Breeding Drought-Tolerant Maize (Zea mays) Using Molecular Breeding Tools: Recent Advancements and Future Prospective
Abstract
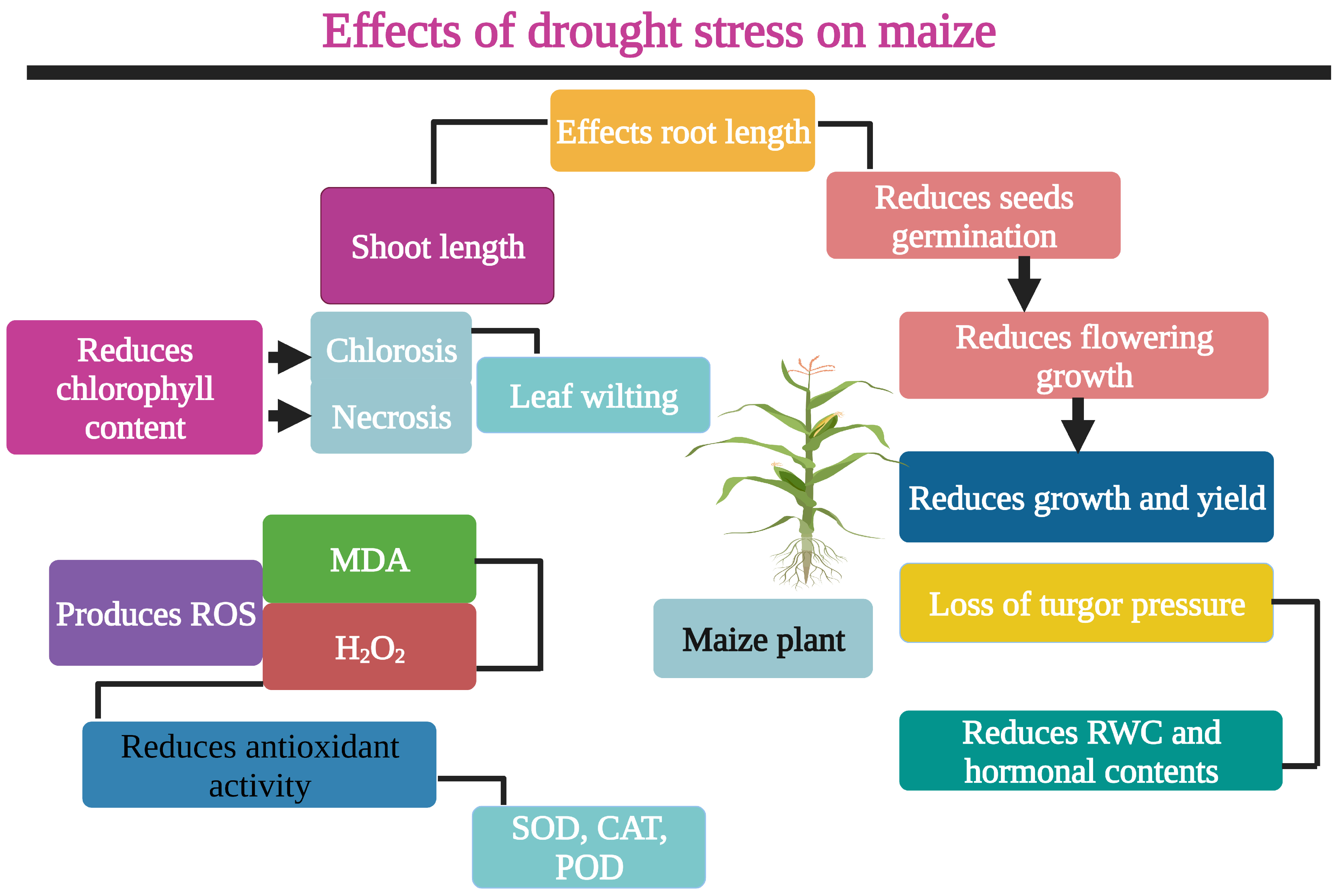
1. Introduction
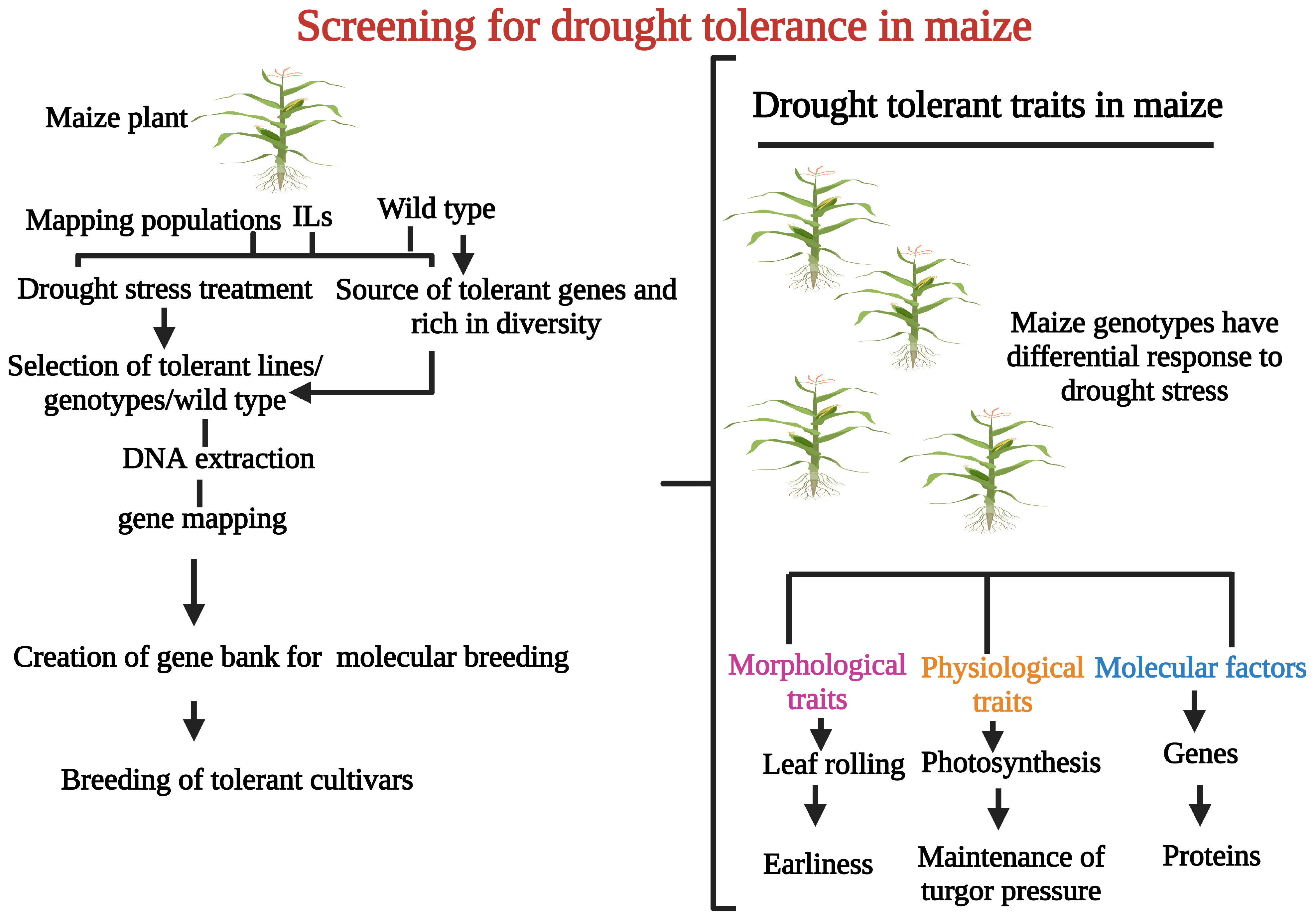
2. Screening of Drought Tolerance in Maize
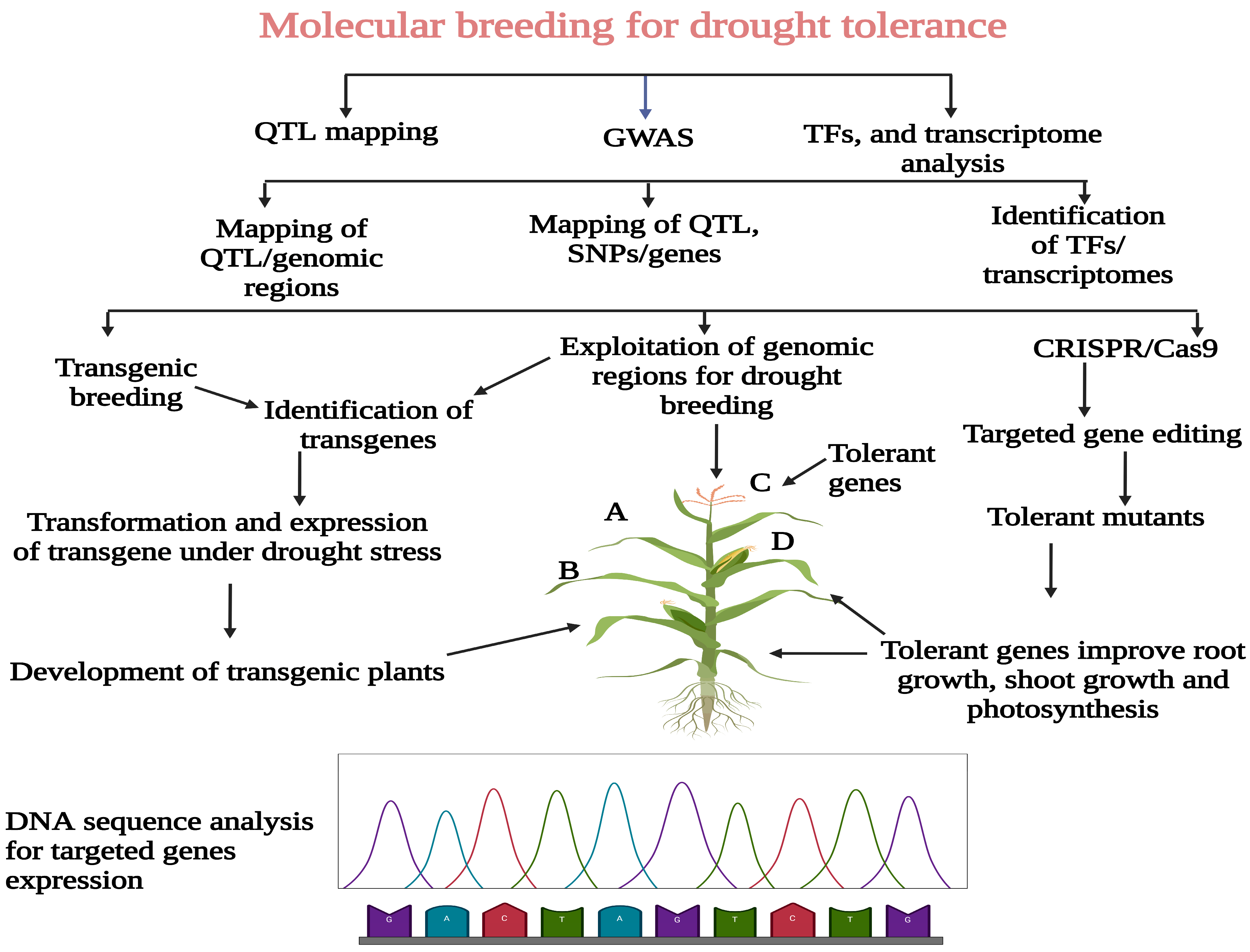
3. QTL Mapping Analysis for Drought Tolerance in Maize
| Parents/Population | QTL | Chromosome | References |
|---|---|---|---|
| 121 RIL | qDTA3-3 and qDTA10 | 3, 10 | [71] |
| 217 DH | qWS-GY3-1 and qWS-ESP3-1 | 3 | [68] |
| 419 F2 individuals | qLRWC2 and qLRWC10a | 2, 10 | [70] |
| 13 F2:3 families | qEL4s and qKW4s | 4 | [72] |
| 234 RIL, 247 F2:3, 300 F2:3 | mQTL_SG_1a and mQTL_EPP_2 | 1, 2 | [75] |
| 234 RIL, 247 F2:3, 300 F2:3 families | qWS-GY-10 and qWS-GY-7 | 10, 7 | [73] |
| 116 F3 families | qGY2 and qGY10 | 2, 10 | [74] |
| 305 maize lines | qPH-HP383-10 | 10 | [76] |
| The population of F2:3 families | qGY1 and qGY9 | 1, 9 | [78] |
| 180 F2:3 families | qES3 | 3 | [77] |
4. Overview of Association Mapping Studies for Drought Tolerance
| Population/Parents | QTL/Genes/SNPs | Function | Reference |
|---|---|---|---|
| 279 maize ILs | 71, 159, and 21 SNPs | ARABIDILLO 1 for ASI and SF16 protein for PH | [84] |
| 420 RIL | 28 and 16 SNPs | Genes improved seedling growth and plant development | [92] |
| 162 tropical maize lines | 66, 27, and 24 SNPs | Four SNPs linked with three traits under CHD | [89] |
| 1326 maize landraces | 5695 SNPs | Two genes linked with drought tolerance | [91] |
| 209 diverse accessions | 62 loci | SRL loci as a potential candidate for drought tolerance | [85] |
| 300 ILs | 688 candidate genes | 46 genes showed significant differential expression | [86] |
| 210 maize ILs | 696 under WS and 413 under WW | The SNPs reflected significant genetic variability | [90] |
| 240 maize lines | 29,619 SNPs | 77 SNPs were significantly related with 10 TFs | [87] |
| 367 inbred lines | ZmVPP1 genes | Enhanced photosynthesis and root development | [79] |
| 318 maize ILs | 123 significant SNPs | 23 metabolic loci linked with drought tolerance | [83] |
| 5000 inbred lines | 365 SNPs located in 354 genes | 52 genes showed differential expression | [27] |
| 201 ILs | 206 significant SNPs in 115 candidate genes | These genes might play a role in drought tolerance | [80] |
| 240 maize lines | 61 SNPs | SNPs related to ABA signaling improved drought tolerance | [88] |
| 350 ILs | 1365 SNPs | 42 SNPs linked with 33 genes and gene GRMZM2G12577 associated with hundred kernel weight | [82] |
| 80 ILs | 1356 SNPs | 29 SNPs linked to phenotypic traits | [81] |
5. TFs Analysis and Their Role in Drought Tolerance
| Families | TFs | Role | Reference |
|---|---|---|---|
| WRKY | ZmWRKY104 | Involved in ABA-induced antioxidant defense system | [97] |
| WRKY | ZmWRKY40 | Enhanced drought tolerance | [98] |
| WRKY | ZmWRKY79 | Boosted the ROS scavenging | [99] |
| bHLH | ZmPTF1 | Enhanced ABA content and improved root system | [100] |
| bHLP | CgbHLH001 | Increases soluble sugar contents | [101] |
| BES1/BZR1s | ZmBES1/BZR1-3, ZmBES1/BZR1-9 | Linked with oxidative stress response and amino acid metabolic process | [102] |
| AP2/ERF | ZmEREBP60 | Alleviated the drought-induced H2O2 content | [103] |
| NAC | ZmNAC55 | Positive regulator of drought tolerance | [105] |
| NAC | ZmNAC49 | Decreased stomatal conductance | [36] |
| HD-ZIP | ZmHDZ9 | Improved antioxidant defense system | [106] |
| HD-ZIP | ATHB-6 | Decreased the content of malondialdehyde | [107] |
| MYB | ZmMYB-CC10 | Reduced oxidative damage and enhanced activity of ZmAPX4 | [108] |
| (NF-Ys) | ZmNF-YB16-, ZmNF-YA1 | ZmNF-YA1 improved root development | [34] |
| ERF | ZmERF21 | Increased antioxidant activities | [109] |
| DREB/CBF | TsCBF1 | Increased relative water content (RWC) and higher grain yield (GY) | [110] |
6. Transcriptome Analysis for Drought Tolerance
7. Transgenic Breeding and CRISPR/Cas9
8. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, M.; Zeng, W.; Chen, F.; Ji, X.; Zhuang, Z.; Jin, B.; Wang, J.; Jia, L.; Peng, Y. Transcriptome expression profiles reveal response mechanisms to drought and drought-stress mitigation mechanisms by exogenous glycine betaine in maize. Biotech. Lett. 2022, 44, 367–386. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, J.; Wang, C.; Wang, Y.; Shang, M.; Zhang, Z.; Chen, F.; Chu, Q. A six-year record of greenhouse gas emissions in different growth stages of summer maize influenced by irrigation and nitrogen management. Field Crops Res. 2023, 290, 108744. [Google Scholar] [CrossRef]
- Notununu, I.; Moleleki, L.; Roopnarain, A.; Adeleke, R. Effects of plant growth-promoting rhizobacteria on the molecular responses of maize under drought and heat stresses: A review. Pedosphere 2022, 32, 90–106. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Liang, X.; Zhuang, J.; Wang, X.; Qin, F.; Jiang, C. A dirigent family protein confers variation of Casparian strip thickness and salt tolerance in maize. Nat. Comm. 2022, 13, 2222. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.-Y.; Lei, L.; Fan, X.-W.; Li, Y.-Z. Effects of high air temperature, drought, and both combinations on maize: A case study. Plant Sci. 2023, 327, 111543. [Google Scholar] [CrossRef]
