Durum Wheat Field Performance and Stability in the Irrigated, Dry and Heat-Prone Environments of Sudan
Abstract
1. Introduction
2. Materials and Methods
2.1. Durum Wheat Genotypes
2.2. Experimental Locations and Conditions
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Effect of Environment on Grain Yield
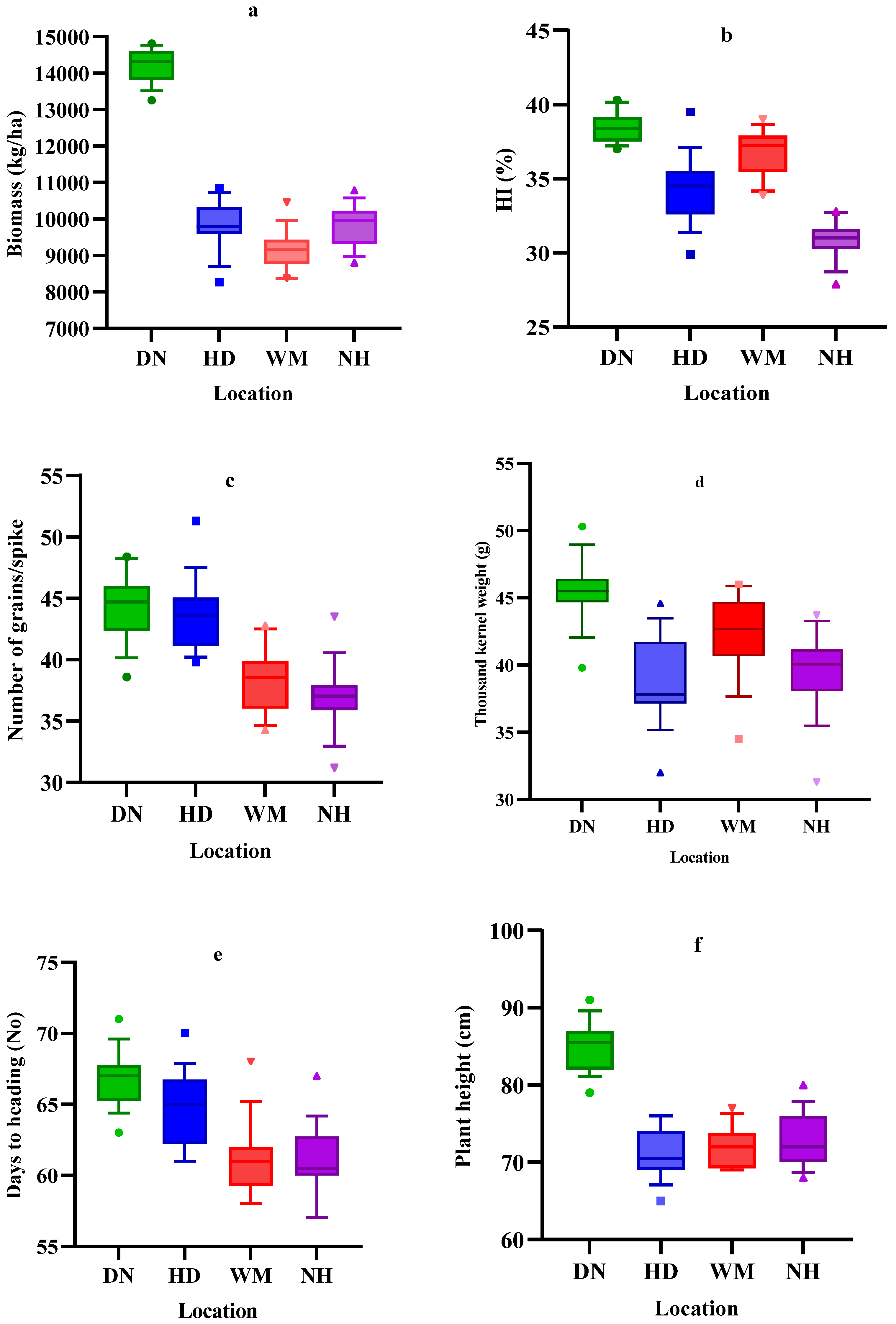
3.2. Grain Yield-Related Traits
3.3. Phenology and Morphology
3.4. Trait Association
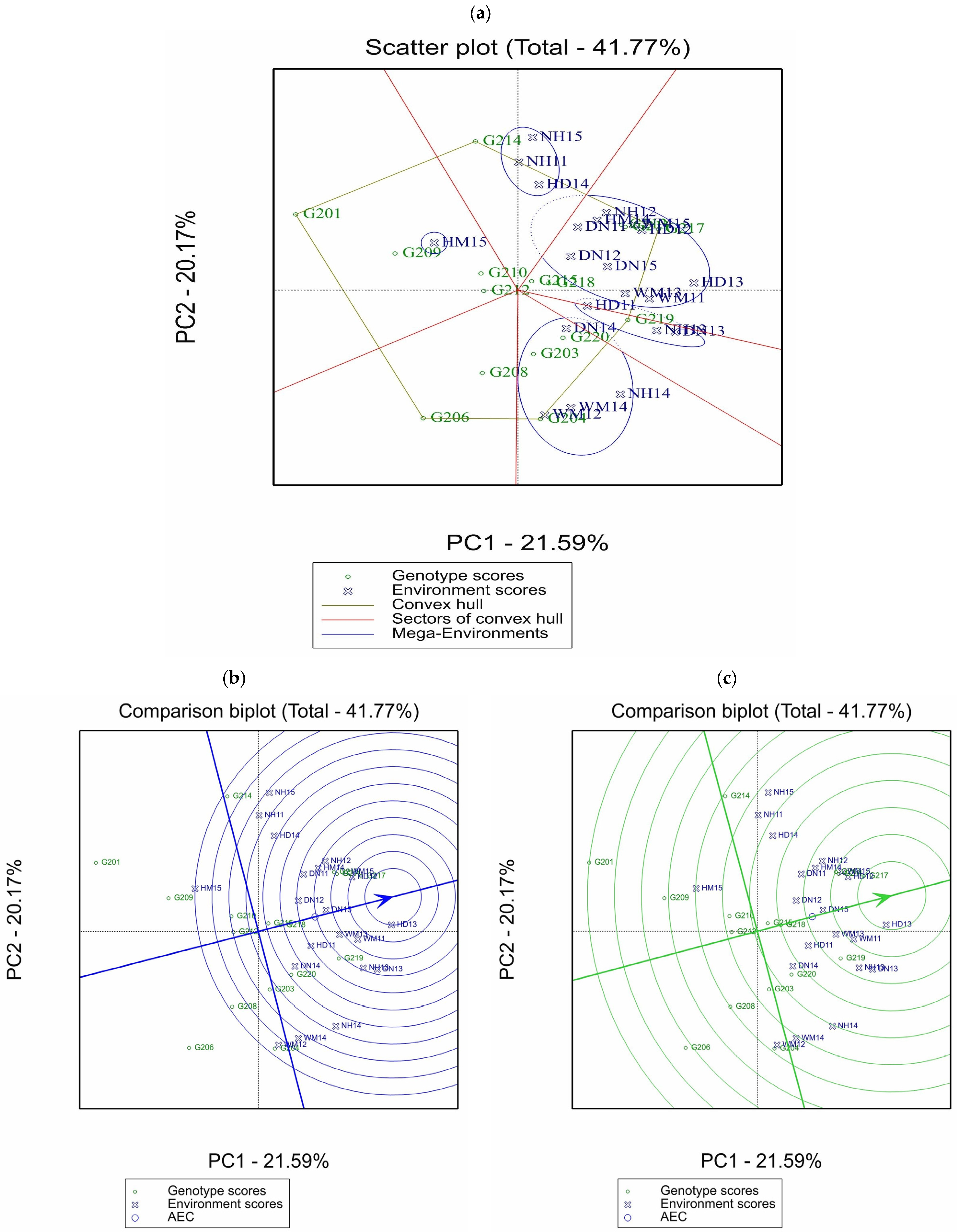
3.5. Genotype × Environment Interaction and the Stability of the Grain Yield
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Royo, C.; Maccaferri, M.; Álvaro, F.; Moragues, M.; Sanguineti, M.C.; Tuberosa, R.; Maalouf, F.; Del Moral, L.F.G.; Demontis, A.; Rhouma, S.; et al. Understanding the relationships between genetic and phenotypic structures of a collection of elite durum wheat accessions. Field Crops Res. 2010, 119, 91–105. [Google Scholar] [CrossRef]
- Abdalla, O.S.; Pena, R.J.; Autrique, J.E.; Nachit, M.M. Durum wheat breeding and quality improvement at CIMMYT Mexico. In Durum Wheat Quality in the Mediterranean Region; Di Fonzo, N., Kaan, F., Nachit, M., Eds.; CIHEAM: Zaragoza, Spain, 1995; pp. 133–141. [Google Scholar]
- Verma, S.R.; Yunus, M.; Sethi, S.K. Breeding for yield and quality in durum wheat. Euphytica 1998, 100, 15–18. [Google Scholar] [CrossRef]
- Bassi, F.M.; Sanchez-Garcia, M. Adaptation and Stability Analysis of ICARDA Durum Wheat Elites across 18 Countries. Crop Sci. 2017, 57, 2419–2430. [Google Scholar] [CrossRef]
- Martínez-Moreno, F.; Ammar, K.; Solís, I. Global Changes in Cultivated Area and Breeding Activities of Durum Wheat from 1800 to Date: A Historical Review. Agronomy 2022, 12, 1135. [Google Scholar] [CrossRef]
