Hydroponic Optimization and Screening of Aluminum Tolerance on Finger Millet (Eleusine coracana (L.) Gaertn.) Accessions and Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Germination Conditions
2.2. Hydroponics Experimental Setup
2.3. Nutrient Solution Culture and Treatment
2.4. Screening of Accessions under Hydroponic Assay
2.5. Data Recording and Analysis
3. Results
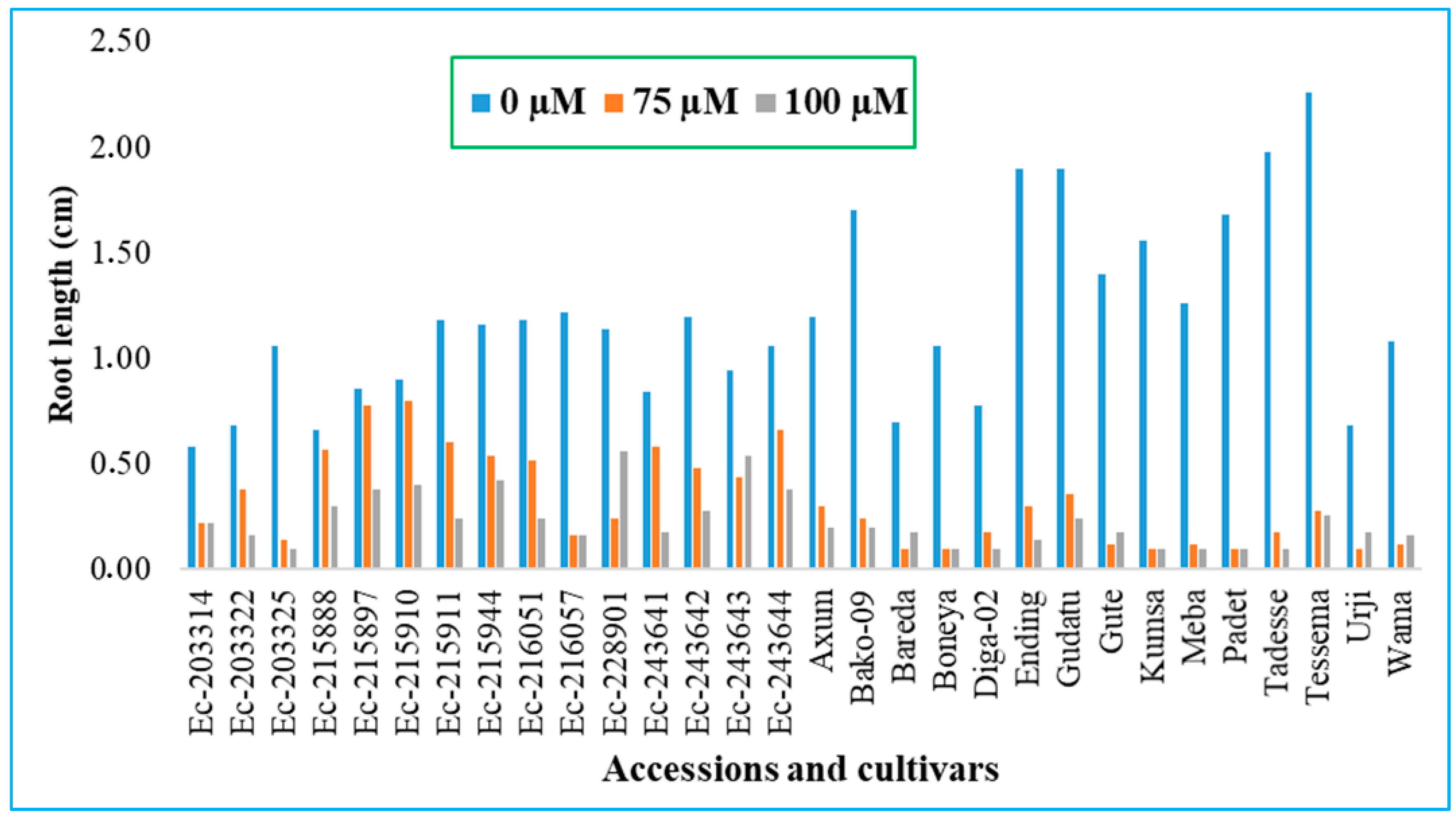
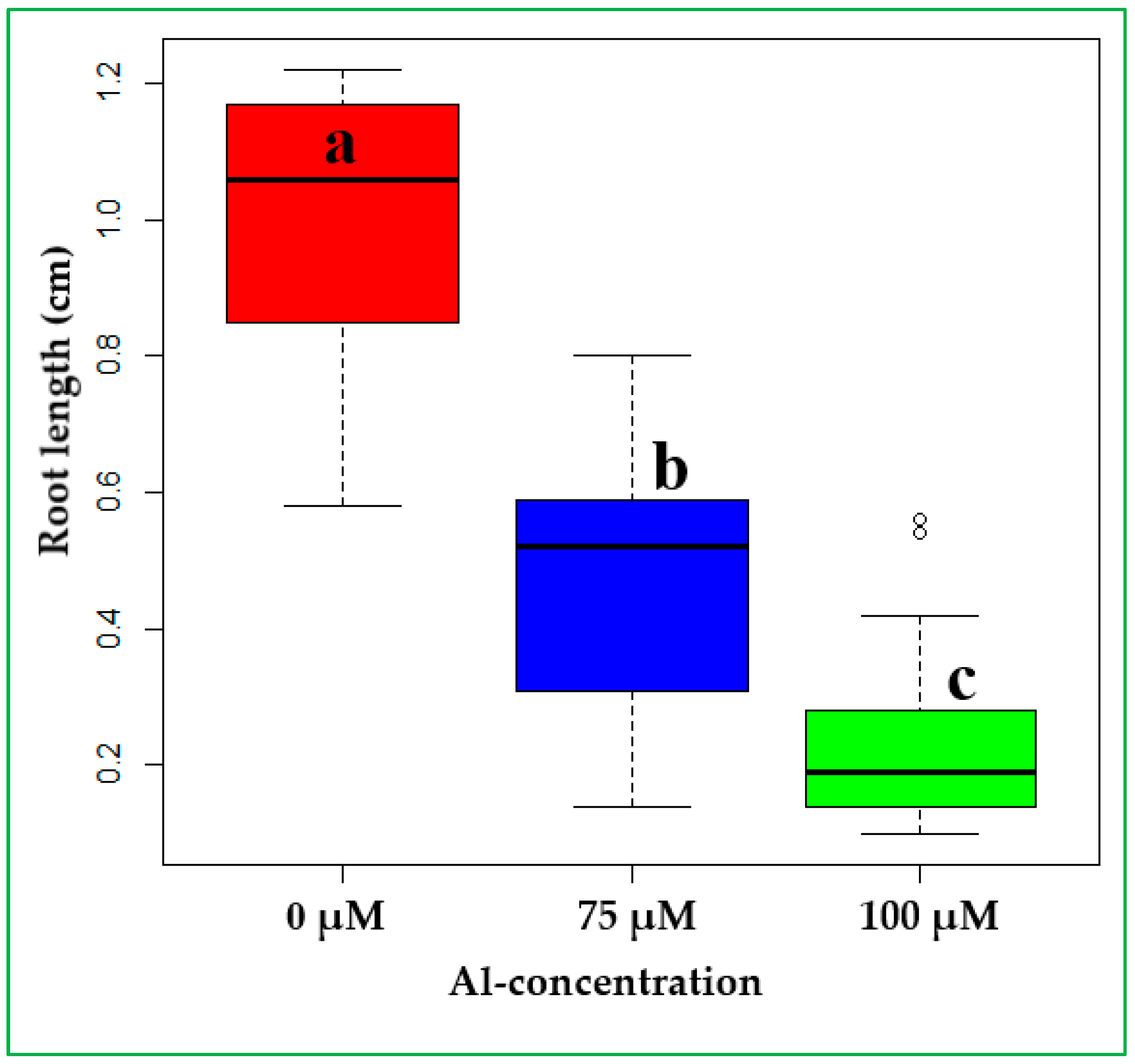
3.1. Optimizing Threshold Level of Al-Toxicity on Finger Millet
3.2. Screening Finger Millet Accessions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Backiyalakshmi, C.; Babu, C.; Reddy, D.N.; Padmakumar, V.; Prasad, K.V.S.V.; Azevedo, V.C.R.; Vetriventhan, M. Assessing Forage Potential of the Global Collection of Finger Millet (Eleusine coracana (L.) Gaertn.) Germplasm Conserved at the ICRISAT Genebank. Agronomy 2021, 11, 1706. [Google Scholar] [CrossRef]
- Hilu, K.W.; De Wet, J.M.J. Domestication of Eleusine coracana. Econ. Bot. 1976, 30, 199–208. [Google Scholar] [CrossRef]
- Goron, T.L.; Raizada, M.N. Genetic diversity and genomic resources available for the small millet crops to accelerate a New Green Revolution. Front. Plant Sci. 2015, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Fakrudin, B.; Kulkarni, R.S.; Shashidhar, H.E.; Hittalmani, S.; Food and Agriculture Organization. Genetic diversity assessment of finger millet, Eleusine coracana (Gaertn), germplasm through RAPD analysis. PGR Newslett. 2004, 138, 50–54. [Google Scholar]
- Admassu, S.; Teamir, M.; Alemu, D. Chemical composition of local and improved finger Millet [Eleusine corocana (L.) Gaetrtin] varieties grown in Ethiopia. Ethiop. J. Health Sci. 2009, 19, 1–8. [Google Scholar]
- Kinfe, H.; Yiergalem, T.; Alem, R.; Redae, W.; Desalegn, Y.; Welegerima, G. Yield performance and adaptability of finger millet landrace in North Western Tigray, Ethiopia. World News Nat. Sci. 2017, 15, 98–111. [Google Scholar]
- Seyoum, A.; Semahegn, Z.; Nega, A.; Gebreyohannes, A. AMMI and GGE Analysis of G × E and yield stability of finger millet [Eleusine coracana (L.) Gaertn] genotypes in Ethiopia. Int. J. Trend Res. 2019, 6, 379–386. [Google Scholar]
- Chauhan, E.S.; Sarita, S. Effects of processing (germination and popping) on the nutritional and anti-nutritional properties of finger millet (Eleusine coracana). Curr. Res. Nutr. Food Sci. J. 2018, 6, 566–572. [Google Scholar] [CrossRef]
- Adugna, A.; Tesso, T.; Degu, E.; Tadesse, T.; Merga, F.; Legesse, W.; Tirfessa, A.; Kidane, H.; Wole, A.; Daba, C. Genotype-by-environment interaction and yield stability analysis in finger millet (Elucine coracana L. Gaertn) in Ethiopia. Am. J. Plant Sci. 2011, 2, 408–415. [Google Scholar] [CrossRef]