- Rahmat, B.P.N.; Octavianis, G.; Budiarto, R.; Jadid, N.; Widiastuti, A.; Matra, D.D.; Ezura, H.; Mubarok, S. SlIAA9 Mutation Maintains Photosynthetic Capabilities under Heat-Stress Conditions. Plants 2023, 12, 378. [Google Scholar] [CrossRef]
- FAO; UNICEF; WFP; WHO. Building Resilience for Peace and Food Security; FAO: Rome, Italy, 2017. [Google Scholar]
- Bellemare, M.F. Rising food prices, food price volatility, and social unrest. Amer. J. Agricul. Econ. 2015, 97, 1–21. [Google Scholar] [CrossRef][Green Version]
- Kalkuhl, M.; Von Braun, J.; Torero, M. Food Price Volatility and Its Implications for Food Security and Policy; Springer Nature: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Hertel, T.W. Food security under climate change. Nat. Clim. Chang. 2016, 6, 10–13. [Google Scholar] [CrossRef]
- Swinnen, J.; Squicciarini, P. Mixed messages on prices and food security. Science 2012, 335, 405–406. [Google Scholar] [CrossRef]
- Ray, D.K.; Gerber, J.S.; MacDonald, G.K.; West, P.C. Climate variation explains a third of global crop yield variability. Nat. Comm. 2015, 6, 5989. [Google Scholar] [CrossRef][Green Version]
- Webber, H.; Ewert, F.; Olesen, J.E.; Müller, C.; Fronzek, S.; Ruane, A.C.; Bourgault, M.; Martre, P.; Ababaei, B.; Bindi, M. Diverging importance of drought stress for maize and winter wheat in Europe. Nat. Comm. 2018, 9, 4249. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wakatsuki, H.; Ju, H.; Nelson, G.C.; Farrell, A.D.; Deryng, D.; Meza, F.; Hasegawa, T. Research trends and gaps in climate change impacts and adaptation potentials in major crops. Curr. Opin. Environ. Sust. 2023, 60, 101249. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, B.; Xie, Y.; Jia, H.; Li, Y.; Xu, M.; Wu, G.; Ma, X.; Li, Q.; Hou, M. The evening complex promotes maize flowering and adaptation to temperate regions. The Plant Cell 2023, 35, 369–389. [Google Scholar] [CrossRef] [PubMed]
- Strable, J.; Scanlon, M.J. Maize (Zea mays): A model organism for basic and applied research in plant biology. Cold Spr. Harb. Prot. 2009, 2009, pdb.emo132. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Palacios-Rojas, N.; McCulley, L.; Kaeppler, M.; Titcomb, T.J.; Gunaratna, N.S.; Lopez-Ridaura, S.; Tanumihardjo, S.A. Mining maize diversity and improving its nutritional aspects within agro-food systems. Compreh. Rev. Food Sci. Food Safety 2020, 19, 1809–1834. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Rezayian, M.; Mousavi, S.H. Drought Stress: Involvement of Plant Hormones in Perception, Signaling, and Response. In Plant Hormones and Climate Change; Springer: Berlin/Heidelberg, Germany, 2023; pp. 227–250. [Google Scholar]
- Adewale, S.; Akinwale, R.; Fakorede, M.; Badu-Apraku, B. Genetic analysis of drought-adaptive traits at seedling stage in early-maturing maize inbred lines and field performance under stress conditions. Euphytica 2018, 214, 145. [Google Scholar] [CrossRef]
- Edmeades, G. Progress in Achieving and Delivering Drought Tolerance in Maize-an Update; ISAAA: Ithaca, NY, USA, 2013; Volume 130. [Google Scholar]
- Tesfaye, K.; Kruseman, G.; Cairns, J.E.; Zaman-Allah, M.; Wegary, D.; Zaidi, P.; Boote, K.J.; Erenstein, O. Potential benefits of drought and heat tolerance for adapting maize to climate change in tropical environments. Clim. Risk Mana. 2018, 19, 106–119. [Google Scholar] [CrossRef]
- Aslam, M.; Zamir, M.; Afzal, I.; Yaseen, M.; Mubeen, M.; Shoaib, A. Drought stress, its effect on maize production and development of drought tolerance through potassium application. Agron. Res. Mol. 2012, 46, 1–16. [Google Scholar]
- Mohammadkhani, N.; Heidari, R. Effects of drought stress on soluble proteins in two maize varieties. Turk. J. Biol. 2008, 32, 23–30. [Google Scholar]
- Khalili, M.; Naghavi, M.R.; Aboughadareh, A.P.; Rad, H.N. Effects of drought stress on yield and yield components in maize cultivars (Zea mays L.). Int. J. Agro. Plant Prod. 2013, 4, 809–812. [Google Scholar]
- Zhang, X.; Lei, L.; Lai, J.; Zhao, H.; Song, W. Effects of drought stress and water recovery on physiological responses and gene expression in maize seedlings. BMC Plant Biol. 2018, 18, 68. [Google Scholar] [CrossRef][Green Version]
- Javed, N.; Ashraf, M.; Akram, N.A.; Al-Qurainy, F. Alleviation of adverse effects of drought stress on growth and some potential physiological attributes in maize (Zea mays L.) by seed electromagnetic treatment. Photochem. Photob. 2011, 87, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, B.; Li, Y.; Liu, C.; Wu, X.; Zhang, D.; Shi, Y.; Song, Y.; Buckler, E.S.; Zhang, Z. Numerous genetic loci identified for drought tolerance in the maize nested association mapping populations. BMC Gen. 2016, 17, 894. [Google Scholar] [CrossRef][Green Version]
- Kakumanu, A.; Ambavaram, M.M.; Klumas, C.; Krishnan, A.; Batlang, U.; Myers, E.; Grene, R.; Pereira, A. Effects of drought on gene expression in maize reproductive and leaf meristem tissue revealed by RNA-Seq. Plant Physiol. 2012, 160, 846–867. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thirunavukkarasu, N.; Sharma, R.; Singh, N.; Shiriga, K.; Mohan, S.; Mittal, S.; Mittal, S.; Mallikarjuna, M.G.; Rao, A.R.; Dash, P.K. Genomewide expression and functional interactions of genes under drought stress in maize. Int. J. Gen. 2017, 2017, 2568706. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophy. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, C.; Chen, F.; Yue, L.; Cao, X.; Li, J.; Zhao, X.; Wu, F.; Wang, Z.; Xing, B. Foliar carbon dot amendment modulates carbohydrate metabolism, rhizospheric properties and drought tolerance in maize seedling. Sci. Total Environ. 2022, 809, 151105. [Google Scholar] [CrossRef] [PubMed]
