Watering Shapes a Robust and Stable Microbial Community under Fusarium Crown Rot Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Inoculum Preparation
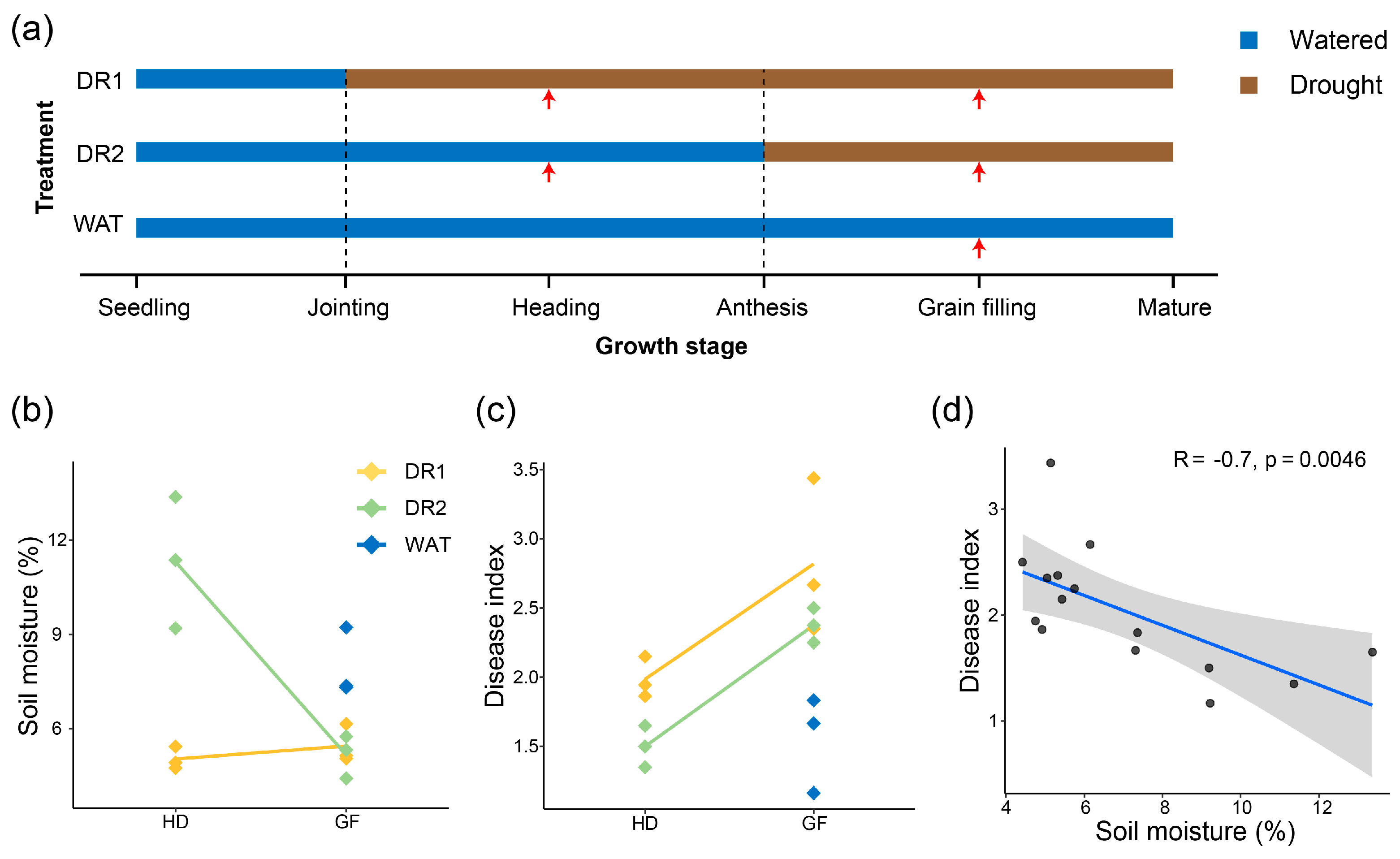
2.2. Greenhouse Experiments Design
2.3. Sample Collection and Processing
2.4. Soil Physicochemical Properties
2.5. Assessment of FCR Disease Severity
2.6. DNA Extraction and Amplicon Sequencing
2.7. Statistical Analyses
3. Results
3.1. The Relationship between Soil Water Content and Disease Severity
3.2. The Effect of Watering Regimes on Soil Physiochemical Properties
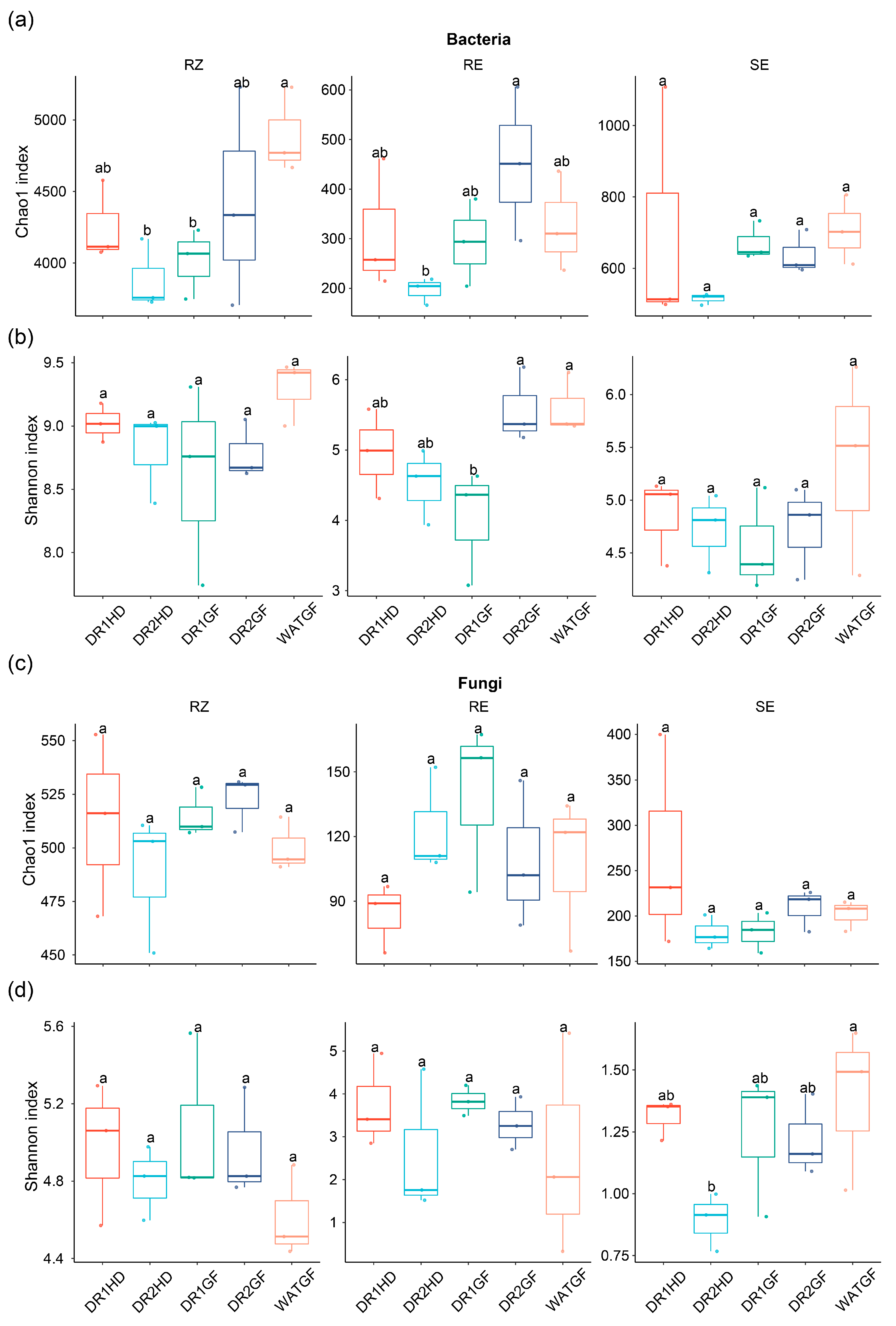
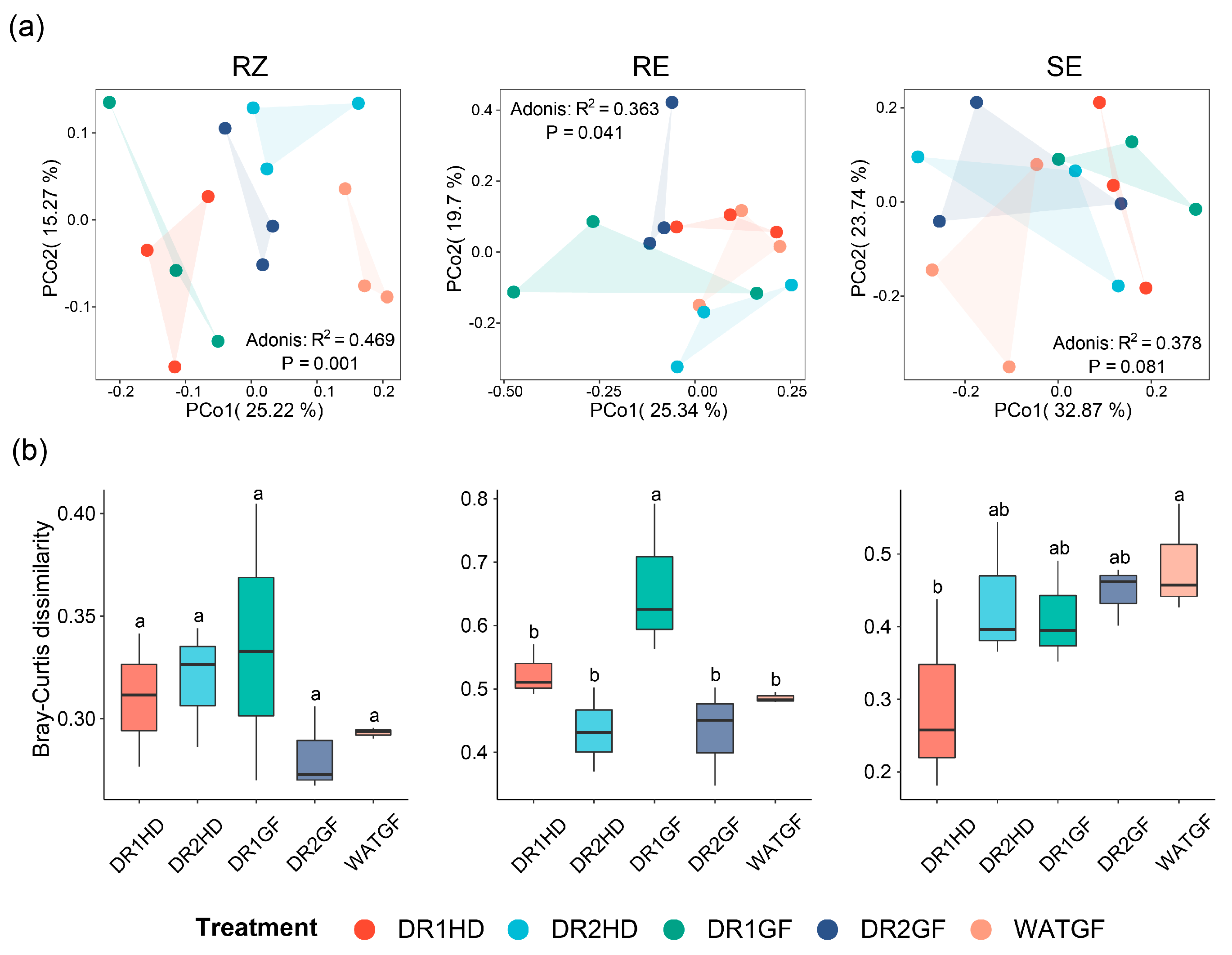
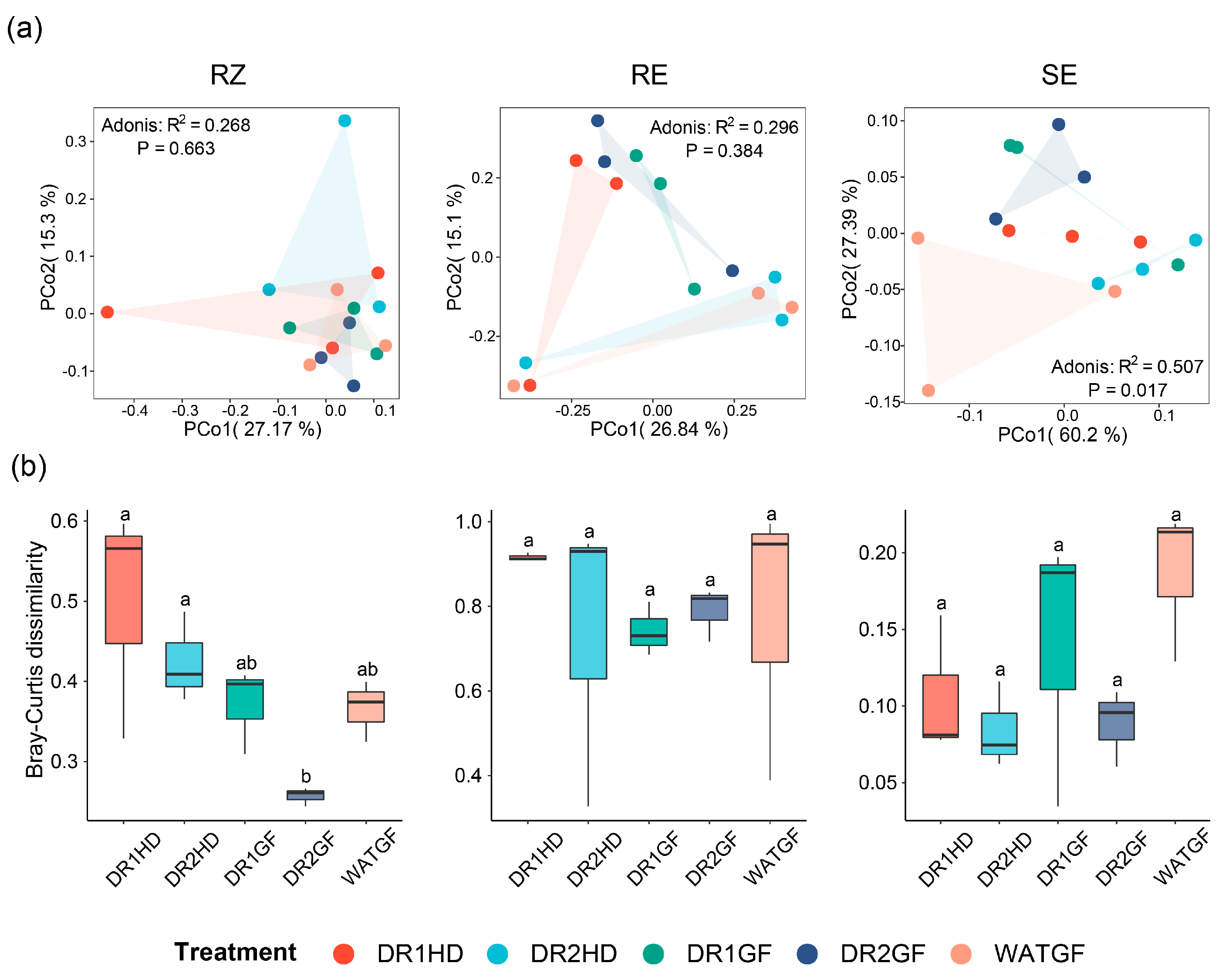
3.3. The Effect of Watering Regimes on Microbial Community Diversity and Composition in Different Compartment Niches
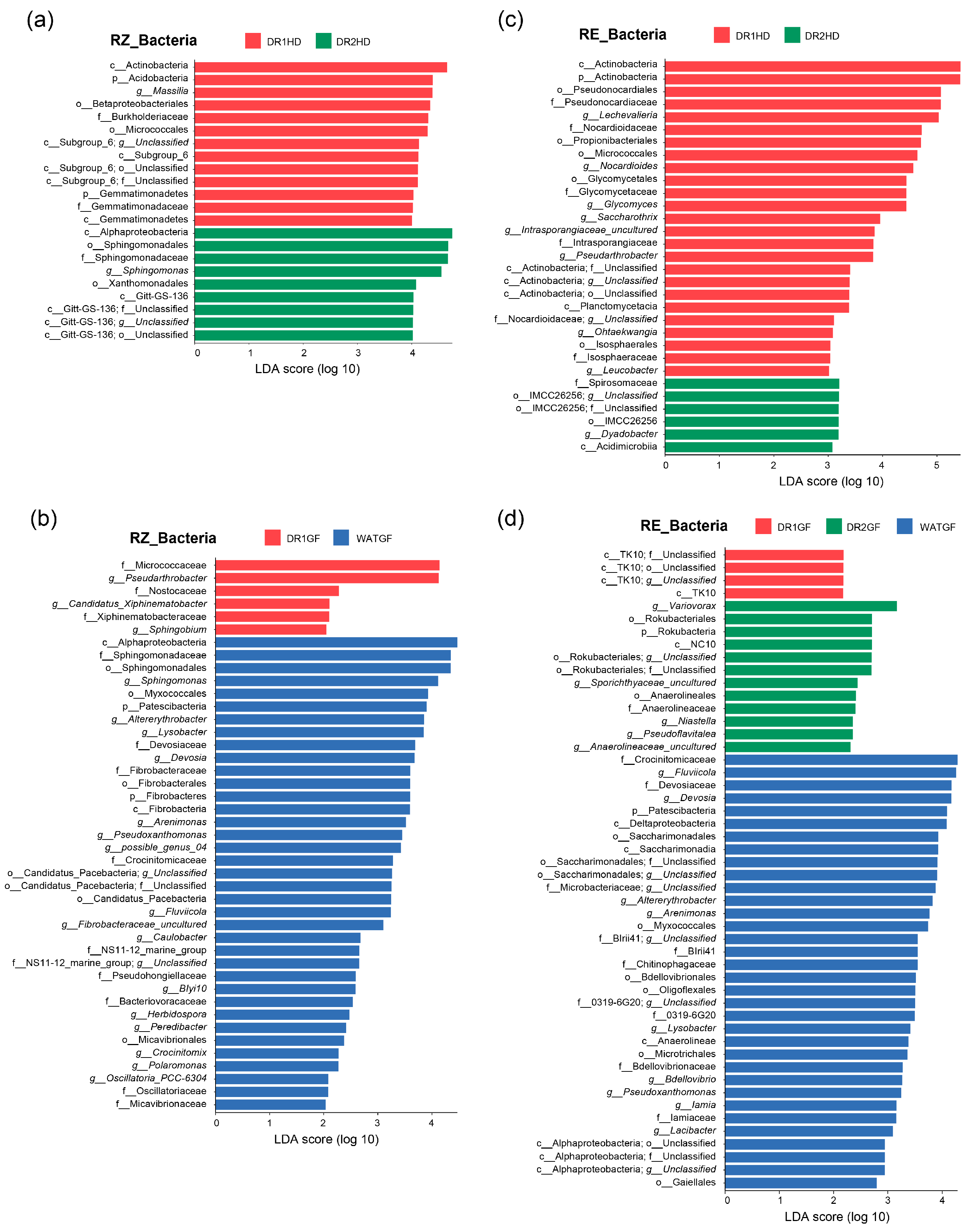
3.4. Microbial Biomarker Enrichment under Different Watering Regimes
3.5. Microbial Co-Occurrence Networks under the Different Watering Regimes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waqas, M.A.; Kaya, C.; Riaz, A.; Farooq, M.; Nawaz, I.; Wilkes, A.; Li, Y. Potential Mechanisms of Abiotic Stress Tolerance in Crop Plants Induced by Thiourea. Front. Plant Sci. 2019, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Soltabayeva, A.; Ongaltay, A.; Omondi, J.O.; Srivastava, S. Morphological, Physiological and Molecular Markers for Salt-Stressed Plants. Plants 2021, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Newton, A.C. Climate Change, Plant Diseases and Food Security: An Overview. Plant Pathol. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Bhargava, S.; Sawant, K. Drought Stress Adaptation: Metabolic Adjustment and Regulation of Gene Expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of Extreme Weather Disasters on Global Crop Production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of Combined Abiotic and Biotic Stresses on Plant Growth and Avenues for Crop Improvement by Exploiting Physio-Morphological Traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef]
- Chilakala, A.R.; Mali, K.V.; Irulappan, V.; Patil, B.S.; Pandey, P.; Rangappa, K.; Ramegowda, V.; Kumar, M.N.; Puli, C.O.R.; Mohan-Raju, B.; et al. Combined Drought and Heat Stress Influences the Root Water Relation and Determine the Dry Root Rot Disease Development Under Field Conditions: A Study Using Contrasting Chickpea Genotypes. Front. Plant Sci. 2022, 13, 890551. [Google Scholar] [CrossRef]