- Sukumaran, S.; Reynolds, M.P.; Sansaloni, C. Genome-Wide Association Analyses Identify QTL Hotspots for Yield and Component Traits in Durum Wheat Grown under Yield Potential, Drought, and Heat Stress Environments. Front. Plant Sci. 2018, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Ceglar, A.; Toreti, A.; Zampieri, M.; Royo, C. Global loss of climatically suitable areas for durum wheat growth in the future. Environ. Res. Lett. 2021, 16, 104049. [Google Scholar] [CrossRef]
- Xynias, I.N.; Mylonas, I.; Korpetis, E.G.; Ninou, E.; Tsaballa, A.; Avdikos, I.D.; Mavromatis, A.G. Durum wheat breeding in the Mediterranean region: Current status and future prospects. Agronomy 2020, 10, 432. [Google Scholar] [CrossRef]
- Subira, J.; Álvaro, F.; García, L.F.; Royo, C. Breeding effects on the cultivar × environment interaction of durum wheat yield. Eur. J. Agron. 2015, 68, 78–88. [Google Scholar] [CrossRef]
- Sheikh Mohamad, A.I. Performance of durum wheat genotypes in Northern Sudan. Rachis 1999, 18, 26–30. [Google Scholar]
- De Vita, P.; Mastrangelo, A.M.; Matteu, L.; Mazzucotelli, E.; Virzì, N.; Palumbo, M.; Storto, M.L.; Rizza, F.; Cattivelli, L. Genetic improvement effects on yield stability in durum wheat genotypes grown in Italy. Field Crops Res. 2010, 119, 68–77. [Google Scholar] [CrossRef]
- Kyratzis, A.C.; Pallides, A.; Katsiotis, A. Investigating Stability Parameters for Agronomic and Quality Traits of Durum Wheat Grown under Mediterranean Conditions. Agronomy 2022, 12, 1774. [Google Scholar] [CrossRef]
- Mazzucotelli, E.; Sciara, G.; Mastrangelo, A.M.; Desiderio, F.; Xu, S.S.; Faris, J.; Hayden, M.J.; Tricker, P.J.; Ozkan, H.; Echenique, V.; et al. The Global Durum Wheat Panel (GDP): An International Platform to Identify and Exchange Beneficial Alleles. Front. Plant Sci. 2020, 11, 9905. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Amri, A. Genotype × environment interaction and genetic improvement for yield and yield stability of rainfed durum wheat in Iran. Euphytica 2013, 192, 227–249. [Google Scholar] [CrossRef]
