Nutritional Characterization Based on Vegetation Indices to Detect Anthocyanins, Carotenoids, and Chlorophylls in Mini-Lettuce
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
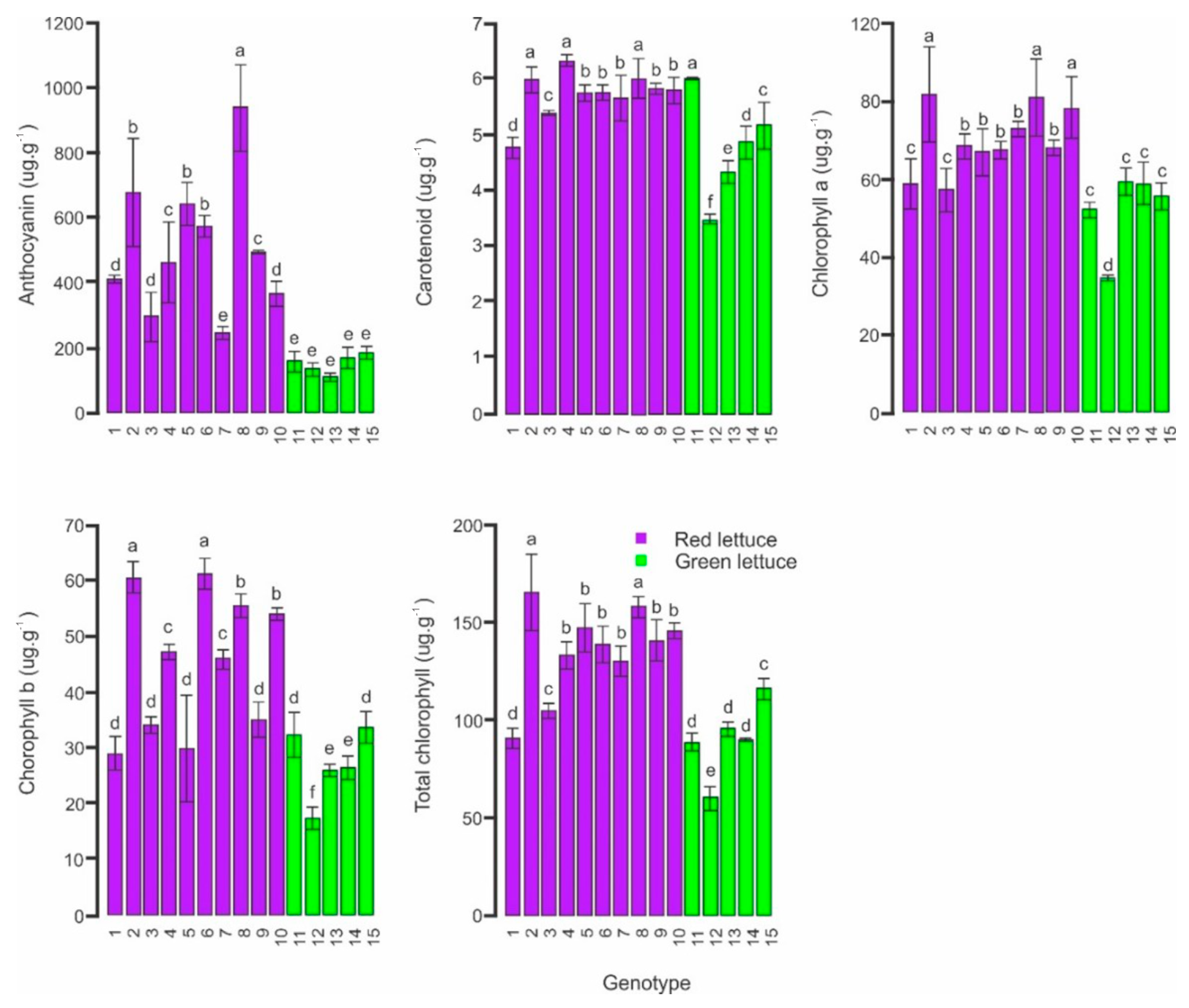
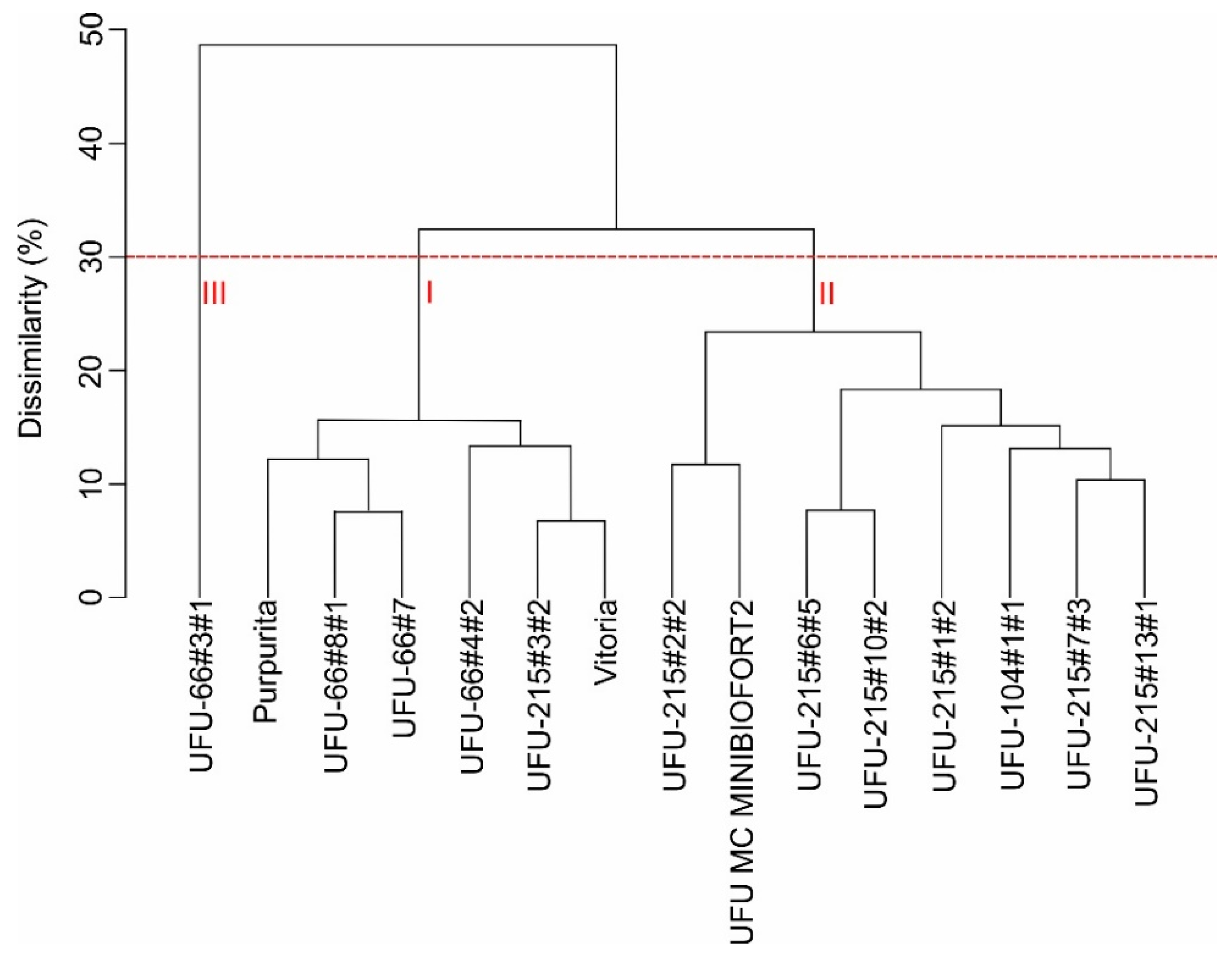
3.1. Nutritional Characterization of Mini-Lettuce Genotypes and Confirmation of Genetic Dissimilarity for Leaf Pigments
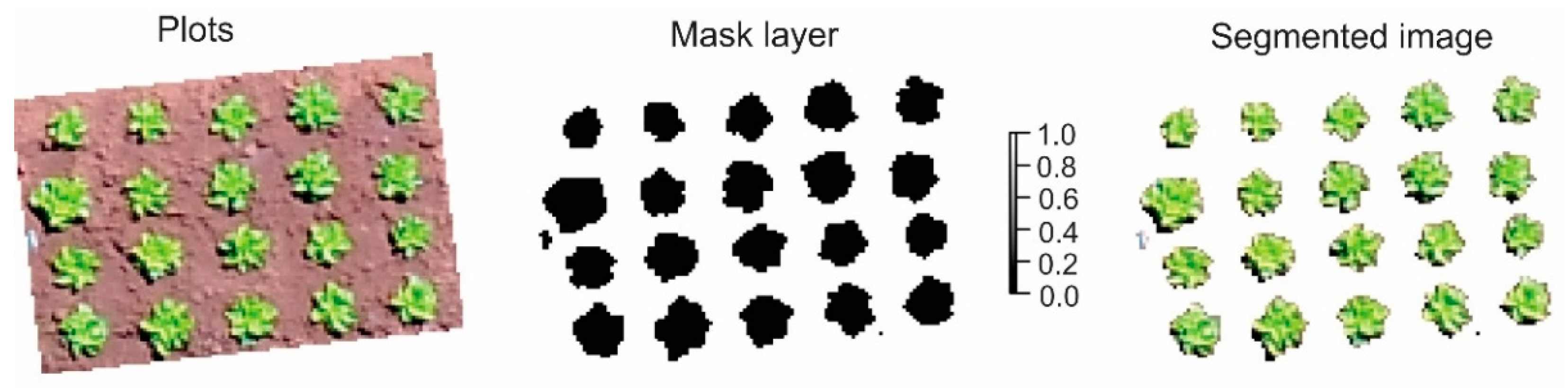
3.2. Segmentation of Plants for RGB Images
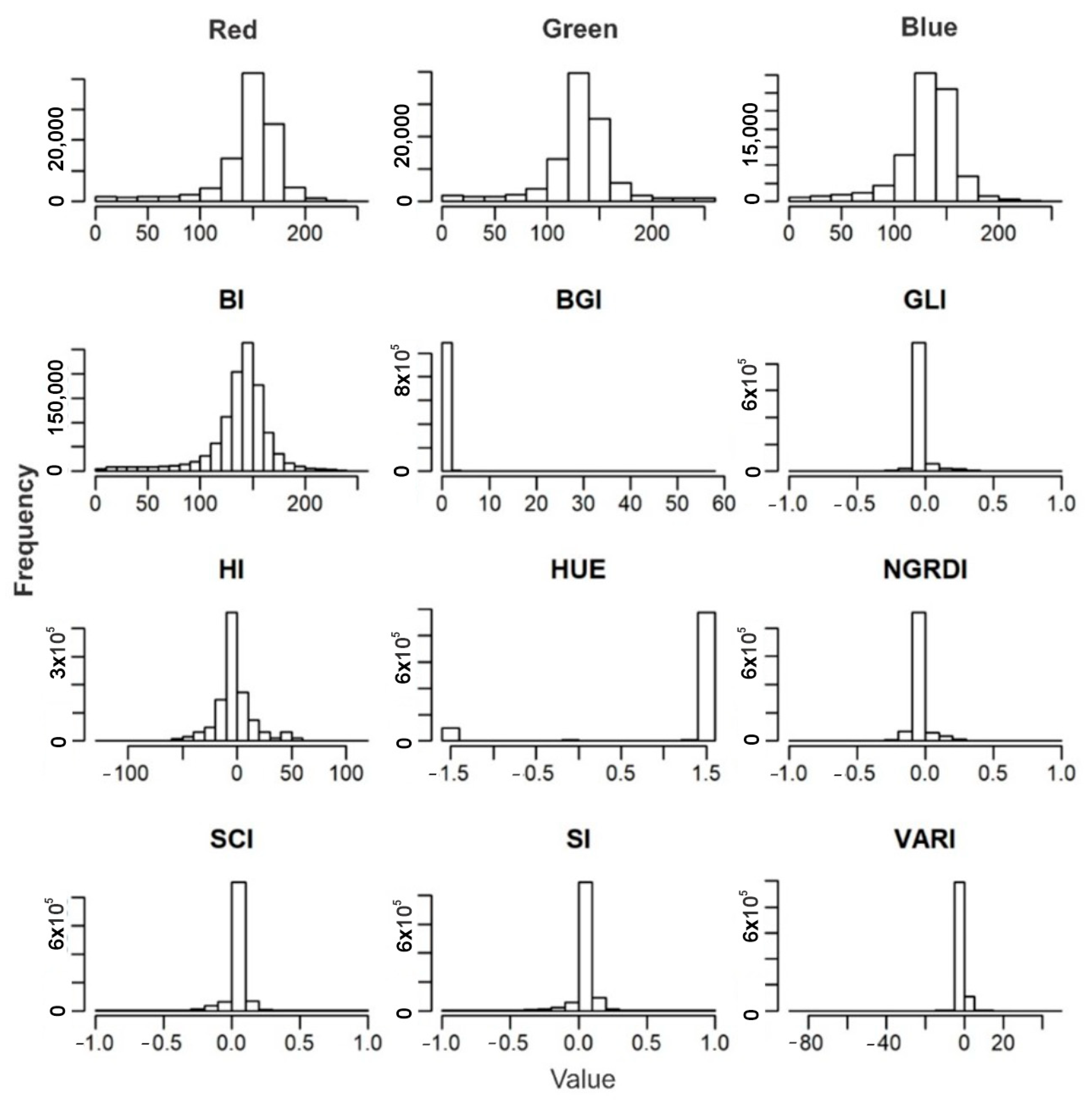
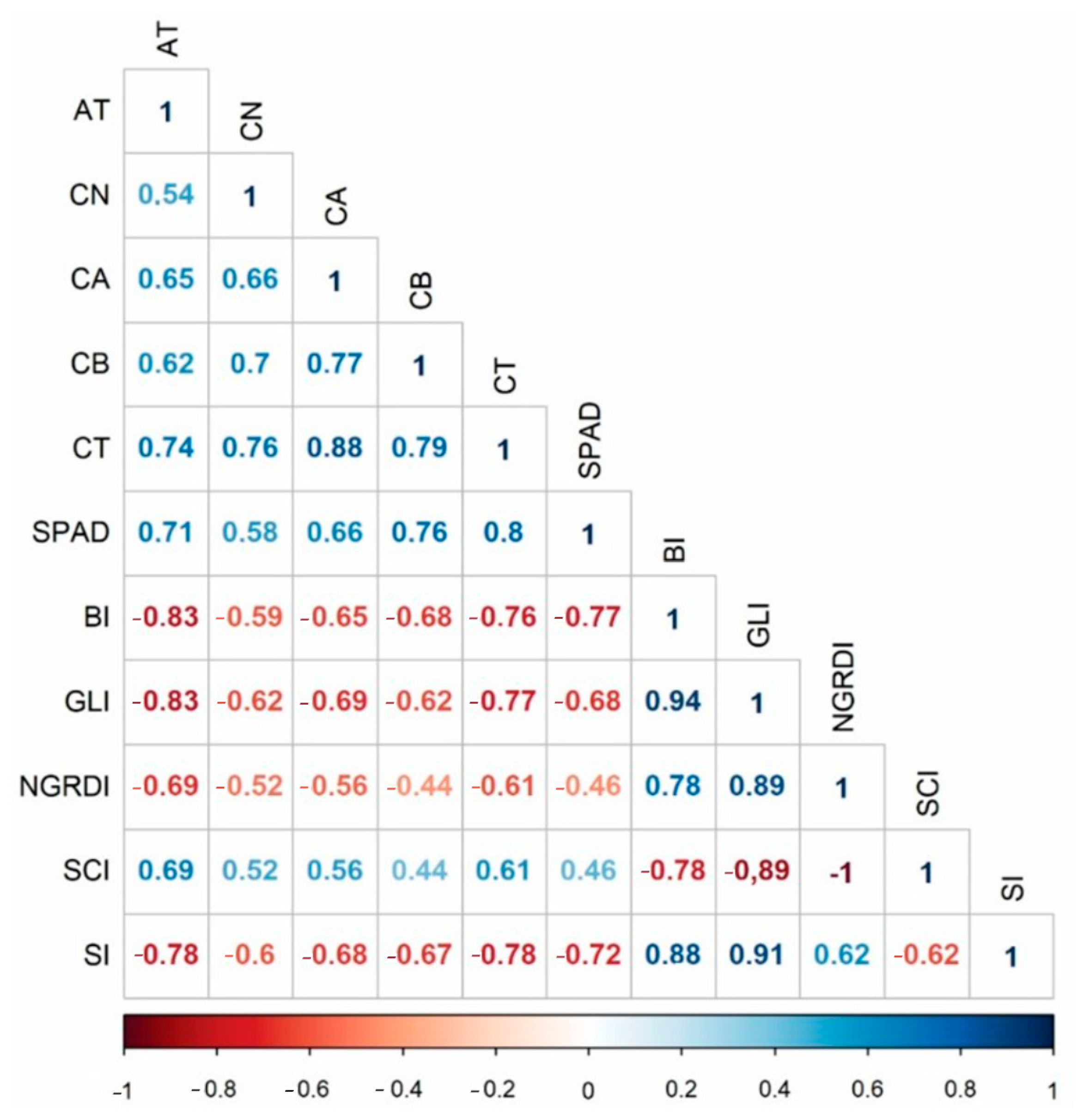
3.3. Validation of High-Performance Phenotyping for Red and Green Lettuce Genotypes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cuenca, M.H.; Proaño, G.V.; Blankenship, J.; Cano-Gutierrez, C.; Chew, S.T.H.; Fracassi, P.; Keller, H.; Mannar, V.; Mastrilli, V.; Milewska, M.; et al. Building global nutrition policies in health care: Insights for tackling malnutrition from the academy of nutrition and dietetics 2019 global nutrition research and policy forum. J. Acad. Nutr. Diet. 2020, 120, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.D.; Kerr, K.W.; Brunton, C.; Williams, J.A.; Dewitt, T.; Wulf, K.L. A First Step Towards Eliminating Malnutrition: A Proposal for Universal Nutrition Screening in Pediatric Practice. Nutr. Diet. Suppl. 2021, 13, 17–24. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2019. Safeguarding against Economic Slowdowns and Downturns. Rome, FAO. 2019. Available online: http://www.fao.org/3/ca5162en/ca5162en.pdf (accessed on 20 September 2020).