- Tuberosa, R.; Salvi, S. Genomics-based approaches to improve drought tolerance of crops. Trend. Plant Sci. 2006, 11, 405–412. [Google Scholar] [CrossRef]
- Hao, Z.; Li, X.; Liu, X.; Xie, C.; Li, M.; Zhang, D.; Zhang, S. Meta-analysis of constitutive and adaptive QTL for drought tolerance in maize. Euphytica 2010, 174, 165–177. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.; Wang, J.; He, C.; Zhang, D.; Li, P.; Zhang, J.; Li, Z. Transcription factors ZmNF-YA1 and ZmNF-YB16 regulate plant growth and drought tolerance in maize. Plant Physiol. 2022, 190, 1506–1525. [Google Scholar] [CrossRef]
- Min, H.; Chen, C.; Wei, S.; Shang, X.; Sun, M.; Xia, R.; Liu, X.; Hao, D.; Chen, H.; Xie, Q. Identification of drought tolerant mechanisms in maize seedlings based on transcriptome analysis of recombination inbred lines. Front. Plant Sci. 2016, 7, 1080. [Google Scholar] [CrossRef][Green Version]
- Xiang, Y.; Sun, X.; Bian, X.; Wei, T.; Han, T.; Yan, J.; Zhang, A. The transcription factor ZmNAC49 reduces stomatal density and improves drought tolerance in maize. J. Exp. Bot. 2021, 72, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; He, C.; Li, B.; Li, N.; Zhang, J. The pyramid of transgenes TsVP and BetA effectively enhances the drought tolerance of maize plants. Plant Biotech. J. 2011, 9, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, H.; Wang, H.; Lafitte, H.R.; Archibald, R.L.; Yang, M.; Hakimi, S.M.; Mo, H.; Habben, J.E. ARGOS 8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotech. J. 2017, 15, 207–216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, X.; Xu, S.; Fuhrmann-Aoyagi, M.B.; Yuan, S.; Iwama, T.; Kobayashi, M.; Miura, K. CRISPR/Cas9 Technique for Temperature, Drought, and Salinity Stress Responses. Curr. Issues Mol. Biol. 2022, 44, 2664–2682. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, K.; An, X. CRISPR/Cas System: Applications and Prospects for Maize Improvement. ACS Agricul. Sci. Technol. 2022, 2, 174–183. [Google Scholar] [CrossRef]
- Khanzada, H.; Wassan, G.M.; He, H.; Mason, A.S.; Keerio, A.A.; Khanzada, S.; Faheem, M.; Solangi, A.M.; Zhou, Q.; Fu, D. Differentially evolved drought stress indices determine the genetic variation of Brassica napus at seedling traits by genome-wide association mapping. J. Adv. Res. 2020, 24, 447–461. [Google Scholar] [CrossRef]
- Oya, T.; Nepomuceno, A.L.; Neumaier, N.; Farias, J.R.B.; Tobita, S.; Ito, O. Drought Tolerance Characteristics of Brazilian Soybean Cultivars—Evaluation and characterization of drought tolerance of various Brazilian soybean cultivars in the field—. Plant Prod. Sci. 2004, 7, 129–137. [Google Scholar] [CrossRef][Green Version]
- Liu, Z.; Li, H.; Wang, X.; Zhang, Y.; Gou, Z.; Zhao, X.; Ren, H.; Wen, Z.; Li, Y.; Yu, L. QTL for yield per plant under water deficit and well-watered conditions and drought susceptibility index in soybean (Glycine max (L.) Merr.). Biotech. Biotechnol. Equip. 2023, 37, 92–103. [Google Scholar] [CrossRef]
- Collins, N.C.; Tardieu, F.; Tuberosa, R. Quantitative trait loci and crop performance under abiotic stress: Where do we stand? Plant Physiol. 2008, 147, 469–486. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Song, H.; Zhou, L.; Xu, Z.; Zhou, G. Vertical distributions of chlorophyll and nitrogen and their associations with photosynthesis under drought and rewatering regimes in a maize field. Agricul. Forest Meteorol. 2019, 272, 40–54. [Google Scholar] [CrossRef]
- Voronin, P.Y.; Maevskaya, S.; Nikolaeva, M. Physiological and molecular responses of maize (Zea mays L.) plants to drought and rehydration. Photosynthetica 2019, 57, 850–856. [Google Scholar] [CrossRef][Green Version]
- Jing, L.; Weng, B.; Yan, D.; Yuan, F.; Zhang, S.; Bi, W.; Yan, S. Assessment of resilience in maize suitable planting areas under drought stress. Agricul. Water Manag. 2023, 277, 108096. [Google Scholar] [CrossRef]
- Hammer, K. Agrarbiodiversität und pflanzengenetische Ressourcen: Herausforderung und Lösungsansatz; Zentralstelle für Agrardokumentation und-information: Bonn, Germany, 1998. [Google Scholar]
- Hammer, K. Checklists and germplasm collecting. FAO/IBPGR Plant Genetic Res. Newslet. 1991, 85, 15–17. [Google Scholar]
- Abdel-Ghani, A.H.; Neumann, K.; Wabila, C.; Sharma, R.; Dhanagond, S.; Owais, S.J.; Börner, A.; Graner, A.; Kilian, B. Diversity of germination and seedling traits in a spring barley (Hordeum vulgare L.) collection under drought simulated conditions. Gen. Res. Crop Evol. 2015, 62, 275–292. [Google Scholar] [CrossRef]
- Thabet, S.G.; Moursi, Y.S.; Karam, M.A.; Graner, A.; Alqudah, A.M. Genetic basis of drought tolerance during seed germination in barley. PLoS ONE 2018, 13, e0206682. [Google Scholar] [CrossRef]
