Molecular Mechanism of Exogenous Selenium Affecting the Nutritional Quality, Species and Content of Organic Selenium in Mustard
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Treatment
2.2. Determination of Growth Indicators
2.3. Determination of Mustard Antioxidant System
2.4. Determination of Total Se and Se Speciation
2.5. Transcriptome Sequencing and Data Analysis
2.6. Statistical Analysis
3. Results
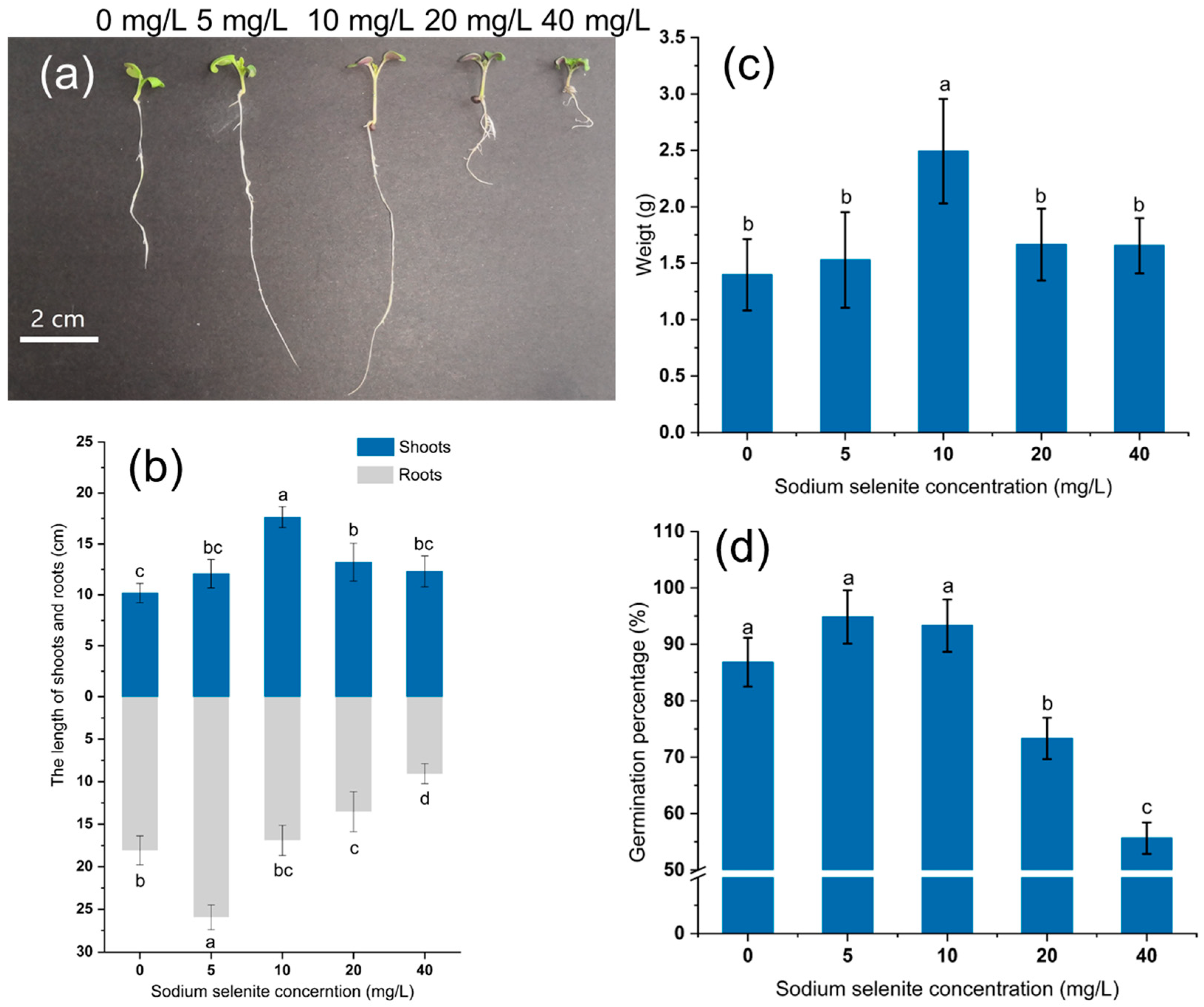
3.1. Effect of Na2SeO3 on the Biomass and Germination Rate of Mustard
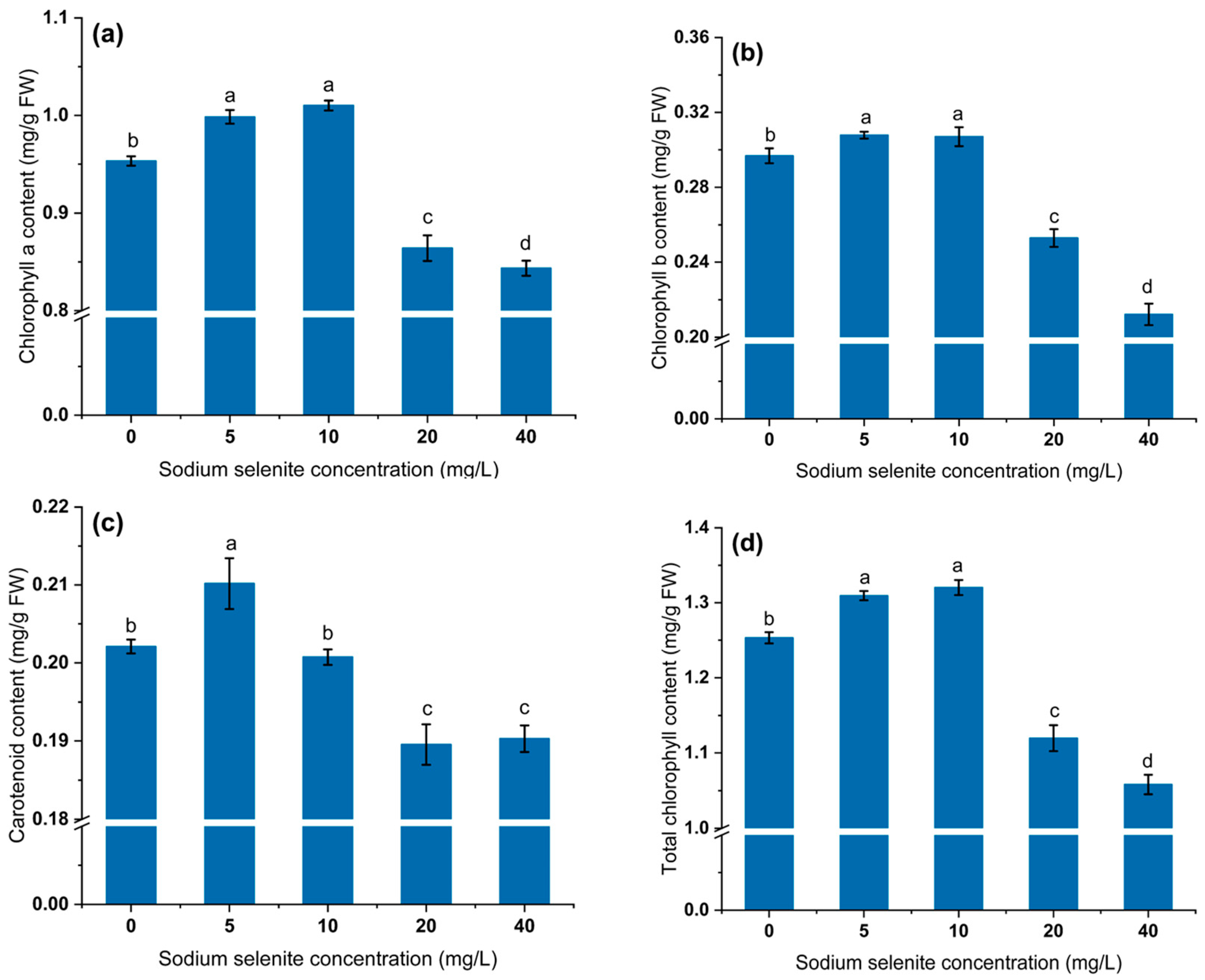
3.2. Effect of Na2SeO3 on Photosynthetic Pigment Content of Mustard
3.3. Effect of Na2SeO3 on Total Se and Se Speciation in Mustard
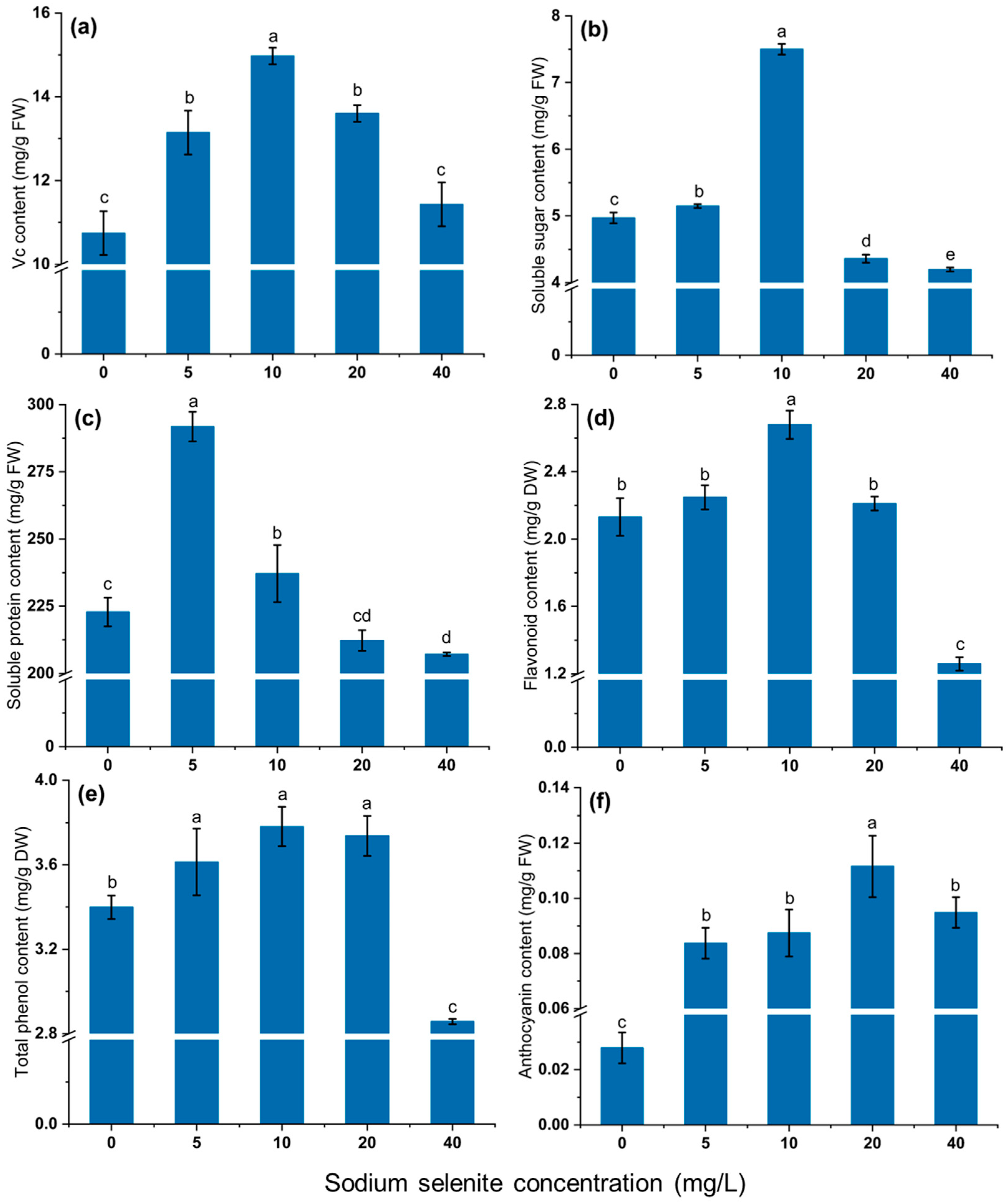
3.4. Effect of Na2SeO3 Treatment on Nutritional Quality of Mustard
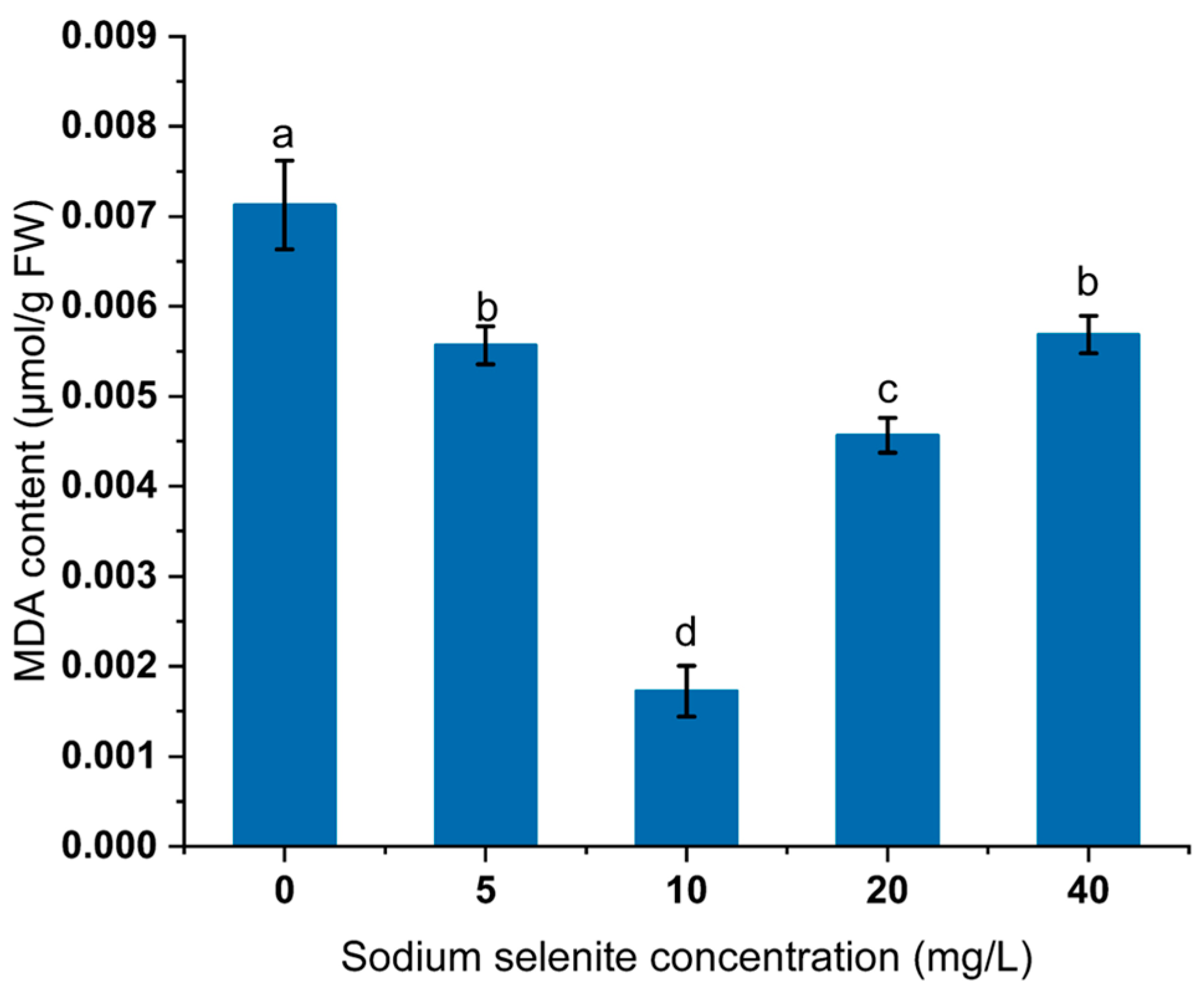
3.5. Effect of Na2SeO3 Treatment on MDA Content of Mustard
3.6. Effect of Na2SeO3 Treatment on Antioxidant System of Mustard
3.7. Analysis of Transcriptome Data of Mustard Treated with Na2SeO3
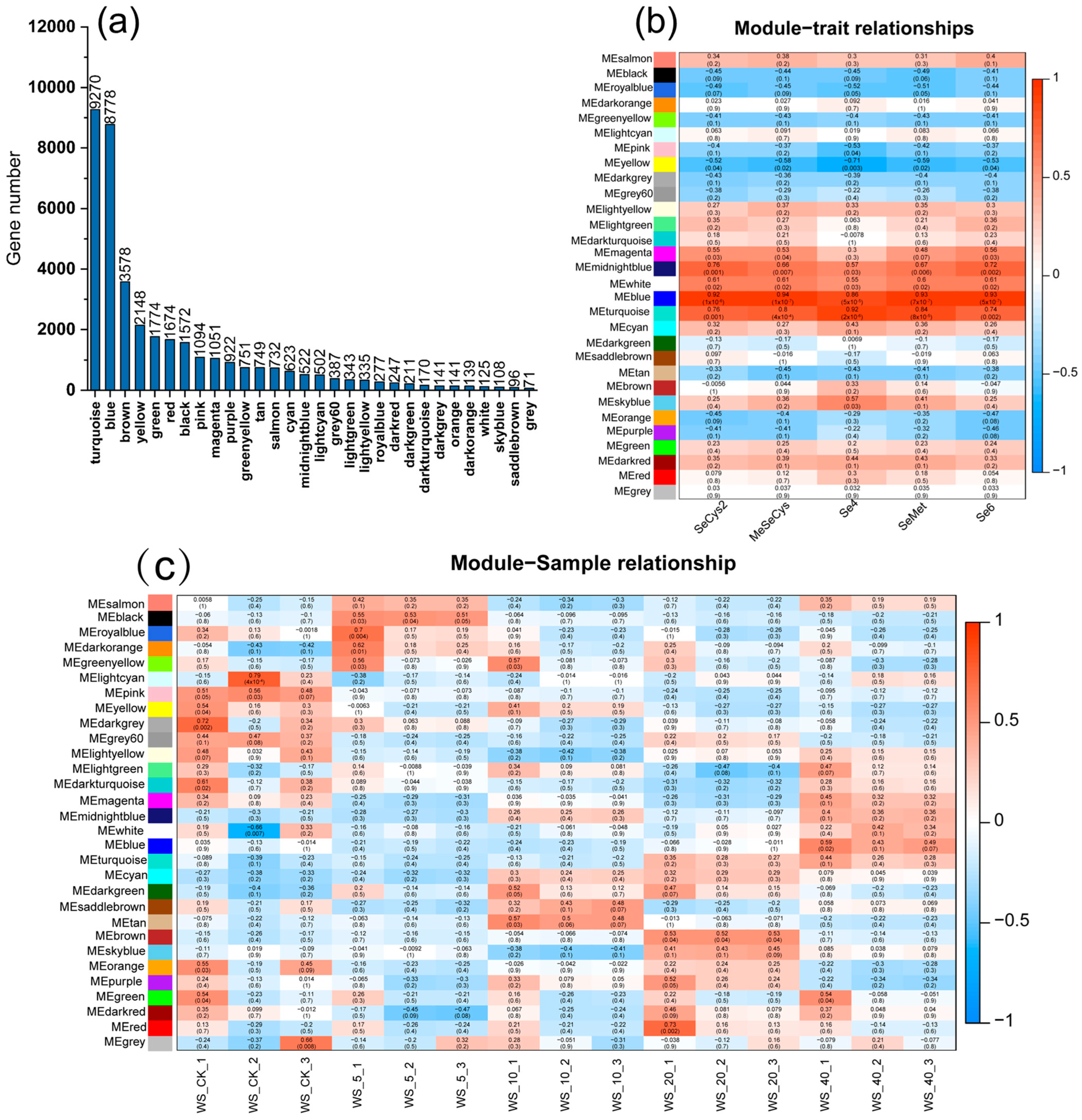
3.8. WGCNA Analysis of Transcriptome Data
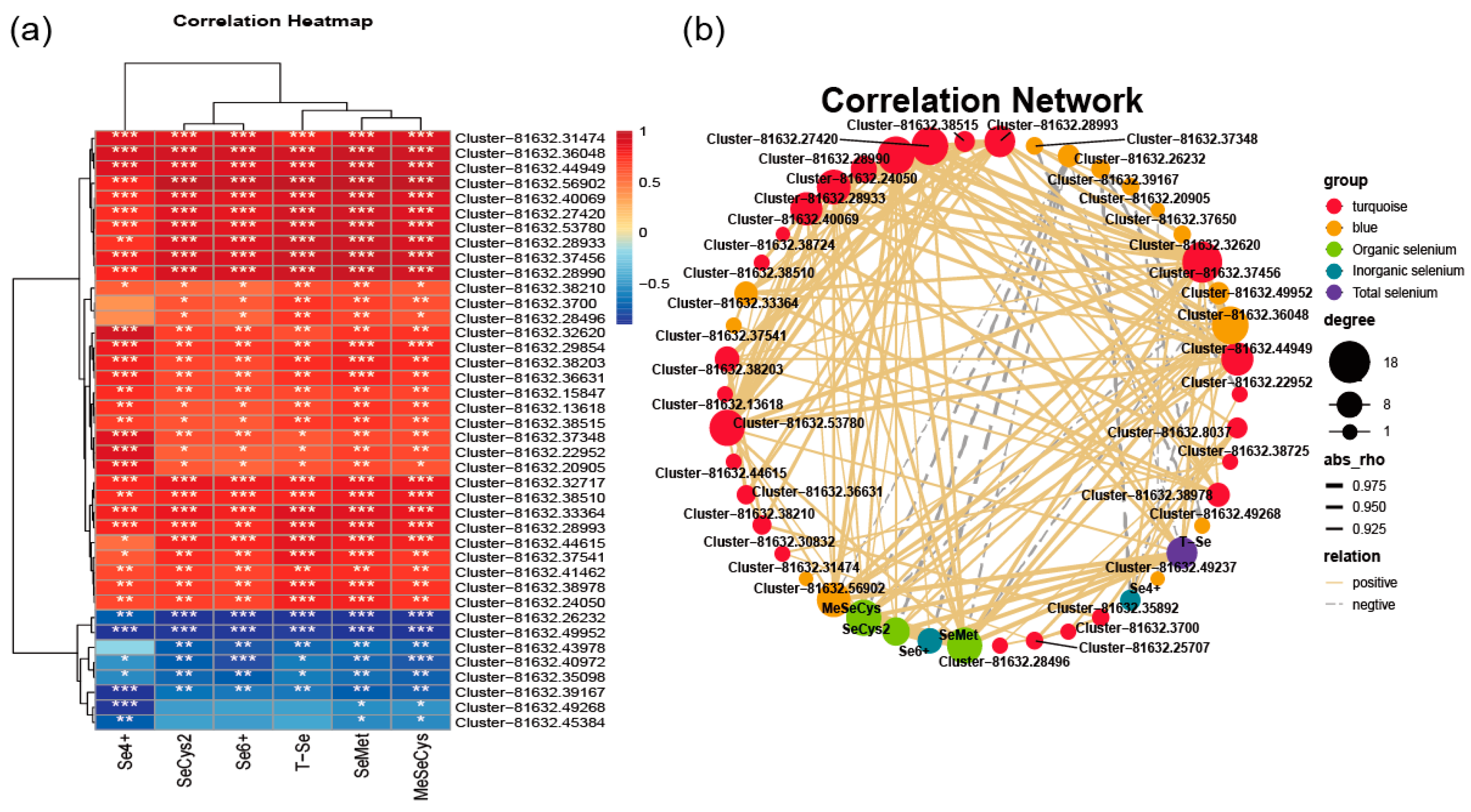
3.9. WGCNA Analysis of Transcriptome Data and Different Se Species
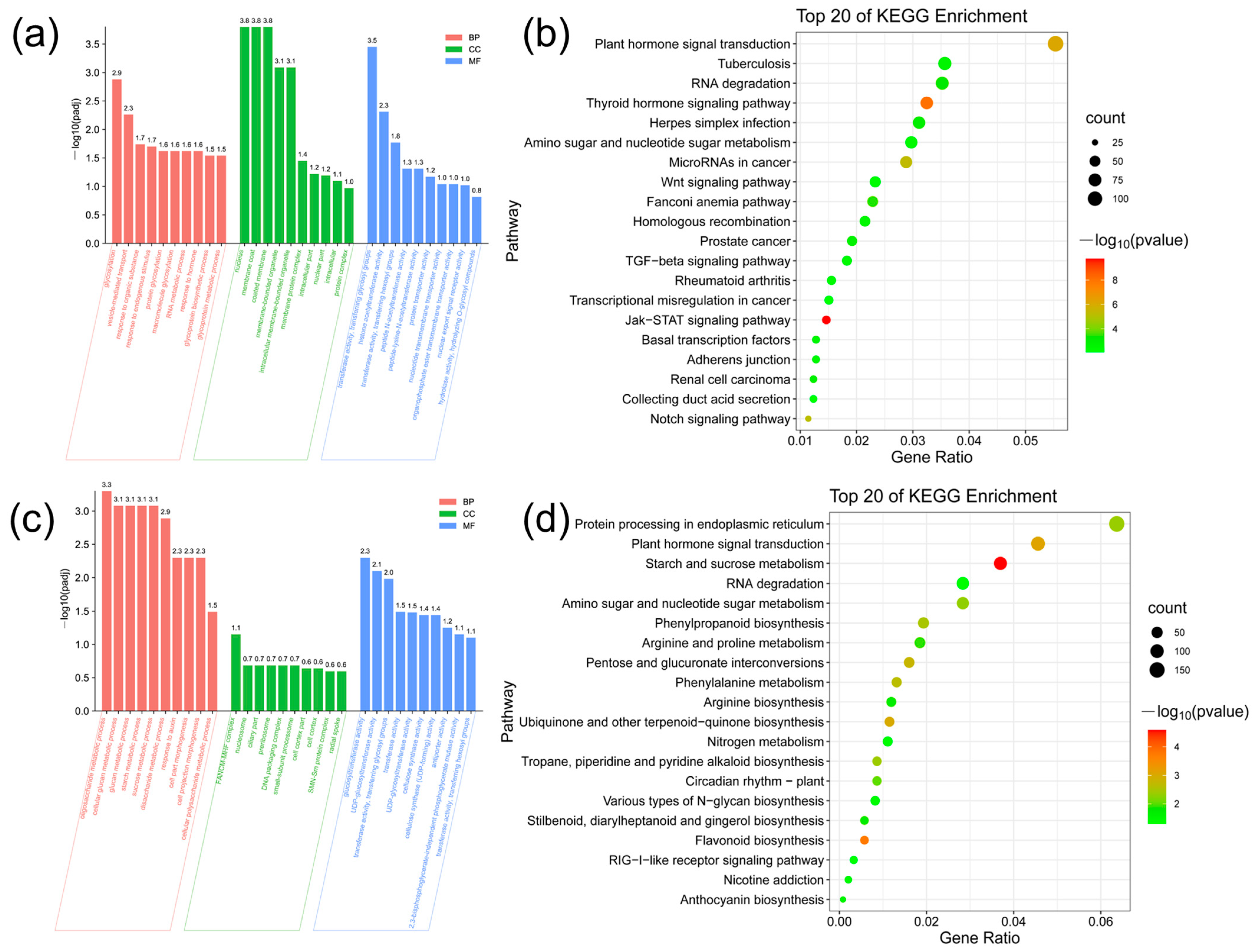
3.10. Analysis of Gene Expression Regulation of Key Modules
4. Discussion
4.1. Effect of Se on the Growth of Mustard
4.2. Effect of Se on Nutritional Quality of Mustard
4.3. Effects of Se on the Antioxidant System of Mustard
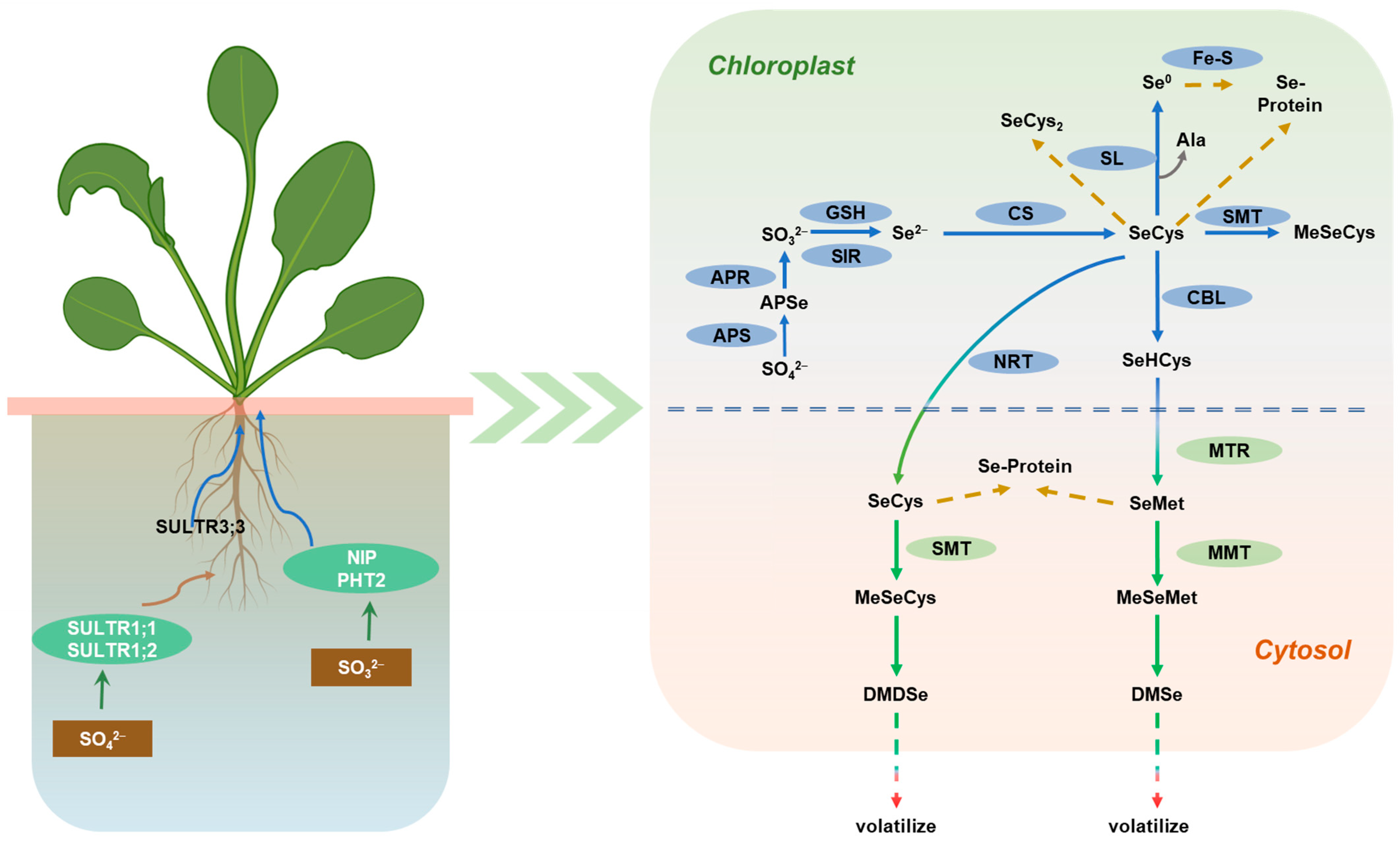
4.4. Molecular Mechanism of Organic Se Transformation in Mustard
