Effect of Different Foliar Fertilizer Applications on Esca Disease of Grapevine: Symptom Expression and Nutrient Content in the Leaf and Composition of the Berry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Leaf Nutrient Applications
2.2. Leaf Symptom Surveys
2.3. Leaf and Grape Berry Sampling
2.4. Chemical Analysis of Leaf and Grape Berry
2.5. Effect of Applications on Vegetative Growth
2.6. Statistical Analysis
3. Results
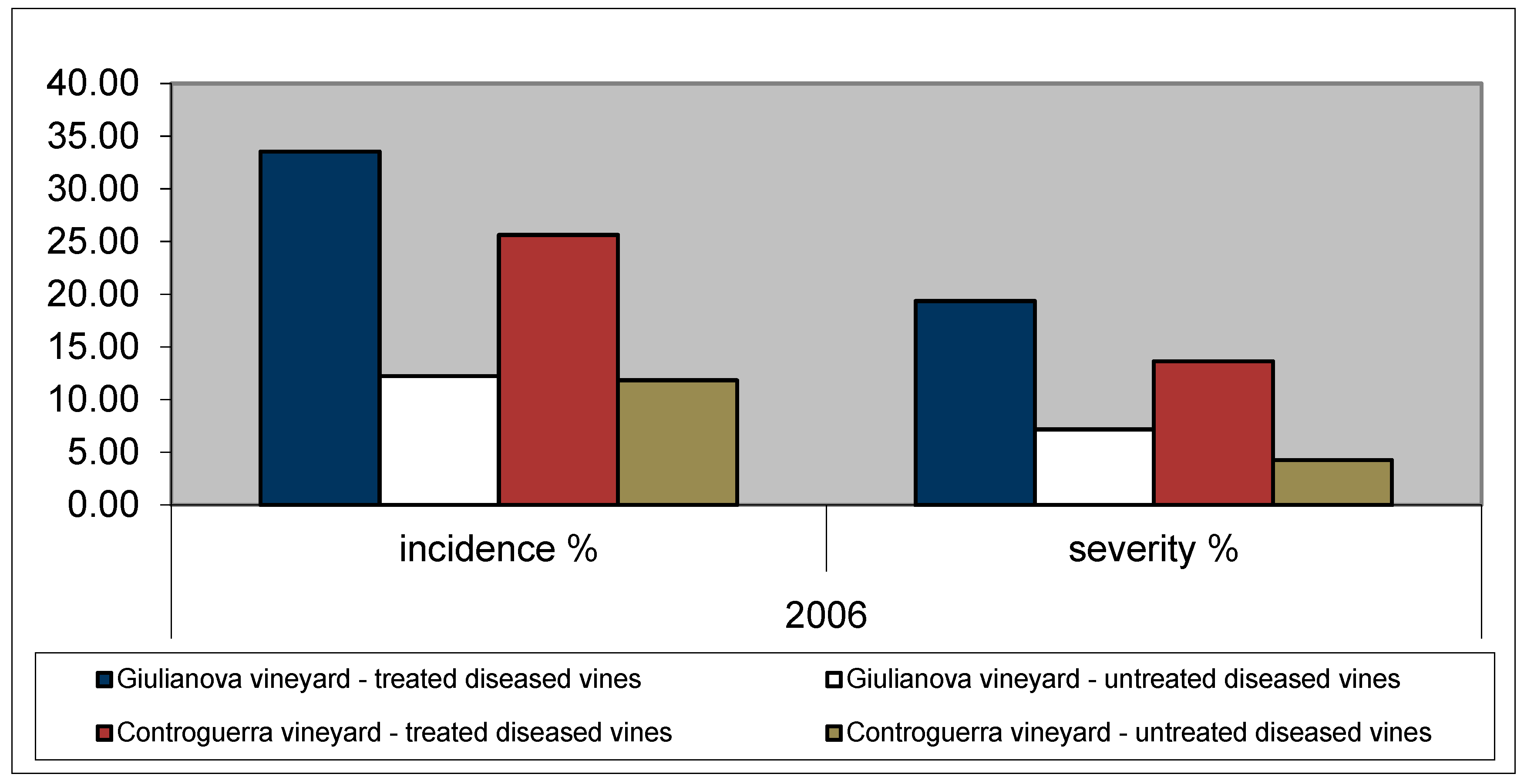
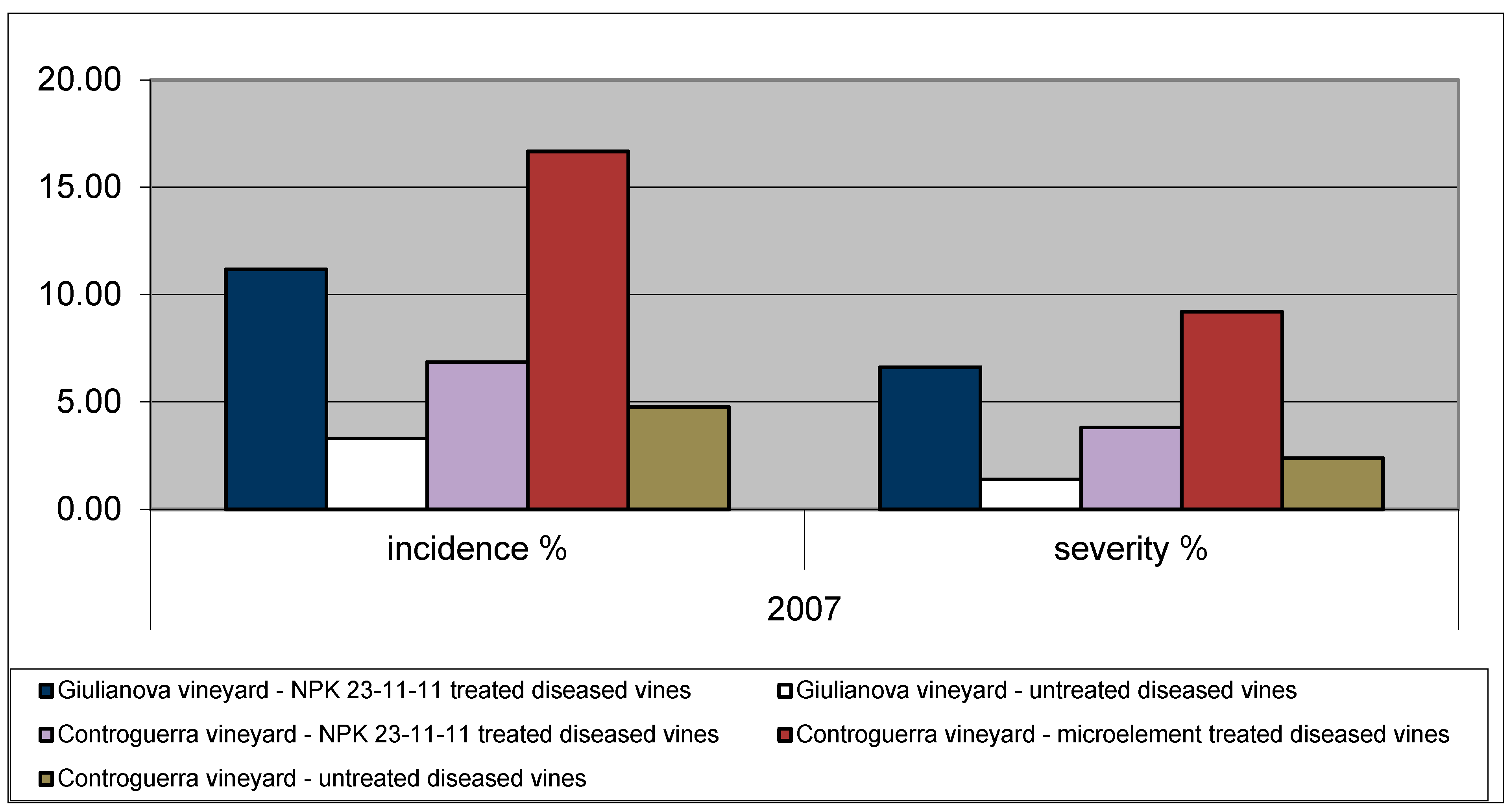
3.1. Incidence and Severity of Esca Foliar Symptoms
3.2. Macro- and Microelements in the Leaf
3.2.1. Analysis of the 2006 Growing Season
3.2.2. Analysis of the 2007 Growing Season
3.3. Macro- and Microelements in the Berry
3.3.1. Analysis of 2006 Growing Season
3.3.2. Analysis of 2007 Growing Season
3.4. Yield Amount and Must Parameters
3.5. Leaf Area Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gramaje, D.; Urbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Baránek, M.; Armengol, J.; Holleinová, V.; Pečenka, J.; Calzarano, F.; Peňázová, E.; Vachůn, M.; Eichmeier, A. Incidence of symptoms and fungal pathogens associated with grapevine trunk diseases in Czech vineyards: First example from a north-eastern European grape-growing region. Phytopathol. Mediterr. 2018, 57, 449–458. [Google Scholar] [CrossRef]
- Moretti, S.; Pacetti, A.; Pierron, R.; Kassemeyer, H.H.; Fischer, M.; Péros, J.P.; Perez-Gonzalez, P.; Bieler, E.; Schilling, M.; Di Marco, S.; et al. Fomitiporia mediterranea M. Fisch., the historical Esca agent: A comprehensive review on the main grapevine wood rot agent in Europe. Phytopathol. Mediterr. 2021, 60, 351–379. [Google Scholar] [CrossRef]
- Mugnai, L.; Graniti, A.; Surico, G. Esca (black measles) and brown wood streaking: Two old and elusive diseases of grapevines. Plant Dis. 1999, 83, 404–418. [Google Scholar] [CrossRef]
- Bertsch, C.; Ramírez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clèment, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2013, 62, 243–265. [Google Scholar] [CrossRef]
- Gramaje, D.; Di Marco, S. Identifying practices likely to have impacts on grapevine trunk disease infections: A European nursery survey. Phytopathol. Mediterr. 2015, 54, 313–324. [Google Scholar]
- Gramaje, D.; Armengol, J. Fungal trunk pathogens in the grapevine propagation process: Potential inoculum sources, detection, identification, and management strategies. Plant Dis. 2011, 95, 1040–1055. [Google Scholar] [CrossRef]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef]
- Di Marco, S.; Osti, F.; Bossio, D.; Nocentini, M.; Cinelli, T.; Calzarano, F.; Mugnai, L. Electrolyzed acid water: A clean technology active on fungal vascular pathogens in grapevine nurseries. Crop Prot. 2019, 119, 88–96. [Google Scholar] [CrossRef]
- Waite, H.; Armengol, J.; Billones-Baaijens, R.; Gramaje, D.; Halleen, F.; Di Marco, S.; Smart, R. A protocol for the management of grapevine rootstock mother vines to reduce latent infections by grapevine trunk pathogens in cuttings. Phytopathol. Mediterr. 2018, 57, 384–398. [Google Scholar]
