Resilience to Terminal Drought, Heat, and Their Combination Stress in Wheat Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Materials
2.2. Field Experiments
2.3. Data Collection
2.4. Statistical Analysis
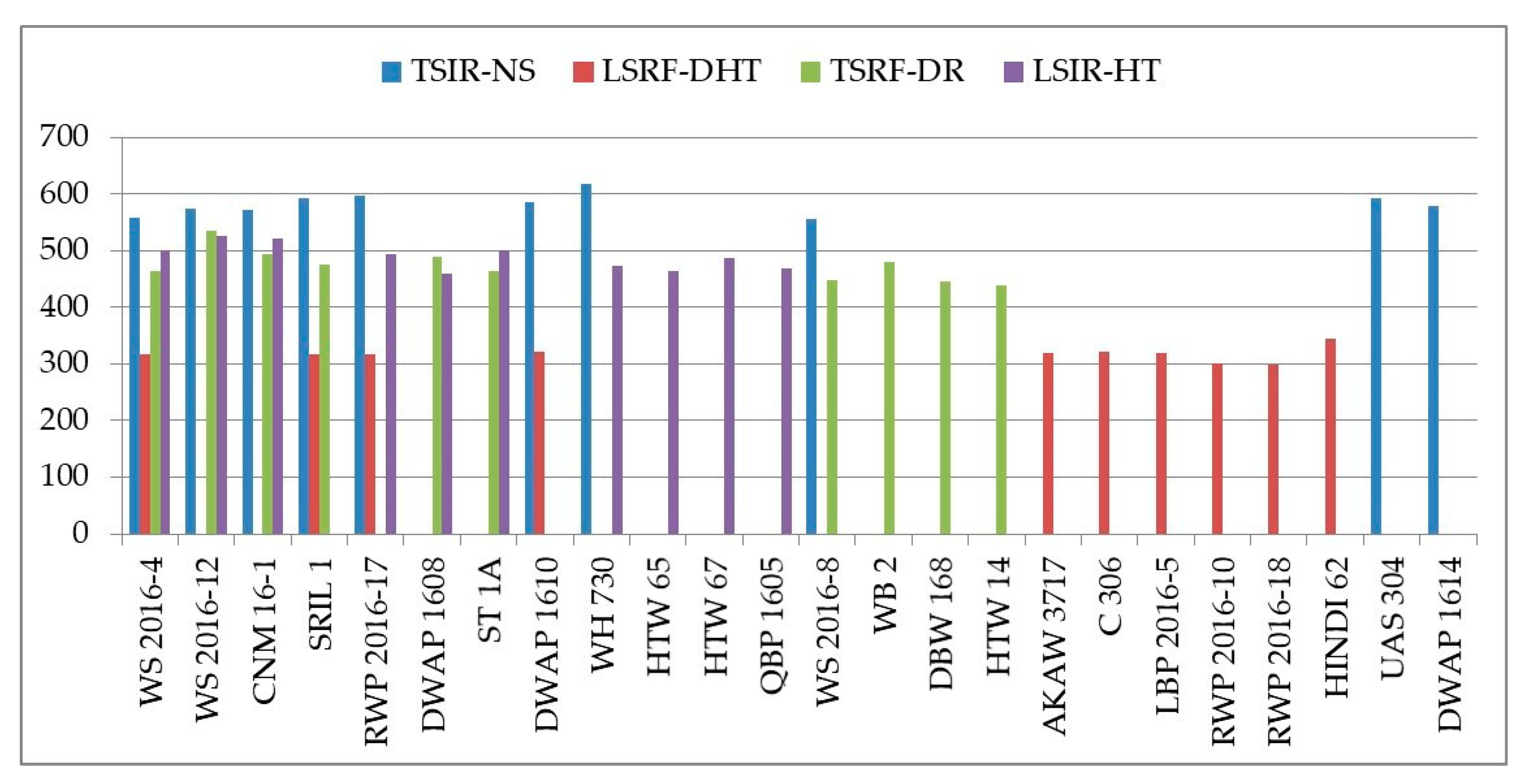
3. Results
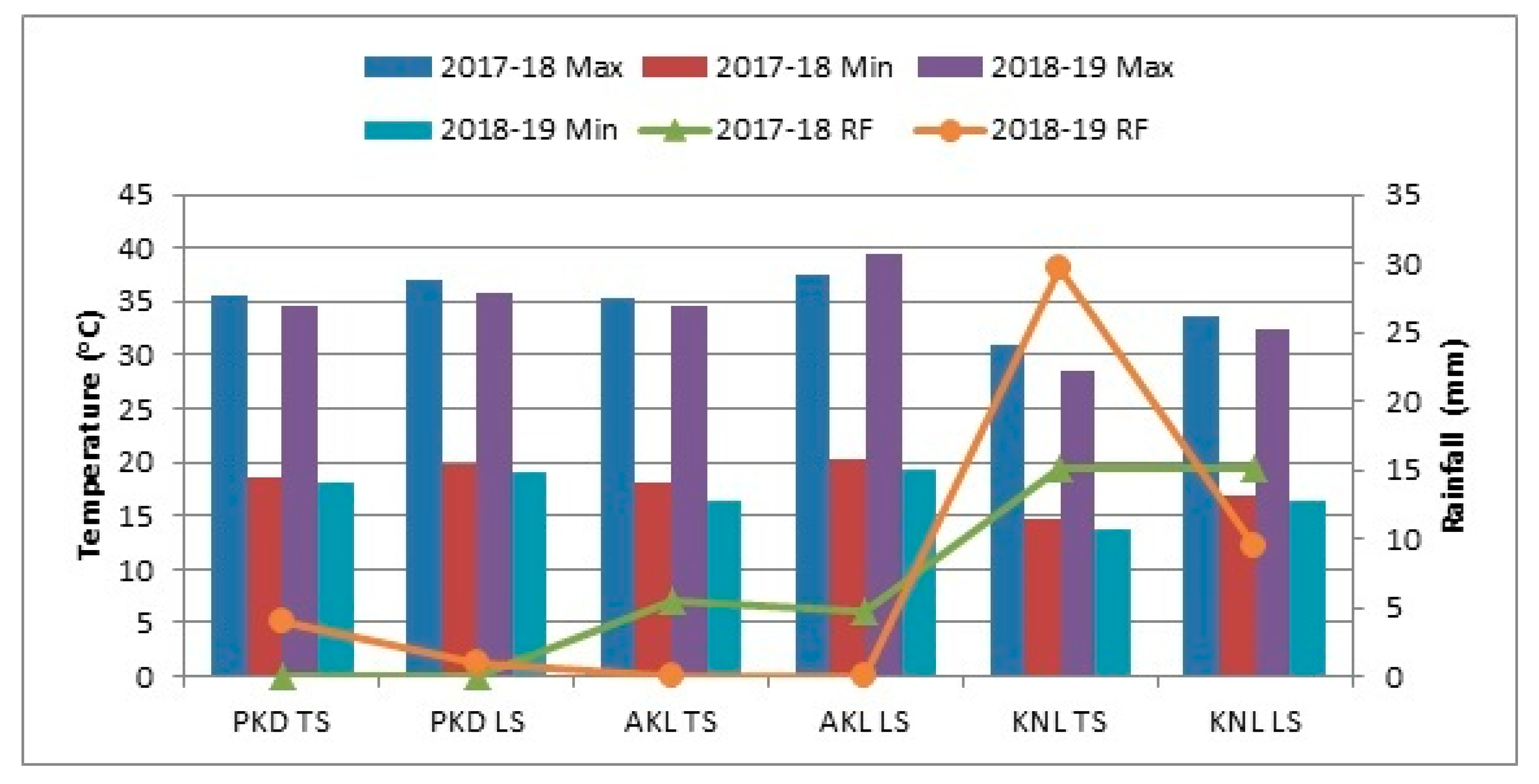
3.1. Exposure to Stress
3.2. Effects of Individual/Combined Heat and Drought Stress on Yield Components
3.3. Correlation among Measured Traits
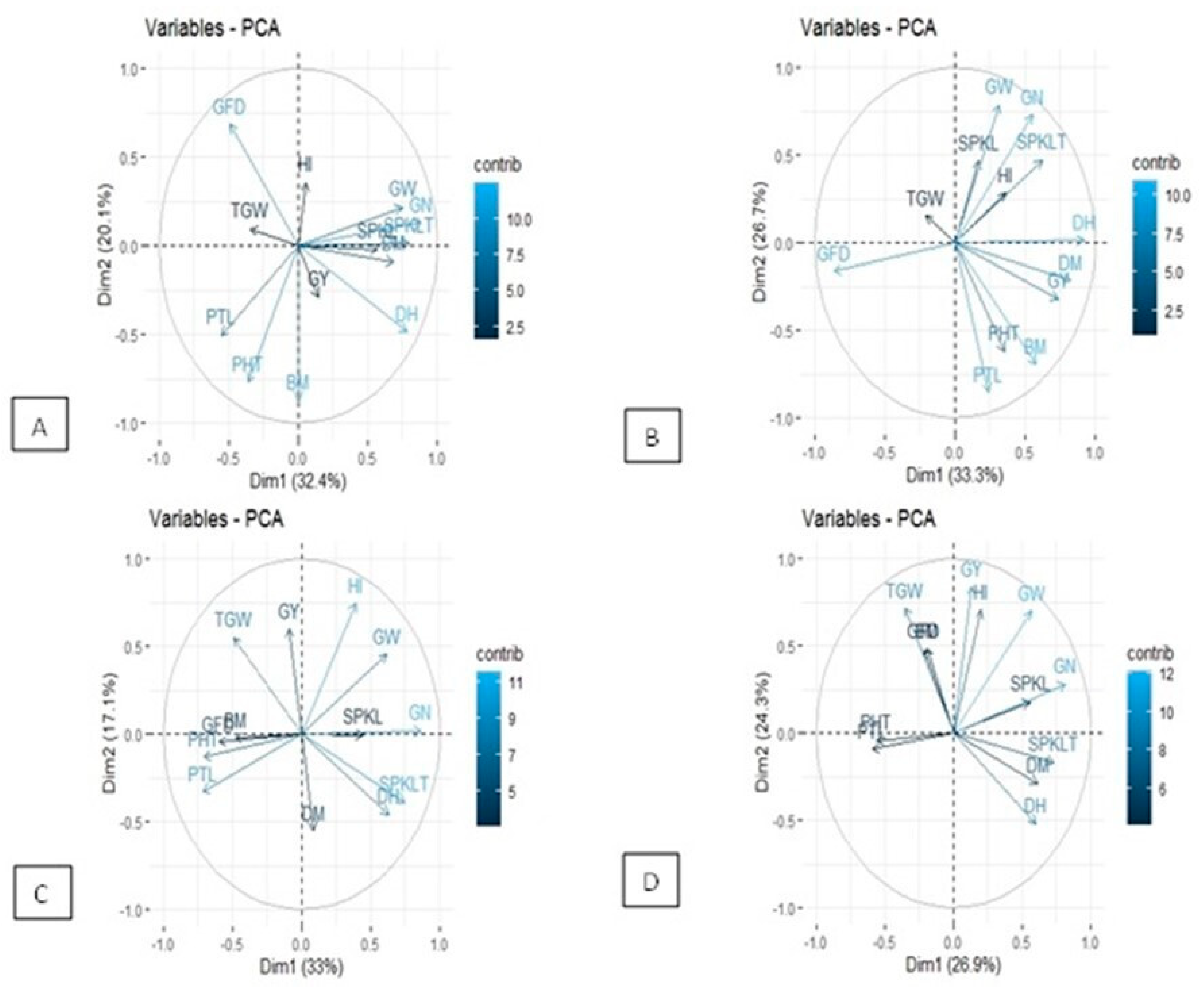
3.4. Principal Component Analysis
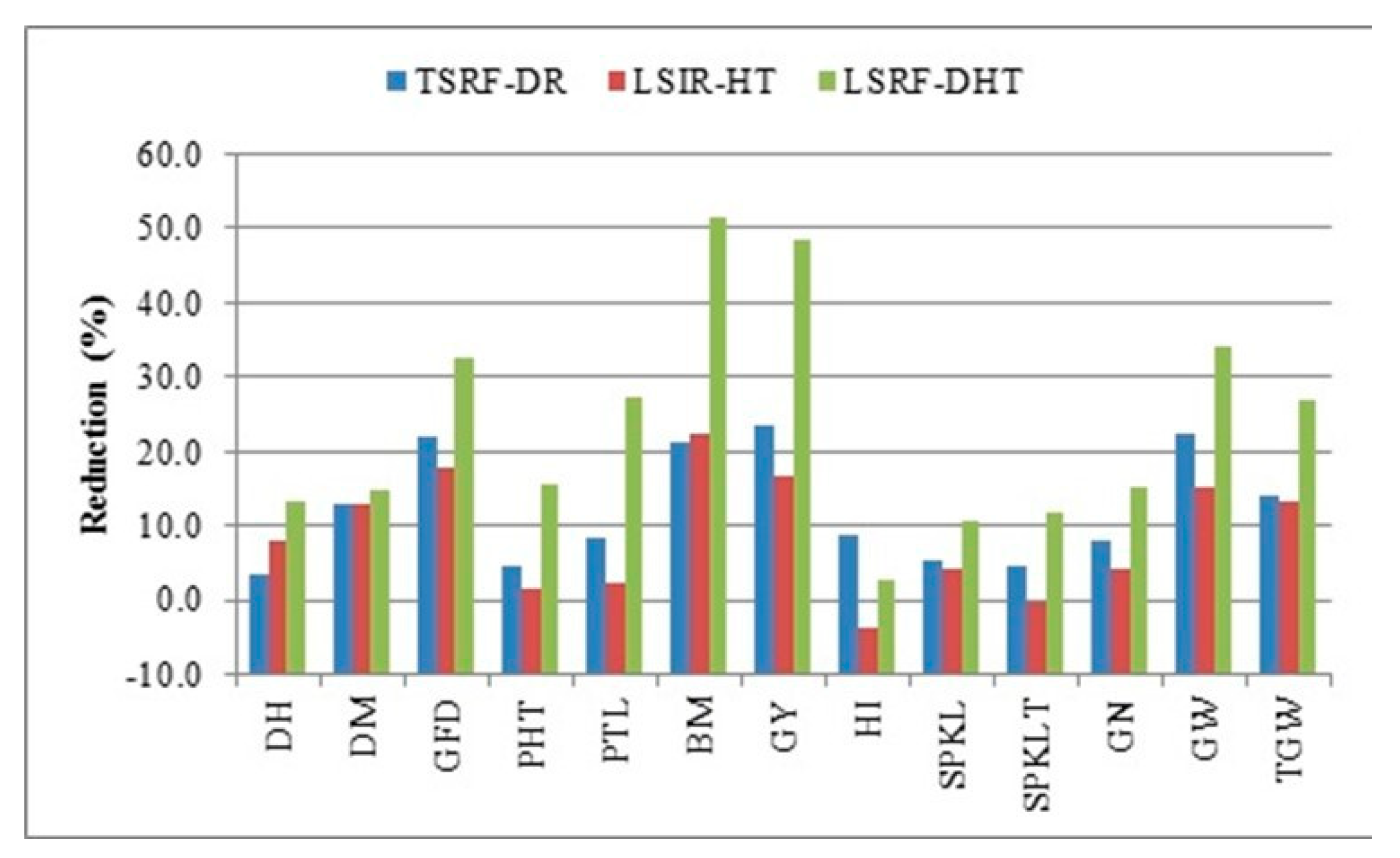
3.5. Contribution of Component Traits to Grain Yield
3.6. Stress Susceptibility and Tolerance Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Raza, A.; Ashraf, F.; Zou, X.; Zhang, X.; Tosif, H. Plant Adaptation and Tolerance to Environmental Stresses: Mechanisms and Perspectives. