0.67 | 0.53 |
| 1 | 9 | 9th rosette node/9th pair of leaves fully developed | - | 0.91 | - | - |
| 1 | 9 | 10th rosette node/10th pair of leaves fully developed | - | 0.83 | - | - |
| 1 | 9 | 11th rosette node/11th pair of leaves fully developed | - | 0.83 | - | - |
| 1 | 9 | 12th rosette node/12th pair of leaves fully developed | - | 0.77 | - | - |
| 1 | 9 | 13th rosette node/13th pair of leaves fully developed | - | 0.71 | - | - |
| 1 | 9 | 14th rosette node/14th pair of leaves fully developed | - | 0.66 | - | - |
| 1 | 9 | 15th rosette node/15th pair of leaves fully developed | - | 0.54 | - | - |

- BBCH Macro Stage 3—Shoot Development
| Code | Description | SP 1. Year | SI 1. Year | SP 2. Year | SI 2. Year | |
|---|---|---|---|---|---|---|
| 3 | 1−x | 1st shoot node/corresponding leaves fully developed | - | - | - | - |
| 3 | 2−x | 2nd shoot node/corresponding leaves fully developed | - | - | - | - |
| 3 | 3−x | 3rd shoot node/corresponding leaves fully developed | - | - | - | - |
| 3 | 4−x | 4th shoot node/corresponding leaves fully developed | - | - | - | - |
| 3 | 5−x | 5th shoot node/corresponding leaves fully developed | - | - | 0.67 | 0.56 |
| 3 | 6−x | 6th shoot node/corresponding leaves fully developed | - | - | 0.86 | 0.92 |
| 3 | 7−x | 7th shoot node/corresponding leaves fully developed | - | - | 0.94 | 1.00 |
| 3 | 8−x | 8th shoot node/corresponding leaves fully developed | - | - | 0.97 | 1.00 |
| 3 | 9−x | 9th shoot node/corresponding leaves fully developed | - | - | 1.00 | 1.00 |
| 3 | 9−x | 10th shoot node/corresponding leaves fully developed | - | 0.57 | 1.00 | 1.00 |
| 3 | 9−x | 11th shoot node/corresponding leaves fully developed | - | 0.71 | 1.00 | 1.00 |
| 3 | 9−x | 12th shoot node/corresponding leaves fully developed | - | 0.89 | 1.00 | 1.00 |
| 3 | 9−x | 13th shoot node/corresponding leaves fully developed | - | 0.89 | 0.89 | 1.00 |
| 3 | 9−x | 14th shoot node/corresponding leaves fully developed | - | 0.89 | 0.61 | 1.00 |
| 3 | 9−x | 15th shoot node/corresponding leaves fully developed | - | 0.89 | - | 0.97 |
| 3 | 9−x | 16th shoot node/corresponding leaves fully developed | - | 0.89 | - | 0.97 |
| 3 | 9−x | 17th shoot node/corresponding leaves fully developed | - | 0.89 | - | 0.97 |
| 3 | 9−x | 18th shoot node/corresponding leaves fully developed | - | 0.89 | - | 0.94 |
| 3 | 9−x | 19th shoot node/corresponding leaves fully developed | - | 0.89 | - | 0.94 |
| 3 | 9−x | 20th shoot node/corresponding leaves fully developed | - | 0.89 | - | 0.94 |
