Abstract
To investigate the amelioration of salt-induced damage on Paeonia ostii ‘Fengdan’ by exogenous silicon, we analyzed the photosynthetic and physiological characteristics of 1.5-year-old ‘Fengdan’ seedlings under NaCl stress by applying exogenous silicon (0, 0.75, and 1.5 mmol/L). Our results showed that the contents of the photosynthetic pigments chlorophyll a, chlorophyll b, and carotene, the transpiration rate, stomatal conductance, and intercellular CO2 were significantly enhanced under salt stress when silicon treatment was applied, implying that the net photosynthetic rate was greatly improved. In addition, the plant height, stem thickness, and above-ground dry biomass of tree peony seedlings were effectively increased under salt stress with low-concentration silicon (0.75 mmol/L) treatment, along with osmotic substance (SS, SP, and Pro) content, total polyamine (TP) contents, and the activities of antioxidant-related enzymes (SOD, POD, and CAT) and polyamine-related synthetases (ADC, ODC, and SAMDC). In the low-concentration silicon treatment, malondialdehyde (MDA), hydrogen peroxide (H2O2), and superoxide anions (O2−) were transformed quickly, which eventually reduced cell oxidative damage and improved seedling tolerance. This is an important finding in the understanding of how exogenous low-concentration silicon can alleviate salt-induced damage and promote the growth of tree peony seedlings, thus providing a new perspective on tree peony cultivation.
1. Introduction
With the increase in global temperatures, stronger evaporation and constant salt accumulation on the soil surface are becoming more obvious, and the increasing salinity level in the natural environment and on cultivated land is becoming an important factor influencing plant growth and agricultural production [1,2]. Overgrazing, intensive cultivation, and unscientific irrigation have led to the accumulation of salt in the soil [3]. Approximately 20% of the world’s irrigated agricultural land is affected by high salinity levels [1,2]. High concentrations of soil salt can cause ion imbalance and osmotic stress and eventually lead to difficulty in water absorption, resulting in plant growth being negatively affected, even to the point of death [4,5,6].
Paeonia ostii ‘Fengdan’ is a perennial plant in the peony family with a strong seed-setting ability. Due to the high seed oil yield and good oil quality, it has recently become a woody oil crop with high economic value [7,8]. Soil salinization has hindered the development of the oil tree peony industry. It has caused a reduction in emergence rate, slow development, and even the death of seedlings, decreasing crop yield and quality and causing huge economic losses to growers [9,10]. Therefore, it is of great importance to carry out research on improving tree peony salt tolerance, which can provide theoretical support for the sustainable development of the tree peony industry.
Silicon (Si) is an important structural and functional substance in plants. As a non-toxic and beneficial element for plants, silicon is becoming more and more widely used in crop production, and research on its mechanism of action has been receiving more and more attention [11]. Silicon can enhance plant photosynthesis efficiency, root activity, and resistance to stress, both biological (e.g., diseases, pests, etc.) [12] and abiotic (e.g., drought, heavy metals, cold damage, etc.) [13,14,15]. Many studies have shown that silicon application can alleviate salt stress damage in crops and improve their resistance [16,17,18,19]. Silicon can inhibit the excessive absorption of sodium ions by plant roots, promote the absorption of potassium, calcium, nitrogen, magnesium, and other nutritional elements, so as to alleviate the nutritional imbalance caused by salt stress [5,16]. Silicon can also improve the ability of plants to absorb and transport water, enhance the activity of antioxidant-related enzymes and polyamine synthase, increase the contents of polyamines and osmotic materials, promote pectin synthesis, pectin methyl esterase, and cation exchange ability, slow the production and accumulation of reactive oxygen species (ROS), reduce malondialdehyde (MDA) content, and maintain the integrity of the cell wall and internal organelles [19,20,21]. It has thus been shown to improve salt tolerance in Hordeum vulgare [22], Zea mays [23], Triticum aestivum [20], and Sorghum bicolor [24].
Previous studies have not yet reported whether exogenous silicon can promote the salt-resistant growth of tree peonies. In this study, we used P. ostii ‘Fengdan’ to analyze the effects of exogenous silicon on seedling growth and physiological metabolism. The main objectives of this study were (ⅰ) to determine the effects of silicon on the photosynthetic characteristics of tree peony under salt stress, including changes in leaf gas exchanges indicators (net photosynthetic rate, transpiration rate, stomatal conductance, and intercellular CO2 concentration) and leaf photosynthetic pigments content (chlorophyll a, chlorophyll b, and carotenoids); (ⅱ) to illustrate the effects of silicon on oxidative stress response under salt stress, including changes in MDA level, ROS levels, and activity of antioxidant-related enzymes, such as superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT); (ⅲ) to explain the changes in leaf osmotic substance levels in seedlings under salt stress, such as soluble sugar (SS), soluble protein (SP), and proline (Pro); and (ⅳ) to illustrate the effects of silicon on polyamine metabolism in the leaves, including changes in total polyamine content and related synthetases activities, such as those of arginine decarboxylase (ADC), ornithine decarboxylase (ODC), and S-adenosylmethionine decarboxylase (SAMDC).
2. Materials and Methods
2.1. Materials
2.1.1. Plant Materials
Seeds of Paeonia ostii ‘Fengdan’ were obtained from the farm of the Agriculture/Tree Peony College of Henan University of Science and Technology, China, in August 2017. After ripening and drying in the shade, tree peony seeds were planted in a field in November 2017. Seedlings were routinely managed and used in later experiments.
2.1.2. Experimental Agent
Potassium silicate (K2SiO3, 280 g/L), a pollution-free and safe product used in modern agriculture, was purchased from Noel Biotechnology Co., Ltd., Shanghai, China.