- Bezaweletaw, K. Genetic diversity of finger millet [Eleusine coracana (L.) Gaertn] landraces characterized by random amplified polymorphic DNA analysis. Innov. Syst. Des. Eng. 2011, 2, 207–218. [Google Scholar]
- Semahegn, Z.; Teressa, T.; Bejiga, T. Finger millet [Eleusinecoracana (L.) Gaertn] breeding in Ethiopia: A review article. Int. J. Res. Stud. Agric. Sci. 2021, 7, 38–42. [Google Scholar] [CrossRef]
- Lule, D.; de Villiers, S.; Fetene, M.; Bogale, T.; Alemu, T.; Geremew, G.; Gashaw, G.; Tesfaye, K. Pathogenicity and yield loss assessment caused by Magnaporthe oryzae isolates in cultivated and wild relatives of finger millet (Eleusine coracana). Indian J. Agric. Res. 2014, 48, 258–268. [Google Scholar] [CrossRef]
- Von Uexküll, H.R.; Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef]
- Kochian, L.V.; Piñeros, M.A.; Hoekenga, O.A. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 2005, 274, 175–195. [Google Scholar] [CrossRef]
- Nogueirol, R.C.; Monteiro, F.A.; Azevedo, R.A. Tropical soils cultivated with tomato: Fractionation and speciation of Al. Environ. Monit. Assess. 2015, 187, 160. [Google Scholar] [CrossRef]
- Gupta, N.; Gaurav, S.S.; Kumar, A. Molecular basis of aluminium toxicity in plants: A review. Am. J. Plant Sci. 2013, 4, 21–37. [Google Scholar] [CrossRef]
- Bose, J.; Babourina, O.; Ma, Y.; Zhou, M.; Shabala, S.; Rengel, Z. Specificity of ion uptake and homeostasis maintenance during acid and aluminium stresses. In Aluminum Stress Adaptation in Plants; Springer: Berlin/Heidelberg, Germany, 2015; pp. 229–251. [Google Scholar]
- Doncheva, S.; Amenós, M.; Poschenrieder, C.; Barceló, J. Root cell patterning: A primary target for aluminium toxicity in maize. J. Exp. Bot. 2005, 56, 1213–1220. [Google Scholar] [CrossRef]
- Mitchell, J.K.; Soga, K. Fundamentals of Soil Behavior; John Wiley & Sons: New York, NY, USA, 2005; Volume 3. [Google Scholar]
- Abdenna, D.; Negassa, C.; Tilahun, G. Inventory of soil acidity status in crop lands of Central and Western Ethiopia. In Utilisation of Diversity in Land Use Systems: Sustainable and Organic Approaches to Meet Human Needs; Tropentag: Berlin, Germany, 2007. [Google Scholar]
- Mendes, P.; Farina, M.P.; Channon, P. Assessment of aluminium tolerance in maize using a rapid screening procedure. S. Afr. J. Plant Soil 1984, 1, 83–86. [Google Scholar] [CrossRef]
- Haftom, B.; Edossa, F.; Teklehaimanot, H.; Kassahun, T. Hydroponic screening and characterization of aluminium tolerance on finger millet (Eleusine coracana (L.) Gaertn) accessions. J. Agric. Biotechnol. Sustain. Dev. 2018, 10, 34–44. [Google Scholar] [CrossRef]