- Tahir, I.S.; Elbashier, E.M.; Mustafa, H.M.; Elhashimi, A.M.; Abdeldaim, M.G.; Hassan, M.K.; Saad, A.S.I.; Idris, A.A.M.; Hussein, A.H.A.; Elbashir, A.A.E.; et al. Field Performance and Stability of Durum Wheat Genotypes in the Irrigated Hot Environments of Sudan: A Proposal for the Release of Four Durum Wheat Varieties; National Variety Release Committee Meeting: Khartoum, Sudan, 2018.
- Mcmaster, G.S.; Wilhelm, W.W. Growing degree-days: One equation, two interpretations. Agric. For. Meteorol. 1997, 87, 300. [Google Scholar] [CrossRef]
- Fernandez, G. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and Other Food Crops in Temperature and Water Stress; Kuo, C.G., Ed.; Asian Vegetable Research and Development Center: Tainan, Taiwan, 1992; pp. 257–270. [Google Scholar]
- Gauch, H.G.; Piepho, H.P.; Annicchiarico, P. Statistical analysis of yield trials by AMMI and GGE: Further considerations. Crop Sci. 2008, 48, 866–889. [Google Scholar] [CrossRef]
- Purchase, J.L.; Hatting, H.; van Deventer, C.S. Genotype × environment interaction of winter wheat (Triticum aestivum L.) in South Africa: II. Stability analysis of yield performance. South Afr. J. Plant Soil 2000, 17, 101–107. [Google Scholar] [CrossRef]
- Farshadfar, E. Incorporation of AMMI Stability Value and Grain Yield in a Single Non-Parametric Index (GSI) in Bread Wheat. Pak. J. Biol. Sci. 2008, 11, 1791–1796. [Google Scholar] [CrossRef]
- Yan, W.; Tinker, N.A. Biplot analysis of multi-environment trial data: Principles and applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar] [CrossRef]
- VSN International. Genstat for Windows, 22nd ed.; VSN International: Hemel Hempstead, UK, 2023; Available online: http://www.genstat.co.uk (accessed on 10 May 2023).
