Abstract
The study was conducted to determine the influence of fluorine soil contamination (100, 200 and 300 mg kg−1 of soil) on the levels of exogenous amino acids (ExAAs) and endogenous amino acids (EnAAs) in the above-ground parts of winter oilseed rape and spring triticale grain. Fluorine soil contamination had a much more pronounced influence on the content of the tested amino acids in spring triticale grain than in the above-ground parts of winter oilseed rape. Soil contamination with fluorine had the greatest influence on leucine (Leu), arginine (Arg) and lysine (Lys), alanine (Ala), glycine (Gly) and glutamic acid (Glu), increasing their content, and on tyrosine (Tyr), methionine (Met) and aspartic acid (Asp), decreasing their concentration in the winter oilseed rape above-ground parts. Under the influence of fluorine soil contamination, an increase in the content of Arg, phenylalanine (Phe), histidine (His), Leu, Tyr, Gly, serine (Ser), Asp and especially, proline (Pro) and Glu, and a decrease in the level of Met and cysteine (Cys) in spring triticale grain have been confirmed. The highest fluorine contamination (300 mg kg−1 of soil) had the most favourable influence on the total amino acid content in the spring triticale. The lowest fluorine dose (100 mg kg−1 of soil) had the same effect in winter oilseed rape. Spring triticale protein had a higher nutritive value than that of winter oilseed rape. Fluorine soil contamination caused a gradual increase in the nutritive value of protein in spring triticale grain (in contrast to rape).
1. Introduction
Hazardous anthropogenic activity activities associated with the development of civilisation increase the migration of heavy metals into the environment. This leads to negative changes in the quality of the soil, its physicochemical and biological properties, which consequently contributes to limiting plant growth and development, even causing their death [1,2,3,4]. Fluorine (F) is an element naturally occurring in the environment (13th in the earth’s crust). Its average content in the earth’s crust is over 600 mg kg−1. Both natural processes of chemical degradation of minerals and human industrial activities release it into the environment [1,2]. Environmental contamination with fluorine compounds caused by natural geological processes and from anthropogenic sources is a global problem. The threat concerns all living organisms, including humans [3,4]. With the development of industry and human activities, the problem of environmental contamination with fluorine compounds is becoming more and more acute. The result is the penetration of fluorine into the air, water, soils and grounds. Even a small but permanent influence of this element leads to its accumulation in the environment, causing a threat to living organisms by including this element in the trophic chain [5,6].
The main source of natural emission of fluorine to the environment is weathering processes of minerals with high fluorine content. The most important of them are fluorapatite, cryolite, topaz and fluorite [7]. The second natural source of emission of fluorine compounds into the atmosphere are volcanic eruptions, releasing fluorine in the form of very toxic hydrogen fluoride (HF). It is estimated that the annual emissions from these two sources alone range from 60,000 up to 6 million Mg of fluorine. The release of fluorine from the surface of seas and oceans in the form of aerosols contributes to the emission of about 20,000 Mg per year [8]. Fluorine poisoning is most common in the vicinity of phosphate fertiliser factories, aluminium, iron and glass smelters, and brick, porcelain and cement factories. The standards for fluorine concentration in the air are constantly exceeded in the vicinity of such plants [9,10]. These crops emit fluorine to the air as aerosols, e.g., HF, F2, SiF2 and H2SiF4, and in solid form, e.g., CaF2, NaF and Na2SiF6 [11]. A significant source of fluorine released to the environment can also be the use of fluorinated insecticides [12,13,14] and preventive drinking water fluoridation programmes [15].
In the scientific literature, we can find many studies [16,17,18,19] informing one about very high concentrations of fluorine in drinking water, which pose a real threat to human health. The fluorine content of drinking water should not be higher than 1.5 mg L−1 [15]. Fluorine is a unique element because in a physiological dose it is essential for humans, as it participates in the proper development of bones and teeth. At the same time, apart from its cariostatic effect on the teeth and positive influence on tissues and organs, it can also be toxic. This is due to the fact that the difference between the optimal and toxic dose is minimal [20]. The toxic properties of fluorine were first observed in Italy in 1867. At that time, a dental disease was found in the population living in coastal areas, which was later called dental fluorosis. In 1912, also in Italy, a cattle disease was observed consisting in insufficient saturation of bones with calcium. The disease was caused by fluorine emissions from a superphosphate plant near which the animals were grazing. During World War II, a similar phenomenon was observed in the area surrounding an aluminium smelter in Switzerland. It was observed that animals near the aluminium smelter had deformed leg bones and thickened skull bones [21]. The harmful influence of fluorine seems to be mainly due to its affinity for calcium ions and its strong negative influence on many enzymes. It should be emphasised that the International Fluoride Research Society publishes a scientific journal on fluorine, Fluoride. This quarterly publishes research on the toxicity of fluorine and its role in the environment, as well as the possibility of using fluorine compounds in practice. According to the European Food Safety Authority, the recommended daily intake of fluorine for humans is 0.05 mg kg−1 of body weight [22]. Fluorine is currently considered to be one of the various trace elements that pose a threat to human health, along with mercury, cadmium and lead [23].
Fluorine is usually considered as a contaminant because it is not an essential element for plants. It is assumed that the content of fluorine in plants growing in areas not contaminated with this element does not exceed 10 mg kg−1 of dry matter (D.M.) [24]. Fluorine is taken up from the soil by the plant’s root system and transported to the leaves. Plants damage under the influence of fluoride is usually found during shoots and flowering. They are expressed by necrotic changes of leaves and inflorescences [25]. Cereals grown near emission sources have a lower weight of grain and straw. Plant responses to fluorine stress are complex and involve many changes. They often show abiotic stress symptoms, mainly oxidative stress [26]. Fluorine alters the course of many physiological and biochemical processes that affect seed germination and plant growth at later stages of vegetation [24,26].
Previous studies [27,28,29] also showed changes in the mineral composition of plants, including protein content and its amino acid composition under the fluorine contamination. Proteins, one of the important organic components of plants, play an important role in cellular metabolism under fluorine stress conditions. In recent years, increasing attention has been paid to obtaining crops with a balanced amino acid composition and high nutritional value in order to meet the needs of human and animal nutrition. Fluorine soil contamination increased the content of nitrogen, including protein nitrogen, in the grain and spring straw of spring triticale, and reduced the concentration of protein nitrogen in the aerial biomass of winter oilseed rape and spring triticale yield [28]. Szostek et al. [27] showed that soil contamination with fluorine at doses of 100 and 200 mg kg−1 had a statistically significant positive effect on the content of exogenous amino acids (ExAAs) and endogenous amino acids (EnAAs) in the protein of maize aboveground mass. At the same time, the content of the analysed amino acids was much higher in yellow lupine plants than in maize. Chakrabarti and Patra [30] also found an increase in the all amino acids content of about 27% in the content of all amino acids compared to non-fluorinated objects. This increase affected all three plant organs (grains, leaves and roots) of Oryza sativa L. var. Swarno and Oryza sativa L. var. IR-3. Hautala and Holopainen [31] also showed an increase in the all amino acids content of the Hordeum vulgare L. leaves. They found the most significant increase in the content of aspartic acid (Asp), threonine (Thr), serine (Ser) and glutamic acid (Glu) in plants. Hanson et al. [32] showed a significant increase in proline (Pro) content in the same plant. Fluorine soil contamination also affected other elements content, e.g., it caused a decrease in sodium and sulphur concentrations in crops [29]. There is only limited information available on the effect of soil contamination with fluorine on the amino acid content of plants.