2.2. Experimental Design
Tree Peony Seedling Selection and Pharmaceutical Treatments
In April 2019, P. ostii ‘Fengdan’ seedlings were transplanted into plastic pots (15 cm diameter, 25 cm height) filled with a mixture of vermiculite and quartz sand (V/V, 1:1), with one plant per pot. Two weeks later, all strong seedlings with consistent growth (based on height and stem diameter) were selected and divided into 4 treatment groups as follows: without salt and silicon (control, CK), salt without silicon (200 mL 200 mmol/L NaCl + 0 mmol/L K2SiO3, Na + Si0), salt with a low concentration of silicon (200 mL 200 mmol/L NaCl + 0.75 mmol/L K2SiO3, Na + Si1), and salt with a high concentration of silicon (200 mL 200 mmol/L NaCl + 1.50 mmol/L K2SiO3, Na + Si2). Water was controlled for several days before salt stress was induced so as to facilitate the rapid diffusion of saline. The experiment was performed with a completely randomized design and in triplicate. Seedlings were cultured in an artificial climate chamber at 25 °C with 16 h of light and 8 h of darkness.
2.3. Parameters Determined
2.3.1. Growth Characteristics
On the 7th day of salt stress treatment, five P. ostii ‘Fengdan’ seedlings were selected from each replicate to determine growth characteristics such as plant height (cm), stem thickness (mm), and above-ground and below-ground dry biomass (g) according to the method of Shi et al. [25].
2.3.2. Determination of Leaf Gas Exchange Indicators and Chlorophyll Levels
Photosynthetic parameters, including the net photosynthetic rate (μmol·m−2·s−1), transpiration rate (mmol·m−2·s−1), stomatal conductance (mol·m−2·s−1), and intercellular CO2 concentration (μmol·m−2·s−1) of the top-down third leaves, were determined using a Li-6400 portable photosynthetic meter (Li-6400, LI-COR, Lincoln, NE, USA) at 09:00–11:00 [25]. The content of chlorophyll a, chlorophyll b, and carotenoids was calculated according to the formula of Zou et al. [26]. The experiment was repeated in triplicate.
2.3.3. Determination of the MDA Level and ROS Levels
The concentration of malondialdehyde (MDA), hydrogen peroxide (H2O2), and superoxide anion (O2−) in fresh leaves was determined using the detection kit produced by Keming Biotechnology Co., Ltd.(Suzhou, China), according to the manufacturer’s instructions. The analysis of each sample was conducted in triplicate.
2.3.4. Determination of Antioxidant Enzymatic Activities
The activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) were determined using the detection kit produced by Keming Biotechnology Co., Ltd.(Suzhou, China), according to the manufacturer’s instructions. The analysis of each sample was conducted in triplicate.
2.3.5. Determination of Osmotic Substance Levels
The contents of soluble sugar (SS), soluble protein (SP), and proline (Pro) were determined according to the manufacturer’s instructions (Suzhou Keming Biotechnology Co., Ltd., Suzhou, China).
2.3.6. Determination of Total Polyamines Contents and Related Synthetases Activities
Appropriate amounts of tree peony leaves were used to determine the total polyamine content using high performance liquid chromatography (HPLC) (HPLC: LC-20A, Pro-minence, Shimadzu Co. Ltd., Shimadzu, Japan), according to Hu et al. [27]. We took 1 g of fresh ground sample and added 0.01 mol·L−1 potassium phosphate buffer (pH 8.0) containing 0.1 mol·L−1 phenyl methyl sulfonyl fluoride, 0.01 mol·L−1 pyridoxal phosphate (PLP), 0.05 mol·L−1 dithiothreitol (DTT), 0.05 mol·L−1 ethylenediaminnetetraacetic acid (EDTA), 0.25 mol·L−1 ascorbic acid, and 0.1% polyvinylpyrrolidone. The homogenate was centrifuged at 12,000× g for 40 min (4 °C). The precipitate was resuspended in 3 mL 1 mol·L−1 phosphate buffer (pH 8.0, containing 0.01 mol·L−1 DTT, 0.05 mol·L−1 PLP, 0.001 mol·L−1 EDTA), dialyzed in the dark for 24 h (4 °C), and the dialysate was collected. The activities of arginine decarboxylase (ADC) and ornithine decarboxylase (ODC) were determined according to the method of Hu et al. [26]. The activity of S-adenosylmethionine decarboxylase (SAMDC) was measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions. The kit was purchased from Hufeng Biotechnology Co., Ltd. (Shanghai, China). Results were expressed as three biological replicates for each treatment.
2.4. Statistical Analysis
The LSD method for multiple comparisons and Duncan’s method for difference significance tests (α = 0.05) were conducted in SPSS 22.0 (SPSS Inc., Chicago, IL, USA). The results were expressed as the mean ± standard deviation (SD). All the visual images were processed with Photoshop 7.0 (Adobe Systems Inc., San Jose, CA, USA) and Origin 9.1 (OriginLab Software Inc., Northampton, MA, USA).
3. Results
3.1. Changes in Growth of P. ostii ‘Fengdan’ Seedlings Affected by Exogenous Silicon under Salt Stress
When salt stress reached 200 mmol/L, the plant height, stem thickness, root crown ratio, and aboveground and underground dry biomass of tree peony seedlings were significantly reduced (p < 0.05) by 23.37%, 23.41%, 13.40%, 16.80%, and 27.99% compared with the CK, respectively. The plant height, stem thickness, and above-ground dry biomass of tree peony seedlings in the Na + Si1 group were significantly increased by 25.29%, 29.88%, and 14.30%, respectively, compared with the Na + Si0 group (p < 0.05). The below-ground dry biomass and root crown ratio in the Na + Si1 group were significantly increased by 26.29% and 10.43% compared to the Na + Si0 group and were significantly lower than CK (p < 0.05). All growth indicators in the Na + Si2 group were significantly lower than the CK. The plant height, stem thickness, and shoot dry biomass in the Na + Si2 group were significantly higher than the Na + Si0 group, while the below-ground dry biomass and root-shoot ratio were similar in both treatment groups (p > 0.05)(Table 1).

Table 1.
Changes in growth of P. ostii ‘Fengdan’ seedlings affected by exogenous silicon under salt stress.