- Ventura, D.D.F.L.; Ribeiro, H.; Giulio, G.M.D.; Jaime, P.C.; Nunes, J.; Bógus, C.M.; Waldman, E.A. Desafios da pandemia de COVID-19: Por uma agenda brasileira de pesquisa em saúde global e sustentabilidade. Cad. Saude Publica 2020, 36, e00040620. [Google Scholar] [CrossRef]
- Giordano, M.; El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Stazi, S.R.; De Pascale, S.; Rouphael, Y. Iron biofortification of red and green pigmented lettuce in closed soilless cultivation impacts crop performance and modulates mineral and bioactive composition. Agronomy 2019, 9, 290. [Google Scholar] [CrossRef]
- Finkelstein, J.L.; Fothergill, A.; Hackl, L.S.; Haas, J.D.; Mehta, S. Iron biofortification interventions to improve iron status and functional outcomes. Proc. Nutr. Soc. 2019, 78, 197–207. [Google Scholar] [CrossRef]
- Lockyer, S.; White, A.; Buttriss, J.L. Biofortified crops for tackling micronutrient deficiencies—What impact are these having in developing countries and could they be of relevance within Europe? Nutr. Bull. 2018, 43, 319–357. [Google Scholar] [CrossRef]
- Almeida, H.J.; Dutra, A.F.; Cecílio Filho, A.B. Biofortificação de hortaliças e saúde global—Um enfoque para selênio, zinco, ferro e iodo. In Nutrição e Adubação de Hortaliças; Prado, R.M., Cecílio Filho, A.B., Eds.; FCAV/Unesp: Jaboticabal, Brazil, 2016; pp. 103–150. [Google Scholar]
- Camejo, D.; Frutos, A.; Mestre, T.C.; Piñero, M.C.; Rivero, R.M.; Martínez, V. Artificial light impacts the physical and nutritional quality of lettuce plants. Hortic. Environ. Biotechnol. 2020, 61, 69–82. [Google Scholar] [CrossRef]