- Delhaize, E.; Ryan, P.R. Aluminum toxicity and tolerance in plants. Plant Physiol. 1995, 107, 315. [Google Scholar] [CrossRef] [PubMed]
- Fikiru, E.; Tesfaye, K.; Bekele, E. Morphological and molecular variation in Ethiopian lentil (Lens culinaris Medikus) varieties. Int. J. Genet. Mol. Biol. 2011, 3, 60–67. [Google Scholar]
- Gallego, F.J.; Benito, C. Genetic control of aluminium tolerance in rye (Secale cereale L.). Theor. Appl. Genet. 1997, 95, 393–399. [Google Scholar] [CrossRef]
- Negusse, H.; Cook, D.R.; Haileselassie, T.; Tesfaye, K. Identification of Aluminum Tolerance in Ethiopian Chickpea (Cicer arietinum L.). Germplasm. Agron. 2022, 12, 948. [Google Scholar] [CrossRef]
- Delhaize, E.; Ryan, P.R.; Hebb, D.M.; Yamamoto, Y.; Sasaki, T.; Matsumoto, H. Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proc. Natl. Acad. Sci. USA 2004, 101, 15249–15254. [Google Scholar] [CrossRef] [PubMed]
- Hede, A.R.; Skovmand, B.; Ribaut, J.-M.; Gonzalez-De-Leon, D.; Stolen, O. Evaluation of aluminium tolerance in a spring rye collection by hydroponic screening. Plant Breed. 2002, 121, 241–248. [Google Scholar] [CrossRef]
- Echart, C.L.; Barbosa-Neto, J.F.; Garvin, D.F.; Cavalli-Molina, S. Aluminum tolerance in barley: Methods for screening and genetic analysis. Euphytica 2002, 126, 309–313. [Google Scholar] [CrossRef]
- Gupta, S.M.; Arora, S.; Mirza, N.; Pande, A.; Lata, C.; Puranik, S.; Kumar, J.; Kumar, A. Finger millet: A “certain” crop for an “uncertain” future and a solution to food insecurity and hidden hunger under stressful environments. Front. Plant Sci. 2017, 8, 643. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Singh, D.; Iquebal, M.A. Selection of pigeonpea genotypes for tolerance to aluminium toxicity. Plant Breed. 2011, 130, 492–495. [Google Scholar] [CrossRef]



| Source | DFZ | MS | |
|---|---|---|---|
| Root Length | Shoot Length | ||
| Concentration | 2 | 8.5 *** | 0.003 ns |
| Residuals | 87 | 0.08 | 0.04 |
| Environmental variance | 2.00 | 42.03 ** | 0.02 ns |
| Replication variance | 12.00 | 0.15 ** | 0.04 ns |
| Genotypic variance | 29.00 | 0.37 ** | 0.18 ** |
| Genotypic X Environment | 58.00 | 0.46 ** | 0.26 ** |
| Residuals | 348.0 | 0.05 | 0.10 |
| No. | Acc. | RRL (%) | RSL (%) | PC | No. | Acc. | RRL (%) | RSL (%) | PC | No. | Acc. | RRL (%) | RSL (%) | PC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 215836 | 127.9 | 98.2 | T | 26 | 215847 | 71.9 | 126.7 | I | 51 | 216031 | 57.6 | 105.4 | I |
| 2 | 215845 | 120.9 | 110.0 | T | 27 | 215896 | 71.4 | 132.5 | I | 52 | 242118 | 57.1 | 59.2 | I |