3.2. Effects of Exogenous Silicon on Leaf Gas Exchange Indicators of P. ostii ‘Fengdan’ Seedlings under Salt Stress
Leaf gas exchange was investigated in P. ostii ‘Fengdan’ seedlings after salt treatment. The net photosynthetic rate, transpiration rate, stomatal conductance, and intercellular CO2 concentration in the Na + Si0 group were found to be significantly reduced by 27.90%, 34.84%, 43.44%, and 16.38% compared to CK, respectively. The application of exogenous silicon can significantly increase the value of each leaf gas exchange indicator in seedlings under salt stress. Additionally, the effects of the low-concentration silicon treatment (0.75 mmol/L K2SiO3) were significantly higher than those of the high-concentration treatment (1.50 mmol/L K2SiO3) (p < 0.05). Compared to the Na + Si0 group, the net photosynthetic rate, transpiration rate, stomatal conductance, and intercellular CO2 concentration in the Na + Si1 group were significantly increased by 38.12%, 50.17%, 76.82%, and 19.75%, respectively, while these indices in the Na + Si2 group were only increased by 19.16%, 25.08%, 53.75%, and 12.90%, respectively (p < 0.05). In brief, the low-concentration silicon treatment was more effective in improving photosynthetic parameters than the high-concentration treatment in P. ostii ‘Fengdan’ seedlings (Figure 1).
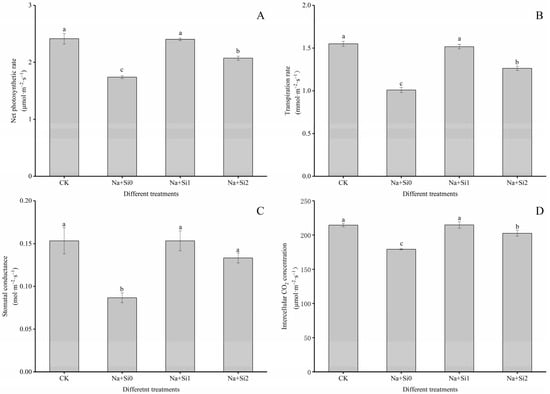
Figure 1.
Effects of exogenous silicon on leaf gas exchange indicators of P. ostii ‘Fengdan’ seedlings under salt stress. (A) Net photosynthetic rate; (B) Transpiration rate; (C) Stomatal conductance; (D) Intercellular CO2 concentration. Note: Data are presented as mean ± SD (n = 3). On each subgraph, different letters indicate significant differences at p < 0.05. Na + Si0, Na + Si1, Na + Si2, and CK refer to salt without silicon (200 mmol/L NaCl + 0 mmol/L K2SiO3), salt with low concentration of silicon (200 mmol/L NaCl + 0.75 mmol/L K2SiO3), salt with high concentration of silicon (200 mmol/L NaCl + 1.5 mmol/L K2SiO3), and without salt and silicon (control, CK), respectively.
3.3. Effects of Exogenous Silicon on Leaf Photosynthetic Pigment Content of P. ostii ‘Fengdan’ Seedlings under Salt Stress
Under salt stress, the photosynthetic pigment content (chlorophyll a, chlorophyll b, and carotenoids) in tree peony seedling leaves dropped significantly by 49.24%, 55.42%, and 16.16%, respectively (p < 0.05) (Figure 2). These reductions were significantly ameliorated by spraying silicon. The photosynthetic pigment content in the Na + Si1 group was significantly higher than that in the Na + Si0 group and the Na + Si2 group (p < 0.05). When compared with the CK, the contents of chlorophyll a and chlorophyll b in the Na + Si2 group were significantly decreased by 17.05% and 10.82%, respectively, while there was no significant difference in carotenoids content between the two groups. This suggested that low concentrations of silicon (0.75 mmol/L K2SiO3) might be more effective to improve photosynthetic pigment content in salt-stressed tree peonies.
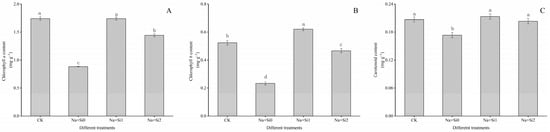
Figure 2.
Effects of exogenous silicon on the assimilatory pigment content of P. ostii ‘Fengdan’ seedling leaves under salt stress. (A) Chlorophyll a content; (B) chlorophyll b content; (C) carotenoid content. Note: Data are presented as mean ± SD (n = 3). On each subgraph, different letters indicate significant differences at p < 0.05. Na + Si0, Na + Si1, Na + Si2, and CK refer to salt without silicon (200 mmol/L NaCl + 0 mmol/L K2SiO3), salt with a low concentration of silicon (200 mmol/L NaCl + 0.75 mmol/L K2SiO3), salt with a high concentration of silicon (200 mmol/L NaCl + 1.5 mmol/L K2SiO3), and without salt and silicon (control, CK), respectively.
3.4. Effects of MDA Level and ROS Levels on P. ostii ‘Fengdan’ Seedlings under Salt Stress
The MDA, H2O2, and O2− contents in tree peony seedling leaves were increased by 93.50%, 70.60%, and 122.68% in the Na + Si0 group compared to the CK, respectively (Figure 3). Exogenous low concentrations of silicon can effectively slow down the increase in membrane lipid peroxide content in comparison with the Na + Si0 group, and the content of MDA, H2O2, and O2− in the leaves was significantly reduced by 43.94%, 38.77%, and 53.40, respectively (p < 0.05). Compared to the Na + Si2 group, the MDA, H2O2, and O2− contents in the Na + Si1 group were significantly reduced by 44.95%, 40.28%, and 56.50%, respectively (p < 0.05). However, these values were not significantly different from the CK. These results indicate that low concentrations of silicon (0.75 mmol/L K2SiO3) could compensate tree peony seedlings to some extent, and return the membrane lipid peroxide content to its normal level. Under salt stress, MDA and H2O2 contents in the Na + Si2 group showed no significant changes compared to the Na + Si0 group, while the O2− content increased greatly. These three parameters were all significantly higher in the Na + Si2 group than in the CK group (p < 0.05).
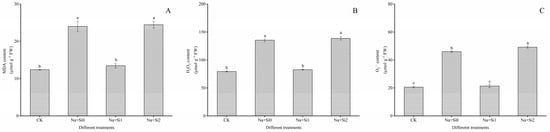
Figure 3.
