Understanding the Physiological Mechanisms of Canopy Light Interception and Nitrogen Distribution Characteristics of Different Maize Varieties at Varying Nitrogen Application Levels
Abstract
1. Introduction
2. Materials and Methods
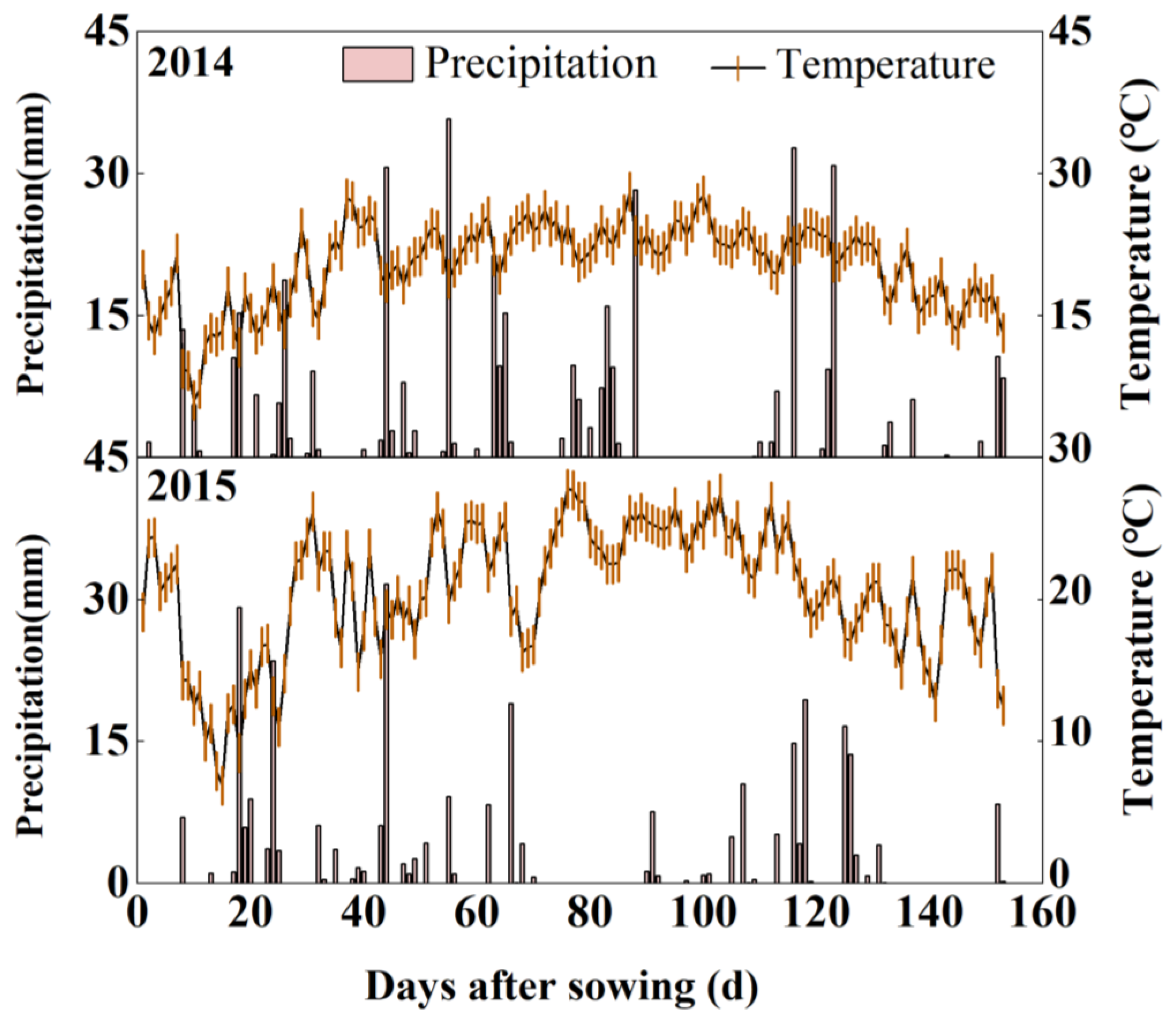
2.1. Site Description
2.2. Experiment Design and Field Management
2.3. Data Collection
2.3.1. Plant Morphology
2.3.2. Leaf Area Index (LAI)
× number of plants per unit area/unit land area
2.3.3. Net Photosynthetic Rate (Pn) and Photosynthetic N Use Efficiency (PNUE)
2.3.4. PAR and PAR Utilization
2.3.5. GS Activity, GOGAT Activity and NR Activity
2.3.6. N Content, Remobilization and Grain Yield
2.4. Statistical Analysis
3. Results
3.1. Grain Yield and Yield Compositions
3.2. Internode Length, Leaf Angle, and Leaf Orientation
3.3. Leaf Area Index (LAI) and Upper Internode Number
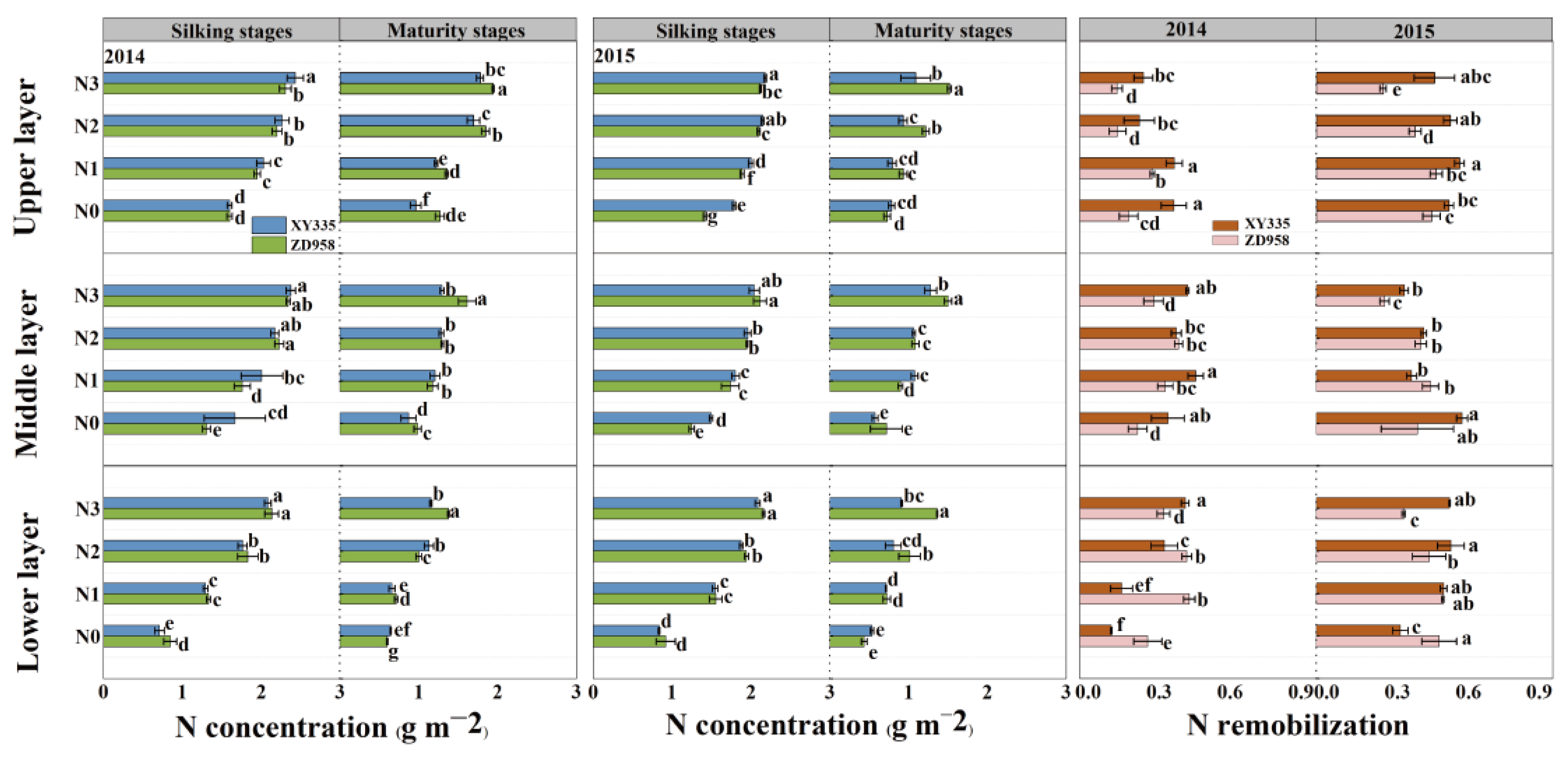
3.4. N Distribution and Remobilization
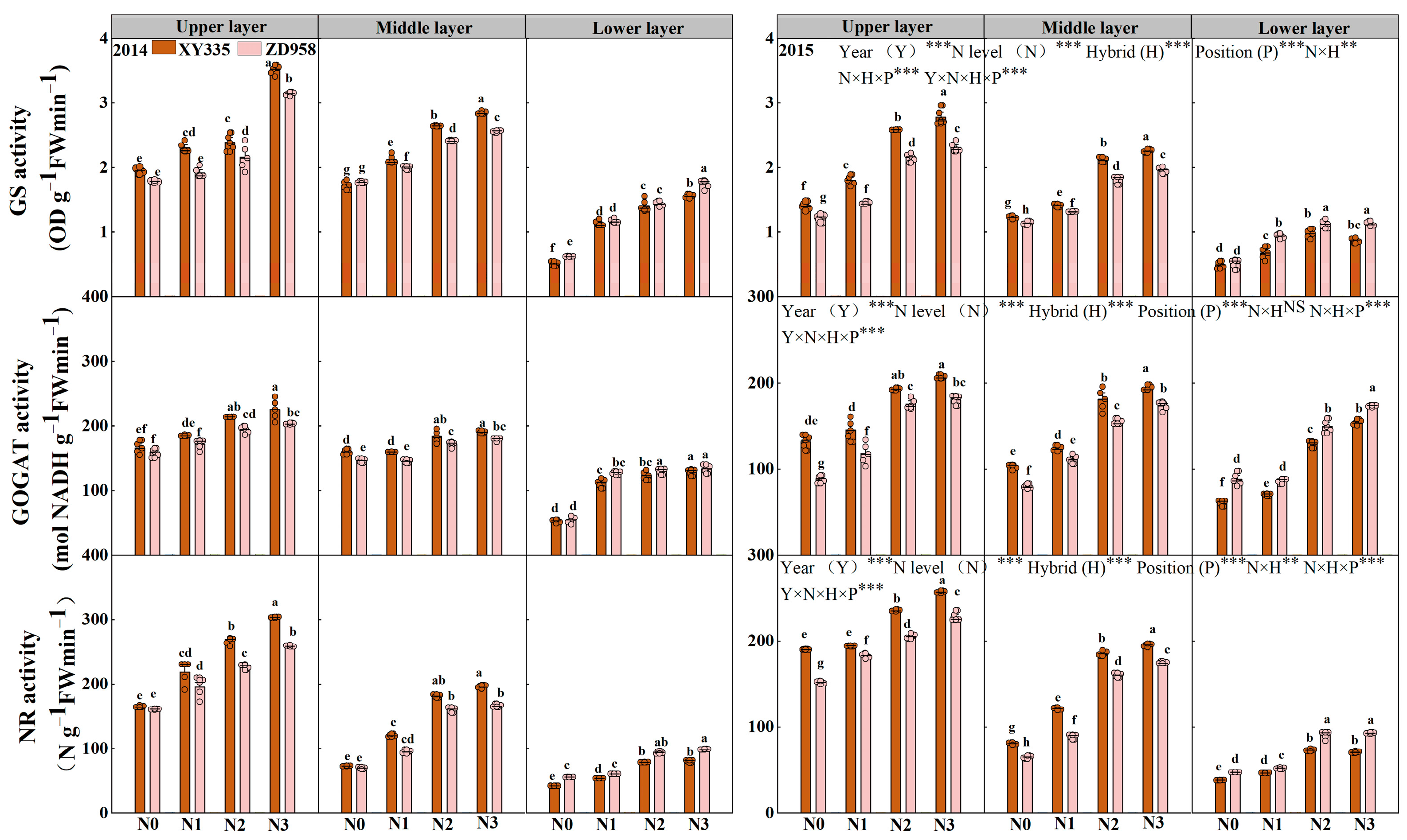
3.5. GS Activity, GOGAT Activity and NR Activity
3.6. Net Photosynthetic Rate (Pn) and Photosynthetic N Use Efficiency (PNUE)
3.7. Light Interception Rate and PAR Utilization (RUE)
3.8. Correlation Analysis
3.9. SEM Explained the RUE and Grain Yield
4. Discussion
4.1. Hybrids × N Management: Grain Yield
4.2. The Relationship between the Canopy Structure and Light Utilization
4.3. The Relationship between Physiological Characteristics and Light Utilization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, S.T.; Gao, R.Q.; Hu, C.H.; Wang, Q.Y.; Wang, K.J. Study of canopy photosynthesis property and high yield potential after a thesis in maize. Acta Agron. Sin. 1997, 23, 318–325. [Google Scholar]
- Li, Y.; Ming, B.; Fan, P.; Liu, Y.; Wang, K.; Hou, P.; Xue, J.; Li, S.; Xie, R. Quantifying contributions of leaf area and longevity to leaf area duration under increased planting density and nitrogen input regimens during maize yield improvement. Field Crop. Res. 2022, 283, 10855. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.T.; Yan, H.; Liu, D.L.; Meinke, H.; Hoogenboom, G.; Bin Wang, B.; Bin Peng, B.; Guan, K.; Jaegermeyr, J.; et al. Silver lining to a climate crisis in multiple prospects for alleviating crop waterlogging under future climates. Nat. Commun. 2023, 14, 765. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Ren, B.; Zhao, B.; Liu, P.; Zhang, J. Optimized agronomic management practices narrow the yield gap of summer maize through regulating canopy light interception and nitrogen distribution. Eur. J. Agron. 2022, 137, 126520. [Google Scholar] [CrossRef]
