Identification of qPSR7-2 as a Novel Cold Tolerance-Related QTL in Rice Seedlings on the Basis of a GWAS
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Evaluation of Cold Tolerance
2.2. Whole-Genome Sequencing Data Analysis
2.3. Analysis of the Population Structure
2.4. Genome-Wide Association Study
2.5. Analysis of Candidate Gene Expression Patterns
2.6. Data Analysis
3. Results
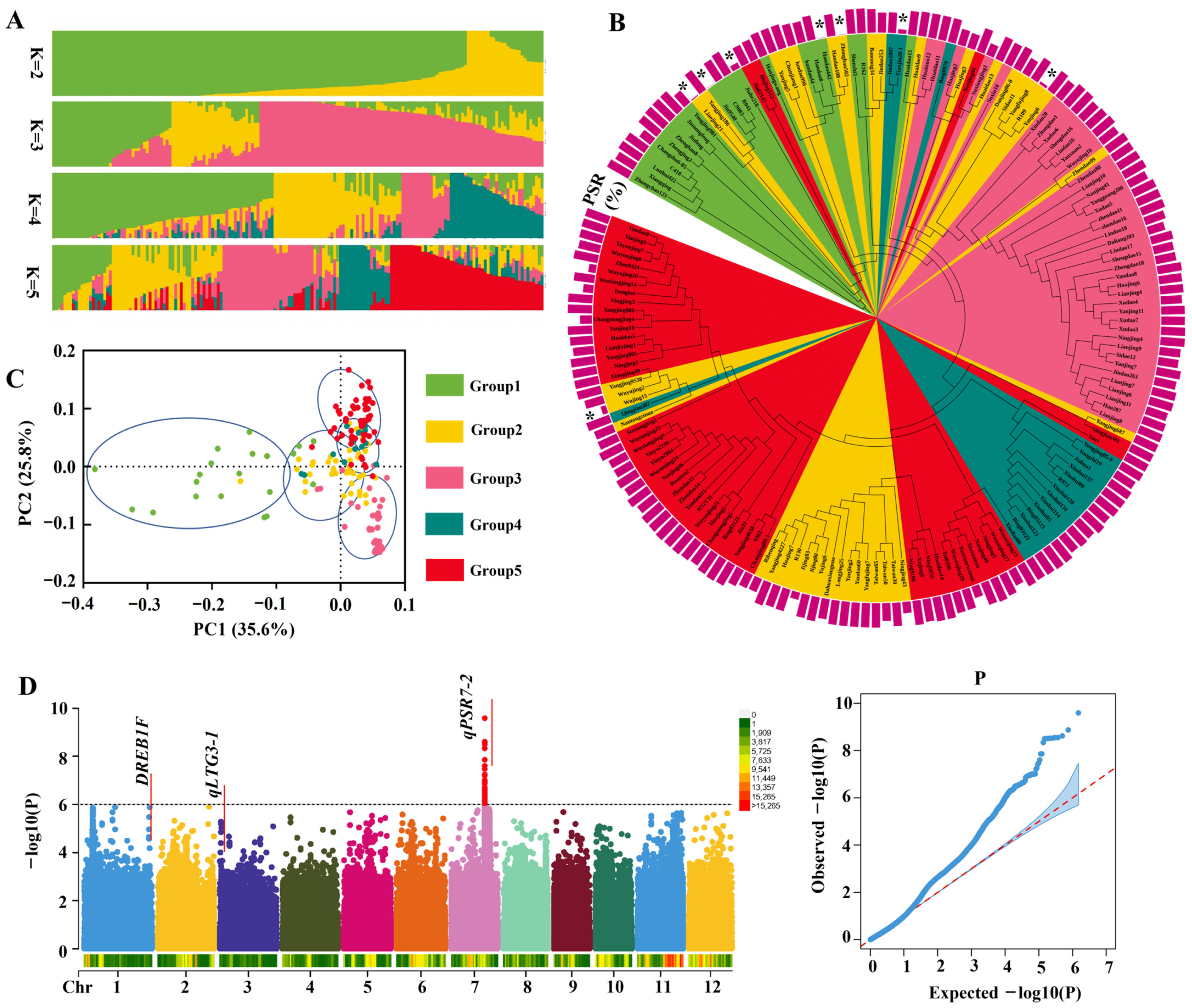
3.1. Genome-Wide Association Study of Cold Tolerance QTLs
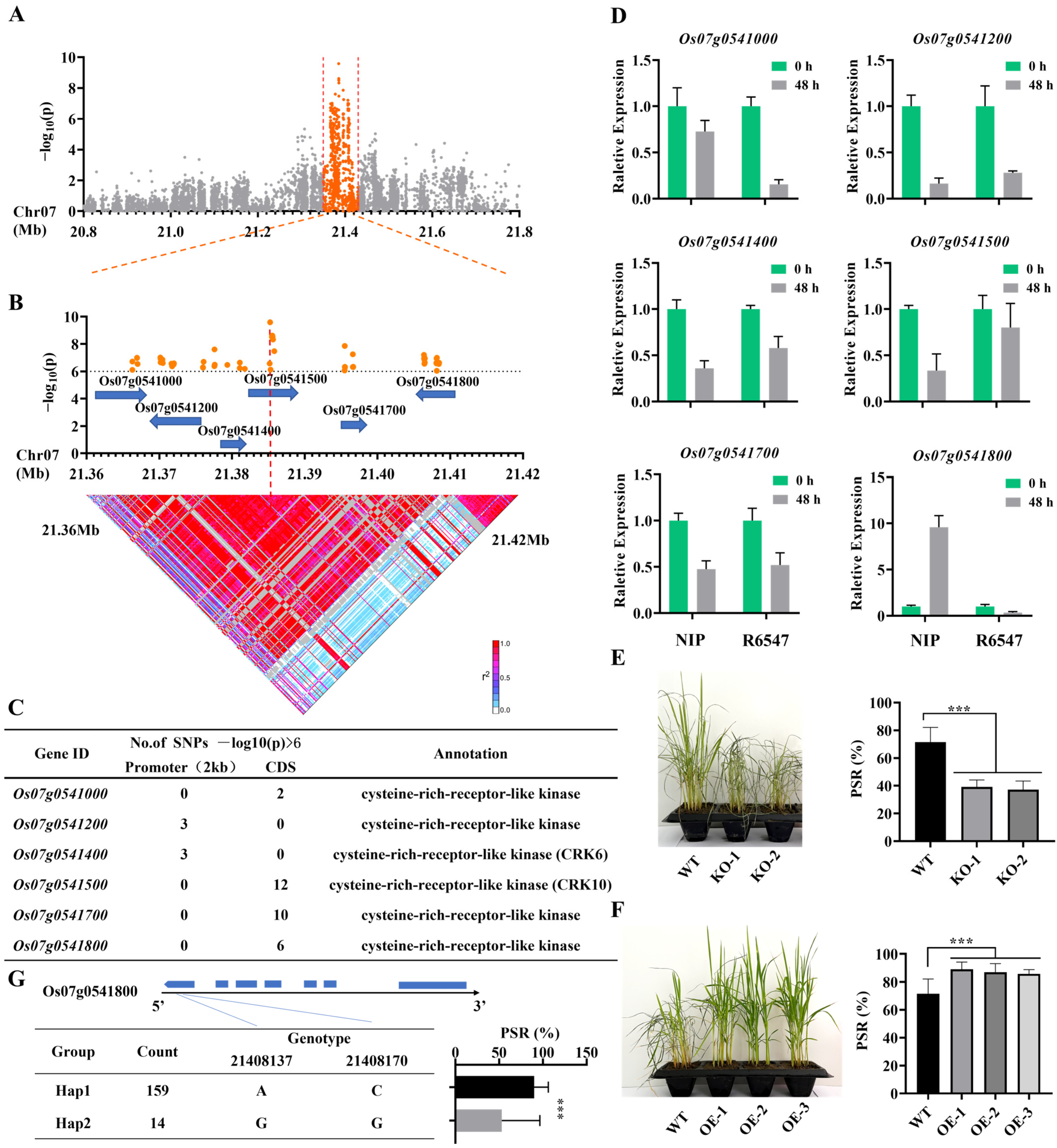
3.2. Fine Mapping and Functional Verification of the Cold Tolerance QTL qPSR7-2
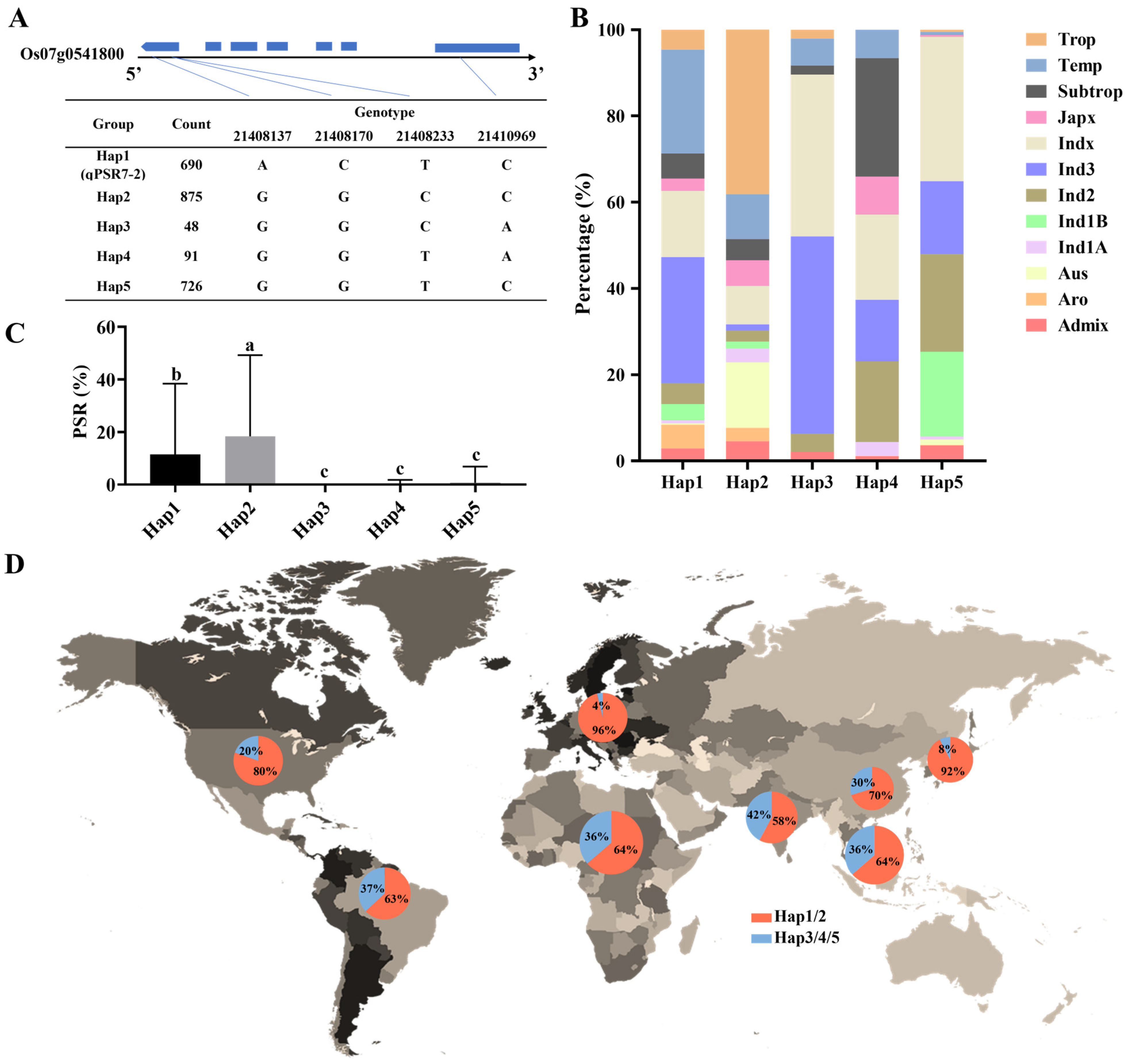
3.3. qPSR7-2 Is Mainly Distributed in Rice Materials from High-Latitude Areas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Zhao, R.; Huang, K.; Huang, S.; Wang, H.; Wei, Z.; Li, Z.; Bian, M.; Jiang, W.; Wu, T.; et al. The OsWRKY63–OsWRKY76–OsDREB1B module regulates chilling tolerance in rice. Plant J. 2022, 112, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Pepijn, A.; Sander, J. Impacts of climate change on rice production in Africa and causes of simulated yield changes. Glob. Chang. Biol. 2018, 24, 1029–1045. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Li, F.; Liu, H.; Yang, W.; Chong, K.; Xu, Y. OsMAPK3 Phosphorylates OsbHLH002/OsICE1 and Inhibits Its Ubiquitination to Activate OsTPP1 and Enhances Rice Chilling Tolerance. Dev. Cell 2017, 43, 731–743. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Bian, J.; Jin, T.; Zou, B.; Liu, S.; Zhang, X.; Wang, P.; Tan, J.; Wu, G.; et al. Identification of cold tolerance QTLs at the bud burst stage in 211 rice landraces by GWAS. BMC Plant Biol. 2021, 21, 542. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Xin, Y.; Tan, Y.; Hu, X.; Bai, J.; Liu, Z.Y.; Yu, Y.; Li, L.; Peng, C.; Fan, T.; et al. Natural variation in the HAN1 gene confers chilling tolerance in rice and allowed adaptation to a temperate climate. Proc. Natl. Acad. Sci USA 2019, 116, 3494–3501. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, S.; Qin, J.; Sun, C.; Liu, F. The lipid transfer protein OsLTPL159 is involved in cold tolerance at the early seedling stage in rice. Plant Biotechnol. J. 2020, 18, 756–769. [Google Scholar] [CrossRef]
- Liu, C.; Ou, S.; Mao, B.; Tang, J.; Wang, W.; Wang, H.; Cao, S.; Schläppi, M.R.; Zhao, B.; Xiao, G.; et al. Early selection of bZIP73 facilitated adaptation of japonica rice to cold climates. Nat. Commun. 2018, 9, 3302. [Google Scholar] [CrossRef]
- Tang, J.; Tian, X.; Mei, E.; He, M.; Gao, J.; Yu, J.; Xu, M.; Liu, J.; Song, L.; Li, X.; et al. WRKY53 negatively regulates rice cold tolerance at the booting stage by fine-tuning anther gibberellin levels. Plant Cell 2022, 34, 4495–4515. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef]
- Feng, J.; Li, Z.; Luo, W.; Liang, G.; Xu, Y.; Chong, K. COG2 negatively regulates chilling tolerance through cell wall components altered in rice. Theor. Appl. Genet. 2023, 136, 19. [Google Scholar] [CrossRef]