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C. Current knowledge on selenium biofortification to improve the nutraceutical profile of food: A comprehensive review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.; Noelia, F.V. Why 21? The significance of selenoproteins for human health revealed by inborn errors of metabolism. FASEB J. 2016, 30, 3669–3681. [Google Scholar]
- Krinsky, N.I.; Beecher, G.R.; Burk, R.F.; Chan, A.C.; Erdman, J.J.; Jacob, R.A.; Jialal, I.; Kolonel, L.N.; Marshall, J.R.; Taylor Mayne, P.R. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Inst. Med. 2000, 19, 95–185. [Google Scholar]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.E.; Leschik-Bonnet, E.; Heseker, H.; German Nutrition, S. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef]
- Silva, V.M.; Boleta, E.H.M.; Lanza, M.G.D.B.; Lavres, J.; Martins, J.T.; Santos, E.F.; dos Santos, F.L.M.; Putti, F.F.; Junior, E.F.; White, P.J.; et al. Physiological, biochemical, and ultrastructural characterization of selenium toxicity in cowpea plants. Environ. Exp. Bot. 2018, 150, 172–182. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A.H. The fascinating facets of plant selenium accumulation–biochemistry, physiology, evolution and ecology. New Phytol. 2017, 213, 1582–1596. [Google Scholar] [CrossRef]
- Gong, R.; Ai, C.; Zhang, B.; Cheng, X. Effect of selenite on organic selenium speciation and selenium bioaccessibility in rice grains of two Se-enriched rice cultivars. Food Chem. 2018, 264, 443–448. [Google Scholar] [CrossRef]
- Nothstein, A.K.; Eiche, E.; Riemann, M.; Nick, P.; Winkel, L.H.E.; Göttlicher, J.; Steininger, R.; Brendel, R.; Von Brasch, M.; Konrad, G. Tracking se assimilation and speciation through the rice plant–nutrient competition, toxicity and distribution. PLoS ONE 2016, 11, e0152081. [Google Scholar] [CrossRef]