| 3 | 9−x | 21th shoot node/corresponding leaves fully developed | - | 0.63 | - | 0.94 |
| 3 | 9−x | 22th shoot node/corresponding leaves fully developed | - | 0.54 | - | 0.86 |
| 3 | 9−x | 23th shoot node/corresponding leaves fully developed | - | 0.51 | - | 0.81 |
| 3 | 9−x | 24th shoot node/corresponding leaves fully developed | - | - | - | 0.75 |
| 3 | 9−x | 25th shoot node/corresponding leaves fully developed | - | - | - | 0.56 |

- BBCH Macro Stage 5–8—Generative Stage
| Macro Stage 5—Bud Formation | ||||||
|---|---|---|---|---|---|---|
| Code | Description | SP 1. Year | SI 1. Year | SP 2. Year | SI 2. Year | |
| 5 | 1 | 1st rank: bud fully developed | - | 0.63 | 1.00 | 1.00 |
| 5 | 2 | 2nd rank: buds fully developed | - | - | 1.00 | 1.00 |
| 5 | 3 | 3rd rank: buds fully developed | - | - | 1.00 | 1.00 |
| 5 | 4 | 4th rank: buds fully developed | - | - | 1.00 | 1.00 |
| 5 | 5 | 5th rank: buds fully developed | - | - | 1.00 | 1.00 |
| 5 | 6 | 6th rank: buds fully developed | - | - | 0.94 | 0.97 |
| 5 | 7 | 7th rank: buds fully developed | - | - | 0.56 | 0.69 |
| Macro stage 6—Flowering | ||||||
| 6 | 1 | 1st rank: Flower fully developed | - | 0.50 | 1.00 | 0.97 |
| 6 | 2 | 2nd rank: Flowers fully developed | - | - | 0.97 | 0.94 |
| 6 | 3 | 3rd rank: Flowers fully developed | - | - | 1.00 | 1.00 |
| 6 | 4 | 4th rank: Flowers fully developed | - | - | 0.97 | 1.00 |
| 6 | 5 | 5th rank: Flowers fully developed | - | - | 0.92 | 0.97 |
| 6 | 6 | 6th rank: Flowers fully developed | - | - | 0.50 | 0.61 |
| Macro stage 7—Seed filling | ||||||
| 7 | 1 | 1st rank: Seed filling fully completed | - | - | 1.00 | 1.00 |
| 7 | 2 | 2nd rank: Seed filling fully completed | - | - | 1.00 | 1.00 |
| 7 | 3 | 3rd rank: Seed filling fully completed | - | - | 1.00 | 1.00 |
| 7 | 4 | 4th rank: Seed filling fully completed | - | - | 1.00 | 1.00 |
| 7 | 5 | 5th rank: Seed filling fully completed | - | - | 0.92 | 0.97 |
| 7 | 6 | 6th rank: Seed filling fully completed | - | - | 0.53 | 0.69 |
| Macro stage 8—Seed ripening | ||||||
| 8 | 1 | 1st rank: Seed ripening completed | - | - | 1.00 | 1.00 |
| 8 | 2 | 2nd rank: Seed ripening completed | - | - | 1.00 | 1.00 |
| 8 | 3 | 3rd rank: Seed ripening completed | - | - | 1.00 | 1.00 |
| 8 | 4 | 4th rank: Seed ripening completed | - | - | 1.00 | 1.00 |
| 8 | 5 | 5th rank: Seed ripening completed | - | - | 0.94 | 0.97 |
| 8 | 6 | 6th rank: Seed ripening completed | - | - | 0.53 | 0.72 |
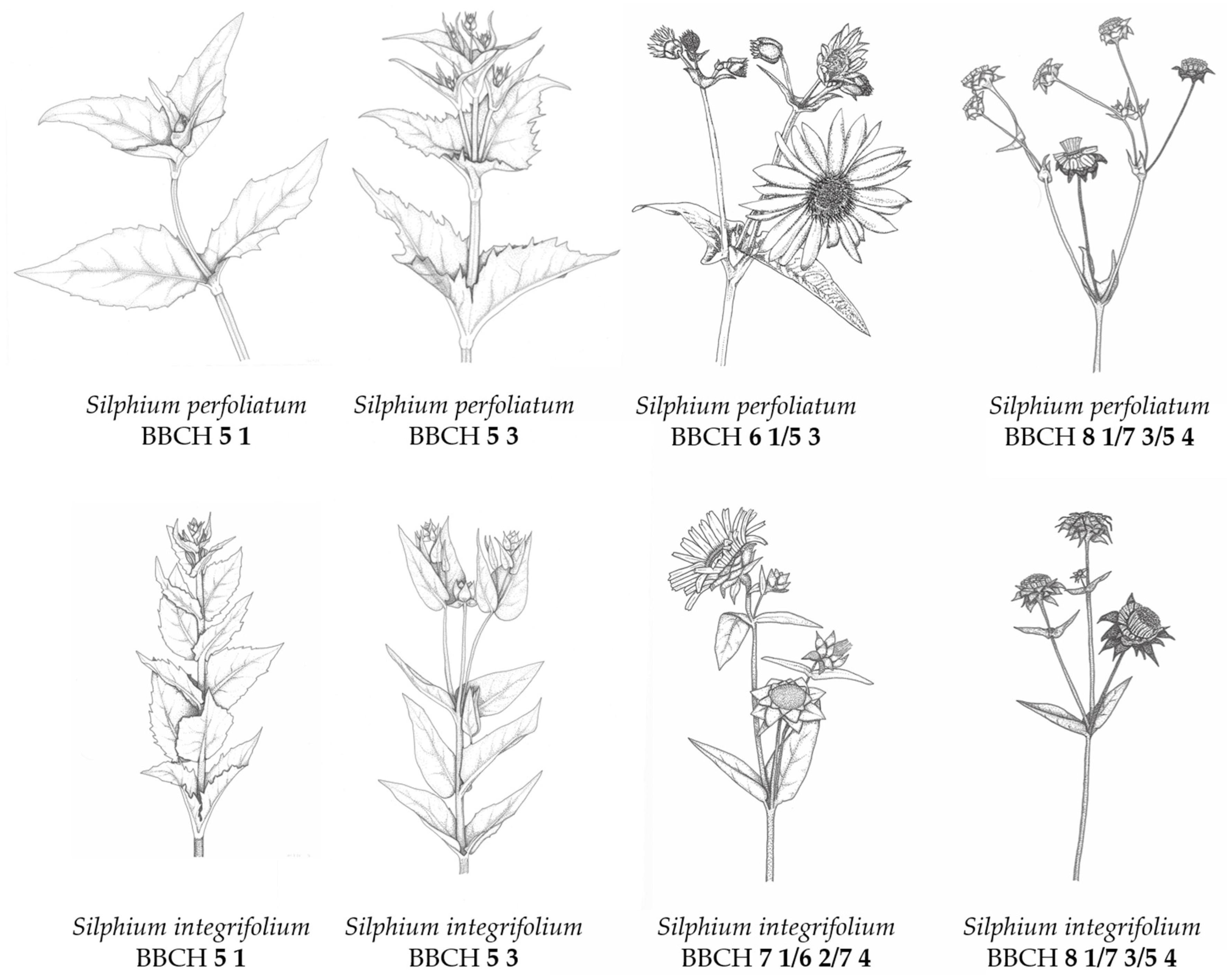
- BBCH Macro Stage 9—Senescence
| Code | Description | SP 1. Year | SI 1. Year | SP 2. Year | SI 2. Year | |