- Shumayla; Tyagi, S.; Sharma, A.; Singh, K.; Upadhyay, S.K. Genomic dissection and transcriptional profiling of Cysteine-rich receptor-like kinases in five cereals and functional characterization of TaCRK68-A. Int. J. Biol. Macromol. 2019, 134, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Liu, M.; Xing, Y.; Chen, X.; Zhang, Y.; Zhu, M.; Lu, X.; Zhang, Q.; Ling, Y.; Sang, X.; et al. Semi-dominant mutation in the cysteine-rich receptor-like kinase gene, ALS1, conducts constitutive defence response in rice. Plant Biol. 2018, 21, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Chern, M.; Xu, Q.; Bart, R.S.; Bai, W.; Ruan, D.; Sze-To, W.H.; Canlas, P.E.; Jain, R.; Chen, X.; Ronald, P.C. Correction: A Genetic Screen Identifies a Requirement for Cysteine-Rich-Receptor-Like Kinases in Rice NH1 (OsNPR1)-Mediated Immunity. PLOS Genet. 2016, 12, e1006182. [Google Scholar] [CrossRef]
- Mansueto, L.; Fuentes, R.R.; Borja, F.N.; Detras, J.; Abriol-Santos, J.M.; Chebotarov, D.; Sanciangco, M.; Palis, K.; Copetti, D.; Poliakov, A.; et al. Rice SNP-seek database update: New SNPs, indels, and queries. Nucl. Acids Res. 2017, 45, D1075–D1081. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Pan, C.; Li, Y.; Wu, Y.; Cai, Y.; Lu, Y.; Wang, R.; Yu, L.; Shi, W.; Kang, H.; et al. Genomic insight into balancing high yield, good quality, and blast resistance of japonica rice. Genome Biol. 2021, 22, 283. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, S. Surviving and thriving: How plants perceive and respond to temperature stress. Dev. Cell 2022, 57, 947–958. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, H.; Wu, S.; Fu, D.; Li, M.; Gong, Z.; Yang, S. CPK28-NLP7 module integrates cold-induced Ca2+ signal and transcriptional reprogramming in Arabidopsis. Sci. Adv. 2022, 8, eabn7901. [Google Scholar] [CrossRef]
- Luo, W.; Huan, Q.; Xu, Y.; Qian, W.; Chong, K.; Zhang, J. Integrated global analysis reveals a vitamin E-vitamin K1 sub-network, downstream of COLD1, underlying rice chilling tolerance divergence. Cell Rep. 2021, 36, 109397. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Pan, Y.; Li, J.; Zhou, L.; Shi, H.; Zeng, Y.; Guo, H.; Yang, S.; Zheng, W.; et al. Natural variation in CTB4a enhances rice adaptation to cold habitats. Nat. Commun. 2017, 23, 14788. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, L.; Zheng, J.; Chen, F.; Wang, T.; Wang, M.; Tao, Y.; Wang, H.; Hong, Z.; Huang, Y.; et al. A missense mutation in Large Grain Size 1 increases grain size and enhances cold tolerance in rice. J. Exp. Bot. 2019, 70, 3851–3866. [Google Scholar] [CrossRef]
- Wang, P.; Xiong, Y.; Gong, R.; Yang, Y.; Fan, K.; Yu, S. A key variant in the cis-regulatory element of flowering gene Ghd8 associated with cold tolerance in rice. Sci. Rep. 2019, 9, 9603. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Meng, X.; Song, X.; Zhang, D.; Kou, L.; Zhang, J.; Jing, Y.; Liu, G.; Liu, H.; Huang, X.; et al. Chilling-induced phosphorylation of IPA1 by OsSAPK6 activates chilling tolerance responses in rice. Cell Discov. 2022, 26, 71. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, N.; Chen, Z.; Wang, Z.; Shi, W.; Cai, Y.; Wu, Y.; Yu, L.; Pan, C.; Li, Y.; Zhou, C.; et al. Identification of qPSR7-2 as a Novel Cold Tolerance-Related QTL in Rice Seedlings on the Basis of a GWAS. Agronomy 2023, 13, 1252. https://doi.org/10.3390/agronomy13051252
Xiao N, Chen Z, Wang Z, Shi W, Cai Y, Wu Y, Yu L, Pan C, Li Y, Zhou C, et al. Identification of qPSR7-2 as a Novel Cold Tolerance-Related QTL in Rice Seedlings on the Basis of a GWAS. Agronomy. 2023; 13(5):1252. https://doi.org/10.3390/agronomy13051252
Chicago/Turabian StyleXiao, Ning, Zichun Chen, Zhiping Wang, Wei Shi, Yue Cai, Yunyu Wu, Ling Yu, Cunhong Pan, Yuhong Li, Changhai Zhou, and et al. 2023. "Identification of qPSR7-2 as a Novel Cold Tolerance-Related QTL in Rice Seedlings on the Basis of a GWAS" Agronomy 13, no. 5: 1252. https://doi.org/10.3390/agronomy13051252
APA StyleXiao, N., Chen, Z., Wang, Z., Shi, W., Cai, Y., Wu, Y., Yu, L., Pan, C., Li, Y., Zhou, C., Zhang, X., Liu, J., Huang, N., Liu, G., Ji, H., Zhu, S., & Li, A. (2023). Identification of qPSR7-2 as a Novel Cold Tolerance-Related QTL in Rice Seedlings on the Basis of a GWAS. Agronomy, 13(5), 1252. https://doi.org/10.3390/agronomy13051252