- Tarawneh, R.A.; Szira, F.; Monostori, I.; Behrens, A.; Alqudah, A.M.; Thumm, S.; Lohwasser, U.; Röder, M.S.; Börner, A.; Nagel, M. Genetic analysis of drought response of wheat following either chemical desiccation or the use of a rain-out shelter. J. App. Gen. 2019, 60, 137–146. [Google Scholar] [CrossRef]
- Badr, A.; El-Shazly, H.H.; Tarawneh, R.A.; Börner, A. Screening for drought tolerance in maize (Zea mays L.) germplasm using germination and seedling traits under simulated drought conditions. Plants 2020, 9, 565. [Google Scholar] [CrossRef]
- Avramova, V.; Nagel, K.A.; AbdElgawad, H.; Bustos, D.; DuPlessis, M.; Fiorani, F.; Beemster, G.T. Screening for drought tolerance of maize hybrids by multi-scale analysis of root and shoot traits at the seedling stage. J. Expe. Bot. 2016, 67, 2453–2466. [Google Scholar] [CrossRef]
- Ali, Q.; Ahsan, M.; Kanwal, N.; Ali, F.; Ali, A.; Ahmed, W.; Ishfaq, M.; Saleem, M. Screening for drought tolerance: Comparison of maize hybrids under water deficit condition. Adv. Life Sci. 2016, 3, 51–58. [Google Scholar]
- Bonea, D. Screening for drought tolerance in maize hybrids using new indices based on resilience and production capacity. Screening 2020, 20, 151–156. [Google Scholar]
- Kumar, B.; Kumar, K.; Jat, S.L.; Srivastava, S.; Tiwari, T.; Kumar, S.; Pradhan, H.R.; Kumar, B.; Chaturvedi, G.; Jha, A.K. Rapid method of screening for drought stress tolerance in maize (Zea mays L.). Ind. J. Gen. Plant Bree. 2020, 80, 16–25. [Google Scholar] [CrossRef]
- Alvi, A.K.; Ahmad, M.S.A.; Rafique, T.; Naseer, M.; Farhat, F.; Tasleem, H.; Nasim, A. Screening of maize (Zea mays L.) genotypes for drought tolerance using photosynthetic pigments and anti-oxidative enzymes as selection criteria. Pak. J. Bot 2022, 54, 33–44. [Google Scholar] [CrossRef]
- Raj, R.N.; Gokulakrishnan, J.; Prakash, M. Assessing drought tolerance using PEG-6000 and molecular screening by SSR markers in maize (Zea mays L.) hybrids. Maydica 2020, 64, 1–7. [Google Scholar]
- Adhikari, B.; Sa, K.J.; Lee, J.K. Drought tolerance screening of maize inbred lines at an early growth stage. Plant Breed. Biotech 2019, 7, 326–339. [Google Scholar] [CrossRef]
- Shahrokhi, M.; Khorasani, S.K.; Ebrahimi, A. Evaluation of drought tolerance indices for screening some of super sweet maize (Zea mays L. var. Saccharata) inbred lines. Agrivita J. Agricul. Sci. 2020, 42, 435–448. [Google Scholar] [CrossRef]
- Balbaa, M.G.; Osman, H.T.; Kandil, E.E.; Javed, T.; Lamlom, S.F.; Ali, H.M.; Kalaji, H.M.; Wróbel, J.; Telesiñski, A.; Brysiewicz, A. Determination of morpho-physiological and yield traits of maize inbred lines (Zea mays L.) under optimal and drought stress conditions. Front. Plant Sci. 2022, 13, 959203. [Google Scholar] [CrossRef]
- Emmanuel, I.; Victor, O.; Caroline, U.; Rizvi, A.H.; Kumar, T.; Alam, A. Screening of some selected indian maize cultivars to simulated drought condition. Ind. J. Agricul. Res. 2020, 54, 465–470. [Google Scholar]
- Masood, M.; Ahsan, M.; Sadaqat, H.; Awan, F. Screening of maize (Zea mays L.) inbred lines under water deficit conditions. Biol. Clin. Sci. Res. J. 2020, 2020, 1–6. [Google Scholar] [CrossRef]
- Ramzan, J.; Aslam, M.; Ahsan, M.; Awan, F.S. Selection of screening criteria against drought stress at early growth stages in maize (Zea mays L.). Pak. J. Agricul. Sci. 2019, 56, 633–643. [Google Scholar]
- Choudhary, M.; Kumar, P.; Kumar, P.; Sheoran, S.; Zunjare, R.U.; Jat, B.S. Molecular breeding for drought and heat stress in maize: Revisiting the progress and achievements. In QTL Mapping in Crop Improvement; Elsevier: Amsterdam, The Netherlands, 2023; pp. 57–74. [Google Scholar]
- Sheoran, S.; Gupta, M.; Kumari, S.; Kumar, S.; Rakshit, S. Meta-QTL analysis and candidate genes identification for various abiotic stresses in maize (Zea mays L.) and their implications in breeding programs. Mol. Breed. 2022, 42, 26. [Google Scholar] [CrossRef]
- Hu, X.; Wang, G.; Du, X.; Zhang, H.; Xu, Z.; Wang, J.; Chen, G.; Wang, B.; Li, X.; Chen, X. QTL analysis across multiple environments reveals promising chromosome regions associated with yield-related traits in maize under drought conditions. Crop J. 2021, 9, 759–766. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, Y. Genetic dissection of the photosynthetic parameters of maize (Zea mays L.) in drought-stressed and well-watered environments. Russ. J. Plant Physiol. 2021, 68, 1125–1134. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, J.; Ma, Z.; Xia, L.; Wang, X.; Zhang, L.; Ding, Y.; Qi, J.; Mu, X.; Zhao, F. Bulk analysis by resequencing and RNA-seq identifies candidate genes for maintaining leaf water content under water deficit in maize. Physiol. Plant. 2021, 173, 1935–1945. [Google Scholar] [CrossRef]
- Leng, P.; Khan, S.U.; Zhang, D.; Zhou, G.; Zhang, X.; Zheng, Y.; Wang, T.; Zhao, J. Linkage Mapping Reveals QTL for Flowering Time-Related Traits under Multiple Abiotic Stress Conditions in Maize. Int. J. Mol. Sci. 2022, 23, 8410. [Google Scholar] [CrossRef]
- Abdelghany, M.; Liu, X.; Hao, L.; Gao, C.; Kou, S.; Su, E.; Zhou, Y.; Wang, R.; Zhang, D.; Li, Y. QTL analysis for yield-related traits under different water regimes in maize. Maydica 2019, 64, 10. [Google Scholar]