Effects of exogenous silicon on lipid peroxidation of P. ostii ‘Fengdan’ seedlings under salt stress. (A) MDA content; (B) H2O2 content; (C) O2− content. Note: Data are presented as mean ± SD (n = 3). On each subgraph, different letters indicate significant differences at p < 0.05. Na + Si0, Na + Si1, Na + Si2, and CK refer to salt without silicon (200 mmol/L NaCl + 0 mmol/L K2SiO3), salt with a low concentration of silicon (200 mmol/L NaCl + 0.75 mmol/L K2SiO3), salt with a high concentration of silicon (200 mmol/L NaCl + 1.5 mmol/L K2SiO3), and without salt and silicon (control, CK), respectively.
3.5. Effects of Exogenous Silicon on Antioxidant-Related Enzyme Activities of P. ostii ‘Fengdan’ Seedlings under Salt Stress
In contrast to CK (without salt and silicon), SOD, POD, and CAT activities in the Na + Si0 group leaves of tree peony seedlings increased by 67.29%, 47.22%, and 43.46%, respectively (Figure 4). Under salt stress, treatment with a low concentration of silicon (0.75 mmol/L K2SiO3) could greatly enhance the activities of antioxidant enzymes in leaves compared to the Na + Si0 group, and the activities of SOD, POD, and CAT significantly increased by 30.45%, 39.12%, and 19.69%, respectively (p < 0.05). However, after spraying high concentrations of silicon (1.50 mmol/L K2SiO3), POD and CAT activity in tree peony leaves increased by 13.21% and 19.89%, respectively, compared to the Na + Si0 group (200 mL, 200 mmol/L NaCl + 0 mmol/L K2SiO3), while SOD activities were not significantly different. Both SOD and POD activities in the high-concentration silicon group were significantly lower than in seedling leaves treated with low concentrations of silicon, while the CAT activity showed little difference (p < 0.05).
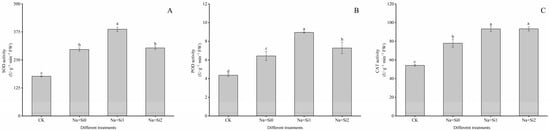
Figure 4.
Effects of exogenous silicon on photosynthetic characteristics of P. ostii ‘Fengdan’ seedlings under salt stress. (A) SOD activity; (B) POD activity; (C) CAT activity. Note: Data are presented as mean ± SD (n = 3). On each subgraph, different letters indicate significant differences at p < 0.05. Na + Si0, Na + Si1, Na + Si2, and CK refer to salt without silicon (200 mmol/L NaCl + 0 mmol/L K2SiO3), salt with a low concentration of silicon (200 mmol/L NaCl + 0.75 mmol/L K2SiO3), salt with a high concentration of silicon (200 mmol/L NaCl + 1.5 mmol/L K2SiO3), and without salt and silicon (control, CK), respectively.
3.6. Effects of Exogenous Silicon on Osmotic Substance Content of P. ostii ‘Fengdan’ Seedlings under Salt Stress
The cumulation of osmotic substances significantly increases in plants under salt stress. According to Figure 5, after salt stress, the contents of soluble sugars, soluble protein, and proline in the leaves of tree peony seedlings significantly increased by 21.74%, 15.72%, and 29.63% compared to the CK, respectively (p < 0.05). This indicates that the tree peony seedlings can resist salt stress damage to a certain extent by increasing soluble sugars, soluble proteins, and proline content. After adding exogenous silicon, the contents of the above three substances accumulated greatly, especially in the low-concentration silicon treatment (0.75 mmol/L K2SiO3). The content of soluble sugars, soluble protein, and proline in the Na + Si1 group increased by 91.30%, 43.56%, and 94.74% compared to the CK group, and 57.14%, 24.06%, and 50.22% compared to the Na + Si0 group, and 21.11%, 2.43%, and 8.05% compared to the Na + Si2 group, respectively.
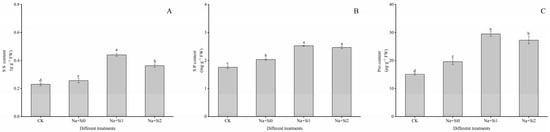
Figure 5.
Effects of exogenous silicon on osmotic substance content of P. ostii ‘Fengdan’ seedlings under salt stress. (A) SS (Soluble sugars) content; (B) SP (Soluble protein) content; (C) Pro (Proline) content. Note: Data are presented as mean ± SD (n = 3). On each subgraph, different letters indicate significant differences at p < 0.05. Na + Si0, Na + Si1, Na + Si2 and CK refer to salt without silicon (200 mmol/L NaCl + 0 mmol/L K2SiO3), salt with low concentration of silicon (200 mmol/L NaCl + 0.75 mmol/L K2SiO3), salt with high concentration of silicon (200 mmol/L NaCl + 1.5 mmol/L K2SiO3), and without salt and silicon (control, CK), respectively.
3.7. Effects of Exogenous Silicon on Total Polyamines Content and Related Synthetase Activities of P. ostii ‘Fengdan’ Seedlings under Salt Stress
The polyamine content of plants is closely related to stress resistance. When the tree peony seedlings were subjected to salt stress, the total polyamine contents in the leaves increased significantly (Figure 6A), and the activity of enzymes involved in polyamine synthesis, ADC, ODC, and SAMDC, were also significantly enhanced (Figure 6B–D) (p < 0.05). This indicated that tree peony seedlings could accumulate these substances by enhancing the activities of polyamines synthesis-related enzymes to increase stress resistance. After adding exogenous silicon, the total polyamine contents and related enzyme activities were greatly increased, and were significantly higher than in the CK (without salt and silicon) and Na + Si0 groups (200 mL 200 mmol/L NaCl + 0 mmol/L K2SiO3). Furthermore, the treatment with low concentrations of silicon (0.75 mmol/L K2SiO3) showed a much greater increase in total polyamine content and ADC and ODC activity than the high-silicon-treatment group (1.50 mmol/L K2SiO3). However, the effect of SAMDC was not significantly different from that in the high-concentration silicon group (p > 0.05). Under the same salt stress conditions, the total polyamine content, ADC, ODC, and SAMDC enzyme activities of the seedlings in the low-concentration silicon group (Na + Si1) were 35.35%, 60.20%, 54.58%, and 16.36% higher, respectively, than in the Na + Si0 group. In the high-concentration silicon group (Na + Si2), these values only increased by 19.92%, 45.20%,, 41.20%, and 15.43%, respectively.