- Calzarano, F.; Pagnani, G.; Pisante, M.; Bellocci, M.; Cillo, G.; Metruccio, E.G.; Di Marco, S. Factors Involved on Tiger-Stripe Foliar Symptom Expression of Esca of Grapevine. Plants 2021, 10, 1041. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.A.; Lawrence, D.P.; Baumgartner, K. Role of basidiomycete fungi in the grapevine trunk disease esca. Plant Pathol. 2020, 69, 205–220. [Google Scholar] [CrossRef]
- Pacetti, A.; Moretti, S.; Pinto, C.; Compant, S.; Farine, S.; Bertsch, C.; Mugnai, L. Trunk Surgery as a Tool to Reduce Foliar Symptoms in Diseases of the Esca Complex and Its Influence on Vine Wood Microbiota. J. Fungi 2021, 7, 521. [Google Scholar] [CrossRef] [PubMed]
- Calzarano, F.; Seghetti, L.; Del Carlo, M.; Cichelli, A. Effect of esca on the quality of berries, musts and wines. Phytopathol. Mediterr. 2004, 43, 125–135. [Google Scholar]
- Lorrain, B.; Ky, I.; Pasquier, G.; Jourdes, M.; Guerin Dubrana, L.; Gény, L.; Rey, P.; Donèche, B.; Teissedre, P.L. Effect of Esca disease on the phenolic and sensory attributes of Cabernet Sauvignon grapes, musts and wines. Aust. J. Grape Wine R. 2012, 18, 64–72. [Google Scholar] [CrossRef]
- Calzarano, F.; Di Marco, S. Wood discoloration and decay in grapevines with esca proper and their relationship with foliar symptoms. Phytopathol. Mediterr. 2007, 46, 96–101. [Google Scholar]
- Calzarano, F.; Osti, F.; Baránek, M.; Di Marco, S. Rainfall and temperature influence expression of foliar symptoms of grapevine leaf stripe disease (esca complex) in vineyards. Phytopathol. Mediterr. 2018, 57, 488–505. [Google Scholar] [CrossRef]
- Bruez, É.; Cholet, C.; Thibon, C.; Redon, P.; Lacampagne, S.; Martignon, T.; Giudici, M.; Darriet, P.; Gény, L. Influence of curettage on Esca-diseased Vitis vinifera L. cv. Sauvignon blanc plants on the quality of musts and wines. OENO One 2021, 55, 171–182. [Google Scholar] [CrossRef]
- Sparapano, L.; Bruno, G.; Graniti, A. Effects on plants of metabolites produced in culture by Phaeoacremonium chlamydosporum, P. aleophilum and Fomitiporia punctata. Phytopathol. Mediterr. 2000, 39, 169–177. [Google Scholar]
- Tabacchi, R.; Fkyerat, A.; Poliart, C.; Dubin, G. Phytotoxins from fungi of esca of grapevine. Phytopathol. Mediterr. 2000, 39, 156–161. [Google Scholar]
- Evidente, A.; Sparapano, L.; Andolfi, A.; Bruno, G. Two naphthalenone pentaketides from liquid cultures of Phaeoacremonium aleophilum, a fungus associated with esca of grapevine. Phytopathol. Mediterr. 2000, 39, 162–168. [Google Scholar]
- Andolfi, A.; Mugnai, L.; Luque, J.; Surico, G.; Cimmino, A.; Evidente, A. Phytotoxins produced by fungi associated with grapevine trunk diseases. Toxins 2011, 3, 1569–1605. [Google Scholar] [CrossRef] [PubMed]
- Magnin-Robert, M.; Letousey, P.; Spagnolo, A.; Rabenoelina, F.; Jacquens, L.; Mercier, L.; Clément, C.; Fontaine, F. Leaf stripe form of esca induces alteration of photosynthesis and defence reactions in presymptomatic leaves. Funct. Plant Biol. 2011, 38, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Peighami Ashnaei, S. Grapevine, esca complex, and environment: The disease triangle. Phytopathol. Mediterr. 2019, 58, 17–37. [Google Scholar] [CrossRef]