- Rai, A.; Irulappan, V.; Senthil-Kumar, M. Dry Root Rot of Chickpea: A Disease Favored by Drought. Plant. Dis. 2022, 106, 346–356. [Google Scholar] [CrossRef]
- Hoheneder, F.; Hofer, K.; Groth, J.; Herz, M.; Heß, M.; Hückelhoven, R. Ramularia Leaf Spot Disease of Barley Is Highly Host Genotype-Dependent and Suppressed by Continuous Drought Stress in the Field. J. Plant Dis. Prot. 2021, 128, 749–767. [Google Scholar] [CrossRef]
- Jones, P.; Garcia, B.J.; Furches, A.; Tuskan, G.A.; Jacobson, D. Plant Host-Associated Mechanisms for Microbial Selection. Front. Plant Sci. 2019, 10, 862. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, X.; Liu, Y.; Xie, S.; Xing, Y.; Dao, J.; Wei, B.; Peng, Y.; Duan, W.; Wang, Z. Response of Sugarcane Rhizosphere Bacterial Community to Drought Stress. Front. Microbiol. 2021, 12, 716196. [Google Scholar] [CrossRef] [PubMed]
- Durán, P.; Thiergart, T.; Garrido-Oter, R.; Agler, M.; Kemen, E.; Schulze-Lefert, P.; Hacquard, S. Microbial Interkingdom Interactions in Roots Promote Arabidopsis Survival. Cell 2018, 175, 973–983.e14. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, F.; Ge, A.; Zhang, H.; Chen, G.; Deng, Y.; Yang, J.; Chen, J.; Ge, T. Enrichment of Microbial Taxa after the Onset of Wheat Yellow Mosaic Disease. Agric. Ecosyst. Environ. 2021, 322, 107651. [Google Scholar] [CrossRef]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of Plant Response to Salt and Drought Stress and Their Alteration by Rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Guo, N.; Zhang, S.; Gu, M.; Xu, G. Function, Transport, and Regulation of Amino Acids: What Is Missing in Rice? Crop J. 2021, 9, 530–542. [Google Scholar] [CrossRef]
- Abdalla, M.; Ahmed, M.A. Arbuscular Mycorrhiza Symbiosis Enhances Water Status and Soil-Plant Hydraulic Conductance Under Drought. Front. Plant Sci. 2021, 12, 722954. [Google Scholar] [CrossRef]
- Deng, J.; Li, F.; Duan, T.Y. Claroideoglomus Etunicatum Reduces Leaf Spot Incidence and Improves Drought Stress Resistance in Perennial Ryegrass. Austral. Plant Pathol. 2020, 49, 147–157. [Google Scholar] [CrossRef]
- Teixeira, P.J.P.; Colaianni, N.R.; Fitzpatrick, C.R.; Dangl, J.L. Beyond Pathogens: Microbiota Interactions with the Plant Immune System. Curr. Opin. Microbiol. 2019, 49, 7–17. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Knight, C.G.; Nicolitch, O.; Williams, A. Harnessing Rhizosphere Microbiomes for Drought-Resilient Crop Production. Science 2020, 368, 270–274. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Spence, C.; Alff, E.; Johnson, C.; Ramos, C.; Donofrio, N.; Sundaresan, V.; Bais, H. Natural Rice Rhizospheric Microbes Suppress Rice Blast Infections. BMC Plant Biol. 2014, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.W.; Raaijmakers, J.M.; de Hollander, M.; Mendes, R.; Tsai, S.M. Influence of Resistance Breeding in Common Bean on Rhizosphere Microbiome Composition and Function. ISME J. 2018, 12, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.-J.; Kong, H.G.; Choi, K.; Kwon, S.-K.; Song, J.Y.; Lee, J.; Lee, P.A.; Choi, S.Y.; Seo, M.; Lee, H.J.; et al. Rhizosphere Microbiome Structure Alters to Enable Wilt Resistance in Tomato. Nat. Biotechnol. 2018, 36, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Akinsanmi, O.A.; Mitter, V.; Simpfendorfer, S.; Backhouse, D.; Chakraborty, S. Identity and Pathogenicity of Fusarium Spp. Isolated from Wheat Fields in Queensland and Northern New South Wales. Aust. J. Agric. Res. 2004, 55, 97. [Google Scholar] [CrossRef]
- Kazan, K.; Gardiner, D.M. Fusarium Crown Rot Caused by Fusarium pseudograminearum in Cereal Crops: Recent Progress and Future Prospects. Mol. Plant Pathol. 2018, 19, 1547–1562. [Google Scholar] [CrossRef]
- Li, H.L.; Yuan, H.X.; Fu, B.; Xing, X.P.; Sun, B.J.; Tang, W.H. First Report of Fusarium pseudograminearum Causing Crown Rot of Wheat in Henan, China. Plant. Dis. 2012, 96, 1065. [Google Scholar] [CrossRef]
- Chakraborty, S.; Liu, C.J.; Mitter, V.; Scott, J.B.; Akinsanmi, O.A.; Ali, S.; Dill-Macky, R.; Nicol, J.; Backhouse, D.; Simpfendorfer, S. Pathogen Population Structure and Epidemiology Are Keys to Wheat Crown Rot and Fusarium Head Blight Management. Austral. Plant Pathol. 2006, 35, 643–655. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C. Effects of Drought-Stress on Fusarium Crown Rot Development in Barley. PLoS ONE 2016, 11, e0167304. [Google Scholar] [CrossRef]
- Khaledi, N.; Taheri, P.; Falahati-Rastegar, M. Evaluation of Resistance and the Role of Some Defense Responses in Wheat Cultivars to Fusarium Head Blight. J. Plant Prot. Res. 2018, 57, 396–408. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Najjar, A.A.; Alzahrani, S.O.; Alkhatib, F.M.; Shafi, M.E.; Selem, E.; Desoky, E.-S.M.; Fouda, S.E.E.; El-Tahan, A.M.; et al. The Use of Biological Selenium Nanoparticles to Suppress Triticum aestivum L. Crown and Root Rot Diseases Induced by Fusarium Species and Improve Yield under Drought and Heat Stress. Saudi J. Biol. Sci. 2021, 28, 4461–4471. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Montville, M.E.; Pinto, J.R. Using Container Weights to Determine Irrigation Needs: A Simple Method. Nativ. Plants J. 2015, 16, 67–71. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Xiang, X.; Sun, R.; Yang, T.; He, D.; Zhang, K.; Ni, Y.; Zhu, Y.-G.; Adams, J.M.; et al. Spatial Scale Affects the Relative Role of Stochasticity versus Determinism in Soil Bacterial Communities in Wheat Fields across the North China Plain. Microbiome 2018, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Corwin, D.L.; Yemoto, K. Measurement of Soil Salinity: Electrical Conductivity and Total Dissolved Solids. Soil Sci. Soc. Am. J. 2019, 83, 1–2. [Google Scholar] [CrossRef]
- Jin, J.; Duan, S.; Qi, Y.; Yan, S.; Li, W.; Li, B.; Xie, C.; Zhen, W.; Ma, J. Identification of a Novel Genomic Region Associated with Resistance to Fusarium Crown Rot in Wheat. Theor. Appl. Genet. 2020, 133, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, C.; Bei, S.; Guangzhou, W.; Geisen, S.; Bedoussac, L.; Christie, P.; Zhang, J. High Bacterial Diversity and Siderophore-Producing Bacteria Collectively Suppress Fusarium oxysporum in Maize/Faba Bean Intercropping. Front. Microbiol. 2022, 13, 972587. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Erdos, P. On Sets of Distances of n Points. Am. Math. Mon. 1970, 77, 738–740. [Google Scholar] [CrossRef]
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial Interactions within the Plant Holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef]
- Ahmadi, M.; Mirakhorli, N.; Erginbas-Orakci, G.; Ansari, O.; Braun, H.-J.; Paulitz, T.; Dababat, A.A. Interactions among Cereal Cyst Nematode Heterodera Filipjevi, Dryland Crown Rot Fusarium culmorum, and Drought on Grain Yield Components and Disease Severity in Bread Wheat. Can. J. Plant Pathol. 2022, 44, 415–431. [Google Scholar] [CrossRef]
- Smiley, W.R.; Collins, H.P.; Rasmussen, P.E. Diseases of Wheat in Long-Term Agronomic Experiments at Pendleton, Oregon. Plant. Dis. 1996, 80, 813. [Google Scholar] [CrossRef]
- Moya-Elizondo, E.A. Fusarium Crown Rot Disease: Biology, Interactions, Management and Function as a Possible Sensor of Global Climate Change. Cienc. Investig. Agrar. 2013, 40, 235–252. [Google Scholar] [CrossRef]
- Saremi, H.; Ammarellou, A.; Jafary, H. Incidence of Crown Rot Disease of Wheat Caused by Fusarium pseudograminearum as a New Soil Born Fungal Species in North West Iran. Pak. J. Biol. Sci. 2007, 10, 3606–3612. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.H.; Hüberli, D.; Sharma, D.L.; D’Antuono, M.F. Soil Salinity Exacerbates Crown Rot in Wheat. Austral. Plant Pathol. 2019, 48, 339–341. [Google Scholar] [CrossRef]