- Iizumi, T.; Ali-Babiker, I.E.A.; Tsubo, M.; Tahir, I.S.A.; Kurosaki, Y.; Kim, W.; Gorafi, Y.S.A.; Idris, A.A.M.; Tsujimoto, H. Rising temperatures and increasing demand challenge wheat supply in Sudan. Nat. Food 2021, 2, 19–27. [Google Scholar] [CrossRef]
- Mohammadi, R.; Abdulahi, A.; Haghparast, R.; Armion, M. Interpreting genotype × environment interactions for durum wheat grain yields using nonparametric methods. Euphytica 2007, 157, 239–251. [Google Scholar] [CrossRef]
- Mohammadi, R.; Amri, A.; Haghparast, R.; Sadeghzadeh, D.; Armion, M.; Ahmadi, M.M. Pattern analysis of genotype-by-environment interaction for grain yield in durum wheat. J. Agric. Sci. 2009, 147, 537–545. [Google Scholar] [CrossRef]
- Chairi, F.; Aparicio, N.; Serret, M.D.; Araus, J.L. Breeding effects on the genotype × environment interaction for yield of durum wheat grown after the Green Revolution: The case of Spain. Crop J. 2020, 8, 623–634. [Google Scholar] [CrossRef]
- Sharifi, P.; Aminpanah, H.; Erfani, R.; Mohaddesi, A.; Abbasian, A. Evaluation of Genotype × Environment Interaction in Rice Based on AMMI Model in Iran. Rice Sci. 2017, 24, 173–180. [Google Scholar] [CrossRef]
- Senguttuvel, P.; Sravanraju, N.; Jaldhani, V.; Divya, B.; Beulah, P.; Nagaraju, P.; Manasa, Y.; Prasad, A.S.H.; Brajendra, P.; Gireesh, C.; et al. Evaluation of genotype by environment interaction and adaptability in lowland irrigated rice hybrids for grain yield under high temperature. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Azrai, M.; Efendi, R.; Muliadi, A.; Aqil, M.; Suwarti; Zainuddin, B.; Syam, A.; Junaedi; Syah, U.T.; Dermail, A.; et al. Genotype by Environment Interaction on Tropical Maize Hybrids Under Normal Irrigation and Waterlogging Conditions. Front. Sustain. Food Syst. 2022, 6, 913211. [Google Scholar] [CrossRef]
- Enyew, M.; Feyissa, T.; Geleta, M.; Tesfaye, K.; Hammenhag, C.; Carlsson, A.S. Genotype by environment interaction, correlation, AMMI, GGE biplot and cluster analysis for grain yield and other agronomic traits in sorghum (Sorghum bicolor L. Moench). PLoS ONE 2021, 16, e0258211. [Google Scholar] [CrossRef]
- Mwiinga, B.; Sibiya, J.; Kondwakwenda, A.; Musvosvi, C.; Chigeza, G. Genotype x environment interaction analysis of soybean (Glycine max (L.) Merrill) grain yield across production environments in Southern Africa. Field Crops Res. 2020, 256, 107922. [Google Scholar] [CrossRef]
- Lopes, M.; Reynolds, M.; Jalal-Kamali, M.; Moussa, M.; Feltaous, Y.; Tahir, I.; Barma, N.; Vargas, M.; Mannes, Y.; Baum, M. The yield correlations of selectable physiological traits in a population of advanced spring wheat lines grown in warm and drought environments. Field Crops Res. 2012, 128, 129–136. [Google Scholar] [CrossRef]
- Tahir, I.S.A.; Elbashier, E.M.E.; Ibrahim, M.A.S.; Saad, A.S.I.; Abdalla, O.S. Genetic Gain in Wheat Grain Yield and Nitrogen Use Efficiency at Different Nitrogen Levels in an Irrigated Hot Environment. Int. J. Agron. 2020, 2020, 9024671. [Google Scholar] [CrossRef]
- Achilli, A.L.; Roncallo, P.F.; Echenique, V. Genetic Gains in Grain Yield and Agronomic Traits of Argentinian Durum Wheat from 1934 to 2015. Agronomy 2022, 12, 2151. [Google Scholar] [CrossRef]
- Chairi, F.; Vergara-Diaz, O.; Vatter, T.; Aparicio, N.; Nieto-Taladriz, M.T.; Kefauver, S.C.; Bort, J.; Serret, M.D.; Araus, J.L. Post-green revolution genetic advance in durum wheat: The case of Spain. Field Crops Res. 2018, 228, 158–169. [Google Scholar] [CrossRef]