In Poland, special attention was paid to the role of oilseed rape in animal nutrition, mainly non-ruminant animals. Oilseed rape is characterised by a well-balanced protein in terms of the content of ExAAs. It contains a large amount of sulphur amino acids and Thr, a significant amount of tryptophan and little undesirable leucine (Leu) [33]. Winter and spring triticale grains are also used for the production of animal feed. The factor determining the nutritional and fodder value of triticale grain is the protein content and its amino acid composition. The key role is played by ExAAs, which must be supplied from the outside, because animals do not have the ability to synthesise them. These amino acids include arginine (Arg), phenylalanine (Phe), histidine (His), isoleucine (Ile), Leu, methionine (Met), Thr, tyrosine (Tyr) and valine (Val). The content of these amino acids determines the nutritional value of the protein. The second group of amino acids are EnAAs that animals produce themselves. These include alanine (Ala), cysteine (Cys), glycine (Gly), Asp, Glu, Ser and Pro. Their content also affects the nutritional value of feeds [34].
With this in mind, research hypotheses were put forward: (1) fluorine soil contamination causes a reduction in the content of individual ExAAs, EnAAs and their sum in winter oilseed rape (Brassica napus L.) and spring triticale (× Triticosecale Wittm. ex A. Camus); (2) fluorine application to the soil is the cause of a reduction in the nutritional value of plant protein.
2. Materials and Methods
2.1. Plant Growth Experiment
The research was based on a pot experiment carried out in the vegetation hall owned by the University of Warmia and Mazury in Olsztyn (53°46′23″ N, 20°28′34″ E, Poland). The soil used in the experiment came from a cultivated field and was taken from a depth of 0–25 cm. It corresponded to the granulometric composition of loamy sand. Table 1 shows the basic physical and chemical properties of soil.

Table 1.
Physico-chemical properties of soil.
The experimental plants were winter oilseed rape (Brassica napus L.)—Bojan variety and spring triticale (× Triticosecale Wittm. ex A. Camus)—Milewo variety. The sensitivity of both plants to fluorine soil contamination was determined on the basis of preliminary pilot studies carried out before the actual experiment was set up. In both experiments, fluorine was applied to the soil at levels of 100, 200 and 300 mg kg−1 of soil. The response of plants to soil contamination with fluorine was compared to the control object, i.e., without fluorine. The fluorine doses were selected on the basis of the average total fluorine content of Polish soils [35]. In all experiments, fluorine was introduced into the soil as potassium fluoride (commercial form) with the following properties: density—2.49 g cm−3; molecular weight—58.09 g mol−1; melting point—858 °C; boiling point—1505 °C; vapour density relative to air—2.01.
The same NPK mineral fertilisation was applied to all experimental objects: 111 mg N, 48 mg P and 111 mg K kg−1 soil. The mineral fertiliser was applied as urea 46%, triple superphosphate 46% and potassium salt 57%. Soil, dried and sieved (sieve of a diameter of 1 cm), was used in the experiment. The soil (9 kg per pot) mixed with fluorine and mineral fertilisers was placed in properly labelled polyethylene pots. Next, experimental plants were sown (13 plants per pot). Soil moisture was maintained at 60% of capillary water capacity throughout the growing season. Weather data during the experiment were typical: average air temperature: minimum—6.0 °C, average—11.3 °C, maximum—16.0 °C, average humidity—62.9%, day length: minimum—9 h 4 min, maximum—16 h 18 min. Winter oilseed rape was harvested at the flowering stage (BBCH 65), while spring triticale was harvested at the full grain maturity stage (BBCH 89).
2.2. Analytical Methods
The plant biomass was weighed, cut and dried (60 °C). The samples were ground after drying. Methods for pre-experiment soil and plant analyses are given in Table 2. The content of the ExAAs: Arg, Phe, His, Ile, Leu, Lys, Met, Thr, Tyr and Val and the EnAAs: Ala, Cys, Gly, Asp, Glu, Pro and Ser were determined. The results of the amino acid content analyses were related to the above-ground parts of winter rape and spring triticale grain. Sigma standard amino acid solution (Sigma-Aldrich Co. LLC, Saint Louis, MO, USA) was used to calibrate the amino acid analyser. The nutritive value of the protein in both plants was assessed on the basis of the content of ExAAs. This was done by calculating the Oser index [36], which compares the percentage content of essential amino acids in plants with the concentration of the same amino acids in the protein of a whole hen’s egg. The nutritional value of the tested protein was expressed as the geometric mean of all the essential amino acids analysed.

Table 2.
Methods of soil and plant analyses.
2.3. Statistical Analysis
The results were statistically calculated using one-way ANOVA and Duncan’s test from the Statistica 13.3 software (Tibco Software Inc.: Palo Alto, CA, USA) [43]. A significance level of p ≤ 0.01 was used to calculate least significant differences (LSD), and levels of * p ≤ 0.05 and ** p ≤ 0.01 were used for correlation coefficients (r).
3. Results
Increasing fluorine soil contamination has a small effect on the content of amino acids in the above-ground parts of winter oilseed rape (Table 3).

Table 3.
The content of amino acids in above-ground parts of winter oilseed rape (Brassica napus L.).
The sum of ExAAs per 100 g of total protein (TotProt) (16 g N) of winter oilseed rape was lowest in the control object and highest in the object contaminated with the fluorine highest dose—300 mg kg−1 soil (Table 3). It should be noted that the growing doses of fluorine did not cause very large changes in the content of the tested amino acids in winter oilseed rape protein. The differences between individual objects were small. The influence of soil contamination contributed to an increase in the content of Arg (0.73 **), Ile (1.00 **), Leu (0.89 **), Lys (0.93 **) and Val (0.99 **). However, a reverse relationship was found for Met (−0.96 **), Thr (−0.95 **) and Tyr (−0.93 **). Fluorine soil contamination had the greatest influence on Leu, Arg and Lys, increasing their content by 8%, 8% and 11%, respectively, as well as Tyr and Met, reducing their concentration by 16 and 26%, compared to the control object.
The sum of ExAAs per 1 kg D.M. winter oilseed rape was lowest in the control object and highest in an object contaminated with 100 mg kg−1 soil. The lowest dose of fluorine was most beneficial to the content of the tested amino acids in the dry matter of above-ground parts of winter oilseed rape. It should be emphasised that the growing soil contamination of fluorine also affected the dry matter content of individual ExAAs, such as winter oilseed rape protein. However, changes in their concentration in dry matter were slightly smaller.
