Effects of Source Strength and Sink Size on Starch Metabolism, Starch Properties and Grain Quality of Rice (Oryza sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Measurements of Grain Quality and Starch Physicochemical Properties
2.3. Total RNA Extraction and qRT-PCR Analysis
2.4. Statistical Analysis
3. Results
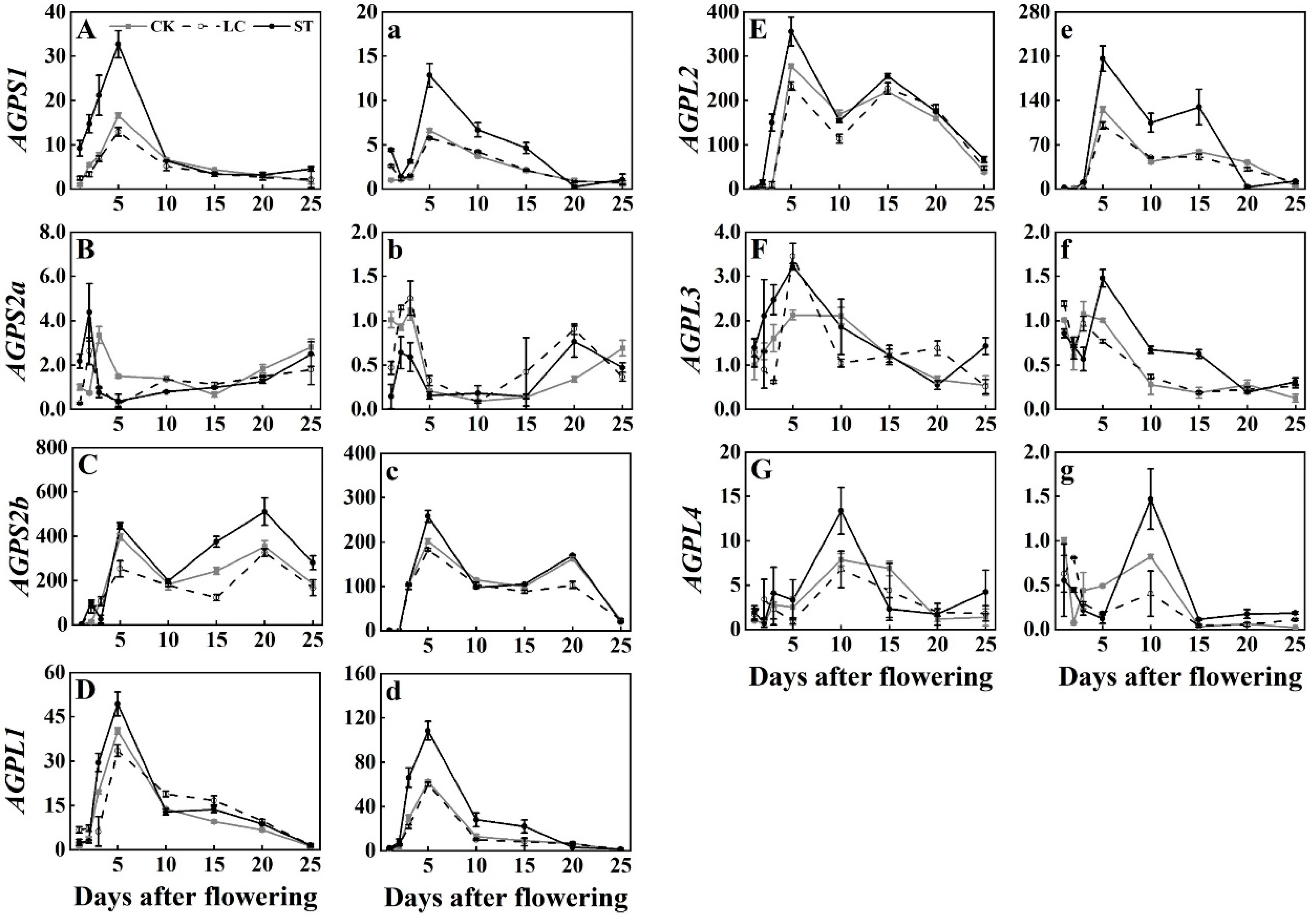
3.1. Expression Profiles of AGPase Genes
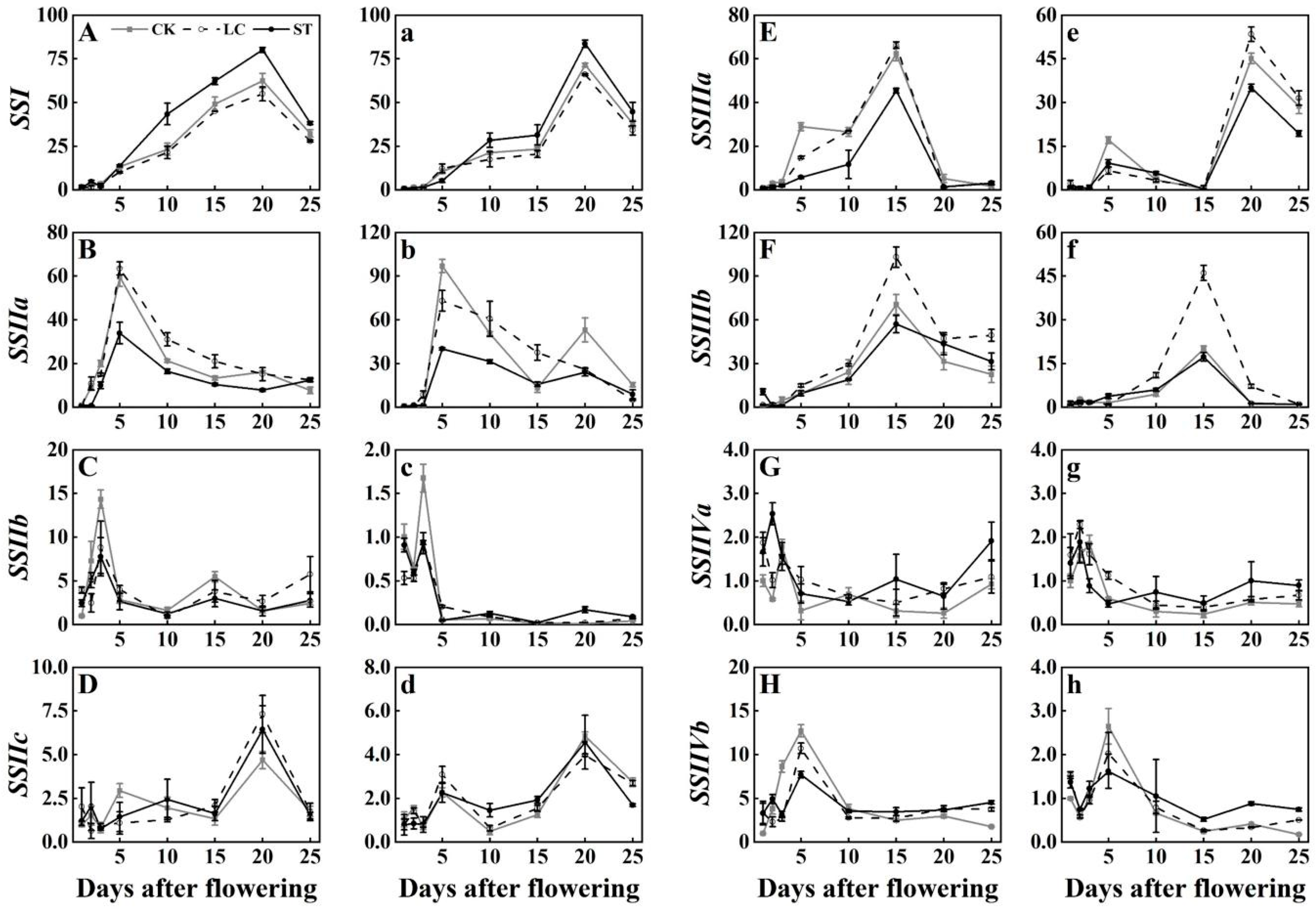
3.2. Expression Profiles of SS Genes
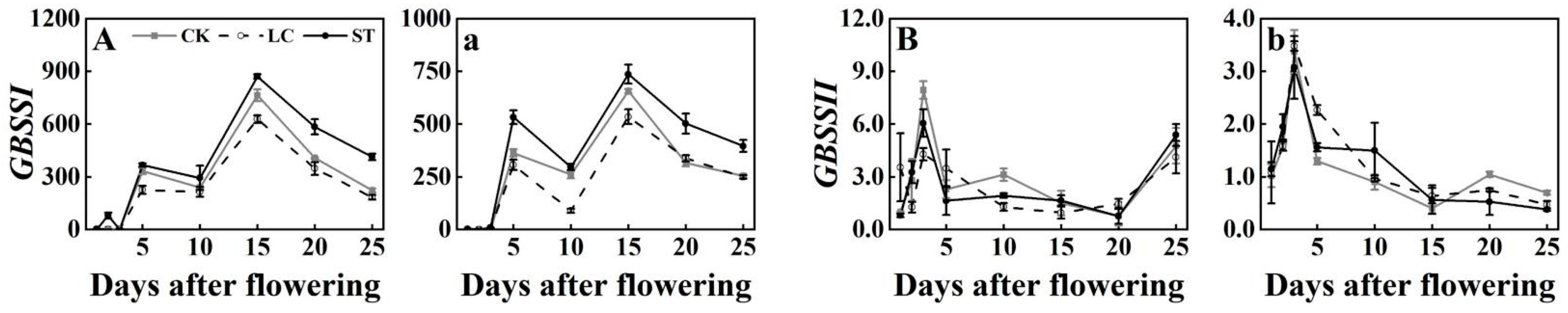
3.3. Expression Profiles of GBSS Genes
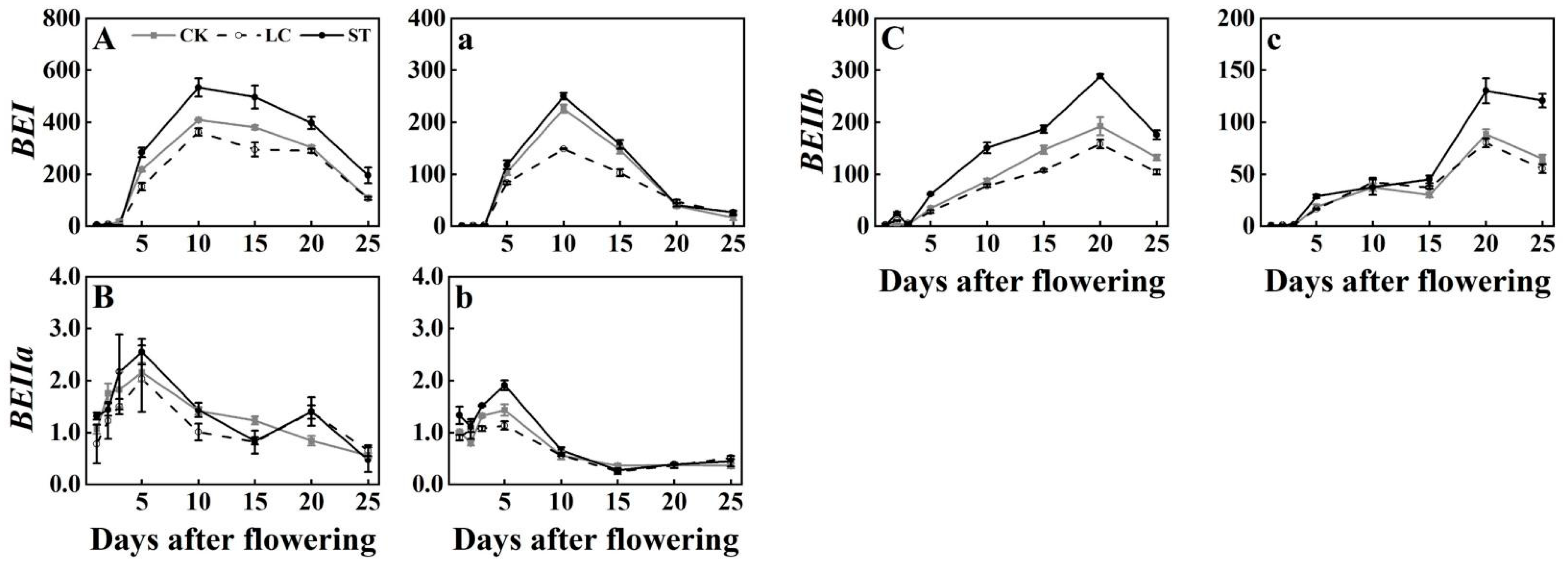
3.4. Expression Profiles of SBE Genes
3.5. Expression Profiles of DBE genes
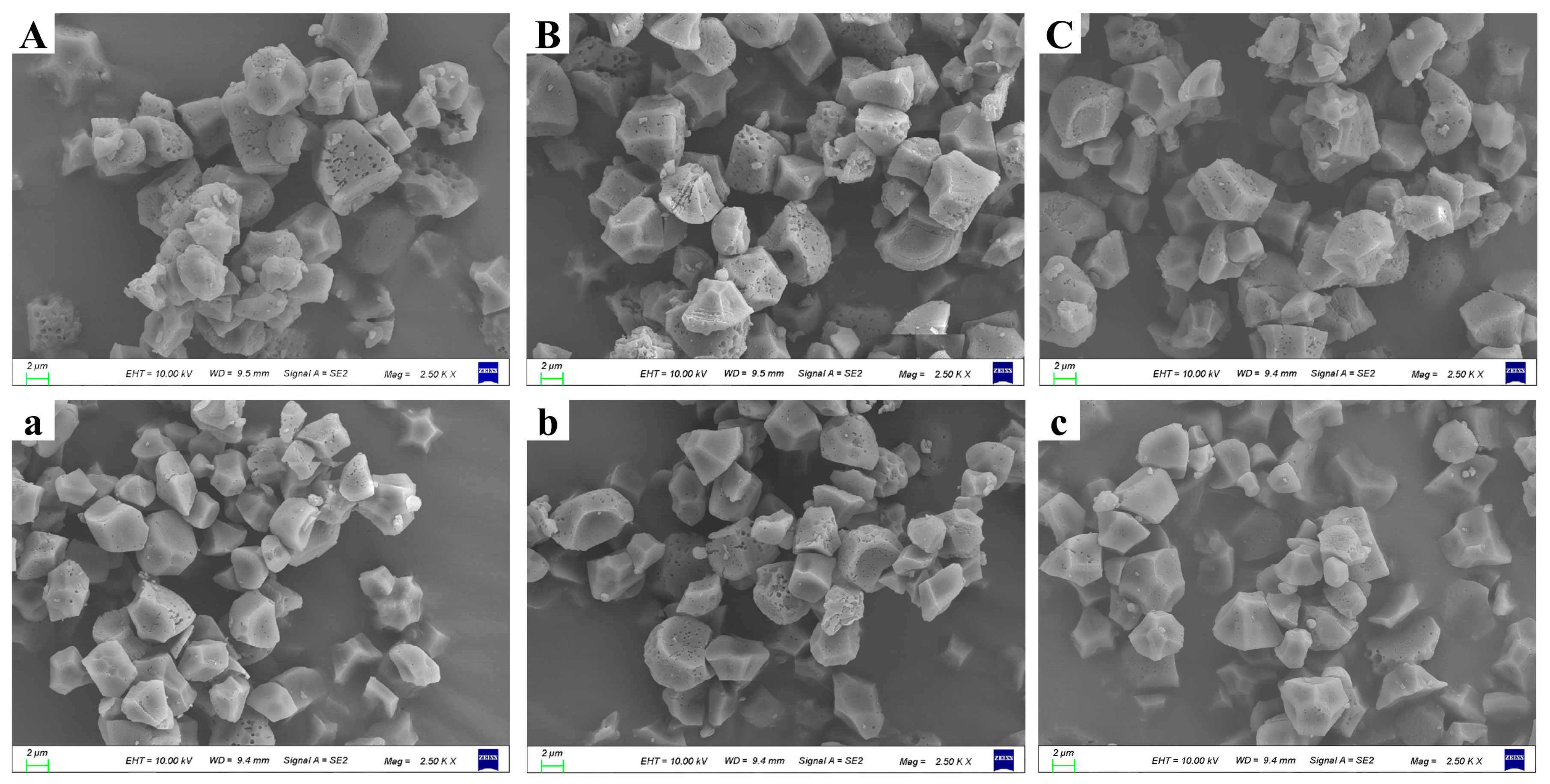
3.6. The Observation of the Morphology of Starch Granules
3.7. The Chain Length Distribution (CLD) of Amylopectin
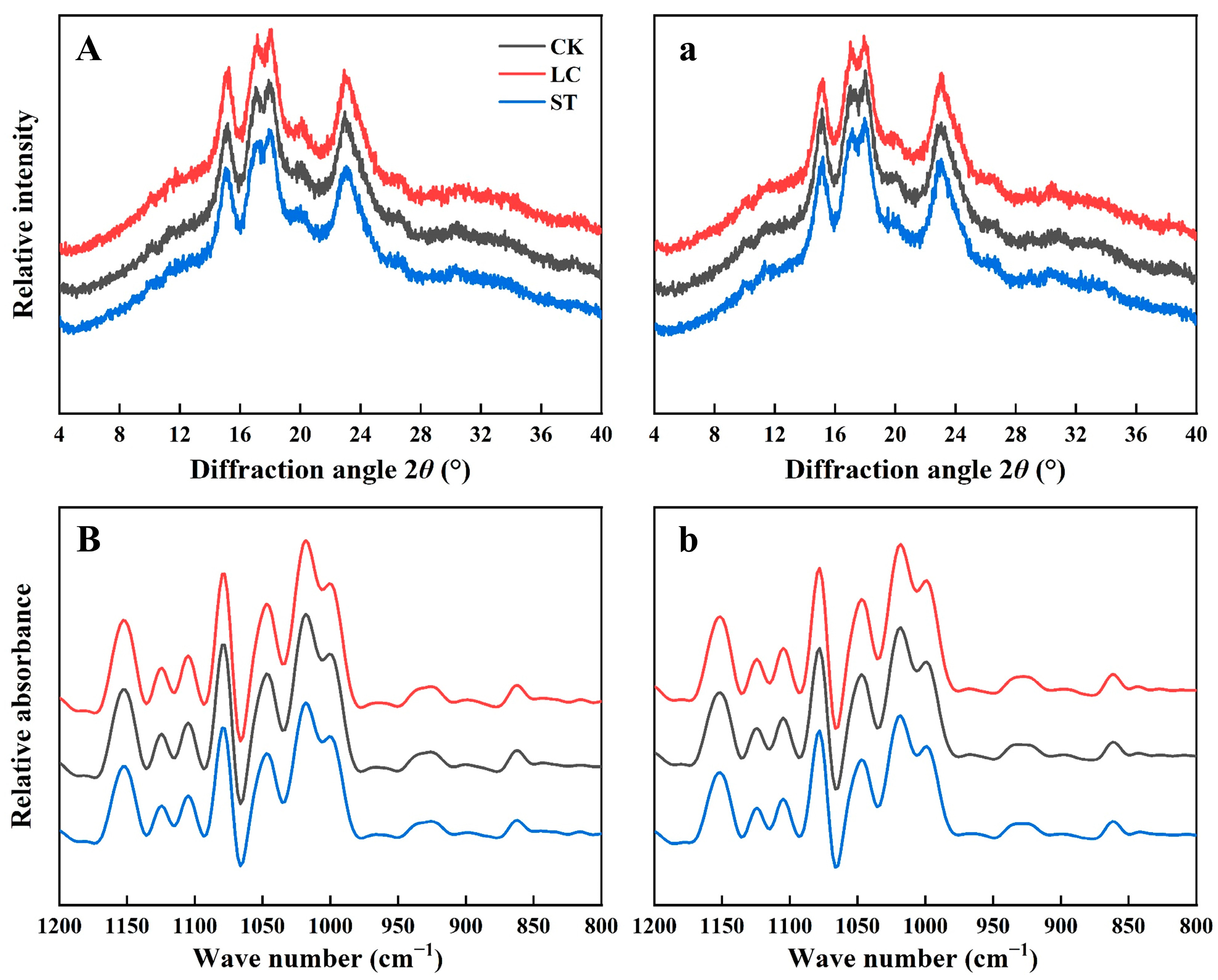
3.8. The XRD Patterns, Relative Crystallinity of Rice Starch and Ordered Structure of Starch External Region
3.9. Starch Pasting Properties
3.10. Processing and Appearance Quality of Rice
3.11. Cooking and Tasting Qualities of Rice
4. Discussion
4.1. Effects of the Transcript Levels of Key Starch Metabolic Genes on Chain Length Distribution (CLD) of Amylopectin
4.2. Effects of Source and Sink Strengths on Grain Quality and Starch Physiochemical Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Motohide, S.; Gabriel, F.F.; Song, X.J.; Motoyuki, A.; Haruka, N.; Keiki, I.; Tomoyuki, Y.; Mayuko, I.; Hidemi, K.; Akiko, S. A mathematical model of phloem sucrose transport as a new tool for designing rice panicle structure for high grain yield. Plant Cell Physiol. 2015, 56, 605–619. [Google Scholar]
- Schnyder, H. The role of carbohydrate storage and redistribution in the source-sink relations of wheat and barley during grain filling-a review. New Phytol. 1993, 123, 233–245. [Google Scholar] [CrossRef]
- Kong, L.G.; Wang, F.H.; Feng, B.; Li, S.D.; Si, J.S.; Zhang, B. The structural and photosynthetic characteristics of the exposed peduncle of wheat (Triticum aestivum L.): An important photosynthate source for grain-filling. BMC Plant Biol. 2010, 10, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.K.; Mondal, S.K. Regulation by kinetin and abcisic acid of correlative senescence in relation to grain maturation, source-sink relationship and yield of rice (Oryza sativa L.). Plant Growth Regul. 1986, 4, 239–245. [Google Scholar] [CrossRef]
- Cui, K.H.; Peng, S.B.; Xing, Y.Z.; Yu, S.B.; Xu, C.G.; Zhang, Q. Molecular dissection of the genetic relationships of source, sink and transport tissue with yield traits in rice. Theor. Appl. Genet. 2003, 106, 649–658. [Google Scholar] [CrossRef]
- Nagato, K.; Chaudhry, F.M. Influence of panicle clipping, flag leaf cutting and shading on ripening of japonica and indica rice. Jpn. J. Crop Sci. 1970, 39, 204–212. [Google Scholar] [CrossRef]