- Cooper, M.; Voss-Fels, K.P.; Messina, C.D.; Tang, T.; Hammer, G.L. Tackling G × E × M interactions to close on-farm yield-gaps: Creating novel pathways for crop improvement by predicting contributions of genetics and management to crop productivity. Theor. Appl. Genet. 2021, 134, 1625–1644. [Google Scholar] [CrossRef]
- Varshney, R.K.; Sinha, P.; Singh, V.K.; Kumar, A.; Zhang, Q.; Bennetzen, J.L. 5Gs for crop genetic improvement. Curr. Opin. Plant Biol. 2020, 56, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.P.; Quilligan, E.; Aggarwal, P.K.; Bansal, K.C.; Cavalieri, A.J.; Chapman, S.C.; Chapotin, S.M.; Datta, S.K.; Duveiller, E.; Gill, K.S.; et al. An integrated approach to maintaining cereal productivity under climate change. Glob. Food Secur. 2016, 8, 9–18. [Google Scholar] [CrossRef]
- Yadav, M.R.; Choudhary, M.; Singh, J.; Lal, M.K.; Jha, P.K.; Udawat, P.; Gupta, N.K.; Rajput, V.D.; Garg, N.K.; Maheshwari, C.; et al. Impacts, Tolerance, Adaptation, and Mitigation of Heat Stress on Wheat under Changing Climates. Int. J. Mol. Sci. 2022, 23, 2838. [Google Scholar] [CrossRef] [PubMed]
- Aberkane, H.; Amri, A.; Belkadi, B.; Filali-Maltouf, A.; Kehel, Z.; Tahir, I.S.A.; Meheesi, S.; Tsivelikas, A. Evaluation of durum wheat lines derived from interspecific crosses under drought and heat stress. Crop Sci. 2021, 61, 119–136. [Google Scholar] [CrossRef]
- Aberkane, H.; Belkadi, B.; Kehel, Z.; Filali-Maltouf, A.; Tahir, I.S.A.; Meheesi, S.; Amri, A. Assessment of drought and heat tolerance of durum wheat lines derived from interspecific crosses using physiological parameters and stress indices. Agronomy 2021, 11, 695. [Google Scholar] [CrossRef]
- Balla, M.Y.; Gorafi, Y.S.A.; Kamal, N.M.; Abdalla, M.G.A.; Tahir, I.S.A.; Tsujimoto, H. Exploiting Wild Emmer Wheat Diversity to Improve Wheat A and B Genomes in Breeding for Heat Stress Adaptation. Front. Plant Sci. 2022, 13, 895742. [Google Scholar] [CrossRef]
- Balla, M.Y.; Gorafi, Y.S.A.; Kamal, N.M.; Abdalla, M.G.A.; Tahir, I.S.A.; Tsujimoto, H. Harnessing the diversity of wild emmer wheat for genetic improvement of durum wheat. Theor. Appl. Genet. 2022, 135, 1671–1684. [Google Scholar] [CrossRef]
- Zaïm, M.; El Hassouni, K.; Gamba, F.; Filali-Maltouf, A.; Belkadi, B.; Sourour, A.; Amri, A.; Nachit, M.; Taghouti, M.; Bassi, F.M. Wide crosses of durum wheat (Triticum durum Desf.) reveal good disease resistance, yield stability, and industrial quality across Mediterranean sites. Field Crops Res. 2017, 214, 219–227. [Google Scholar] [CrossRef]
- Beres, B.L.; Rahmani, E.; Clarke, J.M.; Grassini, P.; Pozniak, C.J.; Geddes, C.M.; Porker, K.D.; May, W.E.; Ransom, J.K. A Systematic Review of Durum Wheat: Enhancing Production Systems by Exploring Genotype, Environment, and Management (G × E × M) Synergies. Front. Plant Sci. 2020, 11, 568657. [Google Scholar] [CrossRef] [PubMed]
- Tahir, A.I.S.; Elbashier, E.M.; Hussein, A.H.; Elhashimi, A.M.; Mustafa, H.M.; Sefyan Saad, A.I.; Abdeldaim, M.G.A.; Idris, A.A.M.; Hassan, M.K.; Elbashir, A.A.E.; et al. Agronomic Performance and Stability of Bread Wheat Genotypes under the Hot Environments of Sudan: A Proposal for the Release of Two Bread Wheat Varieties; National Variety Release Committee Meeting: Khartoum, Sudan, 2013.