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I: General Consequences and Plant Responses; Hasanuzzaman, M., Ed.; Springer: Amsterdam, The Netherlands, 2020; pp. 117–145. [Google Scholar] [CrossRef]
- Raza, A.; Mubarik, M.S.; Sharif, R.; Habib, M.; Jabeen, W.; Zhang, C.; Chen, H.; Chen, Z.; Siddique, K.H.M.; Zhuang, W.; et al. Developing drought-smart, ready-to-grow future crops. Plant Genome 2022, e20279. [Google Scholar] [CrossRef] [PubMed]
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Gahlaut, V.; Jaiswal, V.; Tyagi, B.S.; Singh, G.; Sareen, S.; Balyan, H.S.; Gupta, P.K. QTL mapping for nine drought-responsive agronomic traits in bread wheat under irrigated and rain-fed environments. PLoS ONE 2017, 12, e0182857. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.K.; Ramankutty, N.; Mueller, N.D.; West, P.C.; Foley, J.A. Recent patterns of crop yield growth and stagnation. Nat. Commun. 2012, 3, 1293. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Pisipati, S.R.; Momcilovic, I.; Ristic, Z. Independent and combined effects of high temperature and drought stress during grain filling on plant yield and chloroplast protein synthesis elongation factor (EF-Tu) expression in spring wheat. J. Agron. Crop Sci. 2011, 197, 430–441. [Google Scholar] [CrossRef]
- ICAR-IIWBR. Director’s Report of AICRP on Wheat and Barley 2021–2022; Singh, G.P., Ed.; ICAR-Indian Institute of Wheat and Barley Research: Karnal, India, 2022; p. 83.