- Akgül, D.S.; Erkilic, A. Effect of Wheat Cultivars, Fertilizers, and Fungicides on Fusarium Foot Rot Disease of Wheat. Turk. J. Agric. For. 2016, 40, 101–108. [Google Scholar] [CrossRef]
- Davis, R.A.; Huggins, D.R.; Cook, J.R.; Paulitz, T.C. Nitrogen and Crop Rotation Effects on Fusarium Crown Rot in No-till Spring Wheat. Can. J. Plant Pathol. 2009, 31, 456–467. [Google Scholar] [CrossRef]
- Xiong, C.; Zhu, Y.; Wang, J.; Singh, B.; Han, L.; Shen, J.; Li, P.; Wang, G.; Wu, C.; Ge, A.; et al. Host Selection Shapes Crop Microbiome Assembly and Network Complexity. New Phytol. 2021, 229, 1091–1104. [Google Scholar] [CrossRef]
- Gao, M.; Xiong, C.; Gao, C.; Tsui Clement, K.M.; Wang, M.-M.; Zhou, X.; Zhang, A.-M.; Cai, L. Disease-Induced Changes in Plant Microbiome Assembly and Functional Adaptation. Microbiome 2021, 9, 187. [Google Scholar] [CrossRef]
- Chen, Y.; Bonkowski, M.; Shen, Y.; Griffiths, B.S.; Jiang, Y.; Wang, X.; Sun, B. Root Ethylene Mediates Rhizosphere Microbial Community Reconstruction When Chemically Detecting Cyanide Produced by Neighbouring Plants. Microbiome 2020, 8, 4. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, J.; Shi, Y.; Wu, F.; Zhou, X. Green Manures of Indian Mustard and Wild Rocket Enhance Cucumber Resistance to Fusarium Wilt through Modulating Rhizosphere Bacterial Community Composition. Plant Soil 2019, 441, 283–300. [Google Scholar] [CrossRef]
- Schmitz, L.; Yan, Z.; Schneijderberg, M.; de Roij, M.; Pijnenburg, R.; Zheng, Q.; Franken, C.; Dechesne, A.; Trindade, L.M.; van Velzen, R.; et al. Synthetic Bacterial Community Derived from a Desert Rhizosphere Confers Salt Stress Resilience to Tomato in the Presence of a Soil Microbiome. ISME J. 2022, 16, 1907–1920. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, Variation, and Assembly of the Root-Associated Microbiomes of Rice. Proc. Natl. Acad. Sci. USA 2015, 112, e911–e920. [Google Scholar] [CrossRef] [PubMed]
- Preece, C.; Verbruggen, E.; Liu, L.; Weedon, J.T.; Peñuelas, J. Effects of Past and Current Drought on the Composition and Diversity of Soil Microbial Communities. Soil Biol. Biochem. 2019, 131, 28–39. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, L.; Yang, S.; Wang, Z.; Tian, R.; Peng, Z.; Chen, Y.; Zhang, X.; Kuang, J.; Ling, N.; et al. Critical Transition of Soil Bacterial Diversity and Composition Triggered by Nitrogen Enrichment. Ecology 2020, 101, e03053. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, F.; Zhang, H.; Chen, G.; Deng, Y.; Chen, J.; Yang, J.; Ge, T. Enrichment of Beneficial Rhizosphere Microbes in Chinese Wheat Yellow Mosaic Virus-Resistant Cultivars. Appl. Microbiol. Biotechnol. 2021, 105, 9371–9383. [Google Scholar] [CrossRef]