- Almeida, G.D.; Makumbi, D.; Magorokosho, C.; Nair, S.; Borém, A.; Ribaut, J.-M.; Bänziger, M.; Prasanna, B.M.; Crossa, J.; Babu, R. QTL mapping in three tropical maize populations reveals a set of constitutive and adaptive genomic regions for drought tolerance. Theoret. App. Gen. 2013, 126, 583–600. [Google Scholar] [CrossRef][Green Version]
- Nikolić, A.; Anđelković, V.; Dodig, D.; Mladenović-Drinić, S.; Kravić, N.; Ignjatović-Micić, D. Identification of QTL-s for drought tolerance in maize, II: Yield and yield components. Genetika 2013, 45, 341–350. [Google Scholar] [CrossRef]
- Almeida, G.D.; Nair, S.; Borém, A.; Cairns, J.; Trachsel, S.; Ribaut, J.-M.; Bänziger, M.; Prasanna, B.M.; Crossa, J.; Babu, R. Molecular mapping across three populations reveals a QTL hotspot region on chromosome 3 for secondary traits associated with drought tolerance in tropical maize. Mol. Breed. 2014, 34, 701–715. [Google Scholar] [CrossRef][Green Version]
- Lu, Y.; Xu, J.; Yuan, Z.; Hao, Z.; Xie, C.; Li, X.; Shah, T.; Lan, H.; Zhang, S.; Rong, T. Comparative LD mapping using single SNPs and haplotypes identifies QTL for plant height and biomass as secondary traits of drought tolerance in maize. Mol. Breed. 2012, 30, 407–418. [Google Scholar] [CrossRef][Green Version]
- Zhu, J.-J.; Wang, X.-P.; Sun, C.-X.; Zhu, X.-M.; Meng, L.; Zhang, G.-D.; Tian, Y.-C.; Wang, Z.-L. Mapping of QTL associated with drought tolerance in a semi-automobile rain shelter in maize (Zea mays L.). Agricul. Sci. Chi. 2011, 10, 987–996. [Google Scholar] [CrossRef]
- Rahman, H.; Pekic, S.; Lazic-Jancic, V.; Quarrie, S.; Shah, S.; Pervez, A.; Shah, M. Molecular mapping of quantitative trait loci for drought tolerance in maize plants. Genet. Mol. Res. 2011, 10, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Liu, S.; Ferjani, A.; Li, J.; Yan, J.; Yang, X.; Qin, F. Genetic variation in ZmVPP1 contributes to drought tolerance in maize seedlings. Nat. Gen. 2016, 48, 1233–1241. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Z.-p.; Liang, X.-l.; Weng, J.-f.; Lv, X.-l.; Zhang, D.-g.; Yang, J.; Yong, H.-j.; Li, M.-s.; Li, F.-h. Identification of loci contributing to maize drought tolerance in a genome-wide association study. Euphytica 2016, 210, 165–179. [Google Scholar] [CrossRef]
- Hao, Z.; Li, X.; Xie, C.; Weng, J.; Li, M.; Zhang, D.; Liang, X.; Liu, L.; Liu, S.; Zhang, S. Identification of functional genetic variations underlying drought tolerance in maize using SNP markers. J. Integrat. Plant Biol. 2011, 53, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Warburton, M.L.; Sawkins, M.; Zhang, X.; Setter, T.; Xu, Y.; Grudloyma, P.; Gethi, J.; Ribaut, J.-M.; Li, W. Genome-wide association analysis for nine agronomic traits in maize under well-watered and water-stressed conditions. Theoret. App. Gen. 2013, 126, 2587–2596. [Google Scholar] [CrossRef]
- Zhang, X.; Warburton, M.L.; Setter, T.; Liu, H.; Xue, Y.; Yang, N.; Yan, J.; Xiao, Y. Genome-wide association studies of drought-related metabolic changes in maize using an enlarged SNP panel. Theoret. App. Gen. 2016, 129, 1449–1463. [Google Scholar] [CrossRef]
- Khan, S.U.; Zheng, Y.; Chachar, Z.; Zhang, X.; Zhou, G.; Zong, N.; Leng, P.; Zhao, J. Dissection of maize drought tolerance at the flowering stage using genome-wide association studies. Genes 2022, 13, 564. [Google Scholar] [CrossRef]
- Guo, J.; Li, C.; Zhang, X.; Li, Y.; Zhang, D.; Shi, Y.; Song, Y.; Li, Y.; Yang, D.; Wang, T. Transcriptome and GWAS analyses reveal candidate gene for seminal root length of maize seedlings under drought stress. Plant Sci. 2020, 292, 110380. [Google Scholar] [CrossRef]
- Yuan, Y.; Cairns, J.E.; Babu, R.; Gowda, M.; Makumbi, D.; Magorokosho, C.; Zhang, A.; Liu, Y.; Wang, N.; Hao, Z. Genome-wide association mapping and genomic prediction analyses reveal the genetic architecture of grain yield and flowering time under drought and heat stress conditions in maize. Front. Plant Sci. 2019, 9, 1919. [Google Scholar] [CrossRef][Green Version]
- Shikha, M.; Kanika, A.; Rao, A.R.; Mallikarjuna, M.G.; Gupta, H.S.; Nepolean, T. Genomic selection for drought tolerance using genome-wide SNPs in maize. Front. Plant Sci. 2017, 8, 550. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thirunavukkarasu, N.; Hossain, F.; Arora, K.; Sharma, R.; Shiriga, K.; Mittal, S.; Mohan, S.; Namratha, P.M.; Dogga, S.; Rani, T.S. Functional mechanisms of drought tolerance in subtropical maize (Zea mays L.) identified using genome-wide association mapping. BMC Gen. 2014, 15, 1182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Osuman, A.S.; Badu-Apraku, B.; Karikari, B.; Ifie, B.E.; Tongoona, P.; Danquah, E.Y. Genome-wide association study reveals genetic architecture and candidate genes for yield and related traits under terminal drought, combined heat and drought in tropical maize germplasm. Genes 2022, 13, 349. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, B.; Liang, X.; Zhou, Y.; Song, J.; Yang, J.; Yong, H.; Weng, J.; Zhang, D.; Li, M. Genome-wide association study and genomic prediction analyses of drought stress tolerance in China in a collection of off-PVP maize inbred lines. Mol. Breed. 2019, 39, 113. [Google Scholar] [CrossRef]