- Danso, O.P.; Asante-Badu, B.; Zhang, Z.; Song, J.; Wang, Z.; Yin, X.; Zhu, R. Selenium biofortification: Strategies, progress and challenges. Agriculture 2023, 13, 416. [Google Scholar] [CrossRef]
- Reis, A.R.D.; El-Ramady, H.; Santos, E.F.; Gratão, P.L.; Schomburg, L. Overview of selenium deficiency and toxicity worldwide: Affected areas, selenium-related health issues, and case studies. In Selenium in Plants; Springer: Berlin/Heidelberg, Germany, 2017; pp. 209–230. [Google Scholar]
- Hamilton, S.J. Review of selenium toxicity in the aquatic food chain. Sci. Total Environ. 2004, 326, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiong, Y.; Wang, Y.; Wu, S.; Xiao, C.; Wang, S.; Cheng, S.; Cheng, H. Effect of nano-selenium on nutritional quality of cowpea and response of ABCC transporter family. Molecules 2023, 28, 1398. [Google Scholar] [CrossRef] [PubMed]
- Lanza, M.G.D.B.; Silva, V.M.; Montanha, G.S.; Lavres, J.; de Carvalho, H.W.P.; Dos Reis, A.R. Assessment of selenium spatial distribution using μ-XFR in cowpea (Vigna unguiculata (L.) Walp.) plants: Integration of physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111216. [Google Scholar] [CrossRef]
- Ahmad, P.; Abd Allah, E.F.; Hashem, A.; Sarwat, M.; Gucel, S. Exogenous application of selenium mitigates cadmium toxicity in Brassica juncea L. (Czern & Cross) by up-regulating antioxidative system and secondary metabolites. J. Plant Growth Regul. 2016, 35, 936–950. [Google Scholar]
- Dixon, G.R. Origins and diversity of Brassica and its relatives. In Vegetable Brassicas and Related Crucifers; CABI: Wallingford, UK, 2006; pp. 1–33. [Google Scholar]
- Kang, L.; Qian, L.; Zheng, M.; Chen, L.; Chen, H.; Yang, L.; You, L.; Yang, B.; Yan, M.; Gu, Y. Genomic insights into the origin, domestication and diversification of Brassica juncea. Nat. Genet. 2021, 53, 1392–1402. [Google Scholar] [CrossRef]