- Tollenaar, M.; Lee, E. Yield potential, yield stability and stress tolerance in maize. Field Crop. Res. 2002, 75, 161–169. [Google Scholar] [CrossRef]
- Moreau, D.; Allard, V.; Gaju, O.; Le Gouis, J.; Foulkes, M.J.; Martre, P. Acclimation of leaf nitrogen to vertical light gradient at anthesis in wheat is a whole-plant process that scales with the size of the canopy. Plant Physiol. 2012, 160, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Zhu, X.G.; Naidu, S.L.; Ort, D.R. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 2006, 29, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Within-Leaf Nitrogen Allocation in Adaptation to Low Nitrogen Supply in Maize during Grain-Filling Stage. Front. Plant Sci. 2016, 7, 699. [Google Scholar] [CrossRef] [PubMed]
- Massignam, A.M.; Chapman, S.C.; Hammer, G.L.; Fukai, S. Effects of nitrogen supply on canopy development of maize and sunflower. Crop. Pasture Sci. 2012, 62, 1045–1055. [Google Scholar] [CrossRef]
- Nasar, J.; Wang, G.-Y.; Ahmad, S.; Muhammad, I.; Zeeshan, M.; Gitari, H.; Adnan, M.; Fahad, S.; Bin Khalid, M.H.; Zhou, X.-B.; et al. Nitrogen fertilization coupled with iron foliar application improves the photosynthetic characteristics, photosynthetic nitrogen use efficiency, and the related enzymes of maize crops under different planting patterns. Front. Plant Sci. 2022, 13, 988055. [Google Scholar] [CrossRef]
- Shen, X.Y.; Dai, J.Y.; Hu, A.C. Studies on relationship among character of canopy light interception and yield in maize populations (Zea mays L.). Acta Agron. Sin. 1993, 19, 246–252, (In Chinese with English Abstract). [Google Scholar]
- Lai, Z.M.; Yang, K.C.; Lei, B.M.; Wen, A.C. Studies on the inheritance of quantitative traits in several inbred lines of maize. Sci. Agri. Sin. 1981, 4, 28–36, (In Chinese with English Abstract). [Google Scholar]
- Hikosaka, K.; Terashima, I.; Katoh, S. Effects of leaf age, nitrogen nutrition and photon flux density on the distribution of nitrogen among leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Oecologia 1994, 97, 451–457. [Google Scholar] [CrossRef]
- Bernhard, B.J.; Below, F.E. Plant population and row spacing effects on corn: Plant growth, phenology, and grain yield. Agron. J. 2020, 112, 2456–2465. [Google Scholar] [CrossRef]