- Barbosa, P.A.M.; Fritsche-Neto, R.; Andrade, M.C.; Petroli, C.D.; Burgueño, J.; Galli, G.; Willcox, M.C.; Sonder, K.; Vidal-Martínez, V.A.; Sifuentes-Ibarra, E. Introgression of maize diversity for drought tolerance: Subtropical maize landraces as source of new positive variants. Front. Plant Sci. 2021, 12, 691211. [Google Scholar] [CrossRef]
- Rida, S.; Maafi, O.; López-Malvar, A.; Revilla, P.; Riache, M.; Djemel, A. Genetics of germination and seedling traits under drought stress in a MAGIC population of maize. Plants 2021, 10, 1786. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, J.; Tang, L.; Zhao, Y.; Gu, X.; Gao, G.; Luo, J. PlantTFDB 2.0: Update and improvement of the comprehensive plant transcription factor database. Nucl. Acids Res. 2011, 39, D1114–D1117. [Google Scholar] [CrossRef][Green Version]
- Ma, J.; Tang, X.; Sun, B.; Wei, J.; Ma, L.; Yuan, M.; Zhang, D.; Shao, Y.; Li, C.; Chen, K.-M. A NAC transcription factor, TaNAC5D-2, acts as a positive regulator of drought tolerance through regulating water loss in wheat (Triticum aestivum L.). Environ. Exp. Bot. 2022, 196, 104805. [Google Scholar] [CrossRef]
- Yu, Y.; Song, T.; Wang, Y.; Zhang, M.; Li, N.; Yu, M.; Zhang, S.; Zhou, H.; Guo, S.; Bu, Y. The wheat WRKY transcription factor TaWRKY1-2D confers drought resistance in transgenic Arabidopsis and wheat (Triticum aestivum L.). Int. J. Biol. Macromol. 2022, 226, 1203–1217. [Google Scholar] [CrossRef]
- Rodriguez-Uribe, L.; O’Connell, M.A. A root-specific bZIP transcription factor is responsive to water deficit stress in tepary bean (Phaseolus acutifolius) and common bean (P. vulgaris). J. Exp. Bot. 2006, 57, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yan, J.; Xiang, Y.; Sun, Y.; Zhang, A. ZmWRKY104 transcription factor phosphorylated by ZmMPK6 functioning in ABA-induced antioxidant defense and enhance drought tolerance in Maize. Biology 2021, 10, 893. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-T.; Ru, J.-N.; Liu, Y.-W.; Yang, J.-F.; Li, M.; Xu, Z.-S.; Fu, J.-D. The maize WRKY transcription factor ZmWRKY40 confers drought resistance in transgenic Arabidopsis. Int. J. Mol. Sci. 2018, 19, 2580. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gulzar, F.; Fu, J.; Zhu, C.; Yan, J.; Li, X.; Meraj, T.A.; Shen, Q.; Hassan, B.; Wang, Q. Maize WRKY transcription factor ZmWRKY79 positively regulates drought tolerance through elevating ABA biosynthesis. Int. J. Mol. Sci. 2021, 22, 10080. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Zhang, Y.; Wang, B.; Ran, Q.; Zhang, J. The bHLH family member ZmPTF1 regulates drought tolerance in maize by promoting root development and abscisic acid synthesis. J. Exp. Bot. 2019, 70, 5471–5486. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, C.; Lan, H. A bHLH transcription factor from Chenopodium glaucum confers drought tolerance to transgenic maize by positive regulation of morphological and physiological performances and stress-responsive genes’ expressions. Mol. Breed. 2021, 41, 74. [Google Scholar] [CrossRef]
- Feng, W.; Liu, Y.; Cao, Y.; Zhao, Y.; Zhang, H.; Sun, F.; Yang, Q.; Li, W.; Lu, Y.; Zhang, X. Maize ZmBES1/BZR1-3 and-9 transcription factors negatively regulate drought tolerance in transgenic Arabidopsis. Int. J. Mol. Sci. 2022, 23, 6025. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Zhou, K.; Tian, C.; Aslam, M.; Zhang, B.; Liu, W.; Zou, H. Overexpression of ZmEREBP60 enhances drought tolerance in maize. J. Plant Physiol. 2022, 275, 153763. [Google Scholar] [CrossRef]
- Wang, G.; Yuan, Z.; Zhang, P.; Liu, Z.; Wang, T.; Wei, L. Genome-wide analysis of NAC transcription factor family in maize under drought stress and rewatering. Physiol. Mol. Biol. Plants 2020, 26, 705–717. [Google Scholar] [CrossRef]
- Mao, H.; Yu, L.; Han, R.; Li, Z.; Liu, H. ZmNAC55, a maize stress-responsive NAC transcription factor, confers drought resistance in transgenic Arabidopsis. Plant Physiol.Biochem. 2016, 105, 55–66. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, G.; Abou-Elwafa, S.F.; Fu, J.; Liu, Z.; Zhang, P.; Xie, X.; Ku, L.; Ma, Y.; Guan, X. Genome-wide identification of HD-ZIP transcription factors in maize and their regulatory roles in promoting drought tolerance. Physiol. Mol. Biol. Plants 2022, 28, 425–437. [Google Scholar] [CrossRef]
- Jiao, P.; Jiang, Z.; Wei, X.; Liu, S.; Qu, J.; Guan, S.; Ma, Y. Overexpression of the homeobox-leucine zipper protein ATHB-6 improves the drought tolerance of maize (Zea mays L.). Plant Sci. 2022, 316, 111159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, G.; Xiang, Y.; Zhang, A. The transcription factor ZmMYB-CC10 improves drought tolerance by activating ZmAPX4 expression in maize. Biochem. Biophy. Res. Comm. 2022, 604, 1–7. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Ren, Z.; Abou-Elwafa, S.F.; Pu, X.; Zhu, Y.; Dou, D.; Su, H.; Cheng, H.; Liu, Z. ZmERF21 directly regulates hormone signaling and stress-responsive gene expression to influence drought tolerance in maize seedlings. Plant, Cell Environ. 2022, 45, 312–328. [Google Scholar] [CrossRef]
- Zhang, S.; Li, N.; Gao, F.; Yang, A.; Zhang, J. Over-expression of TsCBF1 gene confers improved drought tolerance in transgenic maize. Mol. Breed. 2010, 26, 455–465. [Google Scholar] [CrossRef]