In the case of EnAAs, their sum per 100 g of TotProt (16 g N) of winter oilseed rape was lowest in a control object and highest in an object contaminated with 100 mg kg−1 soil (Table 3). The obtained results indicate that all doses of fluorine have a positive influence on the amino acids content in relation to 100 g of TotProt of the above-ground parts of winter oilseed rape, especially the lowest dose of fluorine. Compared to the control object, an increase in the amino acids content after application of 100 mg kg−1 soil was 5%, including Pro by 18%. The influence of increasing fluorine soil contamination increased in the content of Ala (0.97 **), Gly (0.84 **) and Glu (1.00 **) in protein of winter oilseed rape by 8%. In the case of Pro (−0.37) and Asp (−0.95 **) along with the increasing fluorine soil contamination, the content of these amino acids was reduced by 5% and 8%, respectively, compared to the control object. The highest dose of fluorine was particularly distinguished in this adverse influence. There was no difference in Cys content in protein of winter oilseed rape under the influence of increasing fluorine doses.
The highest sum of EnAAs per 1 kg D.M. of winter oilseed rape was shown in an object contaminated with 100 mg kg−1 soil. Changes in the EnAAs content in the dry matter of winter oilseed rape under the influence of increasing fluorine soil contamination were similar to those for 100 g of TotProt. Increasing fluorine soil contamination caused an increase in Ala (0.90 **), Gly (0.85 **) and Glu (0.98 **) by 7–8%. Under the influence of increasing soil contamination with fluorine, the content of Asp (−0.95 **) in the dry matter of winter rape gradually decreased, by a maximum of 8%. The content of Cys and Pro was not varied or the change in their concentration was parabolic. Fluorine soil contamination had a small influence on the EnAAs content in both TotProt and dry matter of winter oilseed rape.
It should be emphasised that the increasing fluorine soil contamination has a positive influence on the sum of total amino acids (TotAAs) in the above-ground parts of winter oilseed rape. The lowest dose of fluorine (100 mg kg−1 soil) was the most beneficial. Fluorine soil contamination had no significant influence on the contribution of ExAAs and EnAAs in the TotAAs in the above-ground parts of winter oilseed rape (Figure 1). However, the highest share of ExAAs in the TotAAs in the above-ground parts of winter oilseed rape was found in the object contaminated with 300 mg kg−1 of soil).
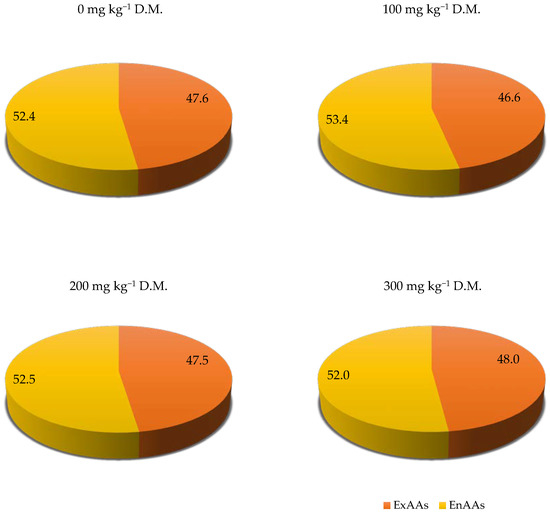
Figure 1.
Contribution of ExAAs and EnAAs to TotAAs in the above-ground parts of winter oilseed rape (Brassica napus L.) depending on soil contamination with fluorine, in %.
The nutritive value of protein in winter oilseed rape above-ground parts determined on the basis of the ExAAs content was similar in most objects (Figure 2). Only the highest dose of fluorine (300 mg kg−1 of soil) contributed to a slight reduction in the nutritive value of winter oilseed rape protein with respect to the control object. The nutritive value of winter oilseed rape protein in objects with the lowest and medium soil contamination was at the same level as in an object not contaminated with this xenobiotic.
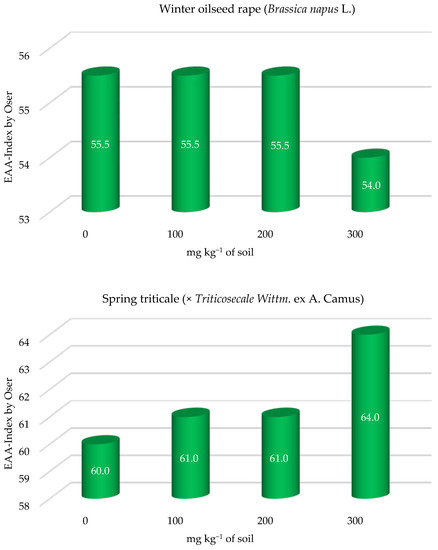
Figure 2.
Nutritive value of protein in the above-ground parts of winter oilseed rape (Brassica napus L.) and grain of spring triticale (× Triticosecale Wittm. ex A. Camus) depending on soil contamination with fluorine.
Fluorine soil contamination caused a greater modifications of the amino acid composition of spring triticale grain than in the amino acid content of above-ground parts of winter oilseed rape. The shaping of TotAAs in spring triticale depending on soil contamination is given in Table 4.

Table 4.
The content of amino acids in the grain of spring triticale (× Triticosecale Wittm. ex A. Camus).
The sum of ExAAs per 100 g of TotProt (16 g N) in the grain of spring triticale was lowest in the control object (not contaminated with fluorine) and highest in the object contaminated with 300 mg kg−1 soil (Table 4).
It should be noted that there is a clear linear increase in the content of these amino acids in the plant grain protein under the influence of growing doses of fluorine. All levels of fluorine soil contamination had a positive influence on the ExAAs content in the grain protein of this plant. This was confirmed by calculated correlation coefficients. Increasing fluorine soil contamination caused a gradual and highly significant increase in the content of Arg by 10% (1.00 **), Phe by 22% (1.00 **), His by 16% (0.97 **), Leu by 14% (0.95 **) and Tyr by 16% (0.98 **) in TotProt of spring triticale grain. A positive correlation was recorded between the growing fluorine soil contamination and the content of Ile (0.66 *), Lys (0.63 *), Thr (0.68 *) and Val (0.18) in spring triticale protein. The shape of their content was similar to a parabola. Only Met was an amino acid for which a linear decrease of content (−0.98 **, up to 35%) was observed under the influence of fluorine soil contamination.
The ExAAs content in the dry matter of spring triticale and their changes almost completely coincided with their content in the grain protein. The sum of ExAAs per 1 kg D.M. was lowest in the control object and highest in the object contaminated with 300 mg kg−1 soil. The TotAAs content in dry matter of spring triticale increased under the influence of increasing fluorine doses. The exception was Met. In the case of Met, the opposite relationship was observed, i.e., its content decreased as a result of growing fluorine soil contamination.