- Goufo, P.; Cortez, I. A lipidomic analysis of leaves of esca-affected grapevine suggests a role for galactolipids in the defense response and appearance of foliar symptoms. Biology 2020, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Heath, M.C. Hypersensitive response-related death. Plant Mol. Biol. 2000, 44, 321–334. [Google Scholar] [CrossRef]
- Lecomte, P.; Darrieutort, G.; Liminana, J.M.; Comont, G.; Muruamendiaraz, A.; Legorburu, F.J.; Choueiri, E.; Jreijiri, F.; El Amil, R.; Fermaud, M. New insights into esca of grapevine: The development of foliar symptoms and their association with xylem discoloration. Plant Dis. 2012, 96, 924–934. [Google Scholar] [CrossRef]
- Pouzoulet, J.; Scudiero, E.; Schiavon, M.; Santiago, L.S.; Rolshausen, P.E. Modeling of xylem vessel occlusion in grapevine. Tree Physiol. 2019, 39, 1438–1445. [Google Scholar] [CrossRef]
- Bortolami, G.; Gambetta, G.A.; Delzon, S.; Lamarque, L.J.; Pouzoulet, J.; Badel, E.; Burlett, R.; Charrier, G.; Cochard, H.; Dayer, S.; et al. Exploring the hydraulic failure hypothesis of esca leaf symptom formation. Plant Physiol. 2019, 181, 1163–1174. [Google Scholar] [CrossRef]
- Larignon, P. Effect of sodium arsenite on the life cycles of the pathogenic agents involved in wood grapevine diseases. Phytopathol. Mediterr. 2017, 56, 537. [Google Scholar]
- Calzarano, F.; Cichelli, A.; Odoardi, M. Preliminary evaluation of variations in composition induced by esca on cv. Trebbiano D’Abruzzo grapes and wines. Phytopathol. Mediterr. 2001, 40, S443–S448. [Google Scholar]
- Guerin-Dubrana, L.; Labenne, A.; Labrousse, J.C.; Bastien, S.; Rey, P.; Gégout-Petit, A. Statistical analysis of grapevine mortality associated with esca or Eutypa dieback foliar expression. Phytopathol. Mediterr. 2013, 52, 276–288. [Google Scholar] [CrossRef]
- Calzarano, F.; D’Agostino, V.; Seghetti, L.; Amalfitano, C. Foliar treatment of esca proper affected vines with nutrients and bioactivators. Phytopathol. Mediterr. 2007, 46, 207–217. [Google Scholar]
- Calzarano, F.; Amalfitano, C.; Seghetti, L.; Cozzolino, V. Nutritional status of vines affected with esca proper. Phytopathol. Mediterr. 2009, 48, 20–31. [Google Scholar]
- Calzarano, F.; Di Marco, S.; D’Agostino, V.; Schiff, S.; Mugnai, L. Grapevine leaf stripe disease (esca complex) are reduced by a nutrients and seaweed mixture. Phytopathol. Mediterr. 2014, 53, 543–558. [Google Scholar]
- Calzarano, F.; Osti, F.; D’Agostino, V.; Pepe, A.; Di Marco, S. Mixture of calcium, magnesium and seaweed affects leaf phytoalexin contents and grape ripening on vines with grapevine leaf stripe disease. Phytopathol. Mediterr. 2017, 56, 445–457. [Google Scholar] [CrossRef]