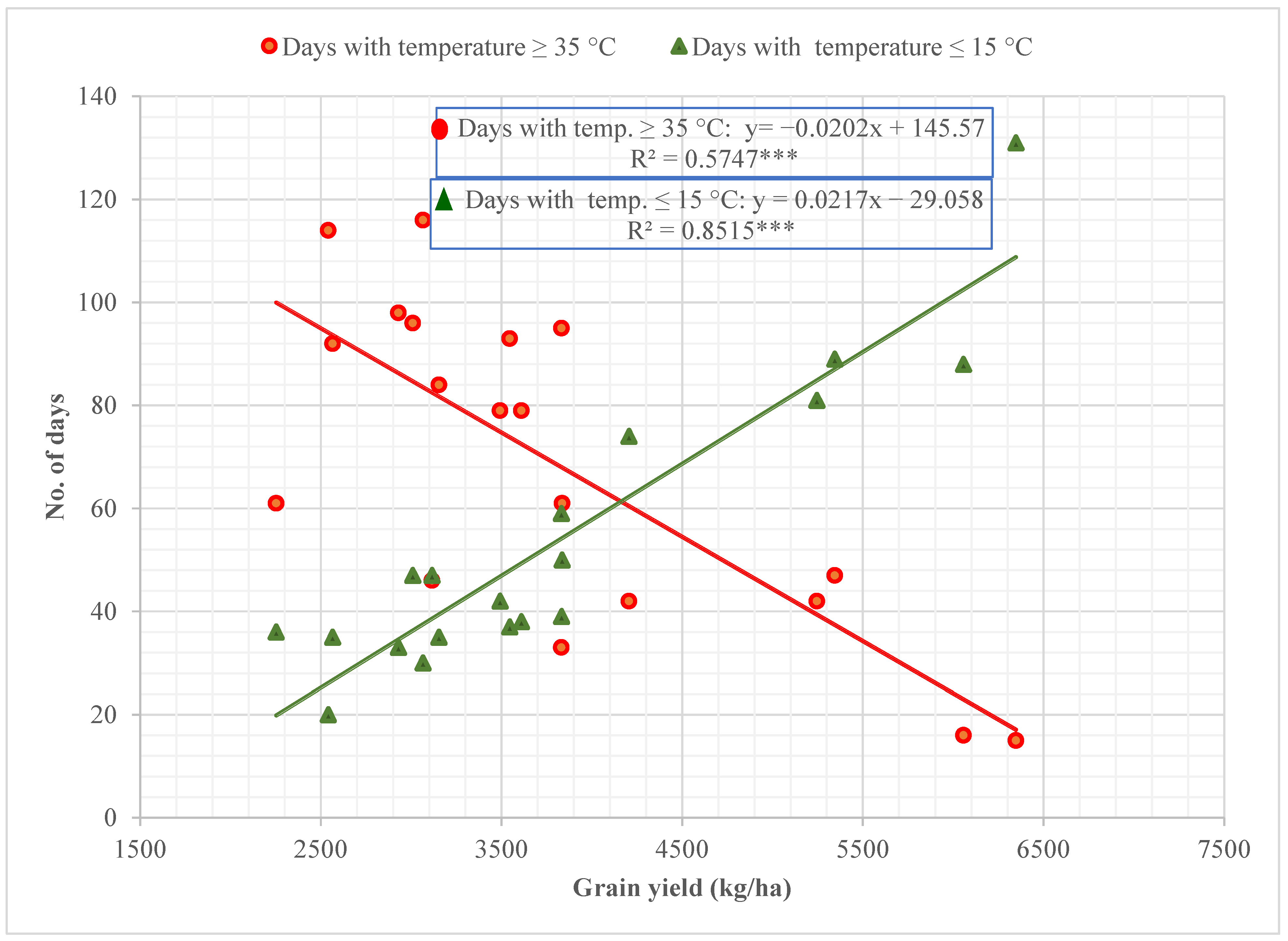

| Season | Experimental Station | Maximum Temperature °C | Days with Temperature ≥ 35 °C | Minimum Temperature °C | Days with Temperature ≤ 15 °C | Mean Temperature (Nov-Mar) °C | GDD to Mean Heading Time (TBase = 10 °C) |
|---|---|---|---|---|---|---|---|
| 2010/2011 | DN | 38.8 | 16 | 6.6 | 88 | 23.0 | 675.7 |
| HD | 39.5 | 46 | 8.4 | 47 | 24.9 | 816.0 | |
| WM | 42.2 | 96 | 7.2 | 47 | 26.7 | 890.6 | |
| NH | 40.8 | 98 | 10.6 | 33 | 26.7 | 896.5 | |
| 2011/2012 | DN | 43.0 | 15 | 4.0 | 131 | 20.8 | 632.7 |
| HD | 41.7 | 33 | 9.0 | 59 | 24.0 | 774.6 | |
| WM | 44.7 | 79 | 8.2 | 42 | 26.8 | 898.0 | |
| NH | 43.8 | 92 | 11.8 | 35 | 26.5 | 885.2 | |
| 2012/2013 | DN | 42.1 | 47 | 5.2 | 89 | 23.1 | 688.3 |
| HD | 41.5 | 61 | 9.0 | 36 | 25.5 | 838.7 | |
| WM | 43.5 | 84 | 8.6 | 35 | 27.1 | 870.7 | |
| NH | 43.5 | 114 | 10.0 | 20 | 27.5 | 918.2 | |
| 2013/2014 | DN | 43.5 | 42 | 6.2 | 81 | 22.9 | 644.5 |
| HD | 42.0 | 61 | 10.0 | 50 | 24.9 | 831.7 | |
| WM | 44.0 | 79 | 8.6 | 38 | 27.1 | 964.4 | |
| NH | 42.5 | 93 | 8.5 | 37 | 26.8 | 834.5 | |
| 2014/2015 | DN | 44.5 | 42 | 2.9 | 74 | 23.2 | 647.5 |
| HD | 44.5 | 76 | 6.7 | 39 | 25.7 | 843.6 | |
| WM | 44.2 | 95 | 4.8 | 39 | 27.5 | 856.8 | |
| NH | 43.6 | 116 | 8.0 | 30 | 27.3 | 866.1 |
| Genotype Code | Name/Cross | Pedigree/Selection History | Source | BLUPs (kg/ha) | % of Check | ASV | GSI |
|---|---|---|---|---|---|---|---|
| G201 | CHAM-1 | Check | Released variety | 3522 | 100 | 25.0 | 26 |
| G203 | Beltagy-2 | ICD97-0396-T-1AP-AP-5AP-0AP-16AP-AP-0SDN | ICARDA | 3771 | 107 | 18.5 | 16 |
| G204 | Gcn/4/D68-1-93A-1A//Ruff/Fg/3/Mtl-5 | ICD95-1302-C-3AP-0AP-1AP-0AP-5AP-AP-3AP-0AP-0SDN | ICARDA | 3760 | 107 | 28.5 | 24 |
| G206 | Msbl-1//Krf/Hcn | ICD95-1133-T-0AP-1AP-0AP-3AP-0TR-2AP-AP-0SDN | ICARDA | 3599 | 102 | 14.9 | 22 |
| G208 | Aghrass-1/Bezaiz98-1 | ICD00-0018-T-21AP-AP-4AP-TR-0SDN | ICARDA | 3730 | 106 | 26.4 | 24 |
| G209 | Maamouri-2/CI115/5/F4 13 J.S/3/Arthur71/Lahn//Blk2/Lahn/4/Quarmal | ICD02-0601-T-TR-7AP-0TR-0SDN | ICARDA | 3719 | 106 | 35.1 | 29 |
| G210 | DCD DW 7/Ter-1 | ICD01-0039-T-14AP-TR-9AP-0AP-0SDN | ICARDA | 3752 | 107 | 26.5 | 24 |
| G211 | SU-ORDEGL3/3/Ch5/20048Traikia (Mor)//STJ3 | ACSAD-8835-27IZ-3IZ-2IZ-0IZ-0SDN | ACSAD | 3952 | 112 | 13.2 | 9 |