- Sala, F.C.; Costa, C.P. Retrospectiva e tendência da alfacicultura brasileira. Hortic. Bras. 2012, 30, 187–194. [Google Scholar] [CrossRef]
- Clemens, S. How metal hyperaccumulating plants can advance Zn biofortification. Plant Soil 2017, 411, 111–120. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Xu, S.Z.; Cheng, Y.W.; Ya, H.Y.; Han, J.M. Transcriptome analysis and anthocyanin-related genes in red leaf lettuce. Genet. Mol. Res. 2016, 15, 10–4238. [Google Scholar] [CrossRef]
- Sala, F.C.; Costa, C.P. Melhoramento de alface. In Melhoramento de Hortaliças, 1st ed.; Nick, C., Borém, A., Eds.; Editora UFV: Viçosa, Brazil, 2016; pp. 95–126. [Google Scholar]
- Lopes, D.C.; Moura, L.O.; Steidle Neto, A.J.; Ferraz, L.C.L.; Carlos, L.A.; Martins, L.M. Spectral indices for non-destructive determination of lettuce pigments. Food Anal. Methods 2017, 10, 2807–2814. [Google Scholar] [CrossRef]
- Bendokas, V.; Skemiene, K.; Trumbeckaite, S.; Stanys, V.; Passamonti, S.; Borutaite, V.; Liobikas, J. Anthocyanins: From plant pigments to health benefits at mitochondrial level. Crit. Rev. Food Sci. Nutr. 2019, 60, 3352–3365. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.R.C.; Murador, D.C.; Mesquita, L.M.S.; Rosso, V.V. Bioavailability of anthocyanins: Gaps in knowledge, challenges and future research. J. Food Compos. Anal. 2018, 68, 31–40. [Google Scholar] [CrossRef]
- Ocean, N.; Howley, P.; Ensor, J. Lettuce be happy: A longitudinal UK study on the relationship between fruit and vegetable consumption and well-being. Soc. Sci. Med. 2019, 222, 335–345. [Google Scholar] [CrossRef]