- Calzarano, F.; Di Marco, S. Further evidence that calcium, magnesium and seaweed mixtures reduce grapevine leaf stripe symptoms and increase grape yield. Phytopathol. Mediterr. 2018, 57, 459–471. [Google Scholar] [CrossRef]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Close, R.; Meier, U.; Weber, E. Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera). Encoding and description of the phenological stages of the grapevine according to the extended BBCH scheme. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- McKinney, H.H. Influence of soil temperature and moisture on infection of wheat seedlings by Helminthosporium sativum. J. Agr. Res. 1923, 26, 195–218. [Google Scholar]
- Commission Regulation (EEC). Community methods for the analysis of wines. In Official Journal of the European Communities, 3 October 1990, No. 2676/90; Commission Regulation (EEC): Brussels, Belgium, 1990. [Google Scholar]
- Lecomte, P.; Diarra, B.; Carbonneau, A.; Rey, P.; Chevrier, C. Esca of grapevine and training practices in France: Results of a 10-year survey. Phytopathol. Mediterr. 2018, 57, 472–487. [Google Scholar]
- Di Marco, S.; Osti, F. Effect of biostimulant sprays on Phaeomoniella chlamydospora and esca proper infected vines under greenhouse and field conditions. Phytopathol. Mediterr. 2009, 48, 47–58. [Google Scholar]
- Lecourieux, D.; Ranjeva, R.; Pugin, A. Calcium in plant defence-signalling pathways. New Phytol. 2006, 171, 249–269. [Google Scholar] [CrossRef]
- Du, L.; Ali, G.S.; Simons, K.A.; Hou, J.; Yang, T.; Reddy, A.S.N.; Poovaiah, B.W. Ca2+/calmodulin regulate salicylic-acid-mediated plant immunity. Nature 2009, 457, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.R.M.; Ferreres, F.; Dias, A.C.P. Response of Vitis vinifera cell cultures to Phaeomoniella chlamydospora: Changes in phenolic production, oxidative state and expression of defence-related genes. Eur. J. Plant Pathol. 2012, 132, 133–146. [Google Scholar] [CrossRef]
- Calzarano, F.; D’Agostino, V.; Pepe, A.; Osti, F.; Della Pelle, F.; de Rosso, M.; Flamini, R.; Di Marco, S. Patterns of phytoalexins in the grapevine leaf stripe disease (esca complex)/grapevine pathosystem. Phytopathol. Mediterr. 2016, 55, 410–426. [Google Scholar] [CrossRef]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. BioMetals 2002, 15, 309–323. [Google Scholar] [CrossRef]
- Marschner, P. (Ed.) Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2011; p. 684. [Google Scholar]
- Colrat, S.; Deswarte, C.; Latché, A.; Klaébe, K.; Bouzayen, M.; Fallot, J.; Roustan, J.P. Enzymatic detoxification of eutypine, a toxin from Eutypa lata, by Vitis vinifera cells: Partial purification of an NADPH-dependent aldehyde reductase. Planta 1999, 207, 544–550. [Google Scholar] [CrossRef]
- Lebon, G.; Wojnarowiez, G.; Holzapfel, B.; Fontaine, F.; Vaillant-Gaveau, N.; Clément, C. Sugars and flowering in the grapevine (Vitis vinifera L.). J. Exp. Bot. 2008, 59, 2565–2578. [Google Scholar] [CrossRef]
- Osti, F.; Di Marco, S. Iron-dependent, non-enzymatic processes promoted by Phaeomoniella chlamydospora and Phaeoacremonium aleophilum, agents of esca in grapevine. Physiol. Mol. Plant Pathol. 2010, 74, 309–316. [Google Scholar] [CrossRef]
- Rengel, Z.; Batten, G.D.; Crowley, D.E. Agronomic approaches for improving the micronutrient density in edible portions of field crops. Field Crops Res. 1999, 60, 27–40. [Google Scholar] [CrossRef]