- Raza, M.A.; Cui, L.; Khan, I.; Din, A.M.U.; Chen, G.; Ansar, M.; Ahmed, M.; Ahmad, S.; Manaf, A.; Titriku, J.K.; et al. Compact maize canopy improves radiation use efficiency and grain yield of maize/soybean relay intercropping system. Environ. Sci. Pollut. Res. 2021, 28, 41135–41148. [Google Scholar] [CrossRef] [PubMed]
- Fromme, D.D.; Spivey, T.A.; Grichar, W.J. Agronomic Response of Corn (Zea mays L.) Hybrids to Plant Populations. Int. J. Agron. 2019, 2019, 3589768. [Google Scholar] [CrossRef]
- Tollenaar, M.; Deen, W.; Echarte, L.; Liu, W. Effect of Crowding Stress on Dry Matter Accumulation and Harvest Index in Maize. Agron. J. 2006, 98, 930–937. [Google Scholar] [CrossRef]
- Li, Y.L.; Huang, X.L.; Xi, Z.Y.; Jia, J.X.; Wang, S.H. Study on the basis of the quantitative inheritance of the simultaneous improvement of corn plant-type and ear-kernel traits. Acta Agric. Uni. Henan. 1996, 30, 319–323. [Google Scholar]
- Maddonni, G.A.; Otegui, M.E.; Cirilo, A.G. Plant population density, rows pacing and hybrid effects on maize canopy architecture and light attenuation. Field Crops Res. 2001, 71, 183–193. [Google Scholar] [CrossRef]
- Pommel, B.; Gallais, A.; Coque, M.; Quilleré, I.; Hirel, B.; Prioul, J.; Andrieu, B.; Floriot, M. Carbon and nitrogen allocation and grain filling in three maize hybrids differing in leaf senescence. Eur. J. Agron. 2006, 24, 203–211. [Google Scholar] [CrossRef]
- Anten, N.P.R.; Schieving, F.; Werger, M.J.A. Patterns of light and nitrogen distribution in relation to whole canopy carbon Sgain in C3 and C4 Mono-and dicotyledonous species. Oecologia 1995, 101, 504–513. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, D.; Mu, X.; Xiao, C.; Chen, F.; Yuan, L.; Mi, G. Vertical Distribution of Photosynthetic Nitrogen Use Efficiency and Its Response to Nitrogen in Field-Grown Maize. Crop. Sci. 2016, 56, 397–407. [Google Scholar] [CrossRef]
- Hirel, B.; Andrieu, B.; Valadier, M.-H.; Renard, S.; Quillere, I.; Chelle, M.; Pommel, B.; Fournier, C.; Drouet, J.-L. Physiology of maize II: Identification of physiological markers representative of the nitrogen status of maize (Zea mays) leaves during grain filling. Physiol. Plant. 2005, 124, 178–188. [Google Scholar] [CrossRef]
- Lin, C.C.; Kao, C.H. Disturbed ammonium assimilation is associated with growth inhibition of roots in rice seedlings caused by NaCl. Plant Growth Regul. 1996, 18, 233–238. [Google Scholar] [CrossRef]