- Lim, H.-S.; Yoo, E.-J.; Choi, M.-R. Changes of physiological activity of mustard leaf during its fermentation period. J. Microbiol. Biotechnol. 2000, 10, 43–47. [Google Scholar]
- Campbell, B.; Han, D.; Triggs, C.M.; Fraser, A.G.; Ferguson, L.R. Brassicaceae: Nutrient analysis and investigation of tolerability in people with Crohn’s disease in a New Zealand study. Funct. Foods Health Dis. 2012, 2, 460–486. [Google Scholar] [CrossRef]
- Bañuelos, G.; Leduc, D.L.; Pilon-Smits, E.A.H.; Terry, N. Transgenic Indian mustard overexpressing selenocysteine lyase or selenocysteine methyltransferase exhibit enhanced potential for selenium phytoremediation under field conditions. Environ. Sci. Technol. 2007, 41, 599–605. [Google Scholar] [CrossRef]
- LeDuc, D.L.; AbdelSamie, M.; Móntes-Bayon, M.; Wu, C.P.; Reisinger, S.J.; Terry, N. Overexpressing both ATP sulfurylase and selenocysteine methyltransferase enhances selenium phytoremediation traits in Indian mustard. Environ. Pollut. 2006, 144, 70–76. [Google Scholar] [CrossRef]
- Hou, F. Experiments in Plant Physiology; Science Press: Beijing, China, 2022; pp. 23–28. [Google Scholar]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Vemuri, H.; Garai, S.; Mondal, M.; Bhatt, R.; et al. Selenium biofortification: Roles, mechanisms, responses and prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef] [PubMed]
- Boldrin, P.F.; de Figueiredo, M.A.; Yang, Y.; Luo, H.; Giri, S.; Hart, J.J.; Faquin, V.; Guilherme, L.R.; Thannhauser, T.W.; Li, L. Selenium promotes sulfur accumulation and plant growth in wheat (Triticum aestivum). Physiol. Plant. 2016, 158, 80–91. [Google Scholar] [CrossRef] [PubMed]