- Claverie, M.; Notaro, M.; Fontaine, F.; Wéry, J. Current knowledge on Grapevine Trunk Diseases with complex etiology: A systemic approach. Phytopathol. Mediterr. 2020, 59, 29–53. [Google Scholar] [CrossRef]
- Masi, M.; Cimmino, A.; Reveglia, P.; Mugnai, L.; Surico, G.; Evidente, A. Advances on fungal phytotoxins and their role in grapevine trunk diseases. J. Agric. Food Chem. 2018, 66, 5948–5958. [Google Scholar] [CrossRef] [PubMed]
- Calzarano, F.; Osti, F.; D’Agostino, V.; Pepe, A.; Della Pelle, F.; de Rosso, M.; Flamini, R.; Di Marco, S. Levels of phytoalexins in vine leaves with different degrees of grapevine leaf stripe disease symptoms (Esca complex of diseases). Phytopathol. Mediterr. 2017, 56, 494–501. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Allen, G.J.; Hugouvieux, V.; Kwak, J.M.; Waner, D. Guard cell signal transduction. Annu. Rev. Plant Biol. 2001, 52, 627–658. [Google Scholar] [CrossRef]
- Lu, Z.; Xie, K.; Pan, Y.; Ren, T.; Lu, J.; Wang, M.; Shen, Q.; Guo, S. Potassium mediates coordination of leaf photosynthesis and hydraulic conductance by modifications of leaf anatomy. Plant Cell Environ. 2019, 42, 2231–2244. [Google Scholar] [CrossRef]
- Maathuis, F.J. Sodium in plants: Perception, signalling, and regulation of sodium fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef]
- Osti, F.; Di Marco, S. Correlation between soil sodicity and foliar symptom of wood decay of kiwifruit. J. Plant Pathol. 2014, 96, 121–131. [Google Scholar]
- Keller, M.; Arnink, K.J.; Hrazdina, G. Interaction of nitrogen availability during bloom and light intensity during veraison. I. Effects on grapevine growth, fruit development, and ripening. Am. J. Enol. Vitic. 1998, 49, 333–340. [Google Scholar] [CrossRef]
- Letousey, P.; Baillieul, F.; Perrot, G.; Rabenoelina, F.; Boulay, M.; Vaillant-Gaveau, N.; Clément, C.; Fontaine, F. Early Events Prior to Visual Symptoms in the Apoplectic Form of Grapevine Esca Disease. Phytopathology 2010, 100, 424–431. [Google Scholar] [CrossRef]
- Fontaine, F.; Pinto, C.; Vallet, J.; Clement, C.; Spagnolo, A. The effects of grapevine trunk diseases (GTDs) on vine physiology. Eur. J. Plant Pathol. 2016, 144, 707–721. [Google Scholar] [CrossRef]
- Jančářová, I.; Jančář, L.; Náplavová, A.; Kubáň, V. Changes of organic acids and phenolic compounds contents in grapevine berries during their ripening. Open Chem. 2013, 11, 1575–1582. [Google Scholar] [CrossRef]
- Rietra, R.P.; Heinen, M.; Dimkpa, C.O.; Bindraban, P.S. Effects of nutrient antagonism and synergism on yield and fertilizer use efficiency. Commun. Soil Sci. Plant Anal. 2017, 48, 1895–1920. [Google Scholar] [CrossRef]
- Miller, G.W.; Huang, I.J.; Welkie, G.W.; Pushmik, J.C. Function of iron in plants with special emphasis on chloroplasts and photosynthetic activity. In Proceedings of the Iron Nutrition in Soils and Plants: Proceedings of the Seventh International Symposium on Iron Nutrition and Interactions in Plants, Zaragoza, Spain, 27 June–2 July 1993; Abadia, J., Ed.; Springer: Dordrecht, Germany, 1995; Volume 59, pp. 19–28. [Google Scholar]