- Tang, T.; Xie, H.; Wang, Y.X.; Lü, B.; Liang, J.S. The effect of sucrose and abscisic acid interaction on sucrose synthase and its relationship to grain filling of rice (Oryza sativa L.). J. Exp. Bot. 2009, 60, 2641–2652. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.J.; Muthurajan, R.; Rahman, H.; Selvam, J.; Peng, S.B.; Zou, Y.B.; Jagadish, K.S.V. Source-sink dynamics and proteomic reprogramming under elevated night temperature and their impact on rice yield and grain quality. New Phytol. 2013, 197, 825–837. [Google Scholar] [CrossRef] [PubMed]
- He, A.B.; Wang, W.Q.; Jiang, G.L.; Sun, H.J.; Jiang, M.; Man, J.G.; Cui, K.H.; Huang, J.L.; Peng, S.B.; Nie, L.X. Source-sink regulation and its effects on the regeneration ability of ratoon rice. Field Crop Res. 2019, 236, 155–164. [Google Scholar] [CrossRef]
- Gao, B.; Hu, S.W.; Jing, L.Q.; Niu, X.C.; Wang, Y.X.; Zhu, J.G.; Wang, Y.L.; Yang, L.X. Alterations in source-sink relations affect rice yield response to elevated CO2: A free-air CO2 enrichment study. Front. Plant Sci. 2021, 12, 700159. [Google Scholar] [CrossRef]
- Liu, K.; Yang, R.; Lu, J.; Wang, X.Y.; Lu, B.L.; Tian, X.H.; Zhang, Y.B. Radiation use efficiency and source-sink changes of super hybrid rice under shade stress during grain-filling stage. Agron J. 2019, 111, 1788–1798. [Google Scholar] [CrossRef]
- Liu, Q.H.; Wu, X.; Chen, B.C.; Ma, J.Q.; Gao, J. Effects of low light on agronomic and physiological characteristics of rice including grain yield and quality. Rice Sci. 2014, 21, 243–251. [Google Scholar] [CrossRef]
- Tao, L.X.; Wang, X.; Liao, X.Y.; Shen, B.; Tan, H.J.; Huang, S.W. Physiological effects of air temperature and sink-source volume at milk-filling stage of rice on its grain quality. Chin. J. Appl. Ecol. 2006, 17, 647–652, (In Chinese with English Abstract). [Google Scholar]
- Yuan, J.C.; Ding, Z.Y.; Zhao, C.; Zhu, Q.S.; Li, J.Q.; Yang, J.C. Effects of sunshine-shading, leaf-cutting and spikelet-removing on yield and quality of rice in the high altitude region. Acta Agron. Sin. 2005, 31, 1429–1436, (In Chinese with English Abstract). [Google Scholar]
- Miao, X.J.; Wang, S.H.; Li, G.H.; Ding, Y.F. Effects of spikelet-removing on non-structural carbohydrate translocation in filling stage and grain quality of hybrid rice. Hybrid Rice 2008, 23, 55–59, (In Chinese with English Abstract). [Google Scholar]
- Ramesh, M.; Ali, S.Z.; Bhattacharya, K.R. Structure of rice starch and its relation to cooked-rice texture. Carbohyd. Polym. 1999, 38, 337–347. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Zhou, L.H.; Lu, Y.; Yang, Y.; Feng, L.H.; Hao, W.Z.; Li, Q.F.; Fan, X.L.; Zhao, D.S.; Liu, Q.Q. Changes in the physicochemical properties and starch structures of rice grains upon pre-harvest sprouting. Carbohyd. Polym. 2020, 234, 115893. [Google Scholar] [CrossRef]
- Yao, D.P.; Wu, J.; Luo, Q.H.; Zhang, D.M.; Zhang, W.; Xiao, G.; Deng, Q.Y.; Bai, B. Effects of salinity stress at reproductive growth stage on rice (Oryza sativa L.) composition, starch structure, and physicochemical properties. Front. Nutr. 2022, 9, 926217. [Google Scholar] [CrossRef]
- Smith, A.M. Prospects for increasing starch and sucrose yields for bioethanol production. Plant J. Cell Mol. Biol. 2008, 54, 546–558. [Google Scholar] [CrossRef]