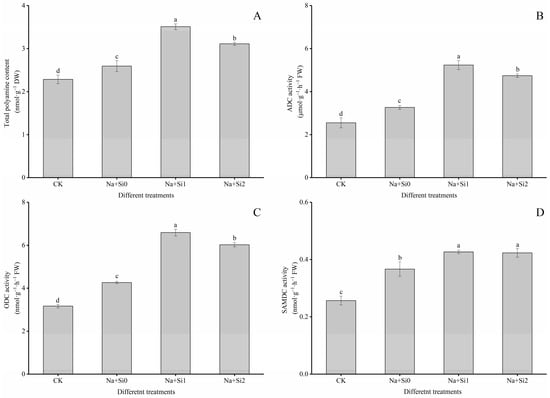
Figure 6.
Effects of exogenous silicon on photosynthetic characteristics of P. ostii ‘Fengdan’ seedlings under salt stress. (A) Total polyamine content; (B) ADC (arginine decarboxylase) activity; (C) ODC (ornithine decarboxylase) activity; (D) SAMDC (S-adenosylmethionine decarboxylase) activity. Note: Data are presented as mean ± SD (n = 3). On each subgraph, different letters indicate significant differences at p < 0.05. Na + Si0, Na + Si1, Na + Si2, and CK refer to salt without silicon (200 mmol/L NaCl + 0 mmol/L K2SiO3), salt with a low concentration of silicon (200 mmol/L NaCl + 0.75 mmol/L K2SiO3), salt with a high concentration of silicon (200 mmol/L NaCl + 1.5 mmol/L K2SiO3), and without salt and silicon (control, CK), respectively.
4. Discussion
Salt stress is one of the main environmental factors limiting agricultural development, as it blocks the growth and development of plants. Root elongation is inhibited, which hinders the uptake of water and nutrients and seriously affects plant growth and crop yield [28,29]. This research also found that plant height, stem thickness, root–crown ratio, and above-ground and below-ground dry biomass of tree peony seedlings (200 mmol/L) were significantly reduced under salt stress compared with the CK (p < 0.05). It has been reported that the water retention capacity of the soil increased after exogenous application of silicon, and the water supply to plants therefore increased [30,31]. Exogenous silicon can be in the form of amorphous silicon precipitated in the soil [32]; thus, the effect of silicon is higher in the soil compared with in plants [33]. Some researchers have reported that amorphous silicon could strongly improve the ability of plants to utilize water [33,34]. Exogenous silicon oxide nanoparticles (SiNPs) were also found to promote root growth in Zingiber officinale. [35]. Here, we investigated the effect of silicon treatment on tree peony seedlings and found that exogenous silicon could greatly promote the development of tree peonies. It was also found that the dry biomass of the below-ground parts increased significantly. Moreover, the crown ratio of the seedlings in the exogenous silicon treatment was significantly greater than that in the silicon-free groups. This indicates that exogenous silicon can affect the above-ground as well as below-ground parts of tree peonies. Additionally, a previous study reported that silicon can reduce salt stress for microbes by increasing water availability [36].
Photosynthesis is an important metabolic process in plant growth and development and provides a major source of material and energy [37]. Photosynthetic parameters are commonly used to detect photosystem damage caused by various abiotic stresses. In this study, salt stress led to a reduction in the maximum net photosynthetic rate, transpiration rate, stomatal conductance, and intercellular CO2 concentration in tree peony leaves, thus significantly decreasing leaf photosynthetic efficiency (p < 0.05). Chloroplasts are an important site of photosynthesis, and the photosynthetic pigment content is positively correlated with the rate of photosynthesis [38]. Salt stress can destroy the structure of chloroplasts, reduce the photosynthetic pigment content, and thus affect photosynthetic growth [39,40,41]. Treatment with a suitable concentration of silicon could significantly slow down the decrease in photosynthetic pigment content [42]. In studies of Oryza sativa [43], Triticum aestivum [44], and Cucumis sativus [19], the content of photosynthetic pigment increased with the addition of silicon. Our study showed that exogenous low concentrations of silicon (0.75 mmol/L K2SiO3) significantly increased the contents of chlorophyll in tree peony leaves and improved the seedlings growth under salt stress, as has been previously observed in Medicago sativa [29]. We described the results of exogenous silicon on leaf gas exchange indicators of P. ostii ‘Fengdan’ seedlings under salt stress, which showed that the photosynthetic index (e.g., the net photosynthetic rate) increased significantly with the application of low concentrations of silicon compared to the CK, thus indicating that adding silicon can help improve photosynthetic abilities under salt stress. In the current study, the net photosynthetic rate of tree peony seedlings was relatively low, even in the control group. This might be related to the fact that the seedlings were potted rather than cultivated in a field; a similar difference has been reported in previous studies of peonies [45].
Salt damage to plants includes a series of physiological and biochemical processes, such as an increase in the production of reactive oxygen species and destruction of the antioxidant metabolic balance, thus causing oxidative damage [46]. Soluble sugars, soluble proteins, and free proline have protective roles in stress resistance and in regulating osmotic pressure and the intracellular environment [47,48]. In our study, the leaf MDA, H2O2, and O2·− contents, as well as the soluble sugar, soluble protein, and proline, were significantly increased in tree peony seedlings under salt stress compared with the control group. Studies on alfalfa have shown that a low concentration of silicon increases soluble sugar content as well as reducing proline and soluble protein contents in alfalfa leaves [29]. However, the content of all three molecules was significantly increased in tree peony seedlings. Therefore, these results may demonstrate that the degree of salinity stress toxicity and the influence of silicon on the permeability content differ between crops.