| BBCH Growth Stages | Application Data | Nutrients | Dose (kg/L ha−1) |
|---|---|---|---|
| Five leaves unfolded—15 | 19 May | Iron humate | 1 |
| NPK 15-36-13 | 1 | ||
| Nine or more leaves unfolded—19 | 26 May | Microelement humate | 1 |
| “S” bioactivator | 0.3 | ||
| Nine or more leaves unfolded—19 | 31 May | Iron-humate | 1 |
| NPK 15-36-13 | 1.5 | ||
| Inflorescens fully developed; flowers separated—57 | 8 June | Microelement humate | 1.5 |
| “S” bioactivator | 0.4 | ||
| NPK 23-11-11 | 1.5 | ||
| Fruit set: young fruit begin to swell—71 | 20 June | Microelement humate | 1.5 |
| “S” bioactivator | 0.4 | ||
| NPK 23-11-11 | 1.5 | ||
| Berries groat-sized—73 | 30 June | Microelement humate | 1.5 |
| “S” bioactivator | 0.4 | ||
| NPK 12-18-32 | 1.5 | ||
| Berries pea-sized—75 | 11 July | Ca-Mg-B solution | 1.5 |
| “S” bioactivator | 0.4 | ||
| Berries beginning to touch—77 | 21 July | NPK 8-16-50 | 1.5 |
| “S” bioactivator | 0.4 |
| BBCH Growth Stages | Application Data | Nutrients | Dose (kg ha−1) | ||
|---|---|---|---|---|---|
| Controguerra Vineyard (Plot 1) | Controguerra Vineyard (Plot 2) | Giulianova Vineyard | |||
| Berries groat-sized—73 | 3 July | NPK 23-11-11 | Microelement humate | NPK 23-11-11 | 1.5 |
| Berries pea-sized—75 | 17 July | NPK 23-11-11 | Microelement humate | NPK 23-11-11 | 1.5 |
| Berries beginning to touch—77 | 31 July | NPK 23-11-11 | Microelement humate | NPK 23-11-11 | 1.5 |
| Vineyard | Comparison among Treatments | 2006 | 2007 | ||
|---|---|---|---|---|---|
| Incidence % | Severity % | Incidence % | Severity % | ||
| Controguerra | Treated diseased vines/untreated diseased vines | 0.0004 | <0.0001 | / | / |
| Microelement treated diseased vines/untreated diseased vines | / | / | 0.0014 | <0.0001 | |
| NPK 23-11-11 treated diseased vines/untreated diseased vines | / | / | 0.4495 | 0.0002 | |
| Giulianova | Treated diseased vines/untreated diseased vines | <0.0001 | <0.0001 | / | / |
| NPK 23-11-11 treated diseased vines/untreated diseased vines | / | / | 0.0043 | <0.0001 | |
| Leaf | N | P | K | Ca | Mg | Fe | Mn | Zn | Na | |
|---|---|---|---|---|---|---|---|---|---|---|
| (g kg−1) | (mg kg−1) | |||||||||
| Untreated vines | Symptomatic | 21.01 a | 1.94 a | 5.99 a | 24.42 b | 2.79 a | 139.92 a | 60.02 a | 24.03 a | 95.33 a |
| Asymptomatic | 21.23 a | 1.98 a | 8.81 a | 31.24 a | 2.82 a | 121.02 a | 67.91 a | 23.82 a | 104.32 a | |
| Healthy | 21.81 a | 1.91 a | 8.97 a | 30.53 a | 2.80 a | 110.11 a | 54.83 a | 24.22 a | 93.27 a | |
| Treated vines | Symptomatic | 24.02 a | 2.15 a | 6.16 a | 19.61 b | 2.29 a | 102.03 a | 45.63 a | 26.12 a | 94.24 a |
| Asymptomatic | 21.32 a | 2.21 a | 6.68 a | 23.64 b | 1.93 a | 105.02 a | 52.44 a | 29.52 a | 93.56 a | |
| Healthy | 22.63 a | 2.25 a | 8.83 a | 21.81 b | 2.02 a | 99.24 a | 53.63 a | 24.74 a | 90.04 a | |
| Leaf | N | P | K | Ca | Mg | Fe | Mn | Zn | Na | |
|---|---|---|---|---|---|---|---|---|---|---|
| (g kg−1) | (mg kg−1) | |||||||||