| 3 | 229722 | 113.3 | 106.7 | T | 28 | 238306 | 70.0 | 81.8 | I | 53 | 215883 | 56.6 | 103.6 | I |
| 4 | 215919 | 107.0 | 41.9 | T | 29 | 215840 | 69.8 | 95.1 | I | 54 | 242610 | 55.9 | 44.6 | I |
| 5 | 216023 | 101.8 | 58.6 | T | 30 | 203336 | 69.8 | 140.0 | I | 55 | 238299 | 55.6 | 135.4 | I |
| 6 | 207962 | 97.8 | 66.7 | T | 31 | 237443 | 68.9 | 91.5 | I | 56 | 215936 | 54.9 | 50.0 | I |
| 7 | 215905 | 93.9 | 47.2 | T | 32 | 215996 | 68.3 | 55.9 | I | 57 | 238342 | 54.8 | 94.5 | I |
| 8 | 203356 | 92.5 | 48.3 | T | 33 | 203364 | 67.7 | 91.7 | I | 58 | 240506 | 54.7 | 64.4 | I |
| 9 | 216055 | 91.9 | 25.0 | T | 34 | 212134 | 67.3 | 51.7 | I | 59 | 203314 | 54.2 | 68.7 | I |
| 10 | 215875 | 90.9 | 47.9 | T | 35 | 203343 | 66.7 | 47.0 | I | 60 | 234147 | 53.6 | 61.5 | I |
| 11 | 215994 | 90.3 | 43.8 | T | 36 | 225893 | 65.9 | 70.8 | I | 61 | 215888 | 53.3 | 136.6 | I |
| 12 | 215841 | 90.0 | 103.8 | T | 37 | 242621 | 64.7 | 70.0 | I | 62 | 215914 | 52.6 | 59.3 | I |
| 13 | 216034 | 86.1 | 68.1 | T | 38 | 235156 | 64.4 | 23.6 | I | 63 | 237971 | 52.6 | 133.3 | I |
| 14 | 216027 | 84.5 | 83.3 | T | 39 | 215930 | 64.3 | 56.1 | I | 64 | 215860 | 52.0 | 85.9 | I |
| 15 | 100093 | 82.9 | 96.5 | T | 40 | 215945 | 63.5 | 53.7 | I | 65 | 238317 | 51.1 | 56.6 | I |
| 16 | 215906 | 81.4 | 89.6 | T | 41 | 245086 | 62.9 | 70.9 | I | 66 | 229724 | 51.0 | 84.0 | I |
| 17 | 215852 | 80.9 | 47.5 | T | 42 | 215831 | 62.1 | 39.6 | I | 67 | 208725 | 50.8 | 55.7 | I |
| 18 | 215868 | 80.6 | 115.1 | T | 43 | 208730 | 61.8 | 46.9 | I | 68 | 215871 | 50.0 | 90.3 | I |
| 19 | 215827 | 80.4 | 47.5 | T | 44 | 243643 | 60.5 | 91.9 | I | 69 | 215944 | 50.0 | 69.0 | I |
| 20 | 245084 | 79.4 | 50.7 | T | 45 | 242116 | 60.3 | 29.8 | I | 70 | 203368 | 50.0 | 83.6 | I |
| 21 | 215910 | 78.9 | 64.7 | I | 46 | 237970 | 60.0 | 62.6 | I | 71 | 215902 | 50.0 | 85.1 | I |
| 22 | 219827 | 75.5 | 37.0 | I | 47 | 215957 | 59.6 | 75.5 | I | 72 | 215880 | 50.0 | 58.9 | I |
| 23 | 242114 | 75.0 | 51.7 | I | 48 | 215877 | 59.0 | 34.2 | I | 73 | 203311 | 49.2 | 41.7 | I |
| 24 | 215942 | 74.1 | 31.1 | I | 49 | 208445 | 58.6 | 63.4 | I | 74 | 215933 | 49.1 | 103.8 | I |
| 25 | 216030 | 73.8 | 59.7 | I | 50 | 215829 | 58.0 | 100.0 | I | 75 | 216025 | 49.0 | 53.3 | I |
| No. | Acc. | RRL (%) | RSL (%) | PC | No. | Acc. | RRL (%) | RSL (%) | PC | No. | Acc. | RRL (%) | RSL (%) | PC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 76 | 211553 | 48.3 | 88.0 | I | 101 | 215870 | 41.9 | 90.2 | I | 126 | 215927 | 37.2 | 85.4 | I |
| 77 | 211506 | 48.3 | 49.0 | I | 102 | 203363 | 41.2 | 38.3 | I | 127 | 215890 | 36.7 | 116.4 | I |