|---|---|---|---|---|---|---|
| 9 | 1 | 10% of the aboveground plant parts are senescent | 0.97 | 0.86 | - | - |
| 9 | 2 | 20% of the aboveground plant parts are senescent | 0.89 | 0.78 | 1.00 | - |
| 9 | 3 | 30% of the aboveground plant parts are senescent | 0.75 | 0.81 | 0.97 | 1.00 |
| 9 | 4 | 40% of the aboveground plant parts are senescent | 0.64 | 0.72 | 0.61 | 0.61 |
| 9 | 5 | 50% of the aboveground plant parts are senescent | 0.64 | 0.75 | 0.94 | 0.69 |
| 9 | 6 | 60% of the aboveground plant parts are senescent | 0.64 | 0.58 | 0.61 | 0.60 |
| 9 | 7 | Leaves completely senescent | 0.72 | 0.72 | 0.78 | 0.75 |
| 9 | 9 | Fully senescent above ground plant parts | 1.00 | 1.00 | 1.00 | 1.00 |
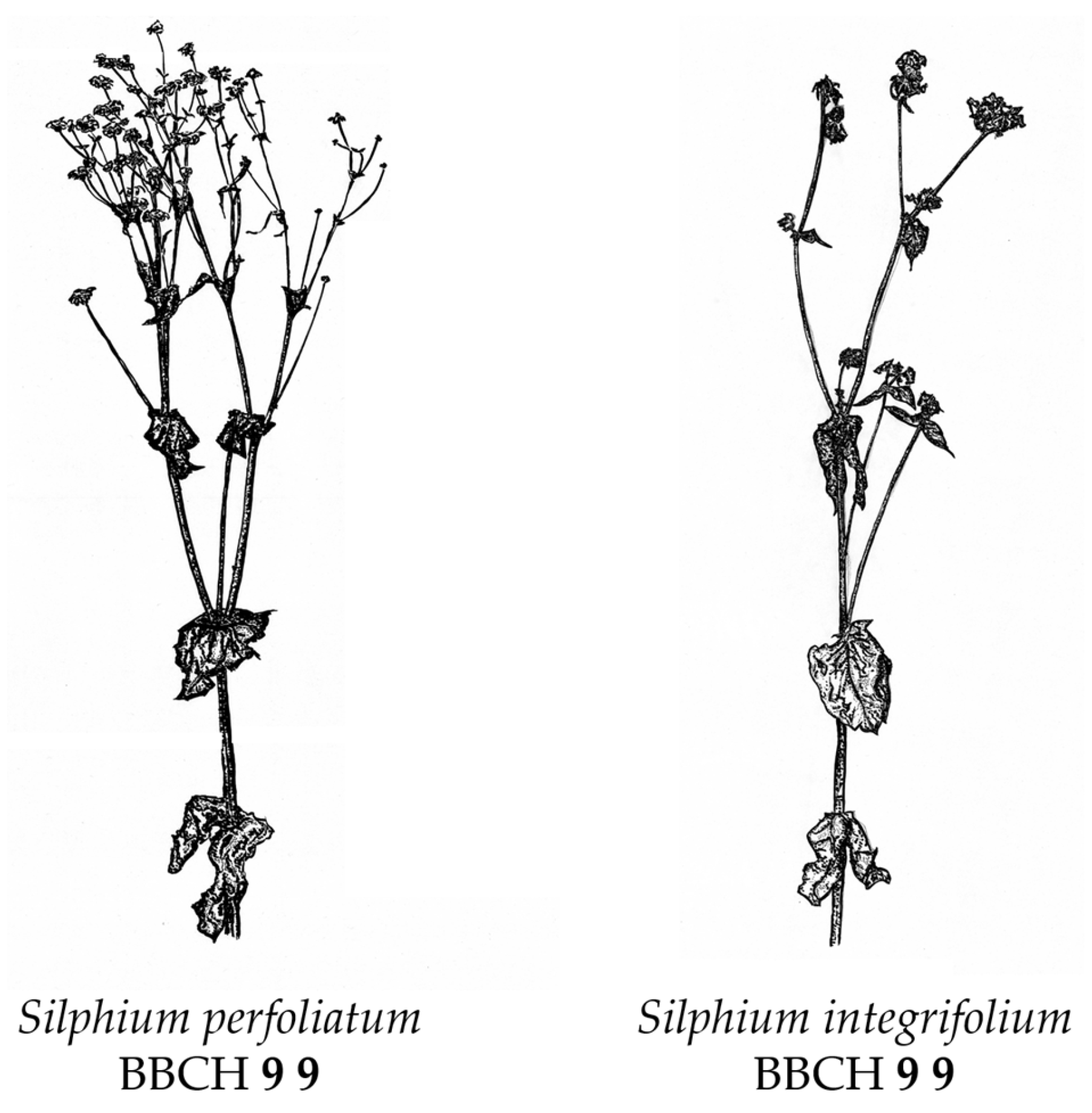
4. Discussion
4.1. Germination Kinetics of Both Silphium Species
Adaption to BBCH Code—Macro Stage 0 [36]
4.2. Rosette Development
4.2.1. Year of Establishment
4.2.2. Second Year of Development
4.2.3. Adaption to BBCH Code—Macro Stage 1 [36]
4.3. General Shoot Development
4.3.1. Year of Establishment
4.3.2. Second Year of Development
4.3.3. Adaption to BBCH Code—Macro Stage 3 [36]
4.4. Generative Phase
4.4.1. Year of Establishment
4.4.2. Second Year of Development
4.4.3. Adaption to BBCH Code—Macro Stage 5–8 [36]
4.5. Senescence
4.5.1. Year of Establishment
4.5.2. Second Year of Development
4.5.3. Adaption to BBCH Code—Macro Stage 9 [36]
4.6. Classification of Phenotypic Traits and Use
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanderson, M.A.; Adler, P.R. Perennial forages as second generation bioenergy crops. Int. J. Mol. Sci. 2008, 9, 768–788. [Google Scholar] [CrossRef] [PubMed]
- Wever, C.; van Tassel, D.L.; Pude, R. Third-Generation Biomass Crops in the New Era of De Novo Domestication. Agronomy 2020, 10, 1322. [Google Scholar] [CrossRef]
- Clevinger, J.A.; Panero, J.L. Phylogenetic analysis of Silphium and subtribe Engelmanniinae (Asteraceae: Heliantheae) based on ITS and ETS sequence data. Am. J. Bot. 2000, 87, 565–572. [Google Scholar] [CrossRef] [PubMed]
- van Tassel, D.L.; Albrecht, K.A.; Bever, J.D.; Boe, A.A.; Brandvain, Y.; Crews, T.E.; Gansberger, M.; Gerstberger, P.; González-Paleo, L.; Hulke, B.S.; et al. Accelerating Domestication: An Opportunity to Develop New Crop Ideotypes and Breeding Strategies Informed by Multiple Disciplines. Crop Sci. 2017, 57, 1274–1284. [Google Scholar] [CrossRef]
- Clevinger, J.A. Silphium integrifolium. Available online: http://floranorthamerica.org/Silphium_integrifolium (accessed on 1 February 2023).