| NPK 23-11-11- treated diseased vines | Symptomatic shoot | 14.41 a | 1.76 a | 4.17 a | 29.02 bc | 3.55 b | 119.00 ab | 64.82 a | 25.42 c | 120.03 a |
| Asymptomatic shoot | 15.02 a | 1.56 ab | 3.60 ab | 33.53 ab | 3.54 b | 118.03 ab | 67.05 a | 24.91 c | 112.01 ab | |
| Microelements- treated diseased vines | Symptomatic shoot | 15.34 a | 1.34 bc | 3.54 ab | 24.82 c | 2.97 b | 132.06 a | 68.13 a | 52.31 a | 102.11 bc |
| Asymptomatic shoot | 16.43 a | 1.24 c | 3.13 ab | 31.43 bc | 3.24 b | 89.92 c | 64.72 a | 40.53 b | 92.42 c | |
| Untreated diseased vines | Symptomatic shoot | 17.62 a | 1.40 bc | 3.71 ab | 28.52 bc | 3.67 b | 113.11 b | 64.51 a | 32.12 bc | 101.03 bc |
| Asymptomatic shoot | 16.10 a | 1.31 bc | 3.21 ab | 37.06 a | 4.94 a | 110.06 b | 68.20 a | 31.73 bc | 89.14 c | |
| Untreated healthy vines | 16.24 a | 1.28 bc | 2.77 b | 31.13 bc | 5.57 a | 87.43 c | 48.23 b | 25.64 c | 104.06 bc | |
| Berry | N | P | K | Ca | Mg | Fe | Mn | Zn | Na | |
|---|---|---|---|---|---|---|---|---|---|---|
| (g kg−1) | (mg kg−1) | |||||||||
| Untreated vines | Symptomatic | 9.58 a | 1.52 a | 12.13 a | 1.75 a | 0.58 b | 22.12 a | 12.21 a | 9.77 a | 38.02 a |
| Asymptomatic | 6.02 c | 1.36 ab | 10.40 ab | 1.10 a | 0.46 bc | 19.61 ab | 7.42 b | 6.18 b | 29.44 a | |
| Healthy | 6.42 bc | 1.18 abc | 10.60 ab | 0.88 a | 0.41 c | 16.11 c | 7.28 b | 6.37 b | 27.06 a | |
| Treated vines | Symptomatic | 8.11 ab | 1.09 abc | 9.87 bc | 1.81 a | 0.84 a | 18.42 bc | 6.48 b | 6.49 b | 38.51 a |
| Asymptomatic | 5.38 c | 0.86 bc | 8.74 bc | 1.26 a | 0.45 bc | 19.34 ab | 4.89 b | 6.36 b | 35.26 a | |
| Healthy | 4.90 c | 0.75 c | 8.26 c | 1.17 a | 0.43 c | 16.15 c | 4.23 b | 4.50 c | 37.03 a | |
| Berry | N | P | K | Ca | Mg | Fe | Mn | Zn | Na |
|---|---|---|---|---|---|---|---|---|---|
| (g kg−1) | (mg kg−1) | ||||||||
| NPK 23-11-11 treated symptomatic vines | 3.93 b | 0.92 ab | 9.48 b | 0.83 a | 0.60 a | 26.03 a | 5.68 c | 5.73 a | 26.14 b |
| Microelements treated symptomatic vines | 3.77 b | 0.83 b | 9.91 b | 0.78 a | 0.50 a | 19.21 b | 7.84 b | 5.75 a | 32.15 b |
| Untreated symptomatic vines | 5.72 a | 1.11 a | 12.8 a | 1.02 a | 0.57 a | 31.53 a | 9.86 a | 5.27 a | 26.33 b |
| Untreated healthy vines | 3.62 b | 0.87 b | 10.29 ab | 1.05 a | 0.56 a | 28.32 a | 9.56 ab | 5.11 a | 51.82 a |
| Berry | Soluble Solids (g L−1) | Total Acidity (g L−1) | Tartaric Acid (g L−1) | Malic Acid (g L−1) | pH | |
|---|---|---|---|---|---|---|
| Untreated vines | Symptomatic | 150 b | 8.18 a | 4.73 bc | 4.46 a | 3.34 c |
| Asymptomatic | 190 a | 6.26 b | 4.45 c | 2.81 b | 3.54 abc | |
| Healthy | 199 a | 5.53 bc | 4.43 c | 2.50 b | 3.57 ab | |
| Treated vines | Symptomatic | 151 b | 6.13 b | 4.94 ab | 2.99 b | 3.39 bc |
| Asymptomatic | 156 b | 5.08 cd | 5.34 a | 1.42 c | 3.48 abc | |
| Healthy | 189 a | 4.53 d | 4.71 bc | 1.23 c | 3.63 a | |
| Berry | Soluble Solids (g L−1) | Total Acidity (g L−1) | Tartaric Acid (g L−1) | Malic Acid (g L−1) | pH |
|---|---|---|---|---|---|
| NPK 23-11-11-treated symptomatic vines | 149 d | 5.50 a | 5.20 a | 0.56 a | 3.23 a |
| Microelements-treated symptomatic vines | 178 b | 5.45 a | 5.28 a | 0.21 b | 3.30 a |