The sum of EnAAs per 100 g of TotProt (16 g N) of spring triticale grain was lowest in an object not contaminated with fluorine and highest in an object contaminated with 300 mg kg−1 soil (Table 4). Fluorine soil contamination has a positive influence on the content of these amino acids in the TotProt of spring triticale. Fluorine soil contamination caused a gradual increase in the content of Gly and Ser by 12% (0.92 ** and 0.99 **), Asp by 15% (0.99 **), Pro by 30% (0.99 **) and Glu by up to 46% (0.97 **). For Ala, the lowest and for Cys, the highest dose of fluorine contributed to a slight reduction in the content of these amino acids in the protein of spring triticale grain in relation to the control object. Other levels of soil contamination have a positive influence on the endogenous amino acid content in the spring triticale grain protein.
The highest EnAAs content per 1 kg D.M. of spring triticale was founded in the object contaminated with 300 mg kg−1 soil. Their sum was 57% higher than in the control object. Increasing fluorine soil contamination has contributed to a linear increase in the content of all tested amino acids (except Cys). The increase was greater than for TotProt. However, the highest dose of fluorine (300 mg kg−1 soil) caused a 21% reduction the Cys content in dry matter of spring triticale, in relation to the variant with a lower dose of fluorine (200 mg kg−1 soil).
To summarise the results obtained in experiment, it should be stated that the sum of TotAAs per 1 kg D.M. of spring triticale was the highest in the object with the highest dose of fluorine (300 mg kg−1 soil). It was 48% higher than in an uncontaminated object. Increasing fluorine soil contamination has a positive influence on the content of analysed amino acids in dry matter of spring triticale grain. Fluorine soil contamination caused a decrease in the contribution of ExAAs to TotAAs in spring triticale grain from 40.4% in the control object to 36.6% in the object with the highest dose of fluorine—300 mg kg−1 of soil (Figure 3). Therefore, it caused a decrease in the nutritional value of the grain of this plant.
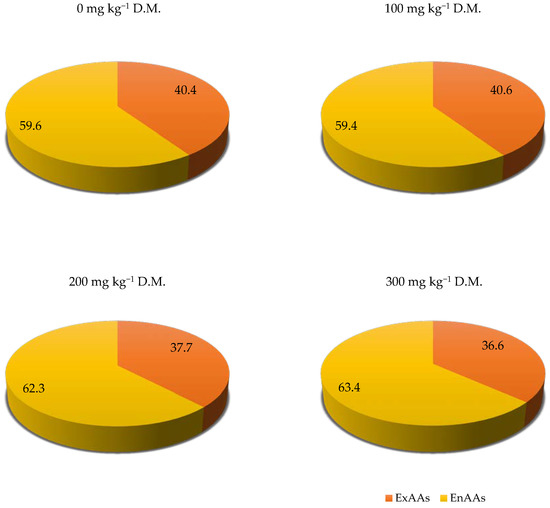
Figure 3.
Contribution of ExAAs and EnAAs to TotAAs in the grain of spring triticale (× Triticosecale Wittm. ex A. Camus) depending on soil contamination with fluorine, in %.
The nutritive index of the protein of spring triticale grain ranged from 60 in the control object (without fluorine) to 64 in the object contaminated with the highest dose of fluorine—300 mg kg−1 soil (Figure 2). Increasing fluorine soil contamination has contributed to a small increase of 7% in the nutritive value of the protein of spring triticale grain.
4. Discussion
In the scientific literature, we can find research reports informing about both the positive influence of fluorine’s natural environment and the negative impact of this contaminant on plants. These changes generally relate to the yield and mineral composition of plants. Such different plant reactions to fluorine stress are caused by many factors, among which the most important are: the level of contamination and the time of fluorine exposure or the physicochemical properties of the soil, mainly its reaction [44]. In slightly acidic or acidic soils, the plant-available forms of trace elements are more concentrated in the soil solution, which increases their content in plants. This is caused by an increase in the solubility of chemical combinations of these elements and a decrease in their binding on soil colloids under low soil pH conditions. The role of an effective material reducing the mobility of heavy metals in the soil–plant system is fulfilled by lime. According to Romar et al. [45], fluorine binding to insoluble compounds such as CaF2 or apatite compounds of similar composition occurs in calcium-rich soils, thus limiting the bioavailability of fluorine to plants. Ruan et al. [44] showed that the application of lime at 1.05 and 2.70 g kg−1 soil contributed to a decrease in fluorine content in tea leaves by 37 and 89%, respectively. In the acidic environments, is generally a greater bioavailability of this element is generally listed. Acid soil was used in our experiment for this reason, that the influence of fluoride on plants was as large as possible. Soil sorption properties and clay mineral content also play an important role. An important role is also played by the individual species characteristics of the plants, due to their different sensitivity to the studied stress factor, i.e., fluorine contamination. The research carried out seems to confirm this hypothesis, as some plant species reacted less well to the stress caused by fluorine contamination, while others reacted more clearly.
The results of our own research showed that fluorine soil contamination was much more clearly modified by the amino acid composition and the biological value of the spring triticale grain protein than the above-ground parts of winter oilseed rape. The reaction of plants to fluorine soil contamination, including its influence on the amino acid profile and protein content in plants, was varied. In Aslam et al. [46], the TotProt content of maize was reduced under the influence of fluorine contamination at a dose of 100 and 200 mg L−1. Pak Afgoi variety was more fluorine-resistant than the Pearl variety. Karmakar et al. [47] reported a significant reduction in the content of TotProt in the leaves of three species of plants. After application of a dose of 3 mg L−1, the reduction of protein content in plants was small, compared to the control. However, the increase in the fluorine dose to the level of 20 mg L−1 has contributed to a clear limitation of protein content in Pistia stratiotes, Eichhornia crassipes and Spirodela polyrhizas by 26, 22 and 28%, respectively. Of the three plant species, Eichhornia crassipes proved to be the most resistant to fluorine stress, as evidenced by the lowest reduction in protein content. The experiment by Pal et al. [48] showed a significant reduction in the protein content of various plant species (there were 13 of them) under the influence of fluorine was demonstrated. Singh et al. [49] stated that plants from control objects (not contaminated with fluorine) were characterised by the highest protein content. After application of fluorine at doses of 100 and 200 mg L−1, the protein content decreased by 10 and 25% in wheat (HUW-234) and by 28 and 34% in barley (EMBSN-34), respectively. A reduction in protein content in plants was also recorded in the experiment of Eyini et al. [50]. At the highest dose of fluorine (50 mg L−1), the greatest reduction in plant protein content was observed (31%).
The decrease in protein content under the influence of fluorine stress can be explained by reduced synthesis, as well as greater degradation of this ingredient. Probably the process of using protein to produce energy in response to metabolic stress occurs. Chang [51] explains it in more detail. He believes that fluorine reduces the number of ribosomes and destroys the structure of ribosomal proteins, which has a negative effect on overall protein synthesis.