- Clevinger, J.A. Silphium perfoliatum. Available online: http://floranorthamerica.org/Silphium_perfoliatum (accessed on 1 February 2023).
- Albrecht, K.A.; Goldstein, W. Silphium perfoliatum: A North American Prairie Plant with Potential as a Forage Crop, 8–19 June 1997, Winnipeg. pp. 167–168. Available online: https://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/reference/ReferencesPapers.aspx?ReferenceID=1472322 (accessed on 11 June 2023).
- Raduski, A.R.; Herman, A.; Pogoda, C.; Dorn, K.M.; van Tassel, D.L.; Kane, N.; Brandvain, Y. Patterns of genetic variation in a prairie wildflower, Silphium integrifolium, suggest a non-prairie origin and locally adaptive variation. Am. J. Bot. 2021, 108, 145–158. [Google Scholar] [CrossRef]
- Assefa, T.; Wu, J.; Albrecht, K.A.; Johnson, P.J.; Boe, A. Genetic Variation for Biomass and Related Morphological Traits in Cup Plant (Silphium perfoliatum L.). Am. J. Plant Sci. 2015, 6, 1098–1108. [Google Scholar] [CrossRef]
- Wever, C.; Höller, M.; Becker, L.; Biertümpfel, A.; Köhler, J.; van Inghelandt, D.; Westhoff, P.; Pude, R.; Pestsova, E. Towards high-biomass yielding bioenergy crop Silphium perfoliatum L.: Phenotypic and genotypic evaluation of five cultivated populations. Biomass Bioenergy 2019, 124, 102–113. [Google Scholar] [CrossRef]
- Mayr, J.; Gansberger, M.; Leonhardt, C.; Moosbeckhofer, R.; Liebhard, P. Durchwachsene Silphie (Silphium perfoliatum L.) eine neue Energiepflanze in Österreich. In Proceedings of the Pflanzenschutz als Beitrag zur Ernährungssicherung, ALVA-Jahrestagung, Klosterneuburg, Austria, 23–24 May 2013. [Google Scholar]
- Clevinger, J.A. New combinations in Silphium (Asteraceae: Heliantheae). Novon 2004, 14, 275–277. [Google Scholar]
- Cumplido-Marin, L.; Graves, A.R.; Burgess, P.J.; Morhart, C.; Paris, P.; Jablonowski, N.D.; Facciotto, G.; Bury, M.; Martens, R.; Nahm, M. Two Novel Energy Crops: Sida hermaphrodita (L.) Rusby and Silphium perfoliatum L.—State of Knowledge. Agronomy 2020, 10, 928. [Google Scholar] [CrossRef]
- Mueller, A.L.; Dauber, J. Hoverflies (Diptera: Syrphidae) benefit from a cultivation of the bioenergy crop Silphium perfoliatum L. (Asteraceae) depending on larval feeding type, landscape composition and crop management. Agric. For. Entomol. 2016, 18, 419–431. [Google Scholar] [CrossRef]
- Reinert, S.; van Tassel, D.L.; Schlautman, B.; Kane, N.C.; Hulke, B.S. Assessment of the biogeographical variation of seed size and seed oil traits in wild Silphium integrifolium Michx. genotypes. Plant Genet. Resour. 2019, 17, 427–436. [Google Scholar] [CrossRef]
- Höller, M.; Lunze, A.; Wever, C.; Deutschle, A.L.; Stücker, A.; Frase, N.; Pestsova, E.; Spiess, A.C.; Westhoff, P.; Pude, R. Meadow hay, Sida hermaphrodita (L.) Rusby and Silphium perfoliatum L. as potential non-wood raw materials for the pulp and paper industry. Ind. Crops Prod. 2021, 167, 113548. [Google Scholar] [CrossRef]
- Peni, D.; Stolarski, M.J.; Bordiean, A.; Krzyżaniak, M.; Dębowski, M. Silphium perfoliatum—A Herbaceous Crop with Increased Interest in Recent Years for Multi-Purpose Use. Agriculture 2020, 10, 640. [Google Scholar] [CrossRef]
- Dauber, J.; Müller, A.L.; Schittenhelm, S.; School, B.; Schorpp, Q.; Schrader, S.; Schroetter, S. Schlussbericht zum Vorhaben. Thema: Agrarökologische Bewertung der Durchwachsenen Silphie (Silphium perfoliatum L.) als eine Biomassepflanze der Zukunft. Teilvorhaben 1: Ober- und unterirdische Biodiversität in Beständen der Durchwachsenen Silphie. Bundesministerium für Ernährung und Landwirtschaft (BMEL). 2016. Available online: http://www.fnr-server.de/ftp/pdf/berichte/22004411.pdf (accessed on 11 June 2023).