- Zheng, H.; Yang, Z.; Wang, W.; Guo, S.; Li, Z.; Liu, K.; Sui, N. Transcriptome analysis of maize inbred lines differing in drought tolerance provides novel insights into the molecular mechanisms of drought responses in roots. Plant Physiol. and Biochem. 2020, 149, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yang, M.; Wu, X.; Wang, Y.; Zhang, R. Physiological and transcriptomic analyses of the effects of exogenous melatonin on drought tolerance in maize (Zea mays L.). Plant Physiol. Biochem. 2021, 168, 128–142. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.; Wu, X.; Wang, W. Identification of drought tolerant mechanisms in a drought-tolerant maize mutant based on physiological, biochemical and transcriptomic analyses. BMC Plant Biol. 2020, 20, 315. [Google Scholar] [CrossRef]
- Waititu, J.K.; Zhang, X.; Chen, T.; Zhang, C.; Zhao, Y.; Wang, H. Transcriptome analysis of tolerant and susceptible maize genotypes reveals novel insights about the molecular mechanisms underlying drought responses in leaves. Int. J. Mol. Sci. 2021, 22, 6980. [Google Scholar] [CrossRef]
- Liu, S.; Zenda, T.; Li, J.; Wang, Y.; Liu, X.; Duan, H. Comparative transcriptomic analysis of contrasting hybrid cultivars reveal key drought-responsive genes and metabolic pathways regulating drought stress tolerance in maize at various stages. PLoS ONE 2020, 15, e0240468. [Google Scholar] [CrossRef]
- Zhang, F.; Ding, Y.; Zhang, J.; Tang, M.; Cao, Y.; Zhang, L.; Ma, Z.; Qi, J.; Mu, X.; Xia, L. Comparative transcriptomic reveals the molecular mechanism of maize hybrid Zhengdan538 in response to water deficit. Physiol. Plant. 2022, 174, e13818. [Google Scholar] [CrossRef]
- Gillani, S.F.A.; Rasheed, A.; Abbasi, A.; Majeed, Y.; Abbas, M.; Hassan, M.U.; Qari, S.H.; Binothman, N.; Al Kashgry, N.A.T.; Tahir, M.M. Comparative gene enrichment analysis for drought tolerance in contrasting maize genotype. Genes 2022, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, C.; Wang, H.; Wang, S.; Yang, S.; Liu, X.; Yan, J.; Li, B.; Beatty, M.; Zastrow-Hayes, G. Mapping regulatory variants controlling gene expression in drought response and tolerance in maize. Genome Biol. 2020, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Zenda, T.; Liu, X.; Wang, Y.; Li, J.; Yang, Y.; Liu, S.; Duan, H. Transcriptome analysis of drought tolerance and utility assessment of DnaJ gene expression as a potential index for drought resistance evaluation in maize. Euphytica 2022, 218, 136. [Google Scholar] [CrossRef]
- Nan, W.; Liang, L.; Gao, W.-W.; WU, Y.-b.; YONG, H.-j.; Weng, J.-F.; LI, M.-S.; Zhang, D.-G.; Hao, Z.-F.; LI, X.-H. Transcriptomes of early developing tassels under drought stress reveal differential expression of genes related to drought tolerance in maize. J. Integrat. Agricul. 2018, 17, 1276–1288. [Google Scholar]
- Zhang, Y.; Soualihou, S.; Li, J.; Xu, Y.; Rose, R.J.; Ruan, Y.-L.; Li, J.; Song, Y. Transcriptome analysis of maize pollen grains under drought stress during flowering. Crop Past. Sci. 2022, 73, 1026–1041. [Google Scholar] [CrossRef]
- Liu, S.; Zenda, T.; Dong, A.; Yang, Y.; Wang, N.; Duan, H. Global transcriptome and weighted gene co-expression network analyses of growth-stage-specific drought stress responses in maize. Front. Gen. 2021, 12, 645443. [Google Scholar] [CrossRef]
- Song, K.; Kim, H.C.; Shin, S.; Kim, K.-H.; Moon, J.-C.; Kim, J.Y.; Lee, B.-M. Transcriptome analysis of flowering time genes under drought stress in maize leaves. Front. Plant Sci. 2017, 8, 267. [Google Scholar] [CrossRef][Green Version]
- Hao, L.-Y.; Liu, X.-Y.; Zhang, X.-J.; Sun, B.-C.; Cheng, L.; Zhang, D.-F.; Tang, H.-J.; LI, C.-H.; Li, Y.-X.; Shi, Y.-S. Genome-wide identification and comparative analysis of drought related genes in roots of two maize inbred lines with contrasting drought tolerance by RNA sequencing. J. Integrat. Agricul. 2020, 19, 449–464. [Google Scholar] [CrossRef]
- Brookes, G.; Barfoot, P. Environmental impacts of genetically modified (GM) crop use 1996–2015: Impacts on pesticide use and carbon emissions. GM Crops Food 2017, 8, 117–147. [Google Scholar] [CrossRef][Green Version]
- ISAAA. Global Status of Commercialized Biotech/GM Crops in 2017: Biotech Crop Adoption Surges as Economic Benefits Accumulate in 22 Years; ISAAA: Ithaca, NY, USA, 2017; Volume 53. [Google Scholar]
- Zeng, T.; Zhang, D.; Li, Y.; Li, C.; Liu, X.; Shi, Y.; Song, Y.; Li, Y.; Wang, T. Identification of genomic insertion and flanking sequences of the transgenic drought-tolerant maize line “SbSNAC1-382” using the single-molecule real-time (SMRT) sequencing method. PLoS ONE 2020, 15, e0226455. [Google Scholar] [CrossRef][Green Version]
- Tao, Y.-B.; He, L.-L.; Niu, L.-J.; Xu, Z.-F. Isolation and characterization of an ubiquitin extension protein gene (JcUEP) promoter from Jatropha curcas. Planta 2015, 241, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, M.; Guo, Z.; Zhu, X.; Xia, Z. Identification of a 119-bp promoter of the maize sulfite oxidase gene (ZmSO) that confers high-level gene expression and ABA or drought inducibility in transgenic plants. Int. J. Mol. Sci. 2019, 20, 3326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, B.; Wei, A.; Song, C.; Li, N.; Zhang, J. Heterologous expression of the TsVP gene improves the drought resistance of maize. Plant Biotech. J. 2008, 6, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, X.; Liu, X.; Zhou, M.; Ren, W.; Zhao, B.; Ju, C.; Liu, Y.; Zhao, J. A leucine-rich repeat-receptor-like kinase gene SbER2–1 from sorghum (Sorghum bicolor L.) confers drought tolerance in maize. BMC Gen. 2019, 20, 737. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.X.; Sticklen, M. Barley HVA1 gene confers drought and salt tolerance in transgenic maize Zea Mays L. Adv. Crop Sci. Tech. 2013, 1, 1–8. [Google Scholar] [CrossRef][Green Version]