| 78 | 215886 | 47.5 | 92.0 | I | 103 | 208444 | 41.2 | 110.5 | I | 128 | 215961 | 36.2 | 56.9 | I |
| 79 | 243635 | 47.5 | 56.7 | I | 104 | 242135 | 41.0 | 85.4 | I | 129 | 215943 | 36.0 | 100.0 | I |
| 80 | 227973 | 47.4 | 52.2 | I | 105 | 225896 | 40.9 | 50.0 | I | 130 | 215865 | 35.9 | 83.3 | I |
| 81 | 208448 | 47.3 | 67.2 | I | 106 | 215952 | 40.7 | 40.0 | I | 131 | 237972 | 35.8 | 144.0 | I |
| 82 | 203312 | 47.1 | 93.0 | I | 107 | 216041 | 40.5 | 66.7 | I | 132 | 238321 | 35.7 | 41.0 | I |
| 83 | 203355 | 46.8 | 89.3 | I | 108 | 237456 | 40.3 | 128.3 | I | 133 | 208427 | 35.3 | 84.6 | I |
| 84 | 203353 | 46.7 | 92.9 | I | 109 | 238308 | 40.2 | 82.4 | I | 134 | 242121 | 35.3 | 36.0 | I |
| 85 | 238313 | 46.4 | 68.8 | I | 110 | 225894 | 39.8 | 48.4 | I | 135 | 215954 | 35.3 | 122.4 | I |
| 86 | 244798 | 45.8 | 103.6 | I | 111 | 215805 | 39.4 | 175.0 | I | 136 | 215913 | 35.0 | 112.2 | I |
| 87 | 215995 | 45.5 | 83.3 | I | 112 | 215837 | 39.3 | 50.0 | I | 137 | 228901 | 34.5 | 51.2 | I |
| 88 | 216050 | 45.5 | 68.4 | I | 113 | 203272 | 39.1 | 43.1 | I | 138 | 216049 | 34.5 | 54.8 | I |
| 89 | 215937 | 45.1 | 83.3 | I | 114 | 215893 | 38.9 | 79.6 | I | 139 | 216032 | 34.4 | 66.2 | I |
| 90 | 242110 | 45.1 | 141.9 | I | 115 | 237969 | 38.9 | 116.7 | I | 140 | 203345 | 33.3 | 88.1 | I |
| 91 | 203354 | 44.7 | 51.4 | I | 116 | 211504 | 38.7 | 107.0 | I | 141 | 220337 | 33.3 | 94.1 | I |
| 92 | 216026 | 43.3 | 70.4 | I | 117 | 203342 | 38.7 | 56.8 | I | 142 | 242613 | 33.3 | 52.9 | I |
| 93 | 215833 | 43.2 | 78.0 | I | 118 | 215932 | 38.6 | 75.8 | I | 143 | 230562 | 33.3 | 97.2 | I |
| 94 | 203322 | 42.9 | 106.0 | I | 119 | 238310 | 38.5 | 45.1 | I | 144 | 212694 | 33.3 | 95.8 | I |
| 95 | 216051 | 42.9 | 47.4 | I | 120 | 215869 | 38.3 | 54.2 | I | 145 | 215978 | 33.3 | 109.4 | I |
| 96 | 216042 | 42.6 | 126.1 | I | 121 | 216057 | 38.3 | 53.3 | I | 146 | 215986 | 32.6 | 37.9 | I |
| 97 | 208443 | 42.4 | 102.5 | I | 122 | 237973 | 38.2 | 54.0 | I | 147 | 216024 | 32.5 | 37.3 | I |
| 98 | 215842 | 42.4 | 55.7 | I | 123 | 203358 | 38.2 | 81.8 | I | 148 | 242637 | 32.3 | 69.8 | I |
| 99 | 216054 | 42.3 | 35.8 | I | 124 | 207460 | 38.1 | 51.9 | I | 149 | 219828 | 32.3 | 70.8 | I |
| 100 | 237458 | 41.9 | 110.5 | I | 125 | 242612 | 37.5 | 90.3 | I | 150 | 215928 | 32.0 | 120.8 | I |
| No. | Acc. | RRL (%) | RSL (%) | PC | No. | Acc. | RRL (%) | RSL (%) | PC | No. | Acc. | RRL (%) | RSL (%) | PC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 151 | 245091 | 31.8 | 69.0 | I | 176 | 215803 | 29.0 | 62.0 | I | 201 | 242689 | 25.9 | 72.9 | I |