- Gansberger, M. Germination characteristic of Silphium perfoliatum L. seeds. J. Land Manag. Food Environ. 2017, 68, 73–79. [Google Scholar] [CrossRef]
- Lunze, A.; Heyman, B.; Chammakhi, Y.; Eichhorn, M.; Büchs, J.; Anders, N.; Spiess, A.C. Investigation of Silphium perfoliatum as Feedstock for a Liquid Hot Water–Based Biorefinery Process Towards 2,3-Butanediol. Bioenergy Res. 2021, 14, 799–814. [Google Scholar] [CrossRef]
- Kowalski, R.; Kędzia, B. Antibacterial Activity of Silphium perfoliatum. Extracts. Pharm. Biol. 2007, 45, 494–500. [Google Scholar] [CrossRef]
- Kowalski, R.; Wolski, T. The chemical composition of essential oils of Silphium perfoliatum L. Flavour Fragr. J. 2005, 20, 306–310. [Google Scholar] [CrossRef]
- Moll, L.; Höller, M.; Hubert, C.; Korte, C.A.C.; Völkering, G.; Wever, C.; Pude, R. Cup Plant (Silphium perfoliatum L.) Biomass as Substitute for Expanded Polystyrene in Bonded Leveling Compounds. Agronomy 2022, 12, 178. [Google Scholar] [CrossRef]
- Mast, B.; Lemmer, A.; Oechsner, H.; Reinhardt-Hanisch, A.; Claupein, W.; Graeff-Hönninger, S. Methane yield potential of novel perennial biogas crops influenced by harvest date. Ind. Crops Prod. 2014, 58, 194–203. [Google Scholar] [CrossRef]
- Chiriac, E.R.; Chiţescu, C.L.; Sandru, C.; Geană, E.-I.; Lupoae, M.; Dobre, M.; Borda, D.; Gird, C.E.; Boscencu, R. Comparative Study of the Bioactive Properties and Elemental Composition of Red Clover (Trifolium pratense) and Alfalfa (Medicago sativa) Sprouts during Germination. Appl. Sci. 2020, 10, 7249. [Google Scholar] [CrossRef]
- Pichard, G. Management, production, and nutritional characteristics of cup-plant (Silphium perfoliatum) in temperate climates of southern Chile. Cienc. Investig. Agrar. 2012, 39, 61–77. [Google Scholar] [CrossRef]
- Gibson, D.R.; Rowe, L.; Isaacs, R.; Landis, D.A. Screening Drought-Tolerant Native Plants for Attractiveness to Arthropod Natural Enemies in the U.S. Great Lakes Region. Environ. Entomol. 2019, 48, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, D.; Panten, K.; Schwarz, A.; Bischoff, W.-A.; Schittenhelm, S. Comparison of maize, permanent cup plant and a perennial grass mixture with regard to soil and water protection. GCB Bioenergy 2020, 12, 694–705. [Google Scholar] [CrossRef]
- Prasifka, J.R.; Peterson, K.; Mallinger, R.E.; van Tassel, D.L. Changes to architecture of Silphium integrifolium Michx. during domestication reveal new trade-offs for yield. Crop Sci. 2022, 62, 1060–1068. [Google Scholar] [CrossRef]
- Kowalski, R. Silphium L. extracts—Composition and protective effect on fatty acids content in sunflower oil subjected to heating and storage. Food Chem. 2009, 112, 820–830. [Google Scholar] [CrossRef]
- Kowalski, R.; Wierciński, J. Evaluation of chemical composition of some Silphium L. species seeds as alternative foodstuff raw materials. Pol. J. Food Nutr. Sci. 2004, 13, 349–354. [Google Scholar]
- Schiffner, S.; Jungers, J.M.; Hulke, B.S.; van Tassel, D.L.; Smith, K.P.; Sheaffer, C.C. Silflower seed and biomass responses to plant density and nitrogen fertilization. Agrosyst. Geosci. Environ. 2020, 3, e20118. [Google Scholar] [CrossRef]
- Reinert, S.; Hulke, B.S.; Prasifka, J.R. Pest potential of Neotephritis finalis (Loew) on Silphium integrifolium Michx., Silphium perfoliatum L., and interspecific hybrids. Agron. J. 2020, 112, 1462–1465. [Google Scholar] [CrossRef]
- Vilela, A.; González-Paleo, L.; Turner, K.; Peterson, K.; Ravetta, D.; Crews, T.; van Tassel, D. Progress and Bottlenecks in the Early Domestication of the Perennial Oilseed Silphium integrifolium, a Sunflower Substitute. Sustainability 2018, 10, 638. [Google Scholar] [CrossRef]
- Hack, H.; Bleiholder, H.; Buhr, L.; Meier, U.; Schnock-Fricke, E.; Weber, E.; Witzenberger, A. Einheitliche Codierung der phänologischen Entwicklungsstadien mono- und dikotyler Pflanzen. -Erweiterte BBCH-Skala, Allgemein. Dtsch. Pflanzenschutzd. 1992, 44, 265–270. [Google Scholar]
- Meier, U. Entwicklungsstadien Mono- Und Dikotyler Pflanzen: BBCH Monografie; Biologische Bundesanstalt für Land und Forstwirtschaft: Bonn, Germany, 2018. [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- University of Bonn, Faculty of Agriculture. Location: Soil. Available online: https://www.aussenlabore.uni-bonn.de/cka/de/standort/copy_of_standortbeschreibung (accessed on 14 January 2020).
- University of Bonn, Faculty of Agriculture. Location: Weather Conditions. Available online: https://www.aussenlabore.uni-bonn.de/cka/de/standort/wetterdaten (accessed on 1 January 2020).