- Aslam, M.; Travis, R.L.; Rains, D. Enhancement of nitrate reductase activity and metabolic nitrate concentration by methionine sulfoximine in barley roots. Plant Sci. 2001, 161, 133–142. [Google Scholar] [CrossRef]
- Zhou, Z.; Struik, P.C.; Gu, J.; van der Putten, P.E.; Wang, Z.; Yin, X.; Yang, J. Leaf-colour modification affects canopy photosynthesis, dry-matter accumulation and yield traits in rice. Field Crop. Res. 2023, 290, 108746. [Google Scholar] [CrossRef]
- Pan, Y.; Cao, Y.; Chai, Y.; Meng, X.; Wang, M.; Wang, G.; Guo, S. Identification of photosynthetic parameters for superior yield of two super hybrid rice varieties: A cross-scale study from leaf to canopy. Front. Plant Sci. 2023, 14, 1110257. [Google Scholar] [CrossRef]
- Parker, G.G. Tamm review: Leaf Area Index (LAI) is both a determinant and a consequence of important processes in vegetation canopies. For. Ecol. Manag. 2020, 477, 118496. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J.; Deng, X. The spike weight contribution of the photosynthetic area above the upper internode in a winter wheat under different nitrogen and mulching regimes. Crop. J. 2018, 7, 89–100. [Google Scholar] [CrossRef]
- Yin, L.; Xu, H.; Dong, S.; Chu, J.; Dai, X.; He, M. Optimised nitrogen allocation favours improvement in canopy photosynthetic nitrogen-use efficiency: Evidence from late-sown winter wheat. Environ. Exp. Bot. 2019, 159, 75–86. [Google Scholar] [CrossRef]
- Wu, B.; Zuo, W.; Yang, P.; Zhang, W. Optimal water and nitrogen management increases cotton yield through improving leaf number and canopy light environment. Field Crop. Res. 2023, 290, 108745. [Google Scholar] [CrossRef]
- Yan, P.; Yue, S.; Qiu, M.; Chen, X.; Cui, Z.; Chen, F. Using maize hybrids and in-season nitrogen management to improve grain yield and grain nitrogen concentrations. Field Crop. Res. 2014, 166, 38–45. [Google Scholar] [CrossRef]
- Plénet, D.; Mollier, A.; Pellerin, S. Growth analysis of maize field crops under phosphorus deficiency. II. Radiation-use efficiency, biomass accumulation and yield components. Plant Soil 2000, 224, 259–272. [Google Scholar] [CrossRef]
- Duan, F.; Wei, Z.; Soualiou, S.; Zhou, W. Nitrogen partitioning in maize organs and underlined mechanisms from different plant density levels and N application rate in China. Field Crop. Res. 2023, 294, 108874. [Google Scholar] [CrossRef]