The ability of plants to resist external stress is often closely related to the activity of enzymatic defense systems such as SOD, POD, and CAT, which can transform O2− and H2O2 into less active substances and reduce or eliminate their ability to attack membrane lipids, thus protecting membrane lipids from oxidation [49,50]. In our study, the activities of SOD, POD, and CAT increased significantly in the Na + Si0 treatment compared to the control (Figure 4). This means that the plant activated its own enzymatic immune system to resist salt damage. Exogenous silicon could improve the activities of antioxidant enzymes in tree peony leaves, especially after treatment with low concentrations of silicon (Na + Si1), which showed a significantly higher increase than that in the salt-stressed groups (Na + Si0) and high-concentration silicon-treated groups (Na + Si2). Additionally, after treatment with high concentrations of silicon, although the activity of POD and CAT was significantly enhanced compared with the salt-stressed group, the activity of SOD varied little (p > 0.05). This indicated that low concentrations of silicon play a greater role in enhancing the activity of tree peony antioxidant enzymes to resist salt stress than high concentrations.
Polyamines are a class of compounds containing two or more amino groups that play an important role in plant responses to stress [51]. ADC, ODC, and SAMDC are the key enzymes for polyamine synthesis in plants [52]. Our study found that tree peony seedlings increased the total polyamine contents and the activity of key enzymes, such as ADC, ODC, and SAMDC, when suffering from salt stress. Additionally, the salt stress damage was offset or alleviated by exogenous silicon, especially low-concentration silicon. For instance, the activities of ADC, ODC, and SAMDC were 35.35%, 54.58%, and 16.36% higher in the low-concentration exogenous silicon group than in the silicon-free group under equivalent salt stress conditions, respectively (Figure 6). This demonstrated that tree peonies can increase the content of polyamine substances by increasing the activities of polyamine synthesis-related enzymes when under stress, thus improving stress resistance.
In our study, a low concentration of silicon (0.75 mmol/L K2SiO3) was found to be the most beneficial treatment for tree peony, which has also been shown for Medicago sativa [29] and Cucumis sativus [53]. As the concentration of silicon increased, the positive effects were reduced. The same phenomenon has also been found in Cucumis sativus [53], Triticum aestivum [54], and Zea mays [55]. Therefore, treatment with low concentrations of silicon is suitable for inducing salt resistance in tree peonies.
5. Conclusions
When tree peonies were subjected to salt stress, the in vivo membrane lipid peroxide content increased, and the content of photosynthetic pigment in the leaves dropped sharply. This seriously affected the photosynthetic efficiency and finally led to a significant reduction in seedling plant height, stem thickness, root crown ratio, and above-ground and below-ground dry biomass (p < 0.05). Under salt stress, the addition of low concentrations of exogenous silicon (0.75 mmol/L K2SiO3) effectively increased the photosynthetic pigment content, greatly promoted photosynthesis efficiency, and provided the plant with more energy material to resist the salt stress. It can also improve the activities of antioxidant-related enzymes and polyamine synthesis-related enzymes, thereby accelerating ROS clearance and maintaining a lower membrane lipid peroxide content. Furthermore, exogenous silicon increased the contents of osmotic substances and polyamines and eventually enabled the plant to resist salt damage and restore normal growth. Although high-concentration silicon (1.50 mmol/L K2SiO3) treatment undoubtedly allows tree peony seedlings to resist salt stress, the positive effect is far less than when treated with low concentrations (0.75 mmol/L K2SiO3). We therefore suggest that low concentrations of silicon can ameliorate salt-induced damage in tree peonies (Figure 7).
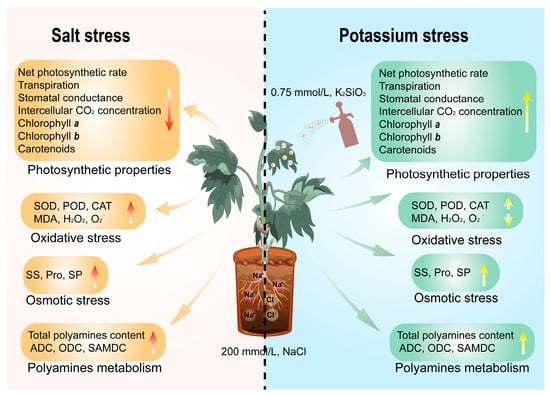
Figure 7.
Summary changes of physiological characteristics in P. ostii ‘Fengdan’ seedlings after exogenous silicon under salt stress.
Author Contributions
X.S., X.X. and X.H. conceived the study and drafted the manuscript. X.S., X.X., H.X., Z.K. and X.H. discussed the writing plan. X.S. and Y.Y. performed the experiments. X.S., Y.Y. and X.H. obtained the experimental materials. X.S. and H.X. analyzed the data. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Zhongyuan Scholars Workstation Project (224400510002), the Luoyang Rural Revitalization Project (2101099A) and the National Natural Science Foundation of China (U2004150).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Wang, W.B.; Kim, Y.H.; Lee, H.S.; Kim, K.Y.; Deng, X.P.; Kwak, S.S. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol. Biochem. 2009, 47, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Ibrahim, M.; Farid, M.; Adress, M. Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: A review. Environ. Sci. Pollut. Res. 2015, 22, 15416–15431. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Zheng, W.; Tian, Y.; Wu, Y.; Zhou, D.W. Effects of various mixed salt-alkaline stresses on growth, photosynthesis, and photosynthetic pigment concentrations of Medicago ruthenica seedlings. Photosynthetica 2011, 49, 275–284. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Gong, H.J.; Yin, J.L. Role of silicon in mediating salt tolerance in plants: A review. Plants 2019, 8, 147. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, W.; Guo, S.; Li, S.; Liu, R. Effects of arbuscular mycorrhizae on photosynthesis of paeonia suffruticosa grown under salt stress. J. Qingdao Agric. Univ. (Nat. Sci.) 2013, 30, 79–83. [Google Scholar]