| G212 | Mrf1/Stj2//Bcrch1 | ICD99-0027-C-0AP-2AP-AP-5AP-AP-0SDN | ICARDA | 3768 | 107 | 4.7 | 11 |
| G213 | Ter-1//Mrf1/Stj2 | ICD99-0866-C-0AP-5AP-AP-4AP-AP-0SDN | ICARDA | 3813 | 108 | 8.8 | 11 |
| G214 | Ter-1/3/Stj3//Bcr/Lks4 | ICD99-1036-T-0AP-8AP-AP-9AP-AP-0SDN | ICARDA | 3797 | 108 | 37.7 | 23 |
| G215 | TARRO | NA | IRAN | 3771 | 107 | 8.2 | 13 |
| G217 | SNITAN*2/RBC | CGSS01B00031T-099Y-099B-099B-1Y-0B-1Y-0B-0SDN | CIMMYT | 4132 | 117 | 6.9 | 4 |
| G218 | P91.272.3.1/3*MEXI75/3/2*STOT//ALTAR 84/ALD | CGSS02Y00103T-099B-099B-52Y-0B-1Y-0B-0SDN | CIMMYT | 3832 | 109 | 30.7 | 19 |
| G219 | CBC 509 CHILE/5/2*AJAIA_16 //HORA/JRO/3/GAN/4/ZAR | CDSS02Y01222T-0TOPB-0Y-0M-1Y-0Y-0SDN | CIMMYT | 3957 | 112 | 20.2 | 11 |
| G220 | HAI-OU_17/PLATA_2//LIRO_3/3/RYPS27_3/SKARV_3 | CDSS02Y00294S-0Y-0M-25Y-0Y-0SDN | CIMMYT | 3835 | 109 | 5.2 | 6 |
| Mean | 3794 | ||||||
| SE± | 71.3 |
| Source of Variation | DF | SS | MS | F | Percent Explained |
|---|---|---|---|---|---|
| Genotypes (G) | 15 | 21233542 | 1415569 | 4.79 *** | 1.3 |
| Environments (E) | 23 | 1515389211 | 65886487 | 46.25 *** | 90.0 |
| Block | 48 | 68376251 | 1424505 | 4.82 *** | |
| GE Interactions | 345 | 146873104 | 425719 | 1.44 *** | 8.7 |
| IPCA1 | 37 | 30182564 | 815745 | 2.76 *** | 20.6 |
| IPCA2 | 35 | 24066602 | 687617 | 2.32 *** | 16.4 |
| IPCA3 | 33 | 18013662 | 545869 | 1.85 ** | 12.3 |
| IPCA4 | 31 | 16765548 | 540824 | 1.83 ** | 11.4 |
| Residuals | 209 | 57844728 | 276769 | 0.94 NS | |
| Error | 720 | 212985199 | 295813 | ||
| Total | 1151 | 1964857307 | 1707087 |
| Number | Environment § | Estimated Yield (kg/ha) | Score | Best Four Genotypes | |||
|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | ||||
| 1 | DN11 | 6057 | −8.939 | G217 | G214 | G219 | G209 |
| 2 | DN12 | 6347 | 7.292 | G214 | G210 | G217 | G219 |
| 3 | DN13 | 5344 | 11.041 | G219 | G217 | G213 | G204 |
| 4 | DN14 | 5244 | 8.359 | G204 | G220 | G203 | G217 |
| 5 | DN15 | 4205 | −6.192 | G218 | G217 | G219 | G209 |
| 6 | HM14 | 5999 | 1.854 | G210 | G214 | G217 | G211 |
| 7 | HM15 | 3707 | −22.171 | G209 | G218 | G201 | G217 |
| 8 | HD11 | 3115 | 17.74 | G210 | G208 | G211 | G213 |
| 9 | HD12 | 3831 | −10.139 | G211 | G213 | G218 | G217 |
| 10 | HD13 | 2253 | 8.666 | G211 | G213 | G219 | G217 |