Sinha et al. [52] also found a reduction in protein content of Hydrilla verticillata. They found a gradual reduction in the protein content in plants, of 41% at the highest dose of fluorine (25 mg L−1), in relation to non-contaminated objects. They indicate a gradual increase in the Cys content of plants with increasing fluorine dose. At the highest level of fluorine contamination, the increase in the Cys content in plants was 39%, in relation to the control. In our own research, only an 8% reduction in the content of Cys in the grain of spring triticale with the highest dose of fluorine (300 mg kg−1 soil) was noted. In the case of winter oilseed rape, the content of Cys was practically the same at all fluorine doses. Our own research has therefore shown a different relationship to that previously cited. Increasing soil contamination has a positive influence on the amino acids sum in both the above-ground parts of winter oilseed rape and the grain of the spring triticale. The lowest dose of fluorine (100 mg kg−1 soil) was the most favourable in the above-ground parts of winter oilseed rape, and the highest level of fluorine contamination in the grain of spring triticale (300 mg kg−1 soil). According to Yang and Miller [53], the increase in amino acid content in plant tissues as a result of exposure to fluorine is due to increased synthesis and a beneficial influence on some cellular enzymes. Yu and Miller [54] also explain this by the fact that fluorine ions in plant tissues can have a positive influence on plant breathing routes. This results in increased synthesis and production of free amino acids. Li and Ni [55] noted an increase in the content of all amino acids (except Tyr and Cys) in the leaves of Camellia sinensis L. As the fluorine concentration in soil increased, the content of Tyr gradually decreased. Similar dependencies were obtained in our own research, as a linear reduction of Tyr content in winter oilseed rape was also demonstrated. In the grain of spring triticale, the Tyr content gradually increased in relation to both dry matter and TotProt against the background of increasing fluorine doses. In the present study, in the experiment with winter rape, the Cys content did not fluctuate against the background of soil contamination with fluorine and practically did not change. In spring triticale, there was a gradual increase in the content of this amino acid compared to the control. Only in relation to TotProt, the highest dose of fluoride slightly decreased the content of this amino acid by 8%.
There are data in the literature confirming that fluorine compounds can significantly increase the content of free amino acids in plants. Li et al. [56] found a gradual increase in the Pro content of Camellia sinensis after the application of increasing fluorine contamination in their experiment with hydroponic cultures. At the highest level of contamination of 0.53 mM F, the content of Pro increased more than 2 times compared to an uncontaminated object. Maitra et. al. [57] showed an increase in the content of Pro in Vigna radiata. Interesting studies on the response of Punica granatum to fluorine emissions from a phosphorus fertiliser factory in Tunisia, depending on the distance from the emission source, were presented by Elloumi et al. [58]. Plants growing around the factory at a distance of only 0.5 km accumulated the largest amounts of Pro. Punica granatum leaves contained significantly larger amounts than the roots. When the emission source was dismissed, the content of Pro in Punica granatum gradually decreased. Elloumi et al. [59] and Mezghani et al. [60] confirmed the same tendency in research with Eriobotrya japonica. Other studies by Elloumi et al. [61] with Helianthus annuus indicate an increase in Pro content and a decrease in protein content after phosphogypsum soil application at a dose corresponding to 5% of soil mass. Elloumi et al. [62] with Nerium oleander and Datta et al. [63] in research with Cicer arietinum L. received the same relationship. Gadi et al. [64], Ahmed et al. [65], Saleh et al. [66], Das et al. [67] and Dey et al. [68] showed an increase in Pro content and a simultaneous reduction in the protein content in plants.
Pro is a special amino acid, because an increase in its content in plant tissues indicates cellular stress caused by various toxic substances. Zouari et al. [69] claim that the increase in Pro content can be used as an important indicator informing about the resistance of plants to fluorine. Cai et al. [70] showed a positive correlation between fluorine concentration and Pro content in Camellia sinensis leaves. In their research, the Pro content of Camellia sinensis leaves increased with the increasing fluorine contamination. The Pro content of Camellia sinensis leaves increased by 113% after application of 50 mg L−1. This relationship is also confirmed by the results of our own research. They showed an increase in Pro content in spring triticale grain under the influence of all doses of fluorine and in the above-ground parts of winter oilseed rape growing on soil with lower soil contamination with fluorine. Spring triticale seems to be more resistant to soil contamination with fluorine than winter oilseed rape. The effect of soil contamination with fluoride on plant yield and fluoride content in crops has been reported in previously published papers [28,71].
In summary, it should be noted that the economic importance of winter oilseed rape is constantly growing every year and this is a global trend. This is due to the fact that oilseed rape is mainly used for the production of oil and its consumption by humans, but also for the production of feed for farm animals, or as a bioenergy source and for cellulose production [72]. The strengthening of the global economic position of oilseed rape, among other crops, is due to the doubling of its yield per hectare. Winter oilseed rape has a long tradition of cultivation in European countries, Canada and Asia. The level of yield obtained per hectare achieved in Europe is well above the world average, particularly in Asia. In the European climate, primarily winter varieties are grown, which are more productive than spring varieties, dominating rather in the Western hemisphere, mainly in Canada [73].
The area under spring triticale in the world is currently about 3.5 million hectares. In Poland, in 2018, triticale was cultivated on an area of 1 million 188 thousand hectares, of which 93% was occupied by winter triticale. Such great interest in the cultivation of this cereal is due to its relatively low soil requirements, resistance to fungal diseases, high yield potential and feed value. Triticale is mainly used as feed grain for poultry and pigs. Triticale grain is characterised by a high protein content with a favourable amino acid composition and high digestibility [74]. Choosing a spring variety was associated with fewer publications about spring triticale than winter triticale.
Therefore, it seems justified to constantly monitor of the quality of agricultural products obtained in terms of food safety, especially those coming from areas exposed to the emissions of fluorine compounds and contamination with this element.
5. Conclusions
Fluorine soil contamination had a much more pronounced effect on the amino acids content of spring triticale grains than on winter oilseed rape. Fluorine soil contamination had the greatest influence on Leu, Arg and Lys, Ala, Gly and Glu, increasing their content, as well as Tyr, Met and Asp, decreasing their concentration in above-ground parts of winter oilseed rape. With the increasing soil contamination, there was an increase in the content of Arg, Phe, His, Leu, Tyr, Gly, Ser, Asp and especially, Pro and Glu, and a reduction in Met and Cys concentration in spring triticale grain. Increasing fluorine doses have contributed to the amino acid sum in both spring triticale and winter oilseed rape. In the case of spring triticale, the highest fluorine dose (300 mg kg−1 soil) was most favourable for the total amino acid content, whereas in winter oilseed rape, the lowest dose (100 mg kg−1 soil) was most favourable. Spring triticale protein had a higher nutritive value than that of winter oilseed rape. The nutritive index of protein, calculated on the basis of the ExAAs content in the above-ground parts of winter oilseed rape, was at a similar level. Increasing soil contamination with fluorine contributed to a slight increase in the nutritive value of spring triticale grain protein. Under its influence, a gradual increase in the nutritional value of protein in spring triticale grain was observed, from 60 in the control object (uncontaminated) to 64 in the object contaminated with 300 mg kg−1 soil.
Author Contributions
Conceptualization and methodology, Z.C.; investigation, R.S.; writing—review and editing, R.S. and M.W.; visualization, R.S. and M.W.; supervision, Z.C. and M.W.; funding acquisition, R.S. and M.W.; M.W., corresponding author. All authors have read and agreed to the published version of the manuscript.