- Gardner, F.P.; McCloud, D.E.; Valle, R. Yield Characteristics of Ancient Races of Maize Compared to a Modern Hybrid. Agron. J. 1990, 82, 864–868. [Google Scholar] [CrossRef]
- Li, H.; Liang, Z.; Ding, G.; Shi, L.; Xu, F.; Cai, H. A Natural Light/Dark Cycle Regulation of Carbon-Nitrogen Metabolism and Gene Expression in Rice Shoots. Front. Plant Sci. 2016, 7, 1318. [Google Scholar] [CrossRef]
- Ning, P.; Yang, L.; Li, C.; Fritschi, F.B. Post-silking carbon partitioning under nitrogen deficiency revealed sink limitation of grain yield in maize. J. Exp. Bot. 2018, 69, 1707–1719. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Y.; Zhu, Y.; Liu, X.; Yu, H.; Li, L.; Qi, M.; Sun, H.; Yin, Z.; Wang, Y.; et al. Precipitation variations, rather than N deposition, determine plant ecophysiological traits in a desert steppe in Northern China. Ecol. Indic. 2022, 141, 109144. [Google Scholar] [CrossRef]
- Ghannoum, O.; Evans, J.R.; Chow, W.S.; Andrews, T.J.; Conroy, J.P.; von Caemmerer, S. Faster Rubisco Is the Key to Superior Nitrogen-Use Efficiency in NADP-Malic Enzyme Relative to NAD-Malic Enzyme C4 Grasses. Plant Physiol. 2005, 137, 638–650. [Google Scholar] [CrossRef]
- Ren, H.; Jiang, Y.; Zhao, M.; Qi, H.; Li, C. Nitrogen Supply Regulates Vascular Bundle Structure and Matter Transport Characteristics of Spring Maize Under High Plant Density. Front. Plant Sci. 2021, 11, 602739. [Google Scholar] [CrossRef]
- Ren, H.; Zhao, M.; Zhou, B.; Zhou, W.; Li, K.; Qi, H.; Jiang, Y.; Li, C. Understanding physiological mechanisms of variation in grain filling of maize under high planting density and varying nitrogen applicate rate. Front. Nutr. 2022, 9, 998946. [Google Scholar] [CrossRef] [PubMed]
- Nasar, J.; Zhao, C.J.; Khan, R.; Gul, H.; Gitari, H.; Shao, Z.; Abbas, G.; Haider, I.; Iqbal, Z.; Ahmed, W.; et al. Maize-soybean intercropping at optimal N fertilization increases the N uptake, N yield and N use efficiency of maize crop by regulating the N assimilatory enzymes. Front. Plant Sci. 2023, 13, 1077948. [Google Scholar] [CrossRef] [PubMed]
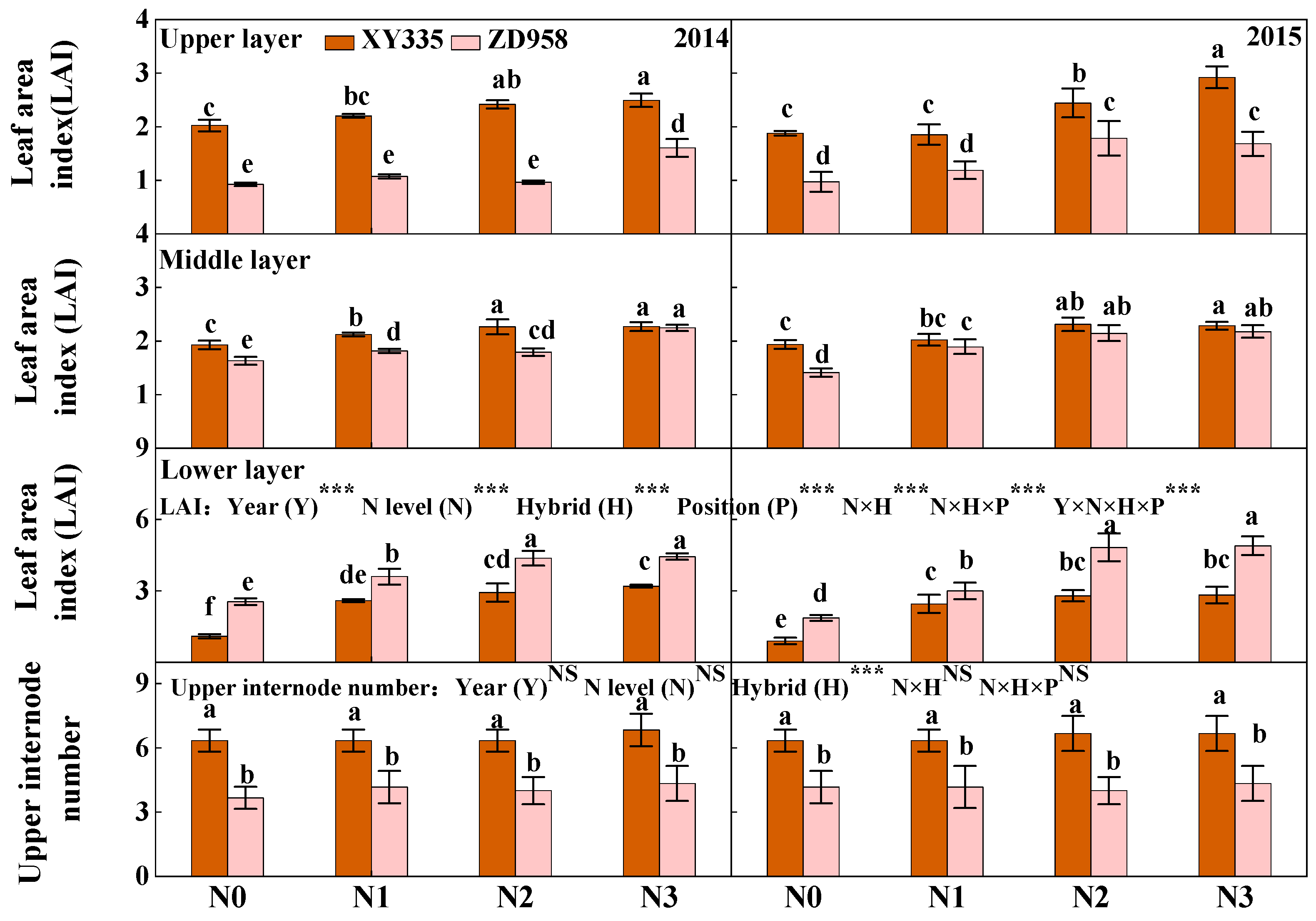
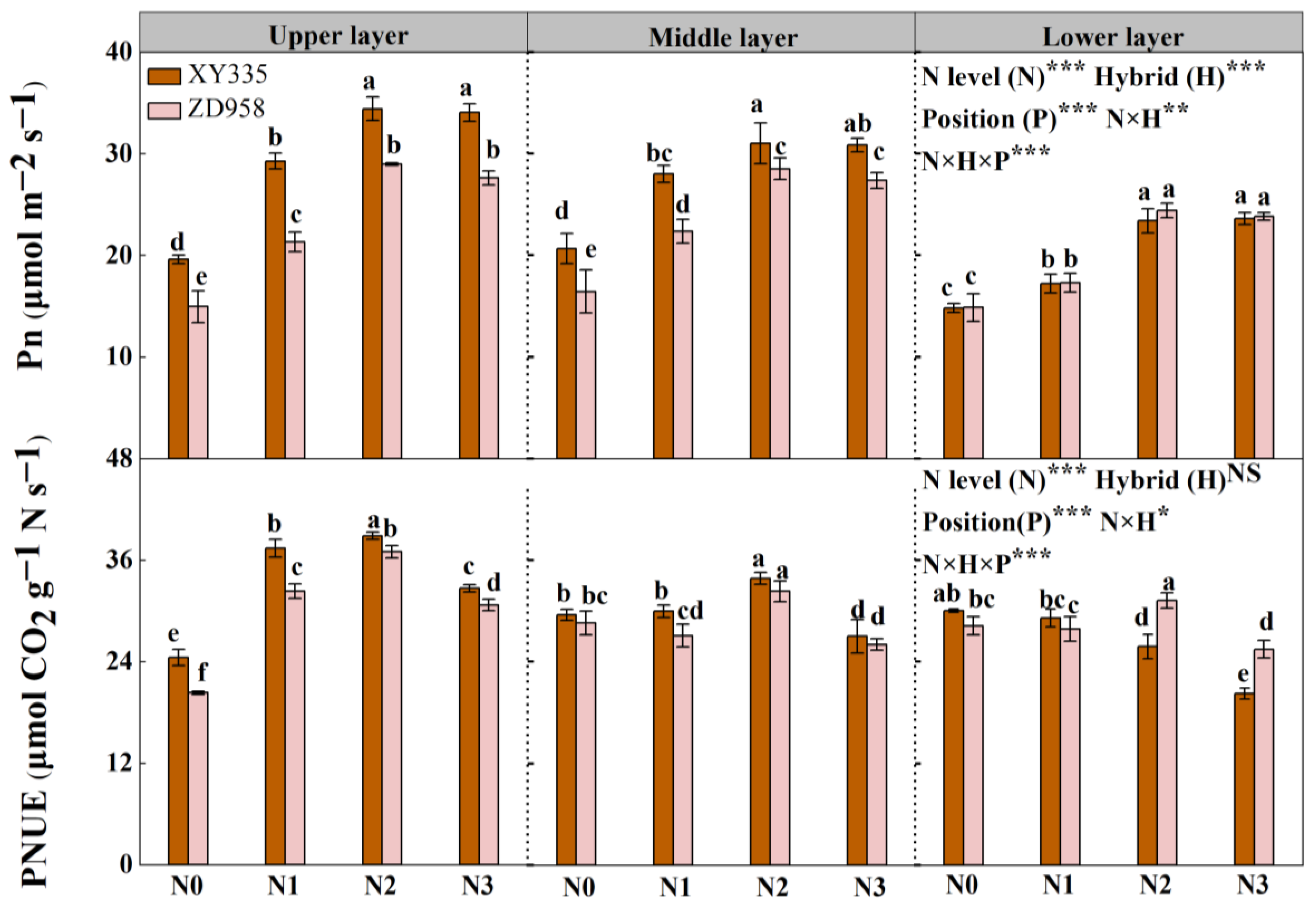
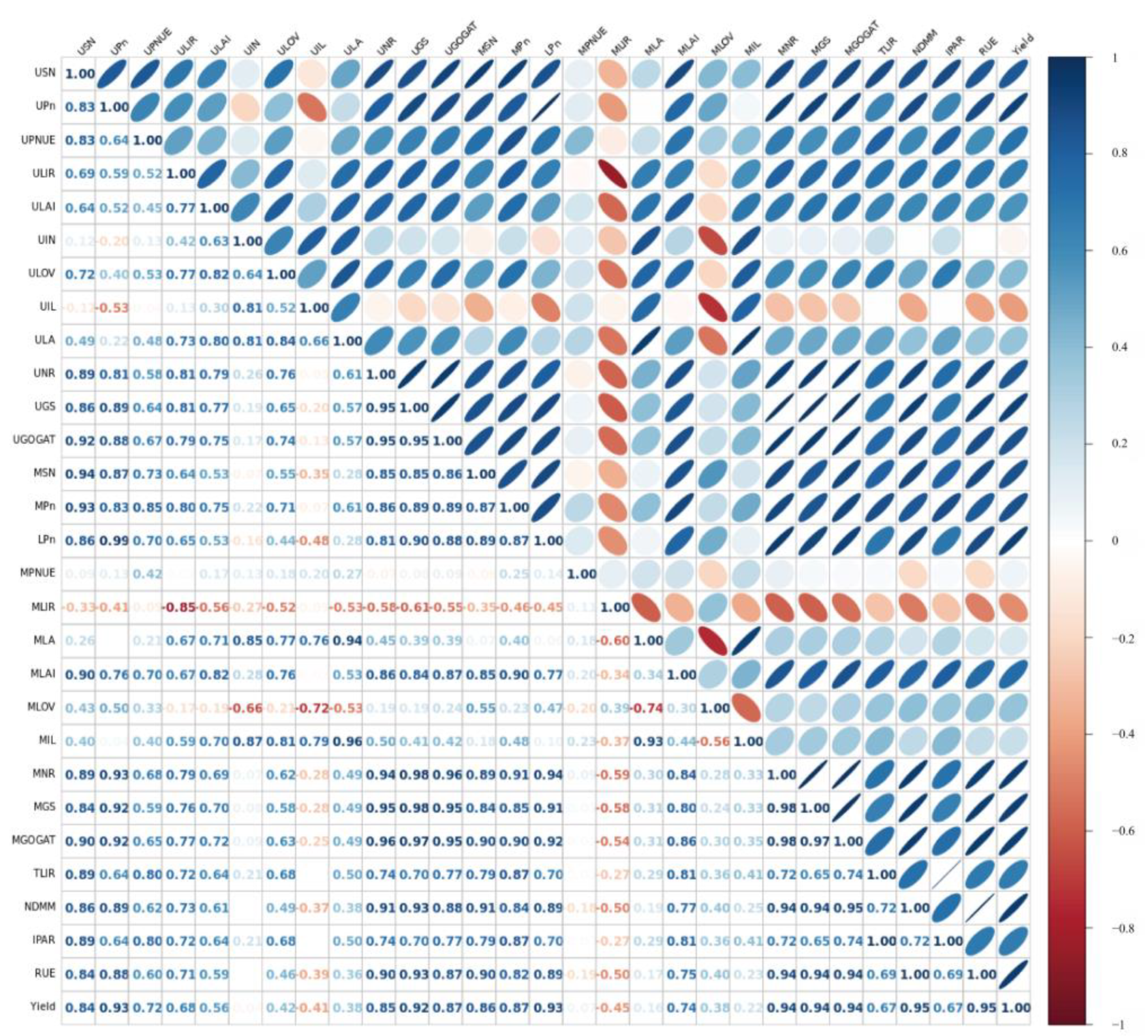
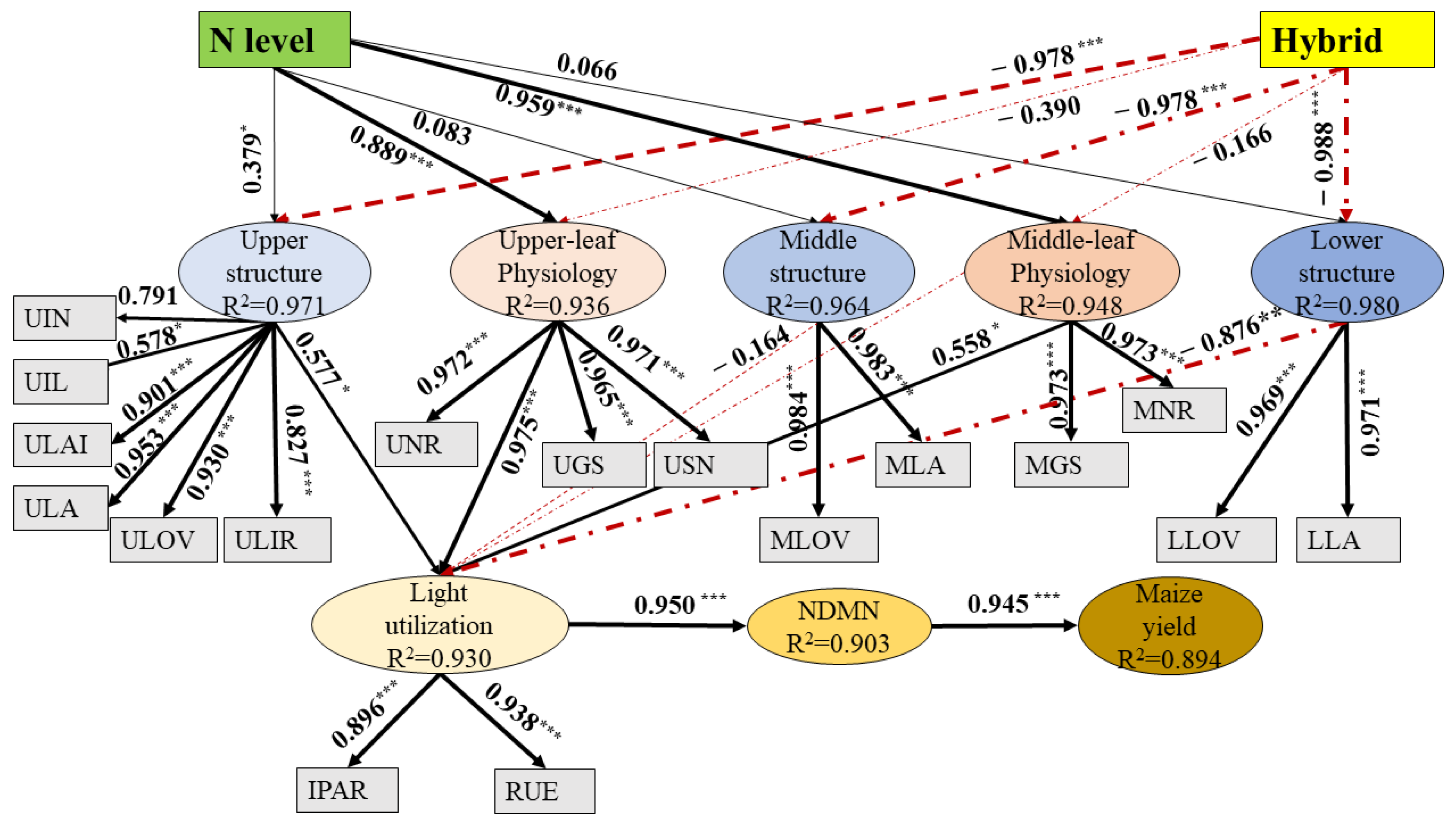
| N Rate (kg N ha−1) | Hybrid | Grain Yield (t ha−1) | Kernel No. per Ear | 1000-Kernel Weight (g) | |||
|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | ||
| 0 | XY335 | 5.1 ± 0.2 f | 4.9 ± 0.7 e | 265.1 ± 2.2 f | 296.9 ± 2.4 g | 298.5 ± 0.9 f | 272.7 ± 1.4 g |