- de Brito Mateus, M.P.; Tavanti, R.F.R.; Tavanti, T.R.; Santos, E.F.; Jalal, A.; Dos Reis, A.R. Selenium biofortification enhances ROS scavenge system increasing yield of coffee plants. Ecotoxicol. Environ. Saf. 2021, 209, 111772. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; de Sousa, G.F.; Corguinha, A.P.B.; de Lima Lessa, J.H.; Dinali, G.S.; Oliveira, C.; Lopes, G.; Amaral, D.; Brown, P.; Guilherme, L.R.G. Selenium biofortification of soybean genotypes in a tropical soil via Se-enriched phosphate fertilizers. Front. Plant Sci. 2022, 13, 988140. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Zhang, X.; Zhang, W.; Huang, L.; Zhang, Z.; Yuan, L.; Liu, X. Effects of foliar application of selenate and selenite at different growth stages on Selenium accumulation and speciation in potato (Solanum tuberosum L.). Food Chem. 2019, 286, 550–556. [Google Scholar] [CrossRef]
- Wang, M.; Li, B.; Li, S.; Song, Z.; Kong, F.; Zhang, X. Selenium in wheat from farming to food. J. Agric. Food Chem. 2021, 69, 15458–15467. [Google Scholar] [CrossRef]
- Taha, R.S.; Seleiman, M.F.; Shami, A.; Alhammad, B.A.; Mahdi, A.H.A. Integrated application of selenium and silicon enhances growth and anatomical structure, antioxidant defense system and yield of wheat grown in salt-stressed. Soil 2021, 10, 1040. [Google Scholar] [CrossRef]
- Zsiros, O.; Nagy, V.; Párducz, Á.; Nagy, G.; Ünnep, R.; El-Ramady, H.; Prokisch, J.; Lisztes-Szabó, Z.; Fári, M.; Csajbók, J.; et al. Effects of selenate and red Se-nanoparticles on the photosynthetic apparatus of Nicotiana tabacum. Photosynth. Res. 2019, 139, 449–460. [Google Scholar] [CrossRef]
- Lehotai, N.; Kolbert, Z.; Pető, A.; Feigl, G.; Ördög, A.; Kumar, D.; Tari, I.; Erdei, L. Selenite-induced hormonal and signalling mechanisms during root growth of Arabidopsis thaliana L. J. Exp. Bot. 2012, 63, 5677–5687. [Google Scholar] [CrossRef]