- Jin, X.; Shi, Y.; Wu, F.; Pan, K.; Zhou, X. Intercropping of Wheat Changed Cucumber Rhizosphere Bacterial Community Composition and Inhibited Cucumber Fusarium Wilt Disease. Sci. Agric. 2019, 77, e20190005. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil Bacterial Networks Are Less Stable under Drought than Fungal Networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
- Li, J.; Luo, C.; Zhang, D.; Cai, X.; Jiang, L.; Zhao, X.; Zhang, G. Diversity of the Active Phenanthrene Degraders in PAH-Polluted Soil Is Shaped by Ryegrass Rhizosphere and Root Exudates. Soil Biol. Biochem. 2019, 128, 100–110. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, N.; Zhang, Y.; Wang, Z.; Zhang, Y.; Yu, J.; Zhang, Y.; Wei, Z.; Xu, Y.; Geisen, S.; et al. The Relative Importance of Soil Moisture in Predicting Bacterial Wilt Disease Occurrence. Soil Ecol. Lett. 2021, 3, 356–366. [Google Scholar] [CrossRef]
- Bortolami, G.; Gambetta, G.A.; Cassan, C.; Dayer, S.; Farolfi, E.; Ferrer, N.; Gibon, Y.; Jolivet, J.; Lecomte, P.; Delmas, C.E.L. Grapevines under Drought Do Not Express Esca Leaf Symptoms. Proc. Natl. Acad. Sci. USA 2021, 118, e2112825118. [Google Scholar] [CrossRef]
- Guo, C.; Yang, M.; Jiang, B.; Ye, C.; Luo, L.; Liu, Y.; Huang, H.; Mei, X.; Zhu, Y.; Deng, W.; et al. Moisture Controls the Suppression of Panax Notoginseng Root Rot Disease by Indigenous Bacterial Communities. mSystems 2022, 7, e00418-22. [Google Scholar] [CrossRef]
- Shi, W.; Li, M.; Wei, G.; Tian, R.; Li, C.; Wang, B.; Lin, R.; Shi, C.; Chi, X.; Zhou, B.; et al. The Occurrence of Potato Common Scab Correlates with the Community Composition and Function of the Geocaulosphere Soil Microbiome. Microbiome 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G. How Plants Recruit Their Microbiome? New Insights into Beneficial Interactions. J. Adv. Res. 2021, 40, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Naylor, D.; DeGraaf, S.; Purdom, E.; Coleman-Derr, D. Drought and Host Selection Influence Bacterial Community Dynamics in the Grass Root Microbiome. ISME J. 2017, 11, 2691–2704. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Delgado-Baquerizo, M.; Trivedi, C.; Hamonts, K.; Anderson, I.C.; Singh, B.K. Keystone Microbial Taxa Regulate the Invasion of a Fungal Pathogen in Agro-Ecosystems. Soil Biol. Biochem. 2017, 111, 10–14. [Google Scholar] [CrossRef]