| 152 | 242117 | 31.7 | 93.0 | I | 177 | 215948 | 28.8 | 96.6 | I | 202 | 216028 | 25.9 | 53.1 | I |
| 153 | 203325 | 31.3 | 87.0 | I | 178 | 241769 | 28.6 | 43.4 | I | 203 | 203344 | 25.8 | 73.5 | I |
| 154 | 203374 | 31.0 | 59.3 | I | 179 | 215926 | 28.6 | 65.4 | I | 204 | 215918 | 25.8 | 72.0 | I |
| 155 | 208728 | 30.8 | 45.3 | I | 180 | 245087 | 28.6 | 104.8 | I | 205 | 234148 | 25.6 | 79.8 | I |
| 156 | 208441 | 30.8 | 78.2 | I | 181 | 203357 | 28.2 | 82.1 | I | 206 | 242108 | 25.5 | 38.8 | I |
| 157 | 215949 | 30.3 | 75.0 | I | 182 | 215889 | 28.2 | 98.1 | I | 207 | 242115 | 25.0 | 80.0 | I |
| 158 | 238312 | 30.2 | 110.0 | I | 183 | 337584 | 28.0 | 87.1 | I | 208 | 203377 | 25.0 | 71.4 | I |
| 159 | 203365 | 30.1 | 109.8 | I | 184 | 229721 | 27.9 | 56.3 | I | 209 | 215915 | 25.0 | 60.6 | I |
| 160 | 237583 | 30.0 | 22.4 | I | 185 | 215862 | 27.8 | 84.6 | I | 210 | 203352 | 25.0 | 59.2 | I |
| 161 | 215857 | 30.0 | 65.4 | I | 186 | 238316 | 27.8 | 69.9 | I | 211 | 237457 | 24.6 | 76.0 | I |
| 162 | 228202 | 29.7 | 75.7 | I | 187 | 207459 | 27.6 | 58.5 | I | 212 | 215959 | 24.6 | 71.7 | I |
| 163 | 215979 | 29.6 | 66.1 | I | 188 | 203388 | 27.6 | 77.3 | I | 213 | 242109 | 24.5 | 70.4 | I |
| 164 | 242624 | 29.6 | 78.9 | I | 189 | 242623 | 27.5 | 51.2 | I | 214 | 215863 | 24.3 | 37.7 | I |
| 165 | 242638 | 29.5 | 97.1 | I | 190 | 215854 | 27.3 | 68.1 | I | 215 | 219829 | 24.1 | 72.5 | I |
| 166 | 215941 | 29.4 | 72.6 | I | 191 | 216052 | 27.2 | 68.9 | I | 216 | 242622 | 24.1 | 45.1 | I |
| 167 | 215848 | 29.4 | 57.1 | I | 192 | 238319 | 27.1 | 86.8 | I | 217 | 216036 | 24.1 | 53.8 | I |
| 168 | 207963 | 29.4 | 54.4 | I | 193 | 203360 | 26.6 | 36.5 | I | 218 | 242119 | 24.0 | 67.6 | I |
| 169 | 228902 | 29.4 | 36.6 | I | 194 | 237447 | 26.5 | 24.0 | I | 219 | 238346 | 24.0 | 118.8 | I |
| 170 | 203386 | 29.4 | 62.2 | I | 195 | 215916 | 26.5 | 93.3 | I | 220 | 215861 | 23.8 | 100.0 | I |
| 171 | 215980 | 29.4 | 105.6 | I | 196 | 215938 | 26.3 | 44.7 | I | 221 | 215920 | 23.7 | 32.8 | I |
| 172 | 203340 | 29.4 | 75.0 | I | 197 | 242111 | 26.2 | 87.5 | I | 222 | 208726 | 23.3 | 88.7 | I |
| 173 | 215901 | 29.4 | 116.5 | I | 198 | 215838 | 26.1 | 98.1 | I | 223 | 230561 | 23.3 | 60.8 | I |
| 174 | 215903 | 29.3 | 68.0 | I | 199 | 216029 | 26.1 | 75.9 | I | 224 | 243641 | 23.1 | 88.0 | I |
| 175 | 203315 | 29.2 | 45.3 | I | 200 | 203335 | 26.0 | 49.1 | I | 225 | 215849 | 23.1 | 83.6 | I |
| No. | Acc. | RRL (%) | RSL (%) | PC | No. | Acc. | RRL (%) | RSL (%) | PC | No. | Acc. | RRL (%) | RSL (%) | PC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 226 | 215895 | 23.1 | 67.4 | I | 251 | 203369 | 20.588 | 67.606 | S | 276 | 203372 | 17.5 | 59.5238 | S |