- Santana, C.V.S.; Almeida, A.C.; Turco, S.H.N. Produção de alface vermelha em ambientes sombreados na região do submédio São Francisco-BA. Rev. Verde 2009, 4, 1–6. [Google Scholar]
- Yang, X.; Wei, S.; Liu, B.; Guo, D.; Zheng, B.; Feng, L.; Liu, Y.; Tomás-Barberán, F.A.; Luo, L.; Huang, D. A novel integrated non-targeted metabolomic analysis reveals significant metabolite variations between different lettuce (Lactuca sativa L.) varieties. Hortic. Res. 2018, 5, 33. [Google Scholar] [CrossRef]
- Maciel, G.M.; Gallis, R.B.A.; Barbosa, R.L.; Pereira, L.M.; Siquieroli, A.C.S.; Peixoto, J.V.M. Image phenotyping of inbred red lettuce lines with genetic diversity regarding carotenoid levels. Int. J. Appl. Earth. Obs. Geoinf. 2019, 81, 154–160. [Google Scholar] [CrossRef]
- Guo, Y.; Yin, G.; Sun, H.; Wang, H.; Chen, S.; Senthilnath, J.; Wang, J.; Fu, Y. Scaling Effects on Chlorophyll Content Estimations with RGB Camera Mounted on a UAV Platform Using Machine-Learning Methods. Sensors 2020, 20, 5130. [Google Scholar] [CrossRef]
- Riccardi, M.; Mele, G.; Pulvento, C.; Lavini, A.; D’andria, R.; Jacobsen, S.E. Non-destructive evaluation of chlorophyll content in quinoa and amaranth leaves by simple and multiple regression analysis of RGB image components. Photosynth. Res. 2014, 120, 263–272. [Google Scholar] [CrossRef]
- Yadav, S.P.; Ibaraki, Y.I.; Gupta, S.D. Estimation of the chlorophyll content of micropropagated potato plants using RGB based image analysis. Plant Cell Tissue Organ Cult. 2010, 100, 183–188. [Google Scholar] [CrossRef]