- Tetlow, I.; Emes, M. Starch biosynthesis in the developing endosperms of grasses and cereals. Agronomy 2017, 7, 81. [Google Scholar] [CrossRef]
- Emes, M.J.; Bowsher, C.G.; Hedley, C.; Burrell, M.M., II; Scrase-Field, E.S.F.; Tetlow, I.J. Starch synthesis and carbon partitioning in developing endosperm. J. Exp. Bot. 2003, 54, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Liu, H.Y.; Jin, Z.X.; Liu, H.L.; Huang, X.; Xu, M.L.; Zhang, F.Z. Changes in activities of key enzymes for starch synthesis and glutamine synthetase in grains of progenies from a rice cross during grain filling. Rice Sci. 2010, 17, 243–246. [Google Scholar] [CrossRef]
- Tuncel, A.; Okita, T.W. Improving starch yield in cereals by over-expression of ADPglucose pyrophosphorylase: Expectations and unanticipated outcomes. Plant Sci. 2013, 211, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Green, T.W.; Hannah, L.C. Adenosine diphosphate glucose pyrophosphorylase, a rate-limiting step in starch biosynthesis. Physiol. Plantarum. 1998, 103, 574–580. [Google Scholar] [CrossRef]
- Lee, S.K.; Hwang, S.K.; Han, M.H.; Eom, J.S.; Kang, H.G.; Han, Y.; Choi, S.B.; Cho, M.H.; Bhoo, S.H.; An, G.; et al. Identification of the ADP-glucose pyrophosphorylase isoforms essential for starch synthesis in the leaf and seed endosperm of rice (Oryza sativa L.). Plant Mol. Biol. 2007, 65, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.M.; Larsen, R.; Laudencia, D.; Kim, W.T.; Morrow, D.; Okita, T.W.; Preiss, J. Molecular characterization of the gene encoding a rice endosperm-specific ADPglucose pyrophosphorylase subunit and its developmental pattern of transcription. Gene 1991, 97, 199–205. [Google Scholar] [CrossRef]
- Fujita, N.; Yoshida, M.; Asakura, N.; Ohdan, T.; Miyao, A.; Hirochika, H.; Nakamura, Y. Function and characterization of starch synthase I using mutants in rice. Plant Physiol. 2006, 140, 1070–1084. [Google Scholar] [CrossRef]
- Nakamura, Y.; Francisco, P.B.; Hosaka, Y.; Sato, A.; Sawada, T.; Kubo, A.; Fujita, N. Essential amino acids of starch synthase IIa differentiate amylopectin structure and starch quality between japonica and indica rice varieties. Plant Mol. Biol. 2005, 58, 213–227. [Google Scholar] [CrossRef]
- Fujita, N.; Yoshida, M.; Kondo, T.; Saito, K.; Utsumi, Y.; Tokunaga, T.; Nishi, A.; Satoh, H.; Park, J.H.; Jane, J.L.; et al. Characterization of SSIIIa-deficient mutants of rice: The function of SSIIIa and pleiotropic effects by SSIIIa deficiency in the rice endosperm. Plant Physiol. 2007, 144, 2009–2023. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, Y.D.; Lu, K.; Wang, C.L. Progress on the functions, allelic variations and interactions of soluble starch synthases SSIIa and SSIIIa genes in rice. Chin. J. Rice Sci. 2022, 36, 227–236, (In Chinese with English Abstract). [Google Scholar]
- Nakamura, T.; Vrinten, P.; Hayakawa, K.; Ikeda, J. Characterization of a granule-bound starch synthase isoform found in the pericarp of wheat. Plant Physiol. 1998, 118, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hasjim, J.; Wu, A.C.; Li, E.P.; Henry, R.J.; Gilbert, R.G. Roles of GBSSI and SSIIa in determining amylose fine structure. Carbohyd. Polym. 2015, 127, 264–274. [Google Scholar] [CrossRef]