- Talbot, M.; Milner, A.D.; Nutkins, M.A.E.; Law, J.R. Effect of interference between plots on yield performance in crop variety trials. J. Agric. Sci. 1995, 124, 335–342. [Google Scholar] [CrossRef]
- Kadereit, J.W.; Körner, C.; Kost, B.; Sonnewald, U. Strasburger—Lehrbuch der Pflanzenwissenschaften; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-54434-7. [Google Scholar]
- Wang, M.; Li, W.; Fang, C.; Xu, F.; Liu, Y.; Wang, Z.; Yang, R.; Zhang, M.; Liu, S.; Lu, S.; et al. Parallel selection on a dormancy gene during domestication of crops from multiple families. Nat. Genet. 2018, 50, 1435–1441. [Google Scholar] [CrossRef]
- Pickersgill, B. Domestication of plants in the Americas: Insights from Mendelian and molecular genetics. Ann. Bot. 2007, 100, 925–940. [Google Scholar] [CrossRef]
- Allaby, R.G. Domestication Syndrome in Plants. In Encyclopedia of Global Archaeology; Smith, C., Ed.; Springer: New York, NY, USA, 2014; pp. 2182–2184. ISBN 978-1-4419-0426-3. [Google Scholar]
- Gansberger, M.; Stüger, H.; Weinhappel, M.; Moder, K.; Liebhard, P.; von Gehren, P.; Mayr, J.; Ratzenböck, A. Seed germination in Silphium perfoliatum L. and Sida hermaphrodita L. and technological measures for its improvement. In Proceedings of the Tagung der Vereinigung der Pflanzenzüchter und Saatgutkaufleute Österreichs, Raumberg-Gumpenstein, Irdning, Austria, 19–21 November 2014. [Google Scholar]
- Reinert, S.; Money, K.L.; Rockstad, G.B.G.; Kane, N.C.; van Tassel, D.L.; Hulke, B.S. Two contrasting laboratory methods improve Silphium integrifolium Michx. germination rate to agronomically acceptable levels. Euphytica 2018, 214, 156. [Google Scholar] [CrossRef]
- Nasreen, S.; Khan, M.A.; Zia, M.; Ishaque, M.; Uddin, S.; Arshad, M.; Rizvi, Z.F. Response of sunflower to various pre-germination techniques for breaking seed dormancy. Pak. J. Bot. 2015, 47, 413–416. [Google Scholar]
- Pude, R. Die Winterfestigkeit von Miscanthus in der Etablierungsphase; Wehle: Bonn, Germany, 1998; ISBN 3895730378. [Google Scholar]
- Jablonowski, N.D.; Kollmann, T.; Nabel, M.; Damm, T.; Klose, H.; Müller, M.; Bläsing, M.; Seebold, S.; Krafft, S.; Kuperjans, I.; et al. Valorization of Sida (Sida hermaphrodita) biomass for multiple energy purposes. GCB Bioenergy 2017, 9, 202–214. [Google Scholar] [CrossRef]
- Kowalski, R. Growth and development of Silphium integrifolium in the first 3 years of cultivation. N. Z. J. Crop Hortic. Sci. 2004, 32, 389–395. [Google Scholar] [CrossRef]
- Stanfort, G. Silphium perfoliatum (Cup-Plant). 1992. Available online: https://images.library.wisc.edu/EcoNatRes/EFacs/NAPC/NAPC12/reference/econatres.napc12.gstanford.pdf (accessed on 11 June 2023).
- Schäfer, A. Verfahrenstechnische Optimierung der Bestandesetablierung von Durchwachsender Silphie (Silphium perfoliatum L.) Mittels Einzelkornsähtechnik unter Berücksichtigung der Korngeometrie. Ph.D. Thesis, Institut für Landtechnik, Rheinische Friedrich-Wilhelms-Universität Bonn, Bonn, Germany, 2019. [Google Scholar]
- Franzaring, J.; Schmid, I.; Bäuerle, L.; Gensheimer, G.; Fangmeier, A. Investigations on plant functional traits, epidermal structures and the ecophysiology of the novel bioenergy species Sida hermaphrodita Rusby and Silphium perfoliatum L. J. Appl. Bot. Food Qual. 2014, 87, 36–45. [Google Scholar] [CrossRef]
- Titei, V. The Evaluation of Biomass of the Sida hermaphrodita and Silphium perfoliatum for Renewable Energy in Moldova. Sci. Pap. Ser. A Agron. 2017, 60, 534–540. [Google Scholar]
- Gansberger, M.; Montgomery, L.F.; Liebhard, P. Botanical characteristics, crop management and potential of Silphium perfoliatum L. as a renewable resource for biogas production: A review. Ind. Crops Prod. 2015, 63, 362–372. [Google Scholar] [CrossRef]
- Conrad, M.; Biertümpfel, A. Abschlussbericht—Optimierung des Anbauverfahrens für Durchwachsene Silphie (Silphium perfoliatum) als Kofermentpflanze in Biogasanlagen Sowie Überführung in Die landwirtschaftliche Praxis; Friedrich-Schiller-Universität Jena: Jena, Germany, 2010. [Google Scholar]
- Kowalski, R.; Wolski, T. Characteristics of growth and development of Silphium perfoliatum L. in the first years cultivation. Ann. Univ. Mariae Curie-Sklodowska Sect. EEE 2001, 9, 311–317. [Google Scholar]
- Schiffner, S.; Jungers, J.M.; van Tassel, D.; Smith, K.P.; Sheaffer, C.C. Seeding date affects seed and biomass yield of Silphium integrifolium Michx. (silflower). Nativ. Plants J. 2021, 22, 30–44. [Google Scholar] [CrossRef]
- Titei, V.; Cîrlig, N.; Guțu, A. Some biological peculiarities and economic value of the cultivation of cup plant, Silphium perfoliatum L. Biologie 2021, 136, 79–82. [Google Scholar] [CrossRef]
- Kowalska, G.; Pankiewicz, U.; Kowalski, R. Evaluation of Chemical Composition of Some Silphium L. Species as Alternative Raw Materials. Agriculture 2020, 10, 132. [Google Scholar] [CrossRef]
- Kowalska, G.; Baj, T.; Kowalski, R.; Hanif, M.A. Characteristics of Selected Silphium Species as Alternative Plants for Cultivation and Industry with Particular Emphasis on Research Conducted in Poland: A Review. Sustainability 2022, 14, 5092. [Google Scholar] [CrossRef]
- Debieu, M.; Tang, C.; Stich, B.; Sikosek, T.; Effgen, S.; Josephs, E.; Schmitt, J.; Nordborg, M.; Koornneef, M.; Meaux, J.d. Co-variation between seed dormancy, growth rate and flowering time changes with latitude in Arabidopsis thaliana. PLoS ONE 2013, 8, e61075. [Google Scholar] [CrossRef]
- Lorenz, M.; Brunotte, J.; Vorderbrügge, T. Anpassung der Lasteinträge landwirtschaftlicher Maschinen an die Verdichtungsempfindlichkeit des Bodens—Grundlagen für ein bodenschonendes Befahren von Ackerland. Landbauforsch. Appl. Agric. For. Res. 2016, 66, 101–144. [Google Scholar] [CrossRef]
- Deutsches Maiskomitee e.V. Bestimmung Reifezahl. Available online: https://www.maiskomitee.de/Produktion/Sorten/Bestimmung-Reifezahl (accessed on 11 June 2023).