Funding
The results presented in this paper were obtained as part of a comprehensive study funded by the University of Warmia and Mazury in Olsztyn, Faculty of Environmental Management and Agriculture, Department of Environmental Chemistry (grant No. 528.1004-0881) and the Faculty of Agriculture and Forestry, Department of Agricultural and Environmental Chemistry (grant No. 30.610.004-110).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available by contacting the authors.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Kumar, R.; Sinha, R.; Sharma, P.K.; Ivy, N.; Kumar, P.; Kant, N.; Jha, A.; Jha, P.K.; Gupta, P.K.; Sharma, P.; et al. Bioaccumulation of fluoride in plants and its microbially assisted remediation: A review of biological processes and technological performance. Processes 2021, 9, 2154. [Google Scholar] [CrossRef]
- Yadav, M.; Singh, G.; Jadeja, R. Fluoride contamination in groundwater, impacts, and their potential remediation techniques. In Groundwater Geochemistry: Pollution and Remediation Methods; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 22–41. [Google Scholar] [CrossRef]
- Vithanage, M.; Rajapaksha, A.U.; Bootharaju, M.S.; Pradeep, T. Surface complexation of fluoride at the activated nano-gibbsite water interface. Colloids Surf. A Physicochem. Eng. Asp. 2014, 462, 124–130. [Google Scholar] [CrossRef]
- Jarosz, Z.; Pitura, K. Fluoride toxicity limit—Can the element exert a positive effect on plants? Sustainability 2021, 13, 12065. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, J.; Du, C.; Yang, H.; Ye, B.-C. Distribution and pollution evaluation of fluoride in a soil–water–plant system in Shihezi, Xinjiang, China. Hum. Ecol. Risk Assess. Int. J. 2018, 24, 445–455. [Google Scholar] [CrossRef]
- Shahab, S.; Mustafa, G.; Khan, I.; Zahid, M.; Yasinzai, M.; Ameer, N.; Asghar, N.; Ullah, I.; Nadhman, A.; Ahmed, A.; et al. Effects of Fluoride Ion Toxicity on Animals, Plants, and Soil Health: A Review. Fluoride 2017, 50, 393–408. Available online: https://www.fluorideresearch.org/504/files/FJ2017_v50_n4_p393-408_sfs.pdf (accessed on 12 December 2022).
- Dehbandi, R.; Moore, F.; Keshavarzi, B.; Abbasnejad, A. Fluoride hydrogeochemistry and bioavailability in groundwater and soil of an endemic fluorosis belt, central Iran. Environ. Earth. Sci. 2017, 76, 177. [Google Scholar] [CrossRef]
- Camargo, J.A. Fluoride toxicity to aquatic organisms: A review. Chemosphere 2003, 50, 251–264. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Dong, T. Removal of fluoride from contaminated field soil by anolyte enhanced electrokinetic remediation. Environ. Earth Sci. 2009, 59, 379–384. [Google Scholar] [CrossRef]
- Ochoa-Herrera, V.; Banihani, Q.; Leon, G.; Khatri, C.; Fidel, A.J.; Sierra-Alvarez, R. Toxicity of fluoride to microorganisms in biological wastewater treatment systems. Water Res. 2009, 43, 3177–3186. [Google Scholar] [CrossRef]
- Ozsvath, L.D. Fluoride and environmental health: A review. Rev. Environ. Sci. Biotechnol. 2009, 8, 59–79. [Google Scholar] [CrossRef]
- Cape, N.J.; Fowler, D.; Davison, A. Ecological effects of sulfur dioxide, fluorides and minor air pollutants: Recent trends and research needs. Environ. Int. 2003, 29, 201–211. [Google Scholar] [CrossRef]
- Mori, T.; Ujihara, K.; Matsumoto, O.; Yanagi, K.; Matsuo, N. Synthetic studies of fluorine containing compounds for household insecticides. J. Fluor. Chem. 2007, 128, 1174–1181. [Google Scholar] [CrossRef]
- Okibe, F.G.; Ekanem, E.J.; Paul, E.D.; Shallangwa, G.A.; Ekwumemgbo, P.A.; Sallau, M.S.; Abanka, O.C. Fluoride content of soil land vegetables from irrigation farms on the bank of river Galma, Zaria, Nigeria. Aust. J. Basic Appl. Sci. 2010, 4, 779–784. [Google Scholar]
- WHO. Fluorides. Environmental Health Criteria; World Health Organisation: Geneva, Switzerland, 2002; Volume 227, p. 268. Available online: https://apps.who.int/iris/bitstream/handle/10665/42415/WHO_EHC_227.pdf (accessed on 18 January 2023).
- Malago, J.; Makoba, E.; Muzuka, A.N. Fluoride levels in surface and groundwater in Africa: A review. Am. J. Water Sci. Eng. 2017, 3, 1–17. [Google Scholar] [CrossRef]
- Ranjith, M.; Sridevi, S.; Jeevanrao, K.; Ramesh, T. Fluoride contamination in the irrigation water, soil and crops of the Rangareddy District of Telangana State, India. Fluoride 2022, 55, 63–80. Available online: https://www.fluorideresearch.online/epub/files/144.pdf (accessed on 16 January 2023).
- Narsimha, A.; Sudarshan, V. Contamination of fluoride in groundwater and its effect on human health: A case study in hard rock aquifers of Siddipet, Telangana State, India. Appl. Water Sci. 2017, 7, 2501–2512. [Google Scholar] [CrossRef]
- Mondal, D.; Gupta, S.; Reddy, D.; Nagabhushanam, P. Geochemical controls on fluoride concentrations in groundwater from alluvial aquifers of the Birbhum district, West Bengal, India. J. Geochem. Explor. 2014, 145, 190–206. [Google Scholar] [CrossRef]
- Kłódka, D.; Musik, D.; Wójcik, K.; Telesiński, A. Fluorine content in selected vegetables grown within the area affected by emission of that element from the ‘Police’ Chemical Plant. Bromatol. Chem. Toksykol. 2008, 61, 964–969. [Google Scholar]
- Zakrzewska, H. Fluorine and its compounds in the natural environment and food. Bromatol. Chem. Toksykol. 1995, 4, 393–398. [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific opinion on Dietary Reference Values for fluoride. EFSA J. 2013, 11, 3332–3378. [Google Scholar] [CrossRef]
- Smolik, B.; Telesiński, A.; Szymczak, J.; Zakrzewska, H. Assessing of humus usefulness in limiting of soluble fluoride content in soil. Environ. Prot. Nat. Resour. 2011, 49, 202–208. [Google Scholar]
- Bauthiyal, M.; Ranghar, S. Physiological and Biochemical Responses of Plants under Fluoride Stress: An Overview. Fluoride 2014, 47, 287–293. Available online: https://www.fluorideresearch.org/474/files/FJ2014_v47_n4_p287-293_sfs.pdf (accessed on 17 January 2023).