- Smith, A.M.; Denyer, K.; Martin, C. The synthesis of the starch granule. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 67–87. [Google Scholar] [CrossRef]
- Tanaka, N.; Fujita, N.; Nishi, A.; Satoh, H.; Hosaka, Y.; Ugaki, M.; Kawasaki, S.; Nakamura, Y. The structure of starch can be manipulated by changing the expression levels of starch branching enzyme IIb in rice endosperm. Plant Biotechnol. J. 2004, 2, 507–516. [Google Scholar] [CrossRef]
- Boyer, C.; Preiss, J. Biosynthesis of bacterial glycogen. Purification and properties of the Escherichia coli B α-1,4-glucan: α-1,4-glucan 6-glycosyltransferase. Biochemistry 1977, 16, 3693–3699. [Google Scholar] [CrossRef]
- Mizuno, K.; Kawasaki, T.; Shimada, H.; Satoh, H.; Kobayashi, E.; Okumura, S.; Arai, Y.; Baba, T. Alteration of the structural properties of starch components by the lack of an isoform of starch branching enzyme in rice seeds. J. Biol. Chem. 1993, 268, 19084–19091. [Google Scholar] [CrossRef]
- Jeon, J.S.; Ryoo, N.; Hahn, T.R.; Walia, H.; Nakamura, Y. Starch biosynthesis in cereal endosperm. Plant Physiol. Biochem. 2010, 48, 383–392. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhang, J.H. Grain-filling problem in ‘super’ rice. J. Exp. Bot. 2010, 61, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.Y.; Zhou, Q.; Li, E.P.; Yuan, L.M.; Wang, W.L.; Zhang, H.; Liu, L.J.; Wang, Z.Q.; Yang, J.C.; Gu, J.F. Effects of nitrogen fertilizer on structure and physicochemical properties of ‘super’ rice starch. Carbohyd. Polym. 2020, 239, 116237. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Zhao, C.; Liu, G.M.; Shi, Y.; Zhao, L.T.; Wang, Y.; Wang, W.L.; Xu, K.; Li, G.H.; et al. The starch physicochemical properties between superior and inferior grains of japonica rice under panicle nitrogen fertilizer determine the difference in eating quality. Foods 2022, 11, 2489. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.K.; Shen, Y.; Zhang, W.Y.; Zhang, H.; Liu, L.J.; Wang, Z.Q.; Gu, J.F.; Yang, J.C. Effects of long-term straw returning on rice yield and soil properties and bacterial community in a rice-wheat rotation system. Field Crops Res. 2023, 291, 108800. [Google Scholar] [CrossRef]
- Tran, T.T.B.; Shelat, K.J.; Tang, D.; Li, E.P.; Gilbert, R.G.; Hasjim, J. Milling of rice grains. The degradation on three structural levels of starch in rice flour can be independently controlled during grinding. J. Agric. Food Chem. 2011, 59, 3964–3973. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.L.; Lu, W.P. Effects of protein removal on the physicochemical properties of waxy maize flours. Starch-Stärke 2012, 64, 874–881. [Google Scholar] [CrossRef]
- Ohdan, T.; Francisco, P.B.; Sawada, T.; Hirose, T.; Terao, T.; Satoh, H.; Nakamura, Y. Expression profiling of genes involved in starch synthesis in sink and source organs of rice. J. Exp. Bot. 2005, 56, 3229–3244. [Google Scholar] [CrossRef]