- Garcia-Caparros, P.; Llanderal, A.; Lao, M.T. Correlations between R, G, and B values, pigment concentration, and nitrogen status in three ornamental potted plants. Agronomy 2023, 13, 177. [Google Scholar] [CrossRef]
- Radočaj, D.; Šiljeg, A.; Marinović, R.; Jurišić, M. State of major vegetation indices in precision agriculture studies indexed in web of science: A review. Agriculture 2023, 13, 707. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Y.; Xie, M.; Zhao, Z.; Yang, L.; Liu, J.; Hou, D. Estimation of Fv/Fm in spring wheat using UAV-Based multispectral and RGB imagery with multiple machine learning methods. Agronomy 2023, 13, 1003. [Google Scholar] [CrossRef]
- Maciel, G.M.; Gallis, R.B.D.A.; Barbosa, R.L.; Pereira, L.M.; Siquieroli, A.C.S.; Peixoto, J.V.M. Image phenotyping of lettuce germplasm with genetically diverse carotenoid levels. Bragantia 2020, 79, 224–235. [Google Scholar] [CrossRef]
- Clemente, A.A.; Maciel, G.M.; Siquieroli, A.C.S.; Gallis, R.B.A.; Pereira, L.M.; Duarte, J.G. High-throughput phenotyping to detect anthocyanins, chlorophylls, and carotenoids in red lettuce germplasm. Int. J. Appl. Earth Obs. Geoinf. 2021, 103, 102533. [Google Scholar] [CrossRef]
- Maciel, G.M.; Siquieroli, A.C.S.; Gallis, R.B.A.; Pereira, L.M.; Sales, V.F. Programa de Computador BG α Biofort. 2019. Depositante: Universidade Federal de Uberlândia. BR512019002403-6. Deposit: 1 February 2019. Concession: 23 October 2019. Available online: https://busca.inpi.gov.br/pePI/servlet/ProgramaServletController (accessed on 10 March 2023).