- Rizzu, M.; Tanda, A.; Canu, L.; Masawe, K.; Mtei, K.; Deroma, M.A.; Roggeroa, P.P.; Seddaiu, G. Fluoride uptake and translocation in food crops grown in fluoride-rich soils. J. Sci. Food Agric. 2020, 100, 5498–5509. [Google Scholar] [CrossRef] [PubMed]
- Mezghani, I.; Elloumi, N.; Abdallah, F.B.; Chaieb, M.; Boukhris, M. Fluoride Accumulation by Vegetation in the Vicinity of a Phosphatate Fertilizer Plant in Tunisia. Fluoride 2005, 38, 69–75. Available online: https://www.fluorideresearch.org/381/files/38169-75.pdf (accessed on 17 January 2023).
- Szostek, R.; Ciećko, Z.; Rolka, E.; Wyszkowski, M. Content of amino acids in maize and yellow lupine after fluorine application to soil. Agriculture 2021, 11, 1120–1131. [Google Scholar] [CrossRef]
- Szostek, R.; Ciećko, Z. Effect of soil contamination with fluorine on the yield and content of nitrogen forms in the biomass of crops. Environ. Sci. Pollut. Res. 2017, 24, 8588–8601. [Google Scholar] [CrossRef]
- Szostek, R.; Wyszkowski, M.; Ciećko, Z.; Rolka, E. Sodium and sulphur content in plants after lime, charcoal, and loam application to soil contaminated with fluorine. Appl. Sci. 2023, 13, 169. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Patra, K.P. Biochemical and Antioxidant Responses of Paddy (Oryza sativa L.) to Fluoride Stress. Fluoride 2015, 48, 56–61. Available online: https://www.fluorideresearch.org/481/files/FJ2015_v48_n1_p056-061_sfs.pdf (accessed on 7 January 2023).
- Hautala, E.L.; Holopainen, J.K. Gramine and free amino acids as indicators of fluoride-induced stress in barley and its consequences to insect herbivory. Ecotoxicol Environ Saf. 1995, 31, 238–245. [Google Scholar] [CrossRef]
- Hanson, A.D.; Nelson, C.E.; Everson, E.H. Evaluation of free proline accumulation as an index of drought resistance using, two contrasting barley cultivars. Crop Sci. 1977, 17, 720–726. [Google Scholar] [CrossRef]
- Jędrusek-Golińska, A.; Korczak, J.; Gliszczyńska-Świgło, A.; Czaczyk, K.; Kmiecik, D. The protein concentrates from defatted rapeseed meal as a raw material for production of protein hydrolysates. Oilseed Crops 2005, 26, 249–259. [Google Scholar]
- Stankiewicz, C. Effect of the Sowing Density and Herbicides on the Composition of Amino Acids and Biological Value of Spring Triticale Protein. Acta Sci. Pol. Agri. 2005, 4, 127–139. Available online: http://old-agricultura.acta.utp.edu.pl/uploads/pliki/000010200500004000010012700139.pdf (accessed on 11 January 2023).
- Kabata-Pendias, A. Trace Elements in Soil and Plants, 4th ed.; CRS Press, Taylor and Francis Group: Boca Raton, FL, USA, 2010; pp. 1–548. [Google Scholar] [CrossRef]
- Oser, B.L. An Integrated Essential Amino Acid Index for Predicting Biological Value of Proteins; Academic Press: New York, NY, USA, 1959; pp. 295–311. [Google Scholar]
- Research Procedure No. 29. Research Procedure; Chemical-Agricultural Station Instruction, II ed.; Chemical-Agricultural Station: Warsaw, Poland, 2008.
- Lityński, T.; Jurkowska, H.; Gorlach, E. Chemical and Agricultural Analysis; PWN Publishing House: Warsaw, Poland, 1976; pp. 129–132. [Google Scholar]
- Shimadzu. Shimadzu Analytical and Measuring Instruments; User’s Manual; Shimadzu Corporation: Kyoto, Japan, 2016. [Google Scholar]
- ISO 11261; Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. ISO: Geneva, Switzerland, 1995.
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods for Analysis and Evaluation of Soil and Plant Properties; Institute of Environmental Protection: Warsaw, Poland, 1991; pp. 1–334. [Google Scholar]
- Amino Acid Analyser AAA400, User Manual; Ingos S.R.O.: Prague, Czech Republic, 2007.
- Tibco Software Inc. Data Analysis Software System, Statistica Version 13.3; Tibco Software Inc.: Palo Alto, CA, USA, 2021. Available online: http://statistica.io (accessed on 23 December 2022).
- Ruan, J.; Ma, L.; Shi, Y.; Han, W. The impact of pH and calcium on the uptake of fluoride by tea plants (Camellia sinensis L.). Ann. Bot. 2004, 93, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Romar, A.; Gago, C.; Fernández-Marcos, L.M.; Álvarez, E. Influence of fluoride addition on the composition of solutions in Equilibrium with acid soils. Pedosphere 2009, 19, 60–70. [Google Scholar] [CrossRef]
- Aslam, A.; Nawaz, H.; Khan, A.; Ghaffar, R.; Abbas, G. Effect of Exogenous Application of Citric Acid on Growth of Maize (Zea mays L.) under Sodium Fluoride Stress. Fluoride 2023, 1–29. Available online: https://www.fluorideresearch.online/epub/files/188.pdf (accessed on 10 November 2022).
- Karmakar, S.; Mukherjee, J.; Mukherjee, S. Removal of fluoride contamination in water by three aquatic plants. Int. J. Phytoremediat. 2016, 18, 222–227. [Google Scholar] [CrossRef]
- Pal, C.K.; Mondal, K.N.; Bhaumik, R.; Banerjee, A.; Datta, K.J. Incorporation of Fluoride in Vegetation and Associated Biochemical Changes due to Fluoride Contamination in Water and Soil: A Comparative Field Study. Ann. Environ. Sci. 2012, 6, 123–139. Available online: https://openjournals.neu.edu/aes/journal/article/view/v6art7/v6p123-139 (accessed on 11 January 2023).
- Singh, U.P.; Rana Yashu, B.; Kumar, S.; Striastava, J.P. Biochemical responses of elevated level of fluoride in nutrient medium on wheat and barley. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3116–3123. [Google Scholar] [CrossRef]
- Eyini, M.; Sujanandini, K.; Pothiraj, C.; Jayakumar, M.; Kil, B.-S. Differental response of Azolla microphylla Kaulf. and Azolla filiculoides Lam. to sodium fluoride. J. Plant Biol. 1999, 42, 299–301. [Google Scholar] [CrossRef]
- Chang, C.W. Effect of fluoride on ribosomes from corn roots. Changes with growth retardation. Physiol. Plant. 1970, 23, 536–543. [Google Scholar] [CrossRef]
- Sinha, S.; Saxena, R.; Singh, S. Fluoride removal from water by Hydrilla verticillata (I.f.) royle and its toxic effects. Bull. Environ. Contam. Toxicol. 2000, 65, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Miller, G.W. Biochemical studies on the effect of fluoride on higher plants. 1. Metabolism of carbohydrates, organic acids and amino acids. Biochem. J. 1963, 88, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Miller, G.W. Effect of fluoride on the respiration of leaves from higher plants. Plant Cell. Physiol. 1967, 8, 484–493. [Google Scholar] [CrossRef]
- Li, C.H.; Ni, D. Effect of Fluoride on the amino Acid Composition of tea Leaves. Fluoride 2016, 49, 274–278. Available online: https://fluorideresearch.org/493Pt1/files/FJ2016_v49_n3Pt1_p274-278_pq.pdf (accessed on 13 January 2023).