- Ali, S.; Liu, Y.; Ishaq, M.; Shah, T.; Abdullah; Ilyas, A.; Din, I.U. Climate Change and Its Impact on the Yield of Major Food Crops: Evidence from Pakistan. Foods 2017, 6, 39. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.T.; Yao, Y.T.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef]
- Hill, C.B.; Li, C. Genetic Improvement of Heat Stress Tolerance in Cereal Crops. Agronomy 2022, 12, 1205. [Google Scholar] [CrossRef]
- Atlin, G.N.; Cairns, J.E.; Das, B. Rapid breeding and varietal replacement are critical to adaptation of cropping systems in the developing world to climate change. Glob. Food Secur. 2017, 12, 31–37. [Google Scholar] [CrossRef]
- Cassia, R.; Nocioni, M.; Correa-Aragunde, N.; LaMattina, L. Climate Change and the Impact of Greenhouse Gasses: CO2 and NO, Friends and Foes of Plant Oxidative Stress. Front. Plant Sci. 2018, 9, 273. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Global Warming of 1.5 °C—An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways; in the Context of Strengthening the Global Response to the Threat of Climate Change; Sustainable Development; and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; World Meteorological Organization: Geneva, Switzerland, 2018; 32p. [Google Scholar]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Liu, B.; Asseng, S.; Müller, C.; Ewert, F.; Elliott, J.; Lobell, D.B.; Martre, P.; Ruane, A.C.; Wallach, D.; Jones, J.W.; et al. Similar estimates of temperature impacts on global wheat yield by three independent methods. Nat. Clim. Chang. 2016, 6, 1130–1136. [Google Scholar] [CrossRef]
- Ortiz, R.; Sayre, K.D.; Govaerts, B.; Gupta, R.; Subbarao, G.V.; Ban, T.; Hodson, D.; Dixon, J.M.; Ortiz-Monasterio, J.I.; Reynolds, M. Climate change: Can wheat beat the heat? Agric. Ecosyst. Environ. 2008, 126, 46–58. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Global Synthesis of Drought Effects on Maize and Wheat Production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, M.; Ceglar, A.; Dentener, F.; Toreti, A. Wheat yield loss attributable to heat waves, drought and water excess at the global, national and subnational scales. Environ. Res. Lett. 2017, 12, 064008. [Google Scholar] [CrossRef]
- Aggarwal, P.K. Global Climate Change and Indian Agriculture—Case Studies from the ICAR Network Project; Indian Council of Agricultural Research: New Delhi, India, 2009.
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Ahmad, M. Overexpression of AtWRKY30 Transcription Factor Enhances Heat and Drought Stress Tolerance in Wheat (Triticum aestivum L.). Genes 2019, 10, 163. [Google Scholar] [CrossRef]
- Toreti, A.; Cronie, O.; Zampieri, M. Concurrent climate extremes in the key wheat producing regions of the world. Sci. Rep. 2019, 9, 5493. [Google Scholar] [CrossRef]
- Bhusal, N.; Sarial, A.K.; Sharma, P.; Sareen, S. Mapping QTLs for grain yield components in wheat under heat stress. PLoS ONE 2017, 12, e0189594. [Google Scholar] [CrossRef]
- Pradhan, G.; Prasad, P.V.V.; Fritz, A.K.; Kirkham, M.B.; Gill, B.S. Effects of drought and high temperature stress on synthetic hexaploid wheat. Funct. Plant Biol. 2012, 39, 190–198. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Rizwan, M.S.; Hussain, M.; Jabran, K.; Cheema, M.A. Terminal drought and heat stress alter physiological and biochemical attributes in flag leaf of bread wheat. PLoS ONE 2020, 15, e0232974. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.J. Identification of genetic diversity and mutations in higher plant acquired thermotolerance. Physiol. Plant. 2001, 112, 167–170. [Google Scholar] [CrossRef]