| 227 | 208447 | 22.8 | 50.0 | I | 252 | 238307 | 20.588 | 82.278 | S | 277 | 215962 | 17.2 | 132.258 | S |
| 228 | 203328 | 22.7 | 101.6 | I | 253 | 215947 | 20.588 | 89.474 | S | 278 | 215985 | 17.1 | 73.7705 | S |
| 229 | 215856 | 22.7 | 64.3 | I | 254 | 215846 | 20 | 151.72 | S | 279 | 215940 | 17.1 | 61.0169 | S |
| 230 | 335141 | 22.6 | 128.0 | I | 255 | 215929 | 20 | 68.889 | S | 280 | 215908 | 17.1 | 78.5714 | S |
| 231 | 216020 | 22.2 | 69.3 | I | 256 | 223146 | 19.355 | 78.571 | S | 281 | 242112 | 17.1 | 76.0563 | S |
| 232 | 216046 | 22.2 | 100.0 | I | 257 | 238322 | 19.231 | 56.522 | S | 282 | 216021 | 16.7 | 64.7059 | S |
| 233 | 215859 | 22.2 | 135.4 | I | 258 | 215934 | 19.231 | 75 | S | 283 | 215993 | 16.7 | 76.5625 | S |
| 234 | 208729 | 21.7 | 54.5 | I | 259 | 203346 | 19.231 | 68.919 | S | 284 | 238460 | 16.7 | 86.1111 | S |
| 235 | 215843 | 21.7 | 55.6 | I | 260 | 203317 | 19.231 | 65 | S | 285 | 215894 | 16.3 | 84.1463 | S |
| 236 | 238300 | 21.7 | 84.4 | I | 261 | 219825 | 18.75 | 32.075 | S | 286 | 208442 | 16.3 | 82.5 | S |
| 237 | 235142 | 21.7 | 53.3 | I | 262 | 219832 | 18.667 | 47.826 | S | 287 | 203347 | 16.2 | 46.0526 | S |
| 238 | 242133 | 21.7 | 87.0 | I | 263 | 245092 | 18.548 | 61.905 | S | 288 | 243642 | 16.2 | 112.903 | S |
| 239 | 203371 | 21.6 | 54.4 | I | 264 | 216038 | 18.519 | 68.919 | S | 289 | 216039 | 16.1 | 123.077 | S |
| 240 | 211505 | 21.3 | 50.0 | I | 265 | 216035 | 18.519 | 35.294 | S | 290 | 203339 | 16.1 | 112.5 | S |
| 241 | 203327 | 21.3 | 37.7 | I | 266 | 242107 | 18.519 | 45.455 | S | 291 | 207964 | 15.6 | 108.333 | S |
| 242 | 215873 | 21.3 | 60.7 | I | 267 | 243640 | 18.519 | 88.406 | S | 292 | 238345 | 15.6 | 58 | S |
| 243 | 245088 | 21.3 | 101.9 | I | 268 | 215904 | 18.421 | 137.74 | S | 293 | 208440 | 15.56 | 70 | S |
| 244 | 215802 | 21.2 | 70.7 | I | 269 | 215834 | 18 | 103.92 | S | 294 | 242120 | 15.15 | 59.155 | S |
| 245 | 238343 | 21.1 | 151.7 | I | 270 | 216033 | 17.857 | 94.444 | S | 295 | 215911 | 15.15 | 136.36 | S |
| 246 | 243644 | 20.9 | 68.2 | S | 271 | 238320 | 17.857 | 82.474 | S | 296 | 215887 | 15.09 | 98.148 | S |
| 247 | 242132 | 20.8 | 43.1 | S | 272 | 203359 | 17.8 | 58.9744 | S | 297 | 245090 | 15 | 67.647 | S |
| 248 | 215832 | 20.8 | 59.3 | S | 273 | 215946 | 17.8 | 28.8462 | S | 298 | 215799 | 14.89 | 78.481 | S |
| 249 | 215867 | 20.7 | 35.8 | S | 274 | 235699 | 17.6 | 62.766 | S | 299 | 203318 | 14.81 | 78.667 | S |
| 250 | 242106 | 20.6 | 69.8 | S | 275 | 243623 | 17.5 | 70.2703 | S | 300 | 215872 | 14.46 | 36.585 | S |
| No. | Acc. | RRL (%) | RSL (%) | PC | No. | Acc. | RRL (%) | RSL (%) | PC |