- Li, C.; Zheng, Y.; Zhou, J.; Xu, J.; Ni, D. Changes of leaf antioxidant system, photosynthesis and ultrastructure in tea plant under the stress of fluorine. Biol. Plantarum 2011, 55, 563–566. [Google Scholar] [CrossRef]
- Maitra, A.; Datta, J.K.; Mondal, N.K. Amelioration of fluoride toxicity with the use of indigenous inputs. J. Stress Physiol. Biochem. 2013, 9, 207–219. [Google Scholar]
- Elloumi, N.; Ben Amor, A.; Zouari, M.; Belhaj, D.; Ben Abdallah, F.; Kallel, M. Adaptive Biochemical Responses of Punicagranatum to Atmospheric Fluoride Pollution. Fluoride 2016, 49, 357–365. Available online: https://www.fluorideresearch.org/493Pt2/files/FJ2016_v49_n3Pt2_p357-365_sfs.pdf (accessed on 9 January 2023).
- Elloumi, N.; Zouari, M.; Mezghani, I.; Abdallah, F.B.; Woodward, S.; Kallei, M. Adaptive biochemical and physiological responses of to fluoride air pollution. Ecotoxicology 2017, 26, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Mezghani, I.; Zouari, M.; Rouina, B.B.; Abdallah, F.B. Mulberry Leaves as a Bioindicator of Fluoride Pollution in the Vicinity of a Phosphate Fertilizer Factory Located in Sfax, Tunisia. Fluoride 2019, 52, 537–545. Available online: https://www.fluorideresearch.online/epub/files/051.pdf (accessed on 23 January 2023).
- Elloumi, N.; Zouari, M.; Chaari, L.; Abdallah, F.B.; Woodward, S.; Kallei, M. Effect of phosphogypsum on growth, physiology, and the antioxidative defense system in sunflower seedlings. Environ. Sci. Pollut. Res. 2015, 22, 14829–14840. [Google Scholar] [CrossRef]
- Elloumi, N.; Belhaj, D.; Mseddi, S.; Zouari, M.; Abdallah, F.B.; Woodward, S.; Kallel, M. Response of Nerium oleander to phosphogypsum amendment and its potential use for phytoremediation. Ecol. Eng. 2017, 99, 164–171. [Google Scholar] [CrossRef]
- Datta, J.K.; Maitra, A.; Mondal, N.K.; Banerjee, A. Studies on the impact of fluoride toxicity on germination and seedling growth of gram seed (Cicer arietinum L. cv. Anuradha). J. Stress Physiol. Biochem. 2012, 8, 194–202. [Google Scholar]
- Gadi, B.R.; Verma, P.; Amra, R. Influence of NaF on seed germination, membrane stability and some biochemical content in Vigna seedlings. J. Chem. Biol. Phys. Sci. 2012, 2, 1371–1378. [Google Scholar]
- Ahmed, S.; Khalid, K.; Jabeen, F.; Ahmad, M.N.; Zia, A.; Haider, A.; Mujahid, M.; Zia, D.; Khan, N.P. The Effects of Fluoride Stress on Okra (Abelmoschus esculentus L.). Fluoride 2019, 52, 354–361. Available online: https://www.fluorideresearch.online/523Pt2/files/FJ2019_v52_n3Pt2_p354-361_sfs.pdf (accessed on 24 January 2023).
- Saleh, A.A.H.; Abdel-Kader, D.Z. Metabolic responses of two Helianthus annuus cultivars to different fluoride concentrations during germination and seedling growth stages. Egypt. J. Exp. Biol. 2003, 5, 43–54. [Google Scholar]
- Das, C.; Dey, U.; Chakraborty, D.; Datta, J.K.; Mondal, N.K. Fluoride Toxicity Effects in Potato Plant (Solanum tuberosum L.) Grown in Contaminated Soils. Octa J. Environ. Res. 2015, 3, 136–143. Available online: http://sciencebeingjournal.com/sites/default/files/03-150606_0302_NKM.pdf (accessed on 20 January 2023).
- Dey, U.; Mondal, N.K.; Das, K.; Datta, J.K. Dual Effect of Fluoride and Calcium on the Uptake of Fluoride, Growth Physiology, Pigmentation and Biochemistry of Bengal Gram Seedlings (Cicer arietinum L.). Fluoride 2012, 45, 389–393. Available online: https://www.fluorideresearch.org/454/files/FJ2012_v45_n4_p389-393_pq.pdf (accessed on 20 January 2023).
- Zouari, M.; Ahmed, C.B.; Elloumi, N.; Rouina, B.B.; Labrousse, P.; Abdallah, F.B. Effects of Irrigation Water Fluoride on Relative Water Content, Photosynthetic Activity, and Proline Accumulation in Young Olive Trees (Olea europaea L. cv. chemlali) in Arid Zones. Fluoride 2016, 49, 303–372. Available online: https://fluorideresearch.org/493Pt2/files/FJ2016_v49_n3Pt2_p366-372_pq.pdf (accessed on 13 January 2023).
- Cai, H.; Dong, Y.; Li, Y.; Li, D.; Peng, C.; Zhang, Z.; Wan, X. Physiological and cellular responses to fluoride stress in tea (Camellia sinensis) leaves. Acta Physiol. Plant. 2016, 38, 144–155. [Google Scholar] [CrossRef]
- Szostek, R.; Ciećko, Z. Content of Fluorine in Biomass of Crops Depending on Soil Contamination by This Element. Fluoride 2014, 47, 294–306. Available online: https://www.fluorideresearch.org/474/files/FJ2014_v47_n4_p294-306_sfs.pdf (accessed on 23 March 2023).
- Dirwai, T.L.; Senzanje, A.; Mabhaudhi, T. Calibration and evaluation of the FAO AquaCrop Model for Canola (Brassica napus) under varied moistube irrigation regimes. Agriculture 2021, 11, 410. [Google Scholar] [CrossRef]
- Zając, T.; Klimek-Kopyra, A.; Oleksy, A.; Lorenc-Kozik, A.; Ratajczak, K. Analysis of yield and plant traits of oilseed rape (Brassica napus L.) cultivated in temperate region in light of the possibilities of sowing in arid areas. Acta Agrobot. 2016, 69, 1696. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Nakielska, M.; Jończyk, K.; Berbeć, A.K.; Kopiński, J. Assessment of the suitability of 10 winter triticale cultivars (x Triticosecale Wittm. ex A. Camus) for organic agriculture: Polish case study. Agronomy 2020, 10, 1144. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).