- Hu, T.; Li, H.; Li, J.; Zhao, G.; Wu, W.; Liu, L.; Wang, Q.; Guo, Y. Absorption and bio-transformation of selenium nanoparticles by wheat seedlings (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.M.; Tavanti, R.F.R.; Gratão, P.L.; Alcock, T.D.; Dos Reis, A.R. Selenate and selenite affect photosynthetic pigments and ROS scavenging through distinct mechanisms in cowpea (Vigna unguiculata (L.) walp) plants. Ecotoxicol. Environ. Saf. 2020, 201, 110777. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Shan, W.; Zhu, G. Effect of foliar application selenium on the yield and quality of soybean. Xinjiang Agric. Sci. 2009, 46, 4. [Google Scholar]
- Liu, G.; Li, P.; Li, Y.; Ren, X.; Wang, J. Effects of sodium selenite treatment on quality and active oxygen of cowpea. J. Gansu Agric. Univ. 2020, 55, 8. [Google Scholar]
- Chan, Q.; Afton, S.E.; Caruso, J.A. Selenium speciation profiles in selenite-enriched soybean (Glycine Max) by HPLC-ICPMS and ESI-ITMS. Metallomics 2010, 2, 147–153. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, N.; Liang, X.; Zheng, L.; Zhang, C.; Li, Y.-F.; Zhang, Z.; Gao, Y.; Zhao, J. A comparative study on the accumulation, translocation and transformation of selenite, selenate, and SeNPs in a hydroponic-plant system. Ecotoxicol. Environ. Saf. 2020, 189, 109955. [Google Scholar] [CrossRef]
- Rady, M.M.; Desoky, E.-S.M.; Ahmed, S.M.; Majrashi, A.; Ali, E.F.; Arnaout, S.M.A.I.; Selem, E. Foliar nourishment with nano-selenium dioxide promotes physiology, biochemistry, antioxidant defenses, and salt tolerance in phaseolus vulgaris. Plants 2021, 10, 1189. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Akbar, A.; Parveen, A.; Rasheed, R.; Hussain, I.; Iqbal, M. Phenological application of selenium differentially improves growth, oxidative defense and ion homeostasis in maize under salinity stress. Plant Physiol. Biochem. 2018, 123, 268–280. [Google Scholar] [CrossRef]