- Sousa, C.S.D.; Bonetti, A.M.; Goulart Filho, L.R.; Machado, J.R.D.A.; Londe, L.N.; Baffi, M.A.; Ramos, R.G.; Vieira, C.U.; Kerr, W.E. Divergência genética entre genótipos de alface por meio de marcadores AFLP. Bragantia 2007, 66, 11–16. [Google Scholar] [CrossRef]
- Burggraaff, O.; Schmidt, N.; Zamorano, J.; Pauly, K.; Pascual, S.; Tapia, C.; Spyrakos, E.; Snik, F. Standardized spectral and radiometric calibration of consumer câmeras. Opt. Express 2019, 27, 19075–19101. [Google Scholar] [CrossRef]
- Francis, F.J. Analysis of anthocyanins. In Anthocyanins as Food Colors, 1st ed.; Markakis, P., Ed.; Academic Press: New York, NY, USA, 1982; pp. 182–208. [Google Scholar]
- Witham, F.H.; Blaydes, D.F.; Devlin, R.M. Experiments in Plant Physiology; Van Nostrand Reinhold: New York, NY, USA, 1971; 245p. [Google Scholar]
- Lichtenthaler, H.; Wellburn, A. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 603, 91–592. [Google Scholar] [CrossRef]
- Minolta. Chlorophyll meter SPAD-502. In Instruction Manual; Minolta Co.: Osaka, Japan, 1989. [Google Scholar]
- Richardson, A.J.; Wiegand, C.L. Distinguishing vegetation from soil background information. Photogramm. Eng. Remote Sens. 1977, 43, 1541–1552. [Google Scholar]
- Zarco-Tejada, P.J.; Berjón, A.; López-Lozano, R.; Miller, J.R.; Martín, P.; Cachorro, M.R.G.; de Frutos, A. Assessing vineyard condition with hyperspectral indices: Leaf and canopy reflectance simulation in a row-structured discontinuous canopy. Remote Sens. Environ. 2005, 99, 271–287. [Google Scholar] [CrossRef]
- Louhaichi, M.; Borman, M.M.; Johnson, D.E. Spatially located platform and aerial photography for documentation of grazing impacts on wheat. Geocarto Int. 2001, 16, 65–70. [Google Scholar] [CrossRef]
- Escadafal, R.; Belghith, A.; Bem, M.H. Indices spectraux pour la télédétection de la dégradation des milieux naturels en Tunisie aride. In Proceedings of the Actes du Sixième Symposium International. Mesures Physiques et Signatures Spectrales en Télédétection, Val d’Isère, France, 17–24 January 1994; pp. 7–21. [Google Scholar]
- Tucker, C. Red and Photographic Infrared Linear Combinations for Monitoring Vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Mathieu, R.; Pouget, M.; Cervelle, B.; Escadafal, R. Relationships between satellite-based radiometric indices simulated using laboratory reflectance data and typic soil color of an arid environment. Remote Sens. Environ. 1998, 66, 17–28. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Hassanein, M.; Lari, Z.; El-Sheimy, N. A new vegetation segmentation approach for cropped fields based on threshold detection from hue histograms. Sensors 2018, 18, 1253. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 10 January 2023).
- Matias, F.I.; Caraza-Harter, M.V.; Endelman, J.B. FIELDimageR: An R package to analyze orthomosaic images from agricultural field trials. Plant. Phenome J. 2020, 3, e20005. [Google Scholar] [CrossRef]
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes: Experimental Designs Package. R Package Version 1.1.2. 2013. Available online: http://CRAN.R-project.org/package=ExpDes (accessed on 10 January 2023).
- Singh, D. The relative importance of characters affecting genetic divergence. Indian J. Genet. Plant. Breed. 1981, 41, 237–245. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-6. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 January 2023).