- Chen, Z.; Liu, Y.; Yin, Y.; Liu, Q.; Li, N.; Li, X.; He, W.; Hao, D.; Liu, X.; Guo, C. Expression of AtGA2ox1 enhances drought tolerance in maize. Plant Growth Reg. 2019, 89, 203–215. [Google Scholar] [CrossRef]
- Gillani, S.F.; Rasheed, A.; Majeed, Y.; Tariq, H.; Yunling, P. Recent advancements on use of CRISPR/Cas9 in maize yield and quality improvement. Not. Bot. Horti Agrobot. Cluj-Nap. 2021, 49, 12459. [Google Scholar] [CrossRef]
- Čermák, T.; Baltes, N.J.; Čegan, R.; Zhang, Y.; Voytas, D.F. High-frequency, precise modification of the tomato genome. Gen. Biol. 2015, 16, 232. [Google Scholar] [CrossRef][Green Version]
- Gao, H.; Smith, J.; Yang, M.; Jones, S.; Djukanovic, V.; Nicholson, M.G.; West, A.; Bidney, D.; Falco, S.C.; Jantz, D. Heritable targeted mutagenesis in maize using a designed endonuclease. Plant J. 2010, 61, 176–187. [Google Scholar] [CrossRef]
- Li, J.-F.; Norville, J.E.; Aach, J.; McCormack, M.; Zhang, D.; Bush, J.; Church, G.M.; Sheen, J. Multiplex and homologous recombination–mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat. Biotech. 2013, 31, 688–691. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, K.; Chen, K.; Gao, C. Targeted mutagenesis in Zea mays using TALENs and the CRISPR/Cas system. J. Gen. Gen. 2014, 41, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Liu, M.; Zhao, H.; Tan, Z.; Liang, K.; Sun, Q.; Gong, D.; He, H.; Zhou, W.; Qiu, F. ZmSRL5 is involved in drought tolerance by maintaining cuticular wax structure in maize. J. Integrat. Plant Biol. 2020, 62, 1895–1909. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Gao, Y.Q.; Wu, W.H.; Chen, L.M.; Wang, Y. Two calcium-dependent protein kinases enhance maize drought tolerance by activating anion channel ZmSLAC1 in guard cells. Plant Biotech. J. 2022, 20, 143–157. [Google Scholar] [CrossRef] [PubMed]
| Genotypes/ILs/Hybrids | DEGs Genes | Role | References |
|---|---|---|---|
| 287M, 753F | 24, 220, 4551 and 16, 29, 2641 | ROS scavenging and hormonal metabolism | [111] |
| Maize seedling | 957 | MT interaction with other hormones enhanced drought tolerance | [112] |
| C7–2t, C7–2 | 4552 | C7–2t had a stable photosynthesis rate | [113] |
| CML69, LX9801 | 4687 genotype-specific and 2219 common drought-tolerant genes | Drought avoidance and osmotic regulation | [114] |
| 224 maize accessions | 73,573 eQTL for 30,000 genes | 97 genes associated with drought tolerance due to expression variant | [118] |
| Maize genotypes | 666, 2417, 7375 | DnaJ and a putative WAK family receptor-like protein kinase improved drought tolerance | [119] |
| 10 ILs | 19,001 | Metabolic regulation at the RNA level | [120] |
| Hybrids ND476 and ZX978 | 3451 and 4088 | Sucrose, starch, pentose, and ribosome | [115] |
| 478, H21 | 68%, 48% and 32% DEGs in 478 | Sucrose and starch metabolism | [117] |
| Chang 7-2, TS141 | 562, 824 | GB-based upregulation and downregulation of DEGs can enhance drought tolerance | [1] |
| Hybrid, ZhongDan909 | 6424 genes and 1302 transcripts | Genes involved in pollen development | [121] |
| Zhengdan538 | 2994–4692 | Energy biosynthesis and photosynthesis | [116] |
| Hybrid ND47 | 3451 | Increased photosynthesis rate and amino acid metabolism | [122] |
| Zea mays cv. B73 | 619 genes and 126 transcripts | 20 drought-tolerant genes involved in flowering time | [123] |
| H082183 (drought tolerant), Lv28 (drought susceptible) | 688 and 3 363 in H082183 and 1428 and 512 in Lv28 | Plant hormone transduction and starch metabolism | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasheed, A.; Jie, H.; Ali, B.; He, P.; Zhao, L.; Ma, Y.; Xing, H.; Qari, S.H.; Hassan, M.U.; Hamid, M.R.; et al. Breeding Drought-Tolerant Maize (Zea mays) Using Molecular Breeding Tools: Recent Advancements and Future Prospective. Agronomy 2023, 13, 1459. https://doi.org/10.3390/agronomy13061459
Rasheed A, Jie H, Ali B, He P, Zhao L, Ma Y, Xing H, Qari SH, Hassan MU, Hamid MR, et al. Breeding Drought-Tolerant Maize (Zea mays) Using Molecular Breeding Tools: Recent Advancements and Future Prospective. Agronomy. 2023; 13(6):1459. https://doi.org/10.3390/agronomy13061459
Chicago/Turabian StyleRasheed, Adnan, Hongdong Jie, Basharat Ali, Pengliang He, Long Zhao, Yushen Ma, Hucheng Xing, Sameer H. Qari, Muhammad Umair Hassan, Muhammad Rizwan Hamid, and et al. 2023. "Breeding Drought-Tolerant Maize (Zea mays) Using Molecular Breeding Tools: Recent Advancements and Future Prospective" Agronomy 13, no. 6: 1459. https://doi.org/10.3390/agronomy13061459
APA StyleRasheed, A., Jie, H., Ali, B., He, P., Zhao, L., Ma, Y., Xing, H., Qari, S. H., Hassan, M. U., Hamid, M. R., & Jie, Y. (2023). Breeding Drought-Tolerant Maize (Zea mays) Using Molecular Breeding Tools: Recent Advancements and Future Prospective. Agronomy, 13(6), 1459. https://doi.org/10.3390/agronomy13061459