- Peni, D.; Dębowski, M.; Stolarski, M.J. Influence of the Fertilization Method on the Silphium perfoliatum Biomass Composition and Methane Fermentation Efficiency. Energies 2022, 15, 927. [Google Scholar] [CrossRef]
- Metzler & Brodmann Saaten GmbH. Das Silphiefaserprojekt. Available online: https://www.donau-silphie.de/faserprojekt/faserprojekt-1 (accessed on 6 November 2022).
- Čater, M.; Zorec, M.; Marinšek Logar, R. Methods for Improving Anaerobic Lignocellulosic Substrates Degradation for Enhanced Biogas Production. Springer Sci. Rev. 2014, 2, 51–61. [Google Scholar] [CrossRef]
- Vilela, A.E.; González-Paleo, L.; Ravetta, D.A.; Murrell, E.G.; van Tassel, D.L. Balancing Forage Production, Seed Yield, and Pest Management in the Perennial Sunflower Silphium integrifolium (Asteraceae). Agronomy 2020, 10, 1471. [Google Scholar] [CrossRef]
- von Cossel, M.; Amarysti, C.; Wilhelm, H.; Priya, N.; Winkler, B.; Hoerner, L. The replacement of maize (Zea mays L.) by cup plant (Silphium perfoliatum L.) as biogas substrate and its implications for the energy and material flows of a large biogas plant. Biofuels Bioprod. Biorefin. 2020, 14, 152–179. [Google Scholar] [CrossRef]
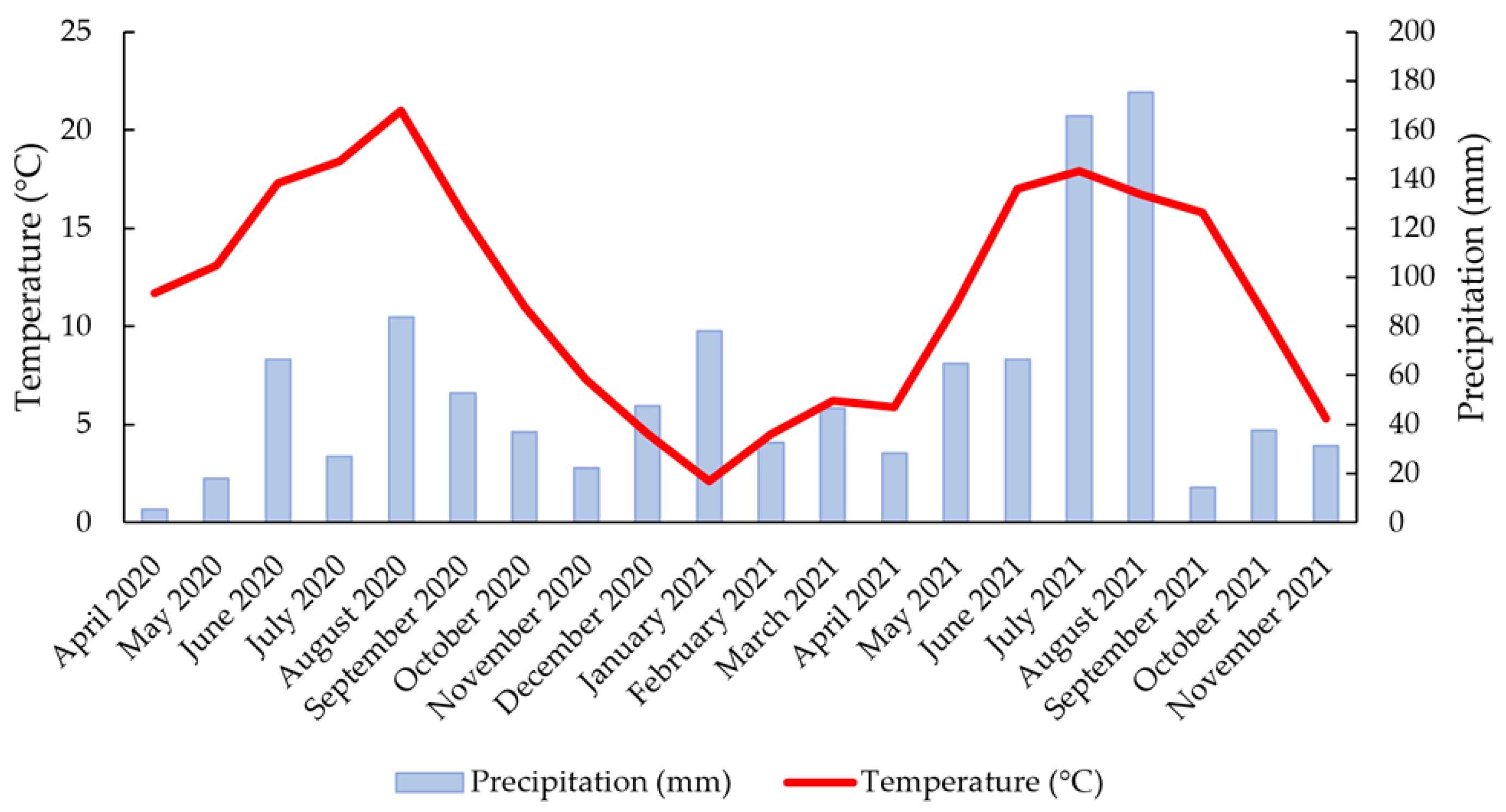

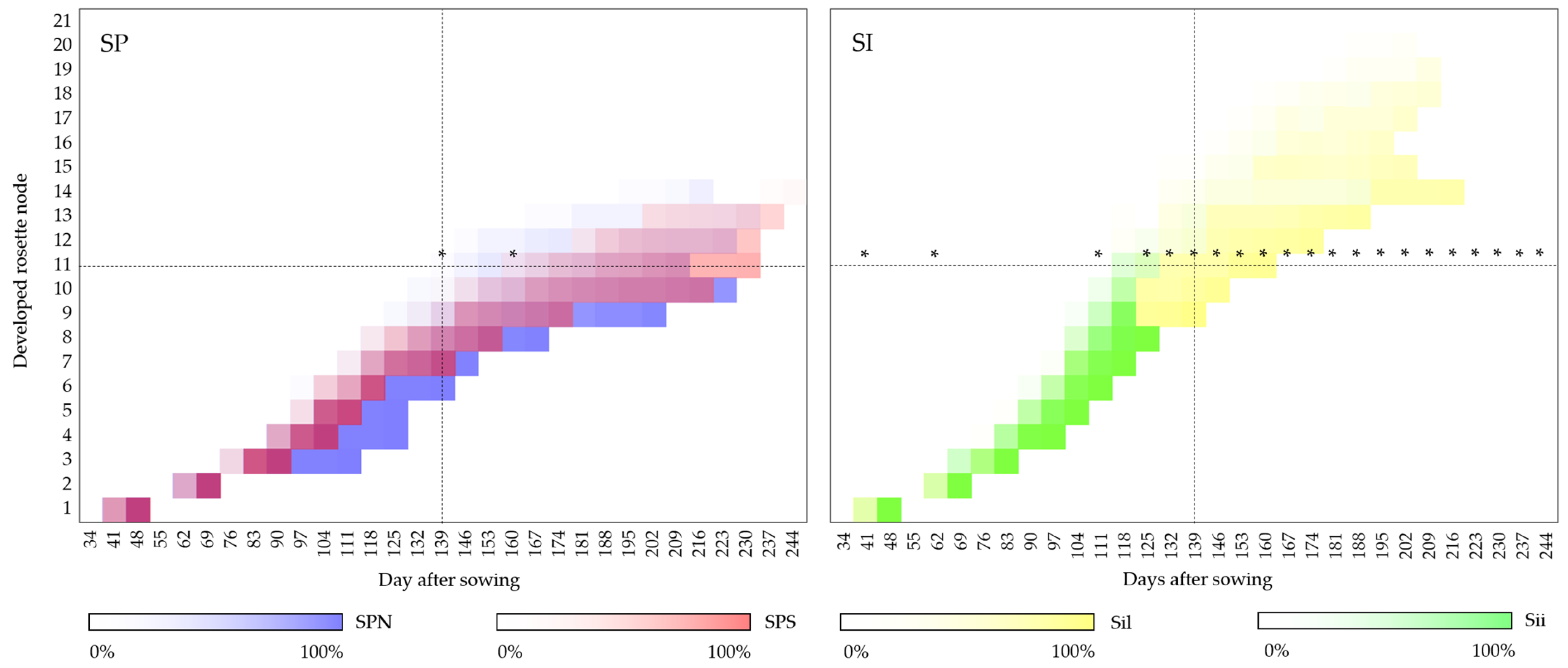



| Accession Name | Accession Description | Latitude (N) | Longitude (E) |
|---|---|---|---|
| SPS | Silphium perfoliatum south | 32.203 | −89.254 |
| SPN | Silphium perfoliatum north | 41.857 | −86.590 |
| Sii | Silphium integrifolium var. integrifolium | 41.776 | −86.404 |
| Sil | Silphium integrifolium var. laeve | 39.106 * | −96.576 * |
| Days after Sowing (DAS) | Gregorian Date | Developmental Occurrences | |
|---|---|---|---|
| First year | 0 | 22 April 2020 | Sowing |