- Alamri, S.; Hu, Y.; Mukherjee, S.; Aftab, T.; Fahad, S.; Raza, A.; Ahmad, M.; Siddiqui, M.H. Silicon-induced postponement of leaf senescence is accompanied by modulation of antioxidative defense and ion homeostasis in mustard (Brassica juncea) seedlings exposed to salinity and drought stress. Plant Physiol. Biochem. 2020, 157, 47–59. [Google Scholar] [CrossRef]
- Li, M.; Yang, Y.; Raza, A.; Yin, S.; Wang, H.; Zhang, Y.; Dong, J.; Wang, G.; Zhong, C.; Zhang, H.; et al. Heterologous expression of Arabidopsis thaliana rty gene in strawberry (Fragaria × ananassa Duch.) improves drought tolerance. BMC Plant Biol. 2021, 21, 57. [Google Scholar] [CrossRef]
- Wasaya, A.; Manzoor, S.; Yasir, T.; Sarwar, N.; Mubeen, K.; Ismail, I.; Raza, A.; Rehman, A.; Hossain, A.; EL Sabagh, A. Evaluation of Fourteen Bread Wheat (Triticum aestivum L.) Genotypes by Observing Gas Exchange Parameters, Relative Water and Chlorophyll Content, and Yield Attributes under Drought Stress. Sustainability 2021, 13, 4799. [Google Scholar] [CrossRef]
- Mondal, S.; Singh, R.P.; Huerta-Espino, J.; Kehel, Z.; Autrique, E. Characterization of Heat- and Drought-Stress Tolerance in High-Yielding Spring Wheat. Crop Sci. 2015, 55, 1552–1562. [Google Scholar] [CrossRef]
- Sukumaran, S.; Reynolds, M.P.; Sansaloni, C. Genome-Wide Association Analyses Identify QTL Hotspots for Yield and Component Traits in Durum Wheat Grown under Yield Potential, Drought, and Heat Stress Environments. Front. Plant Sci. 2018, 9, 81. [Google Scholar] [CrossRef]
- Fischer, R.A.; Maurer, R. Drought resistance in spring wheat cultivars. I. Grain yield responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Fernandez, G.C.J. Effective Selection Criteria for Assessing PLant Stress Toerance. Adaptation of food crops to temperature and water stress. In Proceedings of the International Symposium on Adaptation of Vegetables and other Food Crops in Temperature and Water Stress, Shanhua, Taiwan, 13–16 August 1992; pp. 257–270. [Google Scholar]
- SAS Institute. SAS, Version 9; SAS Institute: Cary, NC, USA, 2014. [Google Scholar]
- R Core Team. Rstudio: Integrated Development for R; Rstudio, Pbc: Boston, MA, USA; Available online: http://www.rstudio.com (accessed on 9 September 2022).
- Acevedo, E.; Silva, P.; Silva, H. Wheat growth and physiology. In Bread Wheat: Improvement and Production; Curtis, B.C., Ed.; (FAO Plant Production and Protection Series No. 30); Food and agriculture organization of the United Nations: Rome, Italy, 2002; p. 567. [Google Scholar]
- Hakim, M.A.; Hossain, A.; Teixeira da Silva, J.A.; Zvolinsky, V.P.; Khan, M.M. Yield; protein and starch content of 20 wheat Triticum aestivum L. genotypes exposed to high temperature under late sowing conditions. J. Sci. Res. 2012, 4, 477–489. [Google Scholar] [CrossRef]
- Khan, M.A.; Tahir, A.; Khurshid, N.; Husnain, M.I.U.; Ahmed, M.; Boughanmi, H. Economic Effects of Climate Change-Induced Loss of Agricultural Production by 2050: A Case Study of Pakistan. Sustainability 2020, 12, 1216. [Google Scholar] [CrossRef]
- Aparicio, A.G.; Zuki, S.M.; Azpilicueta, M.M.; Barbero, F.Á.; Pastorino, M.J. Genetic versus environmental contributions to variation in seedling resprouting in Nothofagus obliqua. Tree Genet. Genomes 2015, 11, 23. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Qureshi, R.; Shaheen, H. Effects of Pre-Anthesis Drought, Heat and Their Combination on the Growth, Yield and Physiology of diverse Wheat (Triticum aestivum L.) Genotypes Varying in Sensitivity to Heat and drought stress. Sci. Rep. 2019, 9, 6955. [Google Scholar] [CrossRef]
