Abstract
Given the ecological and epidemiological specialization of Hyalesthes obsoletus, the principle vector of ‘Candidatus Phytoplasma solani’, the primary objective of this study was to develop molecular tools for discriminating three host plant associations of the vector populations: (i) Convolvulus arvensis–Urtica dioica (Ca–Ud), (ii) Vitex agnus-castus (Vac), and (iii) Crepis foetida (Cf). The genetic diversity of the nearly full-length mitochondrial COI gene (1467 bp) was analyzed and compared among previously reported and newly collected individuals of the three host plant associations on a wide geographic range. Multiplex PCR was designed and evaluated for discriminating H. obsoletus host plant associations based on the size of amplified fragments: 1084 bp for the Cf association, 645 bp for the Ca–Ud association, and 355 bp for the Vac association. Examples of the epidemiological value of combining data on the genetic characteristics of the vector and the pathogen are provided. The method is intended to facilitate an accurate identification of the vector’s phylogenetic lineage, natural host plant preference, and epidemiological transmission routes of ‘Ca. P. solani’. When applied to H. obsoletus specimens collected from cultivated plants within an agroecosystem and combined with ‘Ca. P. solani’ genotyping, the method should provide valuable information on disease epidemiology, source(s) of emergence, and transmission routes.
1. Introduction
The use of molecular methods to describe, determine, or precisely identify biological species has been one of the most notable advantages of their incorporation into taxonomical or applied biological studies (e.g., [1,2,3,4]). Molecular determination and identification of species type material (e.g., holotype, paratype/s, syntype/s) (e.g., [5,6]), cryptic species characterized by the absence of stable morphological traits for identification, and ecological host races or biotypes resulting from ecological specialization have increased the value of fundamental and applied taxonomic studies and natural world research [7,8]. This is due to the fact that molecular methods can acquire an enormous amount of data—much more than those available in the morphological characteristics of the species, i.e., select the species-specific ones, and define them as the reliable and repeatable tool for identification. Combined with other characteristics of the species in question (morphology, biology, geography, ecology, etc.), molecular methods are especially useful and valuable [9]. When dealing with the need for a precise, specific, and reliable determination and identification of agricultural pests, beneficial organisms, or endangered species, molecular methods have frequently proven to be both reliable and the most cost-effective option (e.g., [10,11,12,13,14]). In addition, the overall limited availability of traditional taxonomists’ expertise on the identification of specific groups of organisms or species has been cited repeatedly as one of the primary reasons for the use of molecular identification methods, DNA barcoding, molecular taxonomy, or even better, an integrative taxonomy, which can lead to the most precise molecular tools for identification [1,9,15].
The cixiid planthopper Hyalesthes obsoletus Signoret, 1865 (Hemiptera: Cixiidae) is a primary natural vector of ‘Candidatus (Ca.) Phytoplasma solani’ (‘Ca. P. solani’)—a major phytoplasma pathogen of agricultural crops in Europe, previously known under its trivial name, “stolbur phytoplasma” [16,17,18]. ‘Ca. P. solani’ affects many economically important plants, causing significant yield losses and increases in agricultural production costs [19], while also occurring in plants in natural environments bordering agricultural fields as reservoirs and inoculum sources [20,21,22,23,24]. ‘Ca. P. solani’ primarily affects all major grape-growing regions in Europe and the Middle East, the lavender fields of France, the maize fields of Serbia, and various solanaceous crops in Western, Central, and Southeastern Europe, Asia Minor, and Transcaucasia [17,25,26,27,28,29,30,31,32,33,34,35,36,37]. In the epidemiological context of ‘Ca. P. solani’ transmission and emergence, H. obsoletus is a major vector of the most prevalent phytoplasma disease of grapevine, the Bois noir (BN) [16,21,38], the vector of lavender decline [33], and has also been shown to vector ‘Ca. P. solani’ to sugar beet, potato, pepper, and natural reservoir plants serving as the vector’s host plant [38,39,40,41]. In the last two decades, several other cixiid planthoppers have been identified as vectors of ‘Ca. P. solani’, namely Pentastiridius leporinus (Linnaeus, 1761), Reptalus panzeri (Löw, 1883), and Reptalus artemisiae (Becker, 1865) (previously known as R. quinquecostatus (Dufour, 1833) [42]) [26,27,29,43,44,45,46,47].
Efforts to design the tools for the molecular identification of ‘Ca. P. solani’ planthopper vectors have so far been made only in three cases: for the differentiation of congeneric species with clearly discriminative morphological features on the male genitalia but a lack of distinctive traits for nymphs and females [10,11], and for the identification and discrimination of three species from distinct genera [48] whose outer morphological features allow differentiation, including both males and females (e.g., [49,50]). The former case develops molecular identification tools for four Reptalus species: R. artemisiae, R. cuspidatus (Fieber, 1876), R. panzeri, and R. quinquecostatus (Dufour, 1833) (previously known as R. melanochaetus (Fieber, 1876) [42]) [11], and three Hyalesthes species (H. obsoletus, H. luteipes Fieber, 1876, and H. scotti Ferrari, 1882) [10], while the latter case develops tools for molecular identification and the discrimination of cixiid vectors in sugar beet fields (H. obsoletus, R. artemisiae, and P. leporinus) [48]. In all three cases, methods were designed to allow scientists and plant protection practitioners to conduct epidemiological surveys, trace transmission pathways, obtain information on the host plant feeding preferences of immature stages of the vectors, and develop more effective control and management strategies for ‘Ca. P. solani’-induced plant diseases.