- Zhu, Y.X.; Xia, Y.C.; Liu, L.C.; Yin, J.L.; Ma, D.F. Beneficial effects of silicon on salt tolerance in plants. J. Plant Nutr. Fertil. 2019, 25, 498–509. [Google Scholar] [CrossRef]
- Guo, L.L.; Guo, D.L.; Zhao, W.; Hou, X.G. Newly developed SSR markers reveal genetic diversity and geographical clustering in Paeonia suffruticosa based on flower colour. J. Hortic. Sci. Biotechnol. 2018, 93, 416–424. [Google Scholar] [CrossRef]
- Guo, N.N.; Wang, T.R.; Liu, B.D.; Li, Q. Progress in extraction and application of tree peony seed oil. Grain Oil Food Technol. 2019, 27, 20–23. [Google Scholar]
- Xie, X.Q.; Lin, Q.J.; Wang, M.S.; Chen, Z.D.; Su, J.Q. NaCl physiological response of stress to six foreign species of wild taxonaceae. Fujian Sci. Technol. Trop. Crop. 2020, 45, 26–28. [Google Scholar]
- Lu, L.; Wang, E.Q.; Ji, H.L.; Pang, J.J. Strategic necessity analysis and countermeasures of oil-use peony industrial development in China. Acta Agric. Jiangxi 2017, 29, 147–150. [Google Scholar]
- Gou, T.Y.; Su, Y.; Chen, X.H.; Zhu, Y.X.; Gong, H.J. Silicon up regulates NHX1 expression to enhance Na+ partitioning into vacuoles in leaf mesophyll cells of cucumber under salt stress. J. Plant Nutr. Fertil. 2020, 26, 1923–1934. [Google Scholar]
- Ma, J.F.; Yamaji, N. Functions and transport of silicon in plants. Cell. Mol. Life Sci. 2008, 65, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wang, S.; Tanaka, K.; Fujihara, S.; Itai, A.; Den, X.; Zhang, S. Silicon-mediated changes in polyamines participate in silicon-induced salt tolerance in Sorghum bicolor L. Plant Cell Environ. 2016, 39, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guo, J.; Hu, Y.; Gong, H. Distinct physiological responses of tomato and cucumber plants in silicon-mediated alleviation of cadmium stress. Front. Plant Sci. 2015, 6, 453. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhu, J.; Li, Z.; Chu, G.; Ding, Y.; Zhang, J.; Sun, W. Role of silicon in enhancing resistance to freezing stress in two contrasting winter wheat cultivars. Environ. Exp. Bot. 2008, 64, 286–294. [Google Scholar] [CrossRef]
- Yin, L.N.; Wang, S.W.; Liu, P.; Wang, W.H.; Cao, D.; Deng, X.P.; Zhang, S.Q. Silicon-mediated changes in polyamine and 1-aminocyclopropane-l-carboxylic acid are involved in Si-induced drought resistance in Sorghum bicolor L. Plant Physiol. Biochem. 2014, 80, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, M.; Rizwan, M.S.; Mushtag, M.A. Silicon occurrence, uptake, transport and mechanisms of heavy metals, minerals and salinity enhanced tolerance in plants with future prospects: A review. J. Environ. Manag. 2016, 183, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ashraf, U.; Chang, C.; Abrar, M.; Cheng, X. Effects of silicon and phosphatic fertilization on rice yield and soil fertility. Soil Sci. Plant Nutr. 2020, 20, 557–565. [Google Scholar] [CrossRef]
- Li, E.B.; Fan, W.H.; Liu, F.W.; Wang, G.L.; Yu, M.M. Effects of silicon on growth and photosynthesis of cucumber seedling under cadmium stress. North. Hortic. 2021, 8, 8–16. [Google Scholar]
- Alzahrani, Y.; Kuşvuran, A.; Alharby, H.F.; Sebnem, K.; Rady, M.M. The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol. Environ. Saf. 2018, 154, 187–196. [Google Scholar] [CrossRef]
- Rahmati, M.; Vercambre, G.; Davarynejad, G.; Bannayan, M.; Azizi, M.; Génard, M. Water scarcity conditions affect peach fruitsize and polyphenol contents more severely than other fruit quality traits. J. Sci. Food Agric. 2015, 95, 1055–1065. [Google Scholar] [CrossRef]
- Liang, Y.C. Effects of silicon on enzyme activity and sodium, potassium and calcium concentration in barley under salt stress. Plant Soil 1999, 209, 217. [Google Scholar] [CrossRef]
- Rohanipoor, A.; Norouzi, M.; Moezzi, A.; Hassibi, P. Effect of silicon on some physiological properties of maize (Zea mays) under salt stress. J. Biodivers. Environ. Sci. 2013, 7, 71–79. [Google Scholar]
- Abdel-Latif, A.; El-Demerdash, F.M. The ameliorative effects of silicon on salt-sressed sorghum seedlings and its influence on the activities of sucrose synthase and PEP carboxylase. J. Plant Physiol. Pathol. 2017, 5, 2–8. [Google Scholar] [CrossRef]
- Shi, X.; Yang, Y.; Xue, X.; Liu, W.; Song, C.; Guo, L.; Hou, X. Effects of chitooligosaccharide on the growth physiology of Paeonia ostii‘Fengdan’seedlings under drought stress. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2021, 45, 120–126. [Google Scholar]
- Zou, Q. Experimental Guidance of Plant Physiology; China Agriculture Press: Beijing, China, 2003. [Google Scholar]
- Hu, X.; Xu, Z.; Xu, W.; Li, J.; Zhao, N.; Zhou, Y. Application of γ-aminobutyric acid demonstrates a protective role of polyamine and GABA metabolism in muskmelon seedlings under Ca (NO3)2 stress. Plant Physiol. Biochem. 2015, 92, 1–10. [Google Scholar] [CrossRef]
- Kochian, L.V.; Piñeros, M.A.; Hoekenga, O.A. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 2005, 274, 175–195. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, Y.; Wu, Y.; Wassie, M.; Li, K.; Wang, Z.; Li, H.; Liu, D. Amelioration of salt-induced damage on alfalfa by exogenous application of silicon. Grassl. Sci. 2021, 2, 44–46. [Google Scholar] [CrossRef]
- Schaller, J.; Andreas, C.; Andrea, C.; Mohsen, Z. Biogenic amorphous silica as main driver for plant available water in soils. Sci. Rep. 2020, 10, 2424. [Google Scholar] [CrossRef] [PubMed]