| Untreated symptomatic vines | 165 c | 5.80 a | 5.47 a | 0.28 ab | 3.23 a |
| Untreated healthy vines | 188 a | 5.67 a | 5.40 a | 0.14 b | 3.33 a |
| Leaf Area | Treatment | Healthy Vines | Asymptomatic Vines | Symptomatic Vines | |
|---|---|---|---|---|---|
| 2006 | Primary shoot leaves | Untreated | 27920 | 28469 | 27778 |
| Treated | 35817 | 34790 | 33790 | ||
| Secondary shoot leaves | Untreated | 30723 | 28674 | 27938 | |
| Treated | 42662 | 41827 | 37206 | ||
| 2007 | Primary shoot leaves | Untreated | 26228 | / | 25921 |
| NPK 23-11-11 | / | / | 33932 | ||
| Microelements | / | / | 25960 | ||
| Secondary shoot leaves | Untreated | 31630 | / | 31362 | |
| NPK 23-11-11 | / | / | 42284 | ||
| Microelements | / | / | 31689 |
| Leaf Area | Comparison among Treatments | p Value | |
|---|---|---|---|
| 2006 | Primary shoot leaves | Untreated healthy vines/Treated healthy vines | 0.01324 |
| Untreated asymptomatic vines/Treated asymptomatic vines | 0.04044 | ||
| Untreated symptomatic vines/Treated symptomatic vines | 0.04503 | ||
| Secondary shoot leaves | Untreated healthy vines/Treated healthy vines | 0.04232 | |
| Untreated asymptomatic vines/Treated asymptomatic vines | 0.04280 | ||
| Untreated symptomatic vines/Treated symptomatic vines | 0.20361 | ||
| 2007 | Primary shoot leaves | Untreated healthy vines/Untreated symptomatic vines | 0.60323 |
| Untreated healthy vines/NPK 23-11-11 treated symptomatic vines | 0.00127 | ||
| Untreated healthy vines/Microelement treated symptomatic vines | 0.89887 | ||
| Secondary shoot leaves | Untreated healthy vines/Untreated symptomatic vines | 0.91395 | |
| Untreated healthy vines/NPK 23-11-11 treated symptomatic vines | 0.02981 | ||
| Untreated healthy vines/Microelement treated symptomatic vines | 0.98092 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calzarano, F.; Amalfitano, C.; Seghetti, L.; Di Marco, S. Effect of Different Foliar Fertilizer Applications on Esca Disease of Grapevine: Symptom Expression and Nutrient Content in the Leaf and Composition of the Berry. Agronomy 2023, 13, 1355. https://doi.org/10.3390/agronomy13051355
Calzarano F, Amalfitano C, Seghetti L, Di Marco S. Effect of Different Foliar Fertilizer Applications on Esca Disease of Grapevine: Symptom Expression and Nutrient Content in the Leaf and Composition of the Berry. Agronomy. 2023; 13(5):1355. https://doi.org/10.3390/agronomy13051355
Chicago/Turabian StyleCalzarano, Francesco, Carmine Amalfitano, Leonardo Seghetti, and Stefano Di Marco. 2023. "Effect of Different Foliar Fertilizer Applications on Esca Disease of Grapevine: Symptom Expression and Nutrient Content in the Leaf and Composition of the Berry" Agronomy 13, no. 5: 1355. https://doi.org/10.3390/agronomy13051355
APA StyleCalzarano, F., Amalfitano, C., Seghetti, L., & Di Marco, S. (2023). Effect of Different Foliar Fertilizer Applications on Esca Disease of Grapevine: Symptom Expression and Nutrient Content in the Leaf and Composition of the Berry. Agronomy, 13(5), 1355. https://doi.org/10.3390/agronomy13051355