- Bowsher, C.G.; Scrase-Field, E.F.A.L.; Esposito, S.; Emes, M.J.; Tetlow, I.J. Characterization of ADP-glucose transport across the cereal endosperm amyloplast envelope. J. Exp. Bot. 2007, 58, 1321–1332. [Google Scholar] [CrossRef]
- Hanashiro, I.; Abe, J.I.; Hizukuri, S. A periodic distribution of the chain length of amylopectin as revealed by high-performance anion-exchange chromatography. Carbohyd. Res. 1996, 283, 151–159. [Google Scholar] [CrossRef]
- Cheetham, N.W.H.; Tao, L.P. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohyd. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- Sevenou, O.; Hill, S.E.; Farhat, I.A.; Mitchell, J.R. Organisation of the external region of the starch granule as determined by infrared spectroscopy. Int. J. Biol. Macromol. 2002, 31, 79–85. [Google Scholar] [CrossRef]
- Jenkins, P.J.; Cameron, R.E.; Donald, A.M. A universal feature in the structure of starch granules from different botanical sources. Starch-Stärke 1993, 45, 417–420. [Google Scholar] [CrossRef]
- Cao, H.P.; James, M.G.; Myers, A.M. Purification and characterization of soluble starch synthases from maize endosperm. Arch. Biochem. Biophys. 2000, 373, 135–146. [Google Scholar] [CrossRef]
- Miura, S.; Crofts, N.; Saito, Y.; Hosaka, Y.; Oitome, N.F.; Watanabe, T.; Kumamaru, T.; Fujita, N. Starch synthase IIa-deficient mutant rice line produces endosperm starch with lower gelatinization temperature than japonica rice cultivars. Front. Plant Sci. 2018, 9, 645. [Google Scholar] [CrossRef]
- Ryoo, N.; Yu, C.; Park, C.S.; Baik, M.Y.; Park, I.M.; Cho, M.H.; Bhoo, S.H.; An, G.; Hahn, T.R.; Jeon, J.S. Knockout of a starch synthase gene OsSSIIIa/Flo5 causes white-core floury endosperm in rice (Oryza sativa L.). Plant Cell Rep. 2007, 26, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Crofts, N.; Abe, N.; Oitome, N.F.; Matsushima, R.; Hayashi, M.; Tetlow, I.J.; Emes, M.J.; Nakamura, Y.; Fujita, N. Amylopectin biosynthetic enzymes from developing rice seed form enzymatically active protein complexes. J. Exp. Bot. 2015, 66, 4469–4482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Chen, Z.J.; Zhang, X.; Guo, X.P.; Su, N.; Jiang, L.; Mao, L.; Wan, J.M. Double repression of soluble starch synthase genes SSIIa and SSIIIa in rice (Oryza sativa L.) uncovers interactive effects on the physicochemical properties of starch. Genome 2011, 54, 448–459. [Google Scholar] [CrossRef]
- Blauth, S.L.; Yao, Y.; Klucinec, J.D.; Shannon, J.C.; Thompson, D.B.; Guilitinan, M.J. Identification of mutator insertional mutants of starch-branching enzyme 2a in corn. Plant Physiol. 2001, 125, 1396–1405. [Google Scholar] [CrossRef]
- Satoh, H.; Nishi, A.; Yamashita, K.; Takemoto, Y.; Tanaka, Y.; Hosaka, Y.; Sakurai, A.; Fujita, N.; Nakamura, Y. Starch-branching enzyme I-deficient mutation specifically affects the structure and properties of starch in rice endosperm. Plant Physiol. 2003, 133, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Zhang, J.; Wang, J.M.; Xia, M.; Zhu, S.W.; Cheng, B.J. RNA interference-mediated silencing of the starch branching enzyme gene improves amylose content in rice. Genet. Mol. Res. 2013, 12, 2800–2808. [Google Scholar] [CrossRef]