- García, G.A.; Hasan, A.K.; Puhl, L.E.; Reynolds, M.P.; Calderini, D.F.; Miralles, D.J. Grain Yield Potential Strategies in an Elite Wheat Double-Haploid Population Grown in Contrasting Environments. Crop Sci. 2013, 53, 2577–2587. [Google Scholar] [CrossRef]
- Kirigwi, F.M.; Van Ginkel, M.; Brown-Guedira, G.; Gill, B.S.; Paulsen, G.M.; Fritz, A.K. Markers associated with a QTL for grain yield in wheat under drought. Mol. Breed. 2007, 20, 401–413. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Qureshi, R.; Shaheen, H.; Shafqat, N. Genome-wide association analyses for yield and yield-related traits in bread wheat (Triticum aestivum L.) under pre-anthesis combined heat and drought stress in field conditions. PLoS ONE 2019, 14, e0213407. [Google Scholar] [CrossRef]
- Sareen, S.; Tyagi, B.S.; Sarial, A.K.; Tiwari, V.; Sharma, I. Trait analysis, diversity, and genotype × environment interaction in some wheat landraces evaluated under drought and heat stress conditions. Chil. J. Agric. Res. 2014, 74, 135–142. [Google Scholar] [CrossRef]
- Fleitas, M.C.; Mondal, S.; Gerard, G.S.; Hernández-Espinosa, N.; Singh, R.P.; Crossa, J.; Guzmán, C. Identification of CIMMYT spring bread wheat germplasm maintaining superior grain yield and quality under heat-stress. J. Cereal Sci. 2020, 93, 102981. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Kro’l, A. The Growth and Water Uptake by Yellow Seed and Black Seed Rape Depending on the State of Soil Compaction. Ph.D. Thesis, Bohdan Dobrzański Institute of Agrophysics PAS, Lublin, Poland, 2013. [Google Scholar]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate Trends and Global Crop Production Since. Science 2011, 333, 616–620. [Google Scholar] [CrossRef]
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiol. Plant. 2021, 171, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Sita, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.; Nayyar, H. Effects of drought; heat and their interaction on the growth; yield and photosynthetic function of lentil Lens culinaris Medikus genotypes varying in heat and drought sensitivity. Front. Plant Sci. 2017, 8, 1776. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qin, J.; Tian, X.; Xu, S.; Wang, Y.; Li, H.; Wang, X.; Peng, H.; Yao, Y.; Hu, Z.; et al. Global profiling of alternative splicing landscape responsive to drought; heat and their combination in wheat Triticum aestivum L. Plant Biotechnol. J. 2018, 16, 714–726. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Pellegrineschi, A.; Skovmand, B. Sink-limitation to yield and biomass: A summary of some investigations in spring wheat. Ann. Appl. Biol. 2005, 146, 39–49. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Charagh, S.; García-Caparrós, P.; Rahman, M.A.; Ogwugwa, V.H.; Saeed, F.; Jin, W. Melatonin-mediated temperature stress tolerance in plants. GM Crops Food 2022, 13, 196–217. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Skovmand, B.; Trethowan, R.; Pfeiffer, W. Evaluating a Conceptual Model for Drought Tolerance in Molecular Approaches for Genetic Improvement of Cereals for Stable Production in Water-Limited Environments; Ribaut, J.-M., Poland, D., Eds.; CIMMYT: Veracruz, Mexico, 2000; pp. 49–53. [Google Scholar]
- Hossain, A.; da Silva, J.A.T.; Lozovskaya, M.V.; Zvolinsky, V.P. High temperature combined with drought affect rainfed spring wheat and barley in South-Eastern Russia: I. Phenology and growth. Saudi J. Biol. Sci. 2012, 19, 473–487. [Google Scholar] [CrossRef]
- Yousaf, M.I.; Maize, A.M.; Maize, W.A. Contribution of Spike-Related Traits for Grain Yield in Spring Wheat. J. Agric. Basic Sci. 2017, 2, 2710–2714. [Google Scholar]
- Leilah, A.; Al-Khateeb, S. Statistical analysis of wheat yield under drought conditions. J. Arid. Environ. 2005, 61, 483–496. [Google Scholar] [CrossRef]