- Charrad, M.; Ghazzali, N.; Boiteau, V.; Niknafs, A.; Charrad, M.M. Package “NbClust”. J. Stat. Softw. 2014, 61, 1–36. [Google Scholar]
- Da Silva, A.R.; Malafaia, G.; Menezes, I.P.P. Biotools: An R function to predict spatial gene diversity via an individual-based approach. Genet. Mol. Res. 2017, 16, gmr16029655. [Google Scholar] [CrossRef]
- Harrell, F.E.E., Jr.; Dupont, M.C. Package ‘hmisc’. R Package Version 4.3-0. 2019. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 10 January 2023).
- Alasalvar, C.; Grigor, J.M.; Zhang, D.; Quantick, P.C.; Shahidi, F. Comparison of volatiles, phenolics, sugars, antioxidant vitamins, and sensory quality of different colored carrot varieties. J. Agric. Food Chem. 2001, 49, 1410–1416. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; Mcewen, J.; O’brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Yoo, K.S.; Bang, H.; Pike, L.; Patil, B.S.; Lee, E.J. Comparing carotene, anthocyanins, and terpenoid concentrations in selected carrot lines of different colors. Hortic. Environ. Biotechnol. 2020, 61, 385–393. [Google Scholar] [CrossRef]
- Da Silveira, A.J.; Finzi, R.R.; Neto, L.D.C.; Maciel, G.M.; Beloti, I.F.; Jacinto, A.C.P. Genetic dissimilarity between lettuce genotypes with different levels of carotenoids biofortification. Nativa 2019, 7, 656–660. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, J.C.; Reacher, M.H.; Dean, W.H.; Ngondi, J. Epidemiology of vitamin A deficiency and xerophthalmia in at-risk populations. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 205–214. [Google Scholar] [CrossRef]
- Vijayageetha, M.; Jasmine, J.; Felicia, C.A.; Manjubala, D. Dark Adaptation—An Emerging Nutritional Problem. J. Nurs. Sci. Prac. Res. Adv. 2019, 1, 7–12. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Y.; Lu, F.; Wang, X.; Liao, X.; Hu, X.; Zhang, Y. Beneficial effects of a chlorophyll-rich spinach extract supplementation on prevention of obesity and modulation of gut microbiota in high-fat diet-fed mice. J. Funct. Foods 2019, 60, 103436. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Queiroz, M.I.; Fernandes, A.S.; Deprá, M.C.; Jacob-Lopes, E.; Zepka, L.Q. Introductory Chapter: Chlorophyll Molecules and Their Technological Relevance Chlorophyll; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef]
- Fontana, L.; Rossi, C.A.; Hubinger, S.Z.; Ferreira, M.D.; Spoto, M.H.; Sala, F.C.; Verruma-Bernardi, M.R. Physicochemical characterization and sensory evaluation of lettuce cultivated in three growing systems. Hortic. Bras. 2018, 36, 20–26. [Google Scholar] [CrossRef]
- Silva, E.; Ferreira, E.A.; Ferreira, M.R. Desempenho da alface americana sob a aplicação de adubos químico e orgânico. Cienc. Prax. 2016, 9, 21–24. Available online: https://revista.uemg.br/index.php/praxys/article/view/2627 (accessed on 15 February 2023).
- Sala, F.C. Melhoramento genético de alface. In Proceedings of the Congresso Brasileiro de Olericultura, Viçosa, Brazil, 25 July 2011. [Google Scholar]
- Sala, F.C.; Costa, C.P. “Brunela”: Cultivar de minialface crocante tropicalizada. In Proceedings of the Congresso Brasileiro de Olericultura, Palmas, Brazil, 28 July 2014. [Google Scholar]
- Milligan, G.W.; Cooper, M.C. An examination of procedures for determining the number of clusters in a data set. Psychometrika 1985, 50, 159–179. [Google Scholar] [CrossRef]
- Beniaich, A.; Silva, M.L.N.; Avalos, F.A.P.; de Menezes, M.D.; Cândido, B.M. Determination of vegetation cover index under different soil management systems of cover plants by using an unmanned aerial vehicle with an onboard digital photographic camera. Semin. Cienc. Agrar. 2019, 40, 49–66. [Google Scholar] [CrossRef]
- Nascimento-Júnior, I.; Moro, G.V.; Moro, F.V. Indirect selection of maize genotypes based on associations between root agronomic and anatomical characters. Chil. J. Agric. Res. 2018, 78, 39–47. [Google Scholar] [CrossRef]
- Reis, M.C.; Cardoso, D.B.O.; Júnior, E.S.; Gomes, B.C.; Pereira, L.T.G.; Gomes, D.A.; Sousa, L.B. Correlation among traits as criterion of cotton genotypes indirect selection. Genet. Mol. Res. 2017, 16, 3–12. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Pattaro, M.; Furlanetto, R.H.; Nanni, M.R.; Antunes, W.C. High resolution leaf spectral signature as a tool for foliar pigment estimation displaying potential for species differentiation. J. Plant Physiol. 2020, 249, 153161. [Google Scholar] [CrossRef]