- Cao, Z.Z.; Pan, G.; Wang, F.B.; Wei, K.S.; Li, Z.W.; Shi, C.H.; Geng, W.; Cheng, F.M. Effect of high temperature on the expressions of genes encoding starch synthesis enzymes in developing rice endosperms. J. Integr. Agric. 2015, 14, 642–659. [Google Scholar] [CrossRef]
- Raju, G.N.; Srinivas, T. Effect of physical, physiological, and chemical factors on the expression of chalkiness in rice. Cereal Chem. 1991, 68, 210–211. [Google Scholar]
- Chun, A.; Song, J.; Kim, K.J.; Lee, H.J. Quality of head and chalky rice and deterioration of eating quality by chalky rice. J. Crop Sci. Biotechnol. 2009, 12, 235–240. [Google Scholar] [CrossRef]
- Jing, L.Q.; Zhen, W.Y.; Zhuang, S.T.; Wang, Y.X.; Zhu, J.G.; Wang, Y.L.; Yang, L.X. Effects of CO2 enrichment and spikelet removal on rice quality under open-air field conditions. J. Integr. Agric. 2016, 15, 2012–2022. [Google Scholar] [CrossRef]
- Tian, Z.X.; Qian, Q.; Liu, Q.Q.; Yan, M.X.; Liu, X.F.; Yan, C.J.; Liu, G.F.; Gao, Z.Y.; Tang, S.Z.; Zeng, D.L.; et al. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities. Proc. Natl. Acad. Sci. USA 2009, 106, 21760–21765. [Google Scholar] [CrossRef] [PubMed]
- Jane, J.; Chen, Y.Y.; Lee, L.F.; Mcpherson, A.E.; Wong, K.S.; Radosavljevic, M.; Kasemsuwan, T. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem. 1999, 76, 629–637. [Google Scholar] [CrossRef]
- Witt, T.; Doutch, J.; Gilbert, E.P.; Gilbert, R.G. Relations between molecular, crystalline, and lamellar structures of amylopectin. Biomacromolecules 2012, 13, 4273–4282. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| OsAGPS1 | F: 5′-GTGCCACTTAAAGGCACCATT-3′ | R: 5′-CCCACATTTCAGACACGGTTT-3′ |
| OsAGPS2a | F: 5′-ACTCCAAGAGCTCGCAGACC-3′ | R: 5′-GCCTGTAGTTGGCACCCAGA-3′ |
| OsAGPS2b | F: 5′-AACAATCGAAGCGCGAGAAA-3′ | R: 5′-GCCTGTAGTTGGCACCCAGA-3′ |
| OsAGPL1 | F: 5′-GGAAGACGGATGATCGAGAAAG-3′ | R: 5′-CACATGAGATGCACCAACGA-3′ |
| OsAGPL2 | F: 5′-AGTTCGATTCAAGACGGATAGC-3′ | R: 5′-CGACTTCCACAGGCAGCTTATT-3′ |
| OsAGPL3 | F: 5′-AAGCCAGCCATGACCATTTG-3′ | R: 5′-CACACGGTAGATTCACGAGACAA-3′ |
| OsAGPL4 | F: 5′-TCAACGTCGATGCAGCAAAT-3′ | R: 5′-ATCCCTCAGTTCCTAGCCTCATT-3′ |
| OsSSI | F: 5′-GGGCCTTCATGGATCAACC-3′ | R: 5′-CCGCTTCAAGCATCCTCATC-3′ |
| OsSSIIa | F: 5′-GCTTCCGGTTTGTGTGTTCA-3′ | R: 5′-CTTAATACTCCCTCAACTCCACCAT-3′ |
| OsSSIIb | F: 5′-TAGGAGCAACGGTGGAAGTGA-3′ | R: 5′-GTGAACGTGAGTACGTGACCAAT-3′ |
| OsSSIIc | F: 5′-GACCGAAATGCCTTTTTCTCG-3′ | R: 5′-GGGCTTGGAGCCTCTCCTTA-3′ |
| OsSSIIIa | F: 5′-GCCTGCCCTGGACTACATTG-3′ | R: 5′-GCAAACATATGTACACGGTTCTGG-3′ |
| OsSSIIIb | F: 5′-ATTCCGCTCGCAAGAACTGA-3′ | R: 5′-CAACCGCAGGATAACGGAAA-3′ |
| OsSSIIVa | F: 5′-GGGAGCGGCTCAAACATAAA-3′ | R: 5′-CCGTGCACTGACTGCAAAAT-3′ |
| OsSSIIVb | F: 5′-ATGCAGGAAGCCGAGATGTT-3′ | R: 5′-ACGACAATGGGTGCCAAGAT-3′ |
| OsGBSSI | F: 5′-AACGTGGCTGCTCCTTGAA-3′ | R: 5′-TTGGCAATAAGCCACACACA-3′ |
| OsGBSSII | F: 5′-AGGCATCGAGGGTGAGGAG-3′ | R: 5′-CCATCTGGCCCACATCTCTA-3′ |
| OsBEI | F: 5′-TGGCCATGGAAGAGTTGGC-3′ | R: 5′-CAGAAGCAACTGCTCCACC-3′ |
| OsBEIIa | F: 5′-GCCAATGCCAGGAAGATGA-3′ | R: 5′-GCGCAACATAGGATGGGTTT-3′ |
| OsBEIIb | F: 5′-ATGCTAGAGTTTGACCGC-3′ | R: 5′-AGTGTGATGGATCCTGCC-3′ |