- Mohammadi, R.; Farshadfar, E.; Amri, A. Path analysis of genotype × environment interactions in rainfed durum wheat. Plant Prod. Sci. 2016, 19, 43–50. [Google Scholar] [CrossRef]
- Lopes, M.S.; Reynolds, M.P.; Manes, Y.; Singh, R.P.; Crossa, J.; Braun, H.J. Genetic yield gains and changes in associated traits of CIMMYT spring bread wheat in a “Historic” set representing 30 years of breeding. Crop Sci. 2012, 52, 1123–1131. [Google Scholar] [CrossRef]
- Pandey, A.K.; Mishra, V.K.; Chand, R.; Navathe, S.; Budhlakoti, N.; Srinivasa, J.; Sharma, S.; Joshi, A.K. Crosses with spelt improve tolerance of South Asian spring wheat to spot blotch, terminal heat stress, and their combination. Sci. Rep. 2021, 11, 6017. [Google Scholar] [CrossRef]
- Mondal, S.; Singh, R.; Mason, E.; Huerta-Espino, J.; Autrique, E.; Joshi, A. Grain yield, adaptation and progress in breeding for early-maturing and heat-tolerant wheat lines in South Asia. Field Crop. Res. 2016, 192, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Campuzano, G.; Miralles, D.J.; Slafer, G.A. Genotypic variability and response to water stress of pre- and post-anthesis phases in triticale. Eur. J. Agron. 2008, 28, 171–177. [Google Scholar] [CrossRef]
- Yin, X.; Guo, W.; Spiertz, J.H. A quantitative approach to characterize sink–source relationships during grain filling in contrasting wheat genotypes. Field Crops Res. 2009, 114, 119–126. [Google Scholar] [CrossRef]
- Bergkamp, B.; Impa, S.; Asebedo, A.; Fritz, A.; Jagadish, S.K. Prominent winter wheat varieties response to post-flowering heat stress under controlled chambers and field based heat tents. Field Crop. Res. 2018, 222, 143–152. [Google Scholar] [CrossRef]
- Madani, A.; Rad, A.S.; Pazoki, A.; Nourmohammadi, G.; Zarghami, R. Wheat Triticum aestivum L grain filling and dry matter partitioning responses to source: Sink modifications under post-anthesis water and nitrogen deficiency. Acta Scientiarum. Agron. 2010, 32, 145–151. [Google Scholar]
- Shirdelmoghanloo, H.; Cozzolino, D.; Lohraseb, I.; Collins, N.C. Truncation of grain filling in wheat (Triticum aestivum) triggered by brief heat stress during early grain filling: Association with senescence responses and reductions in stem reserves. Funct. Plant Biol. 2016, 43, 919–930. [Google Scholar] [CrossRef]
- Wei, T.-M.; Chang, X.-P.; Min, D.-H.; Jing, R.-L. Analysis of Genetic Diversity and Trapping Elite Alleles for Plant Height in Drought-Tolerant Wheat Cultivars. Acta Agron. Sin. 2010, 36, 895–904. [Google Scholar] [CrossRef]
- Talukder, S.K.; Babar, A.; Vijayalakshmi, K.; Poland, J.; Prasad, P.V.V.; Bowden, R.; Fritz, A. Mapping QTL for the traits associated with heat tolerance in wheat (Triticum aestivum L.). BMC Genet. 2014, 15, 97. [Google Scholar] [CrossRef]
- Rehman, H.; Tariq, A.; Ashraf, I.; Ahmed, M.; Muscolo, A.; Basra, S.; Reynolds, M. Evaluation of Physiological and Morphological Traits for Improving Spring Wheat Adaptation to Terminal Heat Stress. Plants 2021, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Talukder, A.S.M.H.M.; McDonald, G.K.; Gill, G.S. Effect of short-term heat stress prior to flowering and at early grain set on the utilization of water-soluble carbohydrate by wheat genotypes. Field Crops Res. 2013, 147, 1–11. [Google Scholar] [CrossRef]
- Garg, D.; Sareen, S.; Dalal, S.; Tiwari, R.; Singh, R. Grain filling duration and temperature pattern influence on the performance of wheat genotypes under late planting. Cereal Res. Commun. 2013, 41, 500–507. [Google Scholar] [CrossRef]