- Dai, Z.; Imtiaz, M.; Rizwan, M.; Yuan, Y.; Huang, H.; Tu, S. Dynamics of selenium uptake, speciation, and antioxidant response in rice at different panicle initiation stages. Sci. Total Environ. 2019, 691, 827–834. [Google Scholar] [CrossRef]
- Wang, M.; Peng, Q.; Zhou, F.; Yang, W.; Dinh, Q.T.; Liang, D. Uptake kinetics and interaction of selenium species in tomato (Solanum lycopersicum L.) seedlings. Environ. Sci. Pollut. Res. 2019, 26, 9730–9738. [Google Scholar] [CrossRef]
- Trippe III, R.C.; Pilon-Smits, E.A. Selenium transport and metabolism in plants: Phytoremediation and biofortification implications. J. Hazard. Mater. 2021, 404, 124178. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Yu, T.; Cong, X.; Lai, X.; Xiang, J.; Cao, J.; Liao, X.; Gou, Y.; Chao, W.; Xue, H. Transcriptome, proteome, and metabolome reveal the mechanism of tolerance to selenate toxicity in Cardamine violifolia. J. Hazard. Mater. 2021, 406, 124283. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liao, X.; Yu, L.; Rao, S.; Chen, Q.; Zhu, Z.; Cong, X.; Zhang, W.; Ye, J.; Cheng, S. Combined metabolome and transcriptome analysis reveal the mechanism of selenate influence on the growth and quality of cabbage (Brassica oleracea var. capitata L.). Food Res. Int. 2022, 156, 111135. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Li, X.; Guo, L.; Wang, L.; Hao, X.; Zeng, J. Integrative transcriptome and proteome analysis reveals the absorption and metabolism of selenium in tea plants [Camellia sinensis (L.) Kuntze]. Front. Plant Sci. 2022, 13, 848349. [Google Scholar] [CrossRef] [PubMed]