- Schaller, J.; Daniel, P.; Danuta, K.; Ruth, E.; Michael, S. Silicon Cycling in Soils Revisited. Plants 2021, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Zarebanadkouki, M.; Bahareh, H.; Horst, H.G.; Schaller, J. Amorphous silica amendment to improve sandy soils’ hydraulic properties for sustained plant root access under drying conditions. Front. Environ. Sci. 2022, 10. [Google Scholar] [CrossRef]
- Kuhla, J.; Johanna, P.; Schaller, J. Effect on soil water availability, rather than silicon uptake by plants, explains the beneficial effect of silicon on rice during drought. Plant Cell Environ. 2021, 44, 3336–3346. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Z.; Yu, W.; Li, J.; Zhou, Y.; Long, M. Effect of silicon on growing photosynthetic characteristics and protective enzyme activity of watermelon seedlings. Anhui Agric. Sci. 2022, 50, 151–153+157. [Google Scholar]
- Gao, W.; Xi, K.; Yin, J.; Liu, Y.; Zhu, Y.; Jia, Q. Effects of exogenous SiNPs on growth and physiological characteristics of ginger seedlings under salts tress. J. Northwest A F Univ. (Nat. Sci. Ed.) 2023, 9, 1–10. [Google Scholar]
- Stimmler, P.; Anders, P.; Elberling, B.; Goeckede, M.; Schaller, J. Arctic soil respiration and microbial community structure driven by silicon and calcium. Sci. Total Environ. 2022, 838, 156152. [Google Scholar] [CrossRef]
- Sun, Z.; Fan, W.J.; Liu, G.L.; Tian, C.G.; Zhang, P.; Liu, H.J.; Yang, J.; Zhao, F.L.; Shi, C.Y. Effects of exogenous ABA on leaf photosynthetic characteristics and associated physiological indexes of sweet potato (Ipomoea batatas) seedlings under drought stress. J. Plant Physiol. (Plant Physiol. Commun.) 2017, 53, 873–880. [Google Scholar]
- Yu, M.; Fan, W.; Liu, F.; Tian, L.; Wang, G.; Meng, Q. Effects of Silicon and Selenium on Photosynthesis and Antioxidant Enzyme System of Cucumber Seedlings under Cadmium Stress. J. Henan Agric. Sci. 2023, 52, 116–124. [Google Scholar]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Pinheiro, H.A.; Silva, J.V.; Endres, L.; Ferreira, V.M.; Câmara, C.D.A.; Cabral, F.F.; Oliveira, J.F.; Carvalho, L.W.T.D.; Santos, J.M.D.; Filho, B.G.D.S. Leaf gas exchange, chloroplastic pigments and dry matter accumulation in castor bean (Ricinus communis L) seedlings subjected to salt stress conditions. Ind. Crop. Prod. 2008, 27, 385–392. [Google Scholar] [CrossRef]
- Tuna, A.L.; Kaya, C.; Dikilitas, M.; Higgs, D. The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ. Exp. Bot. 2008, 62, 1–9. [Google Scholar] [CrossRef]
- Gou, T.; Yang, L.; Hu, W.; Chen, X.; Gong, H. Silicon improves the growth of cucumber under excess nitrate stress by enhancing nitrogen assimilation and chlorophyll synthesis. Plant Physiol. Biochem. 2020, 152, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q. Study on the effects of silicon on rice growth and development. Agric. Technol. 2022, 42, 46–49. [Google Scholar]
- Zhang, Y.; Fang, H.; Chen, C.; Nie, S.; Sai, L.; Xu, Q.; Chen, X.; Lei, J. Effect of Exogenous Silicon Drip Application on Growth and Physiological Characteristics of Winter Wheat under Low Light Stress. Xinjiang Agric. Sci. 2023, 60, 336–343. [Google Scholar]
- Zhai, M.; Li, Y.; Yang, Q. Comparison of photosynthetic characteristics between potted and field Paeonia suffruticosa Andr. J. Hortic. 2008, 2, 251–256. [Google Scholar]
- Mora, M.L.; Alfaro, M.A.; Jarvis, S.C.; Demanet, R.; Cartes, P. Soil aluminium availability in andisols of southern chile and its effect on forage production and animal metabolism. Soil Use Manag. 2006, 22, 95–101. [Google Scholar] [CrossRef]
- Jia, S.; Zheng, Y.; Qiu, S.; Zhang, W. Effects of aluminum stress on growth and physiological characteristics of watermelon seedlings. J. Agric. Environ. Sci. 2014, 33, 1485–1492. [Google Scholar]
- Mukhopadyay, M.; Bantawa, P.; Das, A.; Sarkar, B.; Bera, B.; Ghosh, P.; Mondal, T.K. Changes of growth, photosynthesis and alteration of leaf antioxidative defence system of tea [Camellia sinensis (L.) O. Kuntze] seedlings under aluminum stress. Biometals 2012, 25, 1141–1154. [Google Scholar] [CrossRef]
- Li, W.; Qian, Y.; Han, L.; Liu, J.; Sun, Z. Response of the enzymatic reactive oxygen species enging system to differential photoperiod. Northwest J. Bot. 2015, 35, 1428–1436. [Google Scholar]
- Shabir, H.W.; Vinay, K.; Varsha, S.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar]
- Alcázar, R.; Marco, F.; Cuevas, J.C. Involvement of polyamines in plant response to abiotic stress. Biotechnol. Lett. 2006, 28, 1867–1876. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, W.; Wu, H.; Gong, X.; Takaya, M. Polyamines function in stress tolerance: From synthesis to regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Yu, J.; Zhang, G.; Jin, N.; Tang, Z.; Zhang, J.; Zhang, X. Effect of exogenous silicon root application on growth physiology of cucumber cultivated in continuous cropping substrate. J. Gansu Agric. Univ. 2020, 55, 121–128+135. [Google Scholar]
- Hao, L.; Yu, L.; Guo, W.; Xue, Y.; Guo, Y. Effects of silicon fertilizer on the growth, development and yield of spring wheat. J. Heilongjiang Bayi Agric. Reclam. Univ. 2013, 25, 16. [Google Scholar]
- Zhang, J.; Zhang, Y.; Wang, H.; Yang, K.; Liu, T.; Sun, Y.; Xu, R.; Du, J.; Peng, C.; Gao, S. Effects of spraying different concentrations of silicon fertilizer on photosynthetic characteristics and nutrient accumulation of maize in cold fields. Mol. Plant Breed. 2022, 20, 7876–7884. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).