- Cassetari, L.S.; Gomes, M.S.; Santos, D.C.; Santiago, W.D.; Andrade, J.; Guimarães, A.C.; Souza, J.A.; Cardoso, M.G.; Maluf, W.R.; Gomes, L.A. β-Carotene and chlorophyll levels in cultivars and breeding lines of lettuce. Acta Hortic. 2015, 1083, 469–473. [Google Scholar] [CrossRef]
- Hlavinka, J.; Nauš, J.; Špundová, M. Anthocyanin contribution to chlorophyll meter readings and its correction. Photosynth. Res. 2013, 118, 277–295. [Google Scholar] [CrossRef]
- León, A.P.; Vina, S.Z.; Frezza, D.; Chaves, A.; Chiesa, A. Estimation of Chlorophyll Contents by Correlations between SPAD-502 Meter and Chroma Meter in Butterhead Lettuce. Commun. Soil Sci. Plant Anal. 2007, 38, 2877–2885. [Google Scholar] [CrossRef]
- Dong, T.; Han, R.; Yu, J.; Zhu, M.; Zhang, Y.; Gong, Y.; Li, Z. Anthocyanins accumulation and molecular analysis of correlated genes by metabolome and transcriptome in green and purple asparaguses (Asparagus officinalis L.). Food Chem. 2019, 271, 18–28. [Google Scholar] [CrossRef]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; Lee, Y.T.; Mele, M.A.; Choi, I.L.; Kang, H.M. The effect of phosphorus and root zone temperature on anthocyanin of red romaine lettuce. Agronomy 2019, 9, 47. [Google Scholar] [CrossRef]
- Mol, J.; Jenkins, G.; Schäfer, E.; Weiss, D.; Walbot, V. Signal perception, transduction, and gene expression involved in anthocyanin biosynthesis. Crit. Rev. Plant. Sci. 1996, 15, 525–557. [Google Scholar] [CrossRef]
- O’Connell, S. Miniature head lettuce yield and anthocyanin concentration under high tunnels and the field in georgia. HortTechnology 2021, 31, 53–63. [Google Scholar] [CrossRef]



| Vegetation Index | Equation 1 | References |
|---|---|---|
| Brightness Index (BI) | √((R2 + G2 + B2)/3) | [36] |
| Blue Green Pigment Index (BGI) | B/G | [37] |
| Green Leaf Index (GLI) | (2G − R − B)/(2G + R + B) | [38] |
| Primary Colors Hue Index (HI) | (2 × R − G − B)/(G − B) | [39] |
| Overall Hue Index (HUE) | atan (2 × (B − G − R)/30.5 × (G − R)) | [39] |
| Normalized Green/Red Difference Index (NGRDI) | (G − R)(G + R) | [40] |
| Soil Color Index (SCI) | (R − G)/(R + G) | [41] |
| Spectral Slope Saturation Index (SI) | (R − B)/(R + B) | [39] |
| Visible Atmospherically Resistant Index (VARI) | (G − R)/(G + R − B) | [42] |
| Leaf Pigment (ug·g−1) | Model | R2 | p-Value | RMSE |
|---|---|---|---|---|
| Anthocyanin | Y = −1205.23 GLI + 360.68 | 0.75 | *** | 134.4 |
| Carotenoid | Y = −2.888 GLI + 5.3251 | 0.43 | *** | 0.6 |
| Chlorophyll a | Y = −52.282 GLI + 62.67 | 0.48 | *** | 26.4 |
| Chlorophyll b | Y = −50.072 GLI + 37.959 | 0.39 | *** | 10.6 |
| Total chlorophyll | Y = −143.23 GLI + 116.666 | 0.65 | *** | 27.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente, A.A.; Maciel, G.M.; Siquieroli, A.C.S.; Gallis, R.B.d.A.; Luz, J.M.Q.; Sala, F.C.; Pereira, L.M.; Yada, R.Y. Nutritional Characterization Based on Vegetation Indices to Detect Anthocyanins, Carotenoids, and Chlorophylls in Mini-Lettuce. Agronomy 2023, 13, 1403. https://doi.org/10.3390/agronomy13051403
Clemente AA, Maciel GM, Siquieroli ACS, Gallis RBdA, Luz JMQ, Sala FC, Pereira LM, Yada RY. Nutritional Characterization Based on Vegetation Indices to Detect Anthocyanins, Carotenoids, and Chlorophylls in Mini-Lettuce. Agronomy. 2023; 13(5):1403. https://doi.org/10.3390/agronomy13051403
Chicago/Turabian StyleClemente, Andressa Alves, Gabriel Mascarenhas Maciel, Ana Carolina Silva Siquieroli, Rodrigo Bezerra de Araujo Gallis, José Magno Queiroz Luz, Fernando César Sala, Lucas Medeiros Pereira, and Rickey Yoshio Yada. 2023. "Nutritional Characterization Based on Vegetation Indices to Detect Anthocyanins, Carotenoids, and Chlorophylls in Mini-Lettuce" Agronomy 13, no. 5: 1403. https://doi.org/10.3390/agronomy13051403
APA StyleClemente, A. A., Maciel, G. M., Siquieroli, A. C. S., Gallis, R. B. d. A., Luz, J. M. Q., Sala, F. C., Pereira, L. M., & Yada, R. Y. (2023). Nutritional Characterization Based on Vegetation Indices to Detect Anthocyanins, Carotenoids, and Chlorophylls in Mini-Lettuce. Agronomy, 13(5), 1403. https://doi.org/10.3390/agronomy13051403