The need for the molecular identification of H. obsoletus individuals and/or populations is more demanding and complex, due to a number of previously established facts: (i) H. obsoletus is a major vector of ‘Ca. Phytoplasma solani’, and a key vector of BN disease of grapevine [16,17,20,21,30,32,34,38,51,52,53]; (ii) H. obsoletus populations show clear signs of host plant-associated specialization and genetic differentiation, leading to the formation of host races [54,55] or cryptic species [56]; (iii) cryptic species of H. obsoletus are morphologically indistinguishable, but delineated by host plant choice and often found in sympatry [38,56], and iv) H. obsoletus host associations show signs of driving segregated epidemiological pathways of ‘Ca. P. solani’ transmission, i.e., separated epidemiological cycles and routes of pathogen dissemination [21,38,47]. In Southeastern Europe, the distribution center of H. obsoletus and the area of most ‘Ca. P. solani’-inflicted crop diseases, specific host plant associations of the vector, and vector-based transmission routes are determined. These are represented by three phylogenetic plant-associated lineages of vector populations that are ecologically delineated by host plant preference as follows: (i) Convolvulus arvensis and Urtica dioica, (ii) Vitex agnus-castus, and (iii) Crepis foetida [56]. In addition, even though the geographic overlapping of the host plant-associated vector populations is evident, there are additional signs of geographic segregation of H. obsoletus populations associated with V. agnus-castus in Greece and in Montenegro [56]. All three cryptic species, or host plant associations, are evidenced as vectors of ‘Ca. P. solani’ (reviewed in [47]). Moreover, the genetic divergence of ‘Ca. P. solani’ strains vectored by H. obsoletus [20] was the first indication of the ecological segregation of vector populations associated with the pathogen’s reservoir plants. Strains of ‘Ca. P. solani’ were initially identified as genetically distinct using tuf gene typing of isolates associated with U. dioica (tuf-a type) and C. arvensis (tuf-b type) [20], and later verified using several other epidemiologically informative genes (secY, vmp1, and stamp) that led to the discovery of more complex strain diversification [57,58,59,60].
Given the ecological and epidemiological specialization of H. obsoletus populations, the primary objective of our study was to develop and evaluate molecular methods of discrimination for the three host plant-associated vector populations (i.e., cryptic species). We intended to design a method that would be reliable, simple, and cost-effective enough to be used routinely. Furthermore, we aimed to link epidemiological data on the genetic characteristics of vector populations to the specific strains of ‘Ca. P. solani’ that they harbor, as well as to combine this with information on host plant association and geographic area of origin.
2. Materials and Methods
2.1. Hyalesthes obsoletus Specimens Used for mtDNA COI Genotyping
For this study we used previously reported DNA material of H. obsoletus individuals originating from plant-specialized populations across a wide geographical area [56] as well as newly collected specimens. From the previously reported material, we used the DNA of selected specimens collected in association with one of the following host plants: C. arvensis, U. dioica, V. agnus-castus, and C. foetida. In total, 55 individuals were chosen for mitochondrial (mtDNA) cytochrome oxidase subunit I (COI) gene characterization and as the basis for the development of a protocol for specific identification of H. obsoletus host plant association (Table 1). The specimens were chosen to represent the majority of the COI-COII gene region diversity identified in our earlier study [56], as well as the geographic variability. They were selected to represent 26 of the 29 COI-COII haplotypes identified in our study, out of a total of 43 up-to-date known haplotypes [56]. These are the H. obsoletus haplotypes identified in association with their natural host plants in Southeastern Europe and Turkey, i.e., the geographic area where at least two out of three host-specialized H. obsoletus lineages have been shown to co-occur in sympatry. The remaining three haplotypes were excluded from analyses because they were collected from crop plants. The other 14 COI-COII haplotypes not included in analyses originate from either the Western European BB COI-COII/16S-ND1phylogeographic haplogroup or Israel.

Table 1.
List of 55 Hyalesthes obsoletus specimens analyzed for genetic diversity of near full-length mitochondrial COI marker gene sorted according to affiliated haplotype and information on their previously determined affiliation to COI-COII (16S-ND1) haplotype, and country, locality, and host plant association of population origin [56].