| 11 | HD14 | 3835 | −19.468 | G214 | G211 | G217 | G209 |
| 12 | KR13 | 3008 | −14.873 | G219 | G218 | G217 | G209 |
| 13 | KR14 | 3748 | 16.539 | G219 | G208 | G217 | G212 |
| 14 | WM11 | 3008 | −0.423 | G211 | G213 | G217 | G218 |
| 15 | WM12 | 3492 | 10.835 | G204 | G220 | G203 | G217 |
| 16 | WM13 | 3153 | −0.487 | G211 | G217 | G220 | G204 |
| 17 | WM14 | 3610 | 14.413 | G204 | G220 | G203 | G217 |
| 18 | WM15 | 3832 | −3.591 | G211 | G217 | G213 | G219 |
| 19 | ML13 | 2631 | −1.416 | G219 | G218 | G213 | G217 |
| 20 | NH11 | 2929 | −13.229 | G211 | G214 | G217 | G213 |
| 21 | NH12 | 2565 | −2.111 | G217 | G214 | G219 | G208 |
| 22 | NH13 | 2541 | 5.132 | G219 | G217 | G211 | G213 |
| 23 | NH14 | 3545 | 14.732 | G219 | G204 | G218 | G217 |
| 24 | NH15 | 3065 | −13.566 | G214 | G217 | G211 | G210 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahir, I.S.A.; Elbashier, E.M.E.; Mustafa, H.M.; Elhashimi, A.M.A.; Abdalla, M.G.A.; Hassan, M.K.; Saad, A.S.I.; Elbashir, A.A.E.; Elsheikh, O.; Meheesi, S. Durum Wheat Field Performance and Stability in the Irrigated, Dry and Heat-Prone Environments of Sudan. Agronomy 2023, 13, 1598. https://doi.org/10.3390/agronomy13061598
Tahir ISA, Elbashier EME, Mustafa HM, Elhashimi AMA, Abdalla MGA, Hassan MK, Saad ASI, Elbashir AAE, Elsheikh O, Meheesi S. Durum Wheat Field Performance and Stability in the Irrigated, Dry and Heat-Prone Environments of Sudan. Agronomy. 2023; 13(6):1598. https://doi.org/10.3390/agronomy13061598
Chicago/Turabian StyleTahir, Izzat S. A., Elfadil M. E. Elbashier, Hala M. Mustafa, Ashraf M. A. Elhashimi, Modather G. A. Abdalla, Mohamed K. Hassan, Abu Sefyan I. Saad, Awad A. E. Elbashir, Omer Elsheikh, and Sara Meheesi. 2023. "Durum Wheat Field Performance and Stability in the Irrigated, Dry and Heat-Prone Environments of Sudan" Agronomy 13, no. 6: 1598. https://doi.org/10.3390/agronomy13061598
APA StyleTahir, I. S. A., Elbashier, E. M. E., Mustafa, H. M., Elhashimi, A. M. A., Abdalla, M. G. A., Hassan, M. K., Saad, A. S. I., Elbashir, A. A. E., Elsheikh, O., & Meheesi, S. (2023). Durum Wheat Field Performance and Stability in the Irrigated, Dry and Heat-Prone Environments of Sudan. Agronomy, 13(6), 1598. https://doi.org/10.3390/agronomy13061598