| 34 | 26 May 2020 | Rosette development | |
| 111 | 11 August 2020 | Shoot development | |
| 146 | 15 September 2020 | Generative stage | |
| 195 | 3 November 2020 | Senescence | |
| Second year | 265 | 3 January 2021 | Rosette development |
| 376 | 3 May 2021 | Shoot development | |
| 426 | 22 June 2021 | Generative stage | |
| 502 | 6 September 2021 | Senescence | |
| 580 | 23 November 2021 | End of experiment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greve, M.; Korte, C.A.C.; Entrup, J.; Altrogge, H.; Bischoff, P.; Elfers, J.; Wever, C.; Pude, R. Evaluation of the Intra- and Interspecific Development of Different Accessions of Silphium perfoliatum L. and Silphium integrifolium Michx. Agronomy 2023, 13, 1601. https://doi.org/10.3390/agronomy13061601
Greve M, Korte CAC, Entrup J, Altrogge H, Bischoff P, Elfers J, Wever C, Pude R. Evaluation of the Intra- and Interspecific Development of Different Accessions of Silphium perfoliatum L. and Silphium integrifolium Michx. Agronomy. 2023; 13(6):1601. https://doi.org/10.3390/agronomy13061601
Chicago/Turabian StyleGreve, Martin, Christoph Anton Conrad Korte, Johanna Entrup, Hanna Altrogge, Philip Bischoff, Julian Elfers, Christian Wever, and Ralf Pude. 2023. "Evaluation of the Intra- and Interspecific Development of Different Accessions of Silphium perfoliatum L. and Silphium integrifolium Michx." Agronomy 13, no. 6: 1601. https://doi.org/10.3390/agronomy13061601
APA StyleGreve, M., Korte, C. A. C., Entrup, J., Altrogge, H., Bischoff, P., Elfers, J., Wever, C., & Pude, R. (2023). Evaluation of the Intra- and Interspecific Development of Different Accessions of Silphium perfoliatum L. and Silphium integrifolium Michx. Agronomy, 13(6), 1601. https://doi.org/10.3390/agronomy13061601