| ZD958 | 6.3 ± 0.3 e | 6.3 ± 0.3 d | 332.8 ± 1.8 e | 318.4 ± 1.1 f | 328.2 ± 1.4 e | 334.4 ± 2.4 e | |
| 100 | XY335 | 9.3 ± 0.2 d | 8.4 ± 0.2 c | 542.4 ± 2.6 b | 485.6 ± 1.2 b | 300.9 ± 1.0 f | 312.4 ± 2.1 f |
| ZD958 | 9.4 ± 0.1 d | 8.2 ± 0.1 c | 507.4 ± 2.1 c | 425.1 ± 0.9 e | 348.0 ± 2.4 d | 351.4 ± 2.6 c | |
| 200 | XY335 | 12.8 ± 0.9 ab | 12.5 ± 0.5 a | 594.6 ± 1.9 a | 516.0 ± 1.2 a | 350.9 ± 1.9 d | 351.0 ± 0.5 c |
| ZD958 | 12.0 ± 0.6 c | 11.0 ± 0.5 b | 504.1 ± 2.0 c | 443.7 ± 3.3 d | 370.6 ± 1.5 c | 364.7 ± 2.0 a | |
| 300 | XY335 | 13.5 ± 0.5 a | 12.5 ± 0.5 a | 590.7 ± 5.7 a | 513.3 ± 3.7 a | 384.9 ± 3.0 b | 342.1 ± 0.6 d |
| ZD958 | 12.7 ± 0.1b c | 11.2 ± 0.1 b | 498.3 ± 2.1 d | 453.3 ± 1.1 c | 395.0 ± 1.3 a | 356.4 ± 1.7 b | |
| ANOVA Year (Y) | *** | *** | *** | ||||
| N level (N) | *** | *** | *** | ||||
| Hybrids (H) | NS | *** | *** | ||||
| N × H | *** | *** | *** | ||||
| Y × N × H | NS | *** | *** | ||||
| Year | N Rate | Hybrid | Internode Length (cm) | Leaf Angle (°) | Leaf Orientation Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (kg N ha−1) | Upper Layer | Middle Layer | Lower Layer | Upper Layer | Middle Layer | Lower Layer | Upper Layer | Middle Layer | Lower Layer | ||
| 2014 | 0 | XY335 | 15.5 ± 0.5 a | 18.0 ± 0.2 a | 16.3 ± 0.2 c | - | - | - | - | - | - |
| ZD958 | 12.3 ± 0.4 d | 12.6 ± 0.4 d | 15.3 ± 0.2 d | - | - | - | - | - | - | ||
| 100 | XY335 | 15.8 ± 0.3 a | 16.3 ± 0.6 b | 18.6 ± 0.2 b | - | - | - | - | - | - | |
| ZD958 | 13.6 ± 0.6 c | 13.7 ± 0.2 c | 14.5 ± 0.2 e | - | - | - | - | - | - | ||
| 200 | XY335 | 15.4 ± 0.2 a | 18.2 ± 0.3 a | 19.2 ± 0.2 a | - | - | - | - | - | - | |
| ZD958 | 10.5 ± 0.4 e | 12.3 ± 0.2 d | 12.4 ± 0.4 f | - | - | - | - | - | - | ||
| 300 | XY335 | 14.6 ± 0.4 b | 18.2 ± 0.2 a | 19.6 ± 0.3 a | - | - | - | - | - | - | |
| ZD958 | 11.9 ± 0.1 e | 12.5 ± 0.1 d | 12.4 ± 0.4 f | - | - | - | - | - | - | ||
| 2015 | 0 | XY335 | 16.6 ± 0.2 a | 16.3 ± 0.3 c | 17.4 ± 0.4 c | 18.4 ± 0.3 c | 33.3 ± 0.2 d | 32.2 ± 0.3 a | 65.4 ± 0.4 c | 47.6 ± 0.4 d | 48.2 ± 0.1 g |
| ZD958 | 12.4 ± 0.3 e | 12.2 ± 0.4 e | 14.4 ± 0.4 d | 15.2 ± 0.2 e | 24.3 ± 0.6 e | 15.2 ± 0.2 e | 47.6 ± 0.2 g | 45.6 ± 0.2 f | 58.6 ± 0.2 d | ||
| 100 | XY335 | 15.6 ± 0.5 b | 17.4 ± 0.4 e | 18.6 ± 0.2 b | 20.0 ± 0.3 b | 36.4 ± 0.4 c | 28.5 ± 0.4 b | 65.1 ± 0.2 c | 47.5 ± 0.4 e | 51.4 ± 0.2 e | |