| OsISA1 | F: 5′-TGCTCAGCTACTCCTCCATCATC-3′ | R: 5′-AGGACCGCACAACTTCAACATA-3′ |
| OsISA2 | F: 5′-TAGAGGTCCTCTTGGAGG-3′ | R: 5′-AATCAGCTTCTGAGTCACCG-3′ |
| OsISA3 | F: 5′-ACAGCTTGAGACACTGGGTTGAG-3′ | R: 5′-GCATCAAGAGGACAACCATCTG-3′ |
| OsPUL | F: 5′-ACCTTTCTTCCATGCTGG-3′ | R: 5′-CAAAGGTCTGAAAGATGGG-3′ |
| Actin | F: 5′-CTGACAGGATGAGCAAGGAG-3′ | R: 5′-GGCAATCCACATCTGCTGGA-3′ |
| Variety | Treatment | Relative Crystallinity (%) | Infrared Ratio | |
|---|---|---|---|---|
| 1045/1022 cm−1 | 1022/995 cm−1 | |||
| YD 6 | CK | 29.01 ± 0.04 b | 0.715 ± 0.002 b | 1.361 ± 0.011 b |
| LC | 29.48 ± 0.05 a | 0.745 ± 0.015 a | 1.328 ± 0.005 c | |
| ST | 27.45 ± 0.07 c | 0.682 ± 0.006 c | 1.420 ± 0.007 a | |
| JXY 1 | CK | 28.82 ± 0.07 b | 0.696 ± 0.006 b | 1.405 ± 0.012 b |
| LC | 29.30 ± 0.06 a | 0.745 ± 0.021 a | 1.355 ± 0.027 c | |
| ST | 27.33 ± 0.02 c | 0.662 ± 0.011 c | 1.444 ± 0.011 a | |
| Variety | Treatment | Peak Viscosity (cP) | Hot Viscosity (cP) | Breakdown (cP) | Final Viscosity (cP) | Setback (cP) | Peaking Time (s) | Pasting Temperature (°C) |
|---|---|---|---|---|---|---|---|---|
| YD 6 | CK | 2715.33 ± 17.04 b | 2146.33 ± 7.09 b | 569.00 ± 14.73 b | 2957.00 ± 20.42 b | 241.67 ± 9.07 b | 7.00 ± 0.23 a | 76.25 ± 0.09 b |
| LC | 2559.00 ± 10.82 c | 2054.33 ± 13.20 c | 504.67 ± 13.05 c | 2856.67 ± 10.69 c | 297.67 ± 26.08 a | 6.97 ± 0.06 a | 77.28 ± 0.16 a | |
| ST | 2845.33 ± 28.01 a | 2214.67 ± 11.50 a | 630.67 ± 8.08 a | 3048.33 ± 26.58 a | 203.00 ± 19.08 b | 6.96 ± 0.04 a | 75.10 ± 0.15 c | |
| JXY 1 | CK | 2787.00 ± 13.00 b | 2189.00 ± 8.72 b | 598.00 ± 13.53 b | 3173.33 ± 11.37 b | 386.33 ± 14.57 b | 6.34 ± 0.22 a | 76.43 ± 0.29 b |
| LC | 2642.33 ± 16.86 c | 2137.00 ± 19.00 c | 505.33 ± 16.86 c | 3078.00 ± 24.27 c | 435.67 ± 7.51 a | 6.27 ± 0.14 a | 77.10 ± 0.11 a | |
| ST | 2903.67 ± 16.17 a | 2249.00 ± 27.07 a | 654.67 ± 19.09 a | 3261.00 ± 17.06 a | 357.33 ± 11.93 c | 6.24 ± 0.16 a | 75.30 ± 0.21 c |
| Variety | Treatment | Processing Quality | Appearance Quality | ||||
|---|---|---|---|---|---|---|---|
| Brown Rice Rate (%) | Milled Rice Rate (%) | Head Rice Rate (%) | Chalkiness Area (%) | Chalky Rice Rate (%) | Chalkiness (%) | ||
| YD 6 | CK | 81.19 ± 0.03 b | 74.12 ± 0.08 b | 69.74 ± 0.04 b | 25.55 ± 0.11 b | 26.43 ± 0.03 b | 7.38 ± 0.06 b |
| LC | 80.54 ± 0.04 c | 73.61 ± 0.04 c | 65.34 ± 0.03 c | 26.56 ± 0.04 a | 27.88 ± 0.10 a | 7.83 ± 0.10 a | |
| ST | 81.64 ± 0.03 a | 74.44 ± 0.08 a | 71.21 ± 0.02 a | 24.46 ± 0.05 c | 25.21 ± 0.09 c | 6.26 ± 0.05 c | |
| JXY 1 | CK | 83.69 ± 0.10 b | 75.80 ± 0.09 b | 71.41 ± 0.05 b | 23.77 ± 0.12 b | 24.92 ± 0.11 b | 6.93 ± 0.06 b |
| LC | 82.47 ± 0.03 c | 74.74 ± 0.04 c | 69.12 ± 0.12 c | 24.83 ± 0.06 a | 25.86 ± 0.06 a | 7.69 ± 0.09 a | |
| ST | 84.34 ± 0.08 a | 76.97 ± 0.04 a | 72.41 ± 0.09 a | 22.08 ± 0.02 c | 23.01 ± 0.12 c | 6.02 ± 0.02 c | |
| Year | Variety | Treatment | Protein Content (%) | Gelatinization Consistency (mm) | Amylose Content (%) | Hardness (g) | Stickiness (g) | Taste Value |
|---|---|---|---|---|---|---|---|---|