| Na2SeO3 Concentration (mg/L) | Content of Different Se Forms (mg/kg DW) | ||||
|---|---|---|---|---|---|
| SeCys2 | MeSeCys | Se4+ | SeMet | Se6+ | |
| 0 | ND | ND | 0.0038 ± 0.0001 d | 0.0160 ± 0.0003 d | ND |
| 5 | 0.0109 ± 0.0009 d | 0.0188 ± 0.0007 b | 0.0016 ± 0.0002 c | 0.0422 ± 0.0012 d | 0.0124 ± 0.0003 c |
| 10 | 0.0328 ± 0.0013 c | 0.0091 ± 0.0008 c | 0.0014 ± 0.0008 cd | 0.1198 ± 0.0027 c | 0.0177 ± 0.0011 c |
| 20 | 0.0592 ± 0.0011 b | 0.1887 ± 0.0028 b | 0.0037 ± 0.0002 b | 0.7126 ± 0.0087 b | 0.0418 ± 0.0019 b |
| 40 | 0.2227 ± 0.0026 a | 0.6469 ± 0.0095 a | 0.0054 ± 0.0002 a | 1.8165 ± 0.0475 a | 0.1854 ± 0.0046 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Wu, S.; Wang, S.; Shi, X.; Cheng, S.; Cheng, H. Molecular Mechanism of Exogenous Selenium Affecting the Nutritional Quality, Species and Content of Organic Selenium in Mustard. Agronomy 2023, 13, 1425. https://doi.org/10.3390/agronomy13051425
Li L, Wu S, Wang S, Shi X, Cheng S, Cheng H. Molecular Mechanism of Exogenous Selenium Affecting the Nutritional Quality, Species and Content of Organic Selenium in Mustard. Agronomy. 2023; 13(5):1425. https://doi.org/10.3390/agronomy13051425
Chicago/Turabian StyleLi, Linling, Shuai Wu, Shiyan Wang, Xinyu Shi, Shuiyuan Cheng, and Hua Cheng. 2023. "Molecular Mechanism of Exogenous Selenium Affecting the Nutritional Quality, Species and Content of Organic Selenium in Mustard" Agronomy 13, no. 5: 1425. https://doi.org/10.3390/agronomy13051425
APA StyleLi, L., Wu, S., Wang, S., Shi, X., Cheng, S., & Cheng, H. (2023). Molecular Mechanism of Exogenous Selenium Affecting the Nutritional Quality, Species and Content of Organic Selenium in Mustard. Agronomy, 13(5), 1425. https://doi.org/10.3390/agronomy13051425