| ZD958 | 12.7 ± 0.1 e | 13.6 ± 0.2 d | 13.4 ± 0.4 e | 15.5 ± 0.3 e | 18.2 ± 0.4 f | 15.9 ± 0.3 d | 52.9 ± 0.4 f | 59.3 ± 0.4 a | 60.3 ± 0.2 c | ||
| 200 | XY335 | 14.4 ± 0.3 c | 18.4 ± 0.2 a | 20.6 ± 0.2 a | 21.3 ± 0.2 a | 37.6 ± 0.1 b | 28.5 ± 0.2 b | 66.5 ± 0.2 b | 48.4 ± 0.2 e | 52.7 ± 0.2 d | |
| ZD958 | 10.6 ± 0.3 f | 12.3 ± 0.2 e | 12.5 ± 0.2 f | 16.1 ± 0.3 d | 19.1 ± 0.3 g | 17.0 ± 0.2 c | 58.6 ± 0.3 e | 59.7 ± 0.31 a | 66.5 ± 0.3 a | ||
| 300 | XY335 | 13.6 ± 0.2 d | 17.5 ± 0.4 b | 20.6 ± 0.4 a | 21.2 ± 0.2 a | 38.3 ± 0.1 a | 31.3 ± 0.3 a | 67.6 ± 0.2 a | 50.5 ± 0.3 c | 48.2 ± 0.34 g | |
| ZD958 | 10.5 ± 0.2 f | 12.7 ± 0.3 e | 12.4 ± 0.4 f | 15.4 ± 0.3 e | 21.7 ± 0.3 f | 17.5 ± 0.2 c | 60.5 ± 0.3 d | 58.7 ± 0.3 b | 63.5 ± 0.3 b | ||
| ANOVA N level (N) | *** | NS | NS | *** | *** | *** | *** | *** | *** | ||
| Hybrids (H) | *** | *** | *** | *** | *** | *** | *** | *** | *** | ||
| N × H | ** | *** | *** | *** | *** | *** | *** | *** | *** | ||
| Year | N Rate (kg N ha−1) | Hybrid | Light Interception Rate (%) | NDMM (g m−2) | IPAR (MJ m−2) | RUE (g MJ−1) | |||
|---|---|---|---|---|---|---|---|---|---|
| Upper | Middle | Lower | Total | ||||||
| 2014 | N0 | XY335 | 57.9 ± 0.8 e | 21.2 ± 0.6 b | 13.5 ± 0.9 a | 92.6 ± 0.5 d | 1253.8 ± 17.3 h | 1151.1 ± 6.6 d | 1.1 ± 0.02 g |
| ZD958 | 55.7 ± 0.7 f | 20.0 ± 0.8 bc | 14.5 ± 0.4 a | 90.3 ± 0.5 e | 1456.4 ± 8.1 g | 1121.9 ± 6.3 e | 1.3 ± 0.01 f | ||
| N1 | XY335 | 68.5 ± 0.9 b | 17.6 ± 1.6 e | 9.1 ± 0.4 c | 95.1 ± 0.6 bc | 2207.3 ± 4.0 f | 1181.7 ± 7.2 c | 1.9 ± 0.01 e | |
| ZD958 | 53.3 ± 1.8 g | 25.9 ± 0.1 a | 14.3 ± 1.0 a | 93.5 ± 0.8 d | 2281.8 ± 11.8 e | 1161.6 ± 9.8 d | 2.0 ± 0.03 d | ||
| N2 | XY335 | 65.6 ± 1.6 c | 19.4 ± 0.3 cd | 9.6 ± 0.7 c | 94.6 ± 0.8 c | 3062.7 ± 8.6 b | 1175.7 ± 9.6 bc | 2.6 ± 0.03 b | |
| ZD958 | 63.1 ± 1.8 d | 20.2 ± 0.9 bc | 12.1 ± 0.9 b | 95.4 ± 0.1 ab | 2779.9 ± 11.6 d | 1185.8 ± 0.6 ab | 2.3 ± 0.01 c | ||
| N3 | XY335 | 73.1 ± 1.0 a | 15.3 ± 1.2 f | 7.9 ± 0.1 d | 96.2 ± 0.2 a | 3245.3 ± 11.4 a | 1196.3 ± 2.9 a | 2.7 ± 0.01 a | |
| ZD958 | 64.9 ± 0.4 cd | 18.1 ± 0.2 de | 11.5 ± 0.2 b | 94.4 ± 0.3 bc | 3024.2 ± 3.2 c | 1173.5 ± 3.3 bc | 2.6 ± 0.01 b | ||
| 2015 | N0 | XY335 | 57.4 ± 1.3 e | 18.3 ± 0.2 b | 15.1 ± 1.0 ab | 90.7 ± 0.6 d | 1532.2 ± 3.1 h | 1127.8 ± 7.0 d | 1.4 ± 0.01 h |
| ZD958 | 55.0 ± 1.6 f | 16.7 ± 0.2 cd | 16.2 ± 0.4 a | 87.9 ± 1.3 e | 1547.9 ± 2.1 g | 1092.3 ± 16.6 e | 1.4 ± 0.02 g | ||