|---|---|---|---|---|---|---|---|---|---|
| 301 | 238311 | 14.29 | 116.67 | S | 325 | 215826 | 10.64 | 95.161 | S |
| 302 | 215931 | 14.04 | 52.083 | S | 326 | 215967 | 10.53 | 69.048 | S |
| 303 | 203326 | 13.89 | 53.488 | S | 327 | 238309 | 10.42 | 51.456 | S |
| 304 | 243639 | 13.56 | 62.069 | S | 328 | 215899 | 9.804 | 90.741 | S |
| 305 | 215966 | 13.33 | 25.263 | S | 329 | 203370 | 9.615 | 50.943 | S |
| 306 | 215956 | 13.33 | 55.172 | S | 330 | 215892 | 9.434 | 82.54 | S |
| 307 | 242614 | 13.33 | 45.882 | S | 331 | 215804 | 7.143 | 62.712 | S |
| 308 | 215897 | 13.16 | 69.643 | S | 332 | 212462 | 6.849 | 154.55 | S |
| 309 | 215992 | 13.16 | 65.909 | S | 333 | 238323 | 4.372 | 77.358 | S |
| 310 | 215955 | 13.11 | 45.455 | S | |||||
| 311 | 215898 | 12.94 | 66.197 | S | |||||
| 312 | 203362 | 12.5 | 71.642 | S | |||||
| 313 | 215858 | 12.07 | 65.591 | S | |||||
| 314 | 215851 | 12 | 69.091 | S | |||||
| 315 | 219826 | 11.9 | 33.898 | S | |||||
| 316 | 245085 | 11.9 | 52.727 | S | |||||
| 317 | 216048 | 11.76 | 75.862 | S | |||||
| 318 | 215876 | 11.63 | 202.13 | S | |||||
| 319 | 203331 | 11.43 | 62.037 | S | |||||
| 320 | 242105 | 11.36 | 81.481 | S | |||||
| 321 | 203338 | 11.32 | 72 | S | |||||
| 322 | 242625 | 10.71 | 52.381 | S | |||||
| 323 | 241768 | 10.64 | 85.714 | S | |||||
| 324 | 215951 | 10.64 | 79.348 | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brhane, H.; Haileselassie, T.; Tesfaye, K.; Hammenhag, C.; Ortiz, R.; Geleta, M. Hydroponic Optimization and Screening of Aluminum Tolerance on Finger Millet (Eleusine coracana (L.) Gaertn.) Accessions and Cultivars. Agronomy 2023, 13, 1596. https://doi.org/10.3390/agronomy13061596
Brhane H, Haileselassie T, Tesfaye K, Hammenhag C, Ortiz R, Geleta M. Hydroponic Optimization and Screening of Aluminum Tolerance on Finger Millet (Eleusine coracana (L.) Gaertn.) Accessions and Cultivars. Agronomy. 2023; 13(6):1596. https://doi.org/10.3390/agronomy13061596
Chicago/Turabian StyleBrhane, Haftom, Teklehaimanot Haileselassie, Kassahun Tesfaye, Cecilia Hammenhag, Rodomiro Ortiz, and Mulatu Geleta. 2023. "Hydroponic Optimization and Screening of Aluminum Tolerance on Finger Millet (Eleusine coracana (L.) Gaertn.) Accessions and Cultivars" Agronomy 13, no. 6: 1596. https://doi.org/10.3390/agronomy13061596
APA StyleBrhane, H., Haileselassie, T., Tesfaye, K., Hammenhag, C., Ortiz, R., & Geleta, M. (2023). Hydroponic Optimization and Screening of Aluminum Tolerance on Finger Millet (Eleusine coracana (L.) Gaertn.) Accessions and Cultivars. Agronomy, 13(6), 1596. https://doi.org/10.3390/agronomy13061596