| 2019 | YD 6 | CK | 10.31 ± 0.09 a | 63.50 ± 0.45 b | 15.43 ± 0.15 b | 6.57 ± 0.06 b | 5.20 ± 0.10 a | 50.00 ± 0.87 a |
| LC | 10.22 ± 0.29 a | 64.88 ± 0.26 a | 14.98 ± 0.11 c | 7.00 ± 0.10 a | 4.63 ± 0.12 b | 42.00 ± 1.73 b | ||
| ST | 10.44 ± 0.23 a | 62.60 ± 0.19 c | 16.04 ± 0.23 a | 6.67 ± 0.06 b | 5.13 ± 0.15 a | 48.00 ± 1.00 a | ||
| JXY 1 | CK | 9.84 ± 0.28 a | 65.38 ± 0.48 a | 13.26 ± 0.14 b | 6.37 ± 0.06 b | 5.27 ± 0.15 a | 54.00 ± 0.50 a | |
| LC | 9.68 ± 0.14 a | 66.04 ± 0.21 a | 13.18 ± 0.13 b | 7.03 ± 0.06 a | 4.70 ± 0.10 b | 46.00 ± 0.50 b | ||
| ST | 9.97 ± 0.10 a | 62.85 ± 0.49 b | 13.73 ± 0.19 a | 6.40 ± 0.10 b | 5.07 ± 0.15 a | 54.00 ± 0.50 a | ||
| 2020 | YD 6 | CK | 10.34 ± 0.17 a | 63.52 ± 0.30 b | 15.58 ± 0.21 b | 6.60 ± 0.10 b | 5.17 ± 0.06 a | 49.50 ± 0.50 a |
| LC | 10.26 ± 0.20 a | 65.18 ± 0.42 a | 15.06 ± 0.20 c | 7.07 ± 0.06 a | 4.53 ± 0.06 b | 41.00 ± 1.00 b | ||
| ST | 10.43 ± 0.21 a | 62.57 ± 0.33 c | 16.17 ± 0.18 a | 6.70 ± 0.10 b | 5.10 ± 0.10 a | 47.83 ± 0.29 a | ||
| JXY 1 | CK | 9.74 ± 0.20 a | 65.54 ± 0.48 a | 13.43 ± 0.15 ab | 6.33 ± 0.06 b | 5.33 ± 0.06 a | 54.33 ± 0.29 a | |
| LC | 9.64 ± 0.20 a | 66.58 ± 0.44 a | 13.30 ± 0.19 b | 7.00 ± 0.10 a | 4.73 ± 0.12 b | 46.50 ± 0.50 b | ||
| ST | 9.80 ± 0.18 a | 63.27 ± 0.31 b | 13.86 ± 0.18 a | 6.33 ± 0.06 b | 5.17 ± 0.06 a | 54.17 ± 0.29 a | ||
| Analysis of variance | ||||||||
| Year (Y) | NS | NS | NS | NS | NS | NS | ||
| Variety (V) | 56.14 ** | 89.14 ** | 301.96 ** | 51.61 ** | 4.71 * | 268.34 ** | ||
| Treatment (T) | NS | 158.60 ** | 55.18 ** | 181.65 ** | 87.89 ** | 263.08 ** | ||
| Y × V | NS | NS | NS | NS | NS | NS | ||
| Y × T | NS | NS | NS | NS | NS | NS | ||
| V × T | NS | 10.65 ** | 5.87 ** | 11.56 ** | NS | 3.56 * | ||
| Y × V × T | NS | NS | NS | NS | NS | NS | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, C.; Jiang, J.; Liu, C.; Fang, X.; Zhou, T.; Xue, Z.; Wang, W.; Zhang, W.; Zhang, H.; Liu, L.; et al. Effects of Source Strength and Sink Size on Starch Metabolism, Starch Properties and Grain Quality of Rice (Oryza sativa L.). Agronomy 2023, 13, 1288. https://doi.org/10.3390/agronomy13051288
Wei C, Jiang J, Liu C, Fang X, Zhou T, Xue Z, Wang W, Zhang W, Zhang H, Liu L, et al. Effects of Source Strength and Sink Size on Starch Metabolism, Starch Properties and Grain Quality of Rice (Oryza sativa L.). Agronomy. 2023; 13(5):1288. https://doi.org/10.3390/agronomy13051288
Chicago/Turabian StyleWei, Chenhua, Jingjing Jiang, Chang Liu, Xinchi Fang, Tianyang Zhou, Zhangyi Xue, Weilu Wang, Weiyang Zhang, Hao Zhang, Lijun Liu, and et al. 2023. "Effects of Source Strength and Sink Size on Starch Metabolism, Starch Properties and Grain Quality of Rice (Oryza sativa L.)" Agronomy 13, no. 5: 1288. https://doi.org/10.3390/agronomy13051288
APA StyleWei, C., Jiang, J., Liu, C., Fang, X., Zhou, T., Xue, Z., Wang, W., Zhang, W., Zhang, H., Liu, L., Wang, Z., Gu, J., & Yang, J. (2023). Effects of Source Strength and Sink Size on Starch Metabolism, Starch Properties and Grain Quality of Rice (Oryza sativa L.). Agronomy, 13(5), 1288. https://doi.org/10.3390/agronomy13051288