- Carrión, V.J.; Perez-Jaramillo, J.; Cordovez, V.; Tracanna, V.; de Hollander, M.; Ruiz-Buck, D.; Mendes, L.W.; van Ijcken, W.F.J.; Gomez-Exposito, R.; Elsayed, S.S.; et al. Pathogen-Induced Activation of Disease-Suppressive Functions in the Endophytic Root Microbiome. Science 2019, 366, 606–612. [Google Scholar] [CrossRef]
- Pang, Z.; Mao, X.; Xia, Y.; Xiao, J.; Wang, X.; Xu, P.; Liu, G. Multiomics Reveals the Effect of Root Rot on Polygonati Rhizome and Identifies Pathogens and Biocontrol Strain. Microbiol. Spectr. 2022, 10, e02385-21. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, M.; Zhang, W.; Gardea-Torresdey, J.L.; White, J.C.; Ji, R.; Zhao, L. Silver Nanoparticles Alter Soil Microbial Community Compositions and Metabolite Profiles in Unplanted and Cucumber-Planted Soils. Environ. Sci. Technol. 2020, 54, 3334–3342. [Google Scholar] [CrossRef]
- Xu, P.; Stirling, E.; Xie, H.; Li, W.; Lv, X.; Matsumoto, H.; Cheng, H.; Xu, A.; Lai, W.; Wang, Y.; et al. Continental Scale Deciphering of Microbiome Networks Untangles the Phyllosphere Homeostasis in Tea Plant. J. Adv. Res. 2022, 44, 13–22. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, N.; Li, Y.; Zhu, C.; Qu, B.; Liu, H.; Li, R.; Bai, Y.; Shen, Q.; Falcao Salles, J. Bio-Organic Soil Amendment Promotes the Suppression of Ralstonia Solanacearum by Inducing Changes in the Functionality and Composition of Rhizosphere Bacterial Communities. New Phytol. 2022, 235, 1558–1574. [Google Scholar] [CrossRef]
- Chen, S.; Waghmode, T.R.; Sun, R.; Kuramae, E.E.; Hu, C.; Liu, B. Root-Associated Microbiomes of Wheat under the Combined Effect of Plant Development and Nitrogen Fertilization. Microbiome 2019, 7, 136. [Google Scholar] [CrossRef]
- Wen, T.; Yuan, J.; He, X.; Lin, Y.; Huang, Q.; Shen, Q. Enrichment of Beneficial Cucumber Rhizosphere Microbes Mediated by Organic Acid Secretion. Hortic. Res. 2020, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Lazcano, C.; Boyd, E.; Holmes, G.; Hewavitharana, S.; Pasulka, A.; Ivors, K. The Rhizosphere Microbiome Plays a Role in the Resistance to Soil-Borne Pathogens and Nutrient Uptake of Strawberry Cultivars under Field Conditions. Sci. Rep. 2021, 11, 3188. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Casa Vargas, J.M.; Schlatter, D.C.; Hagerty, C.H.; Hulbert, S.H.; Paulitz, T.C. Rhizosphere Community Selection Reveals Bacteria Associated with Reduced Root Disease. Microbiome 2021, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Sucharzewska, E.; Dynowska, M. Preliminary Evaluation of the Effect of Ampelomyces Quisqualis on the Degree of Plant Infestation with Selected Erysiphales Species Proposed as Potential Bioindicators. Plant Prot. Sci. 2017, 38, 436–438. [Google Scholar] [CrossRef]