- Mwadzingeni, L.; Shimelis, H.; Tesfay, S.; Tsilo, T.J. Screening of Bread Wheat Genotypes for Drought Tolerance Using Phenotypic and Proline Analyses. Front. Plant Sci. 2016, 7, 1276. [Google Scholar] [CrossRef]
- Griffiths, C.A.; Reynolds, M.P.; Paul, M.J. Combining yield potential and drought resilience in a spring wheat diversity panel. Food Energy Secur. 2020, 9, e241. [Google Scholar] [CrossRef] [PubMed]
- Cattivelli, L.; Rizza, F.; Badeck, F.-W.; Mazzucotelli, E.; Mastrangelo, A.M.; Francia, E.; Marè, C.; Tondelli, A.; Stanca, A.M. Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Field Crop. Res. 2008, 105, 1–14. [Google Scholar] [CrossRef]
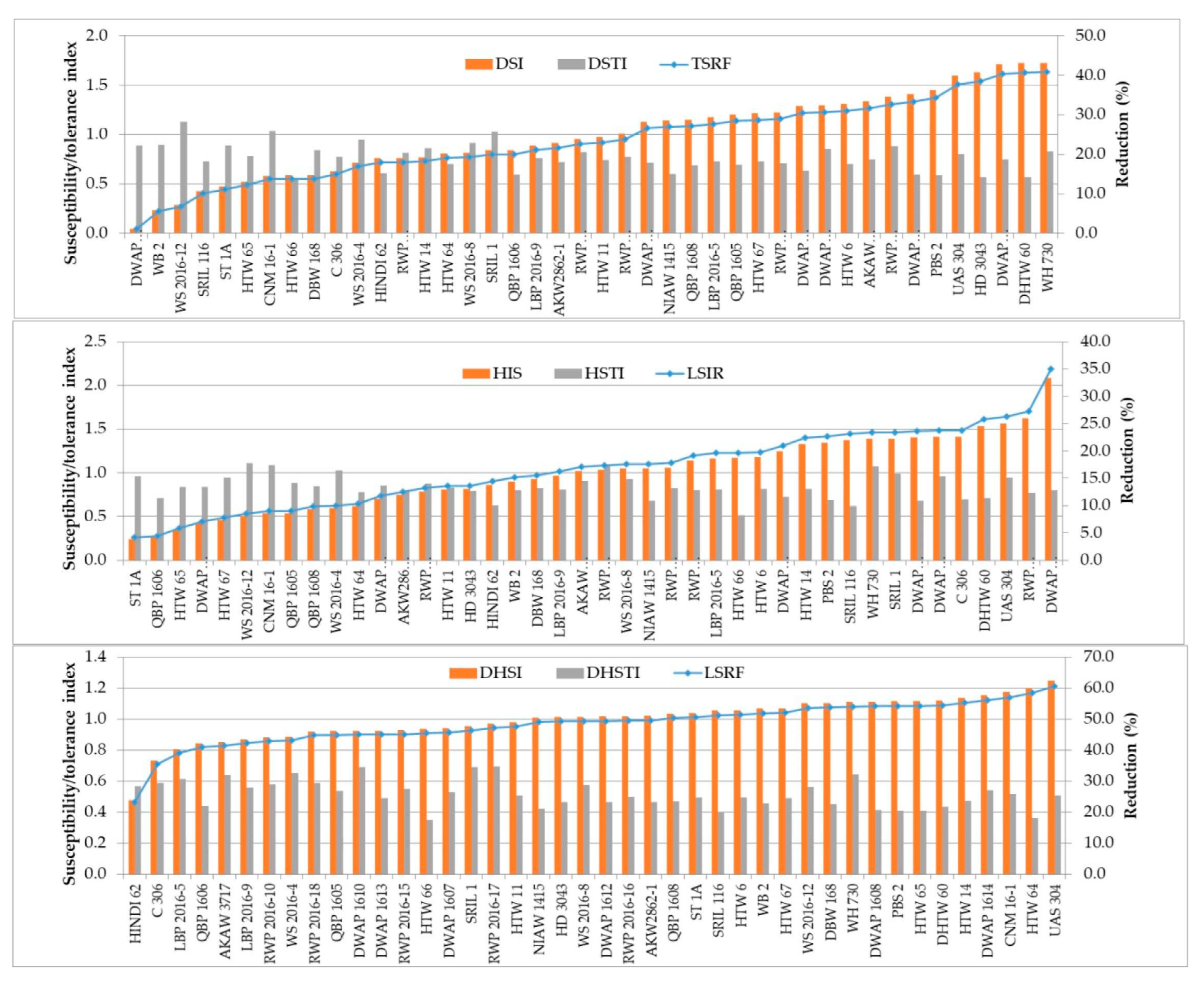

| Genotype | Stress | Susceptibility Index | Grain Yield Rank | Trait | |
|---|---|---|---|---|---|
| Stress | TSIR-NS | ||||
| WS 2016-12 | TSRF-DR | 0.29 | 1 | 7 | GFD, PTL, HI, GN, GW (Escape) |
| CNM 16-1 | TSRF-DR | 0.58 | 2 | 8 | HI, GW |
| DWAP 1608 | TSRF-DR | 0.04 | 3 | 33 | GFD, HI, GN, GW (Escape) |
| WB 2 | TSRF-DR | 0.23 | 4 | 27 | DH, DM, PTL, HI, GN, GW (Escape) |
| ST 1A | TSRF-DR | 0.47 | 6 | 19 | DH, DM, PTL, GN, GW (Escape) |
| DBW 168 | TSRF-DR | 0.59 | 9 | 21 | PTL, HI, GN, GW |
| HTW 65 | TSRF-DR | 0.52 | 11 | 35 | DM, PTL, HI, GN, GW (Escape) |
| C 306 | TSRF-DR | 0.63 | 13 | 32 | DH, DM, GFD, PTL, GN, GW (Escape) |
| SRIL 116 | TSRF-DR | 0.43 | 14 | 39 | DH, DM, PTL, HI, (Escape) |
| HTW 66 | TSRF-DR | 0.59 | 35 | 42 | DM, GFD, PTL, HI, GW (Escape) |
| WS 2016-12 | LSIR-HT | 0.51 | 1 | 7 | GFD.GN (Escape) |
| CNM 16-1 | LSIR-HT | 0.53 | 2 | 8 | PTL, TGW |
| WS 2016-4 | LSIR-HT | 0.60 | 3 | 9 | DH, DM, (Escape) |
| ST 1A | LSIR-HT | 0.25 | 4 | 19 | HI, GN |
| HTW 67 | LSIR-HT | 0.46 | 6 | 14 | HI, GN, TGW |
| QBP 1605 | LSIR-HT | 0.54 | 8 | 22 | DH, PTL, GN, TGW (Escape) |
| HTW 65 | LSIR-HT | 0.35 | 9 | 35 | DM, GFD, GN (Escape) |
| DWAP 1608 | LSIR-HT | 0.42 | 10 | 28 | PTL, GN |
| QBP 1608 | LSIR-HT | 0.58 | 12 | 28 | GFD, TGW (Escape) |
| QBP 1606 | LSIR-HT | 0.27 | 25 | 40 | DH, (Escape) |
| HINDI 62 | LSIR-DHT | 0.48 | 1 | 41 | PTL, HI |
| C 306 | LSIR-DHT | 0.73 | 3 | 32 | PTL, HI, GN, GW |
| AKAW 3717 | LSIR-DHT | 0.85 | 4 | 11 | PTL, HI, |
| LBP 2016-5 | LSIR-DHT | 0.80 | 5 | 17 | DH, DM, GFD, HI (Escape) |
| WS 2016-4 | LSIR-DHT | 0.89 | 6 | 9 | DH, DM, PTL, GN (Escape) |
| RWP 2016-10 | LSIR-DHT | 0.88 | 9 | 16 | DH, DM, HI, GN, GW (Escape) |
| RWP 2016-18 | LSIR-DHT | 0.92 | 10 | 12 | HI, GN, GW |
| LBP 2016-9 | LSIR-DHT | 0.87 | 11 | 25 | HI, GN |
| QBP 1605 | LSIR-DHT | 0.92 | 13 | 22 | DH, DM, PTL, GW (Escape) |
| QBP 1606 | LSIR-DHT | 0.84 | 20 | 40 | DM, GFD, PTL, GN, GW (Escape) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sareen, S.; Budhlakoti, N.; Mishra, K.K.; Bharad, S.; Potdukhe, N.R.; Tyagi, B.S.; Singh, G.P. Resilience to Terminal Drought, Heat, and Their Combination Stress in Wheat Genotypes. Agronomy 2023, 13, 891. https://doi.org/10.3390/agronomy13030891
Sareen S, Budhlakoti N, Mishra KK, Bharad S, Potdukhe NR, Tyagi BS, Singh GP. Resilience to Terminal Drought, Heat, and Their Combination Stress in Wheat Genotypes. Agronomy. 2023; 13(3):891. https://doi.org/10.3390/agronomy13030891
Chicago/Turabian StyleSareen, Sindhu, Neeraj Budhlakoti, K K Mishra, Swati Bharad, N R Potdukhe, Bhudeva Singh Tyagi, and Gyanendra Pratap Singh. 2023. "Resilience to Terminal Drought, Heat, and Their Combination Stress in Wheat Genotypes" Agronomy 13, no. 3: 891. https://doi.org/10.3390/agronomy13030891
APA StyleSareen, S., Budhlakoti, N., Mishra, K. K., Bharad, S., Potdukhe, N. R., Tyagi, B. S., & Singh, G. P. (2023). Resilience to Terminal Drought, Heat, and Their Combination Stress in Wheat Genotypes. Agronomy, 13(3), 891. https://doi.org/10.3390/agronomy13030891