| N1 | XY335 | 66.4 ± 0.3 b | 15.5 ± 1.1 de | 11.3 ± 0.8 d | 93.2 ± 0.5 ab | 2114.9 ± 4.2 f | 1157.9 ± 6.4 ab | 1.8 ± 0.01 f | |
| ZD958 | 52.4 ± 1.2 g | 23.0 ± 0.4 a | 16.3 ± 0.8 a | 91.7 ± 0.7 cd | 2152.3 ± 3.6 e | 1139.8 ± 9.3 cd | 1.9 ± 0.02 e | ||
| N2 | XY335 | 64.4 ± 2.1 bc | 16.4 ± 0.5 de | 11.5 ± 1.0 d | 92.3 ± 1.0 bc | 2668.9 ± 12.8 c | 1147.0 ± 12.6 bc | 2.3 ± 0.04 c | |
| ZD958 | 61.0 ± 1.6 d | 17.7 ± 0.7 bc | 14.3 ± 0.9 bc | 93.0 ± 0.1 ab | 2407.4 ± 15.1 d | 1156.5 ± 1.0 ab | 2.1 ± 0.01 d | ||
| N3 | XY335 | 71.5 ± 1.3 a | 12.4 ± 1.3 f | 10.0 ± 0.1 e | 93.9 ± 0.3 a | 3155.7 ± 5.4 a | 1167.0 ± 3.6 a | 2.7 ± 0.01 a | |
| ZD958 | 63.1 ± 0.3 cd | 15.4 ± 0.2 de | 13.7 ± 0.2 c | 92.2 ± 0.2 bc | 2832.0 ± 6.5 b | 1145.6 ± 2.7 bc | 2.5 ± 0.01 b | ||
| ANOVA Year(Y) | *** | *** | *** | *** | *** | *** | *** | ||
| N rate (N) | *** | *** | *** | *** | *** | *** | *** | ||
| Hybrid (H) | *** | *** | *** | *** | *** | *** | *** | ||
| N × H | *** | *** | *** | *** | *** | *** | *** | ||
| Y × N × H | NS | NS | NS | NS | *** | NS | *** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.; Zhou, P.; Zhou, B.; Li, X.; Wang, X.; Ge, J.; Ding, Z.; Zhao, M.; Li, C. Understanding the Physiological Mechanisms of Canopy Light Interception and Nitrogen Distribution Characteristics of Different Maize Varieties at Varying Nitrogen Application Levels. Agronomy 2023, 13, 1146. https://doi.org/10.3390/agronomy13041146
Ren H, Zhou P, Zhou B, Li X, Wang X, Ge J, Ding Z, Zhao M, Li C. Understanding the Physiological Mechanisms of Canopy Light Interception and Nitrogen Distribution Characteristics of Different Maize Varieties at Varying Nitrogen Application Levels. Agronomy. 2023; 13(4):1146. https://doi.org/10.3390/agronomy13041146
Chicago/Turabian StyleRen, Hong, Peilu Zhou, Baoyuan Zhou, Xiangling Li, Xinbing Wang, Junzhu Ge, Zaisong Ding, Ming Zhao, and Congfeng Li. 2023. "Understanding the Physiological Mechanisms of Canopy Light Interception and Nitrogen Distribution Characteristics of Different Maize Varieties at Varying Nitrogen Application Levels" Agronomy 13, no. 4: 1146. https://doi.org/10.3390/agronomy13041146
APA StyleRen, H., Zhou, P., Zhou, B., Li, X., Wang, X., Ge, J., Ding, Z., Zhao, M., & Li, C. (2023). Understanding the Physiological Mechanisms of Canopy Light Interception and Nitrogen Distribution Characteristics of Different Maize Varieties at Varying Nitrogen Application Levels. Agronomy, 13(4), 1146. https://doi.org/10.3390/agronomy13041146