- Gilbert, K.B.; Holcomb, E.E.; Allscheid, R.L.; Carrington, J.C. Hiding in Plain Sight: New Virus Genomes Discovered via a Systematic Analysis of Fungal Public Transcriptomes. PLoS ONE 2019, 14, e0219207. [Google Scholar] [CrossRef]
- Santos-Medellín, C.; Liechty, Z.; Edwards, J.; Nguyen, B.; Huang, B.; Weimer, B.C.; Sundaresan, V. Prolonged Drought Imparts Lasting Compositional Changes to the Rice Root Microbiome. Nat. Plants 2021, 7, 1065–1077. [Google Scholar] [CrossRef]
- Yegorenkova, I.V.; Tregubova, K.V.; Krasov, A.I.; Evseeva, N.V.; Matora, L.Y. Effect of Exopolysaccharides of Paenibacillus Polymyxa Rhizobacteria on Physiological and Morphological Variables of Wheat Seedlings. J. Microbiol. 2021, 59, 729–735. [Google Scholar] [CrossRef]



| Treatment | ST | pH | NO3−-N | EC |
|---|---|---|---|---|
| DR1HD | 26.33 ± 0.33 a | 7.46 ± 0.02 ab | - | - |
| DR2HD | 24.00 ± 0.58 b | 7.57 ± 0.05 ab | - | - |
| DR1GF | 23.67 ± 0.44 bc | 7.43 ± 0.03 b | 27.88 ± 0.95 a | 365.00 ± 0.58 a |
| DR2GF | 22.50 ± 0.58 c | 7.47 ± 0.15 ab | 26.88 ± 1.03 a | 352.67 ± 8.09 a |
| WATGF | 20.13 ± 0.19 d | 7.73 ± 0.08 a | 16.77 ± 1.07 b | 290.33 ± 16.48 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Du, C.; Gao, Y.; Zhou, X.; Ejaz, I.; Guo, J.; Chen, K.; Ma, J.; Zhang, Y.; Wang, Z.; et al. Watering Shapes a Robust and Stable Microbial Community under Fusarium Crown Rot Infection. Agronomy 2023, 13, 1356. https://doi.org/10.3390/agronomy13051356
Xu R, Du C, Gao Y, Zhou X, Ejaz I, Guo J, Chen K, Ma J, Zhang Y, Wang Z, et al. Watering Shapes a Robust and Stable Microbial Community under Fusarium Crown Rot Infection. Agronomy. 2023; 13(5):1356. https://doi.org/10.3390/agronomy13051356
Chicago/Turabian StyleXu, Runlai, Chenghang Du, Yutian Gao, Xiaohan Zhou, Irsa Ejaz, Jieru Guo, Kunhu Chen, Jun Ma, Yinghua Zhang, Zhimin Wang, and et al. 2023. "Watering Shapes a Robust and Stable Microbial Community under Fusarium Crown Rot Infection" Agronomy 13, no. 5: 1356. https://doi.org/10.3390/agronomy13051356
APA StyleXu, R., Du, C., Gao, Y., Zhou, X., Ejaz, I., Guo, J., Chen, K., Ma, J., Zhang, Y., Wang, Z., & Sun, Z. (2023). Watering Shapes a Robust and Stable Microbial Community under Fusarium Crown Rot Infection. Agronomy, 13(5), 1356. https://doi.org/10.3390/agronomy13051356