During 2018 and 2019, additional surveys and a sampling of H. obsoletus populations were conducted to gain a more comprehensive understanding of haplotype diversity and to further examine the genetic segregation of plant-specialized populations in Greece versus Montenegro. The majority of the sampling was performed on the western coast of mainland Greece and on the Peloponnese, with a focus on populations associated with V. agnus-castus. In addition, specimens were collected from U. dioica in the southern Peloponnese, V. agnus-castus on Krk island in the northern Adriatic Sea (Croatia), and Convolvulus cantabrica in Southeastern Serbia, a novel potential host plant of H. obsoletus (Table 2). Six to twenty specimens were collected per location and host plant, and screened for COI genetic diversity. Unique specimens carrying distinct haplotypes per location were chosen for genetic comparison. In total, nine new localities were surveyed, resulting in the addition of 20 extra H. obsoletus individuals for COI gene diversity analysis. Newly collected specimens were kept in 96% ethanol and stored at −20 °C until DNA extraction. Total DNA was extracted from each individual insect specimen using the previously described non-destructive sodium dodecyl sulfate (SDS) extraction method [56].

Table 2.
List of 20 newly collected specimens of Hyalesthes obsoletus analyzed for genetic diversity of near full-length mitochondrial COI marker gene sorted according to affiliated haplotype and information on their country, region, location, and host plant of collection.
Several mitochondrial markers, including the COI-COII and 16S-ND1 gene regions as well as COI gene fragments, have been utilized in previous studies for genetic identification or phylogenetic analyses of H. obsoletus [10,38,54,56,60,61,62]. However, we have chosen a nearly full-length COI gene for this study because it encompasses a barcoding region that is widely used for species molecular identification [1,7,8,15,63], and the gene’s length (approximately 1550 bp) is sufficient for the analysis of a longer fragment of a single gene to provide the required amount of data for primer design and method development (e.g., [64]). In addition, we aimed to correlate previously obtained information on the diversity of COI-COII and 16S-ND1 H. obsoletus haplotypes [56] with the diversity of the COI gene, so that future research can rely on these results regardless of the marker employed.
2.2. PCR Amplification and Sequencing of COI Gene
The nearly full-length COI gene was amplified from the DNA extracts of 75 individuals of H. obsoletus using a combination of four previously published [64,65] and three newly designed primers, resulting in overlapping amplicons that allowed us to read each nucleotide position at least twice. LCO1490 [65] and PAT (i.e., TL2-N-3014) primers [64] were used to amplify ~1500 bp-long fragment, LCO1490 and HCO2198 primer pair [65] was used to generate ~700 bp-long amplicons on the 5′-end of the gene, and CO1-RLR (i.e., C1-J-2195) and PAT primers [64] were used to amplify ~900 bp-long fragment on the 3′-end. After obtaining the initial sequences of the nearly complete COI gene of H. obsoletus, the identities of primer sequences and their binding positions were compared. In response, we designed three new primers for use in amplification and/or sequencing reactions. HCO2198-Ho (5′-TAAACTTCTGGATGACCAAAGAATCA-3′) was designed to be used as a reverse primer in place of HCO2198 [65] in amplification reactions, whereas HCOf-Ho (5’-TGATTCTTTGGTCATCCAGAAGTTTA-3′) was designed as the reverse complement of HCO2198-Ho for sequencing reactions. The third primer, PAT-Ho (5′-TTCATAGCACTTTTCTGCCATTTTA-3′), was designed to replace PAT [64] in both amplification and sequencing reactions. All three newly designed primers are identical to the COI sequence reads of H. obsoletus haplotypes obtained in this study or to the sequences of the tRNA-Leu region between the COI and COII genes in haplotypes obtained in our earlier study [56].
Polymerase chain reactions (PCR) for all primer pairs were performed under the same conditions in a 20-µL reaction volume containing high yield reaction buffer A with 1.5 mM MgCl2 (1×), an additional 2.25 mM MgCl2, 0.6 mM of each dNTP, 0.5 μM of each primer, 1 U of FastGene Taq DNA polymerase (NIPPON Genetics Europe, Dueren, Germany) and 1 µL of template DNA. Amplification was conducted in a Mastercycler ep gradient S (Eppendorf, Hamburg, Germany) applying the following thermal steps: initial denaturation for 5 min at 95 °C, followed by 35 cycles of denaturation at 95 °C for 60 s, annealing at 52 °C for 60 s, and elongation at 72 °C for 90 s (150 s for longer LCO1490/PAT-Ho fragments); final elongation was performed at 72 °C for 7 min. The obtained amplicons were sequenced using the primers LCO1490 and HCOf-Ho for forward reads, and HCO2198-Ho and PAT-Ho for reverse reads. Sequencing was performed by Macrogen Europe (Amsterdam, The Netherlands). Sequences were edited using FinchTV v.1.4.0 (http://www.geospiza.com) and aligned with ClustalW [66] within the MEGA 7 software [67]. Haplotypic nucleotide sequence data were deposited in the NCBI GenBank database under the accession numbers MK172874-91 (Table 1) and OQ372231-42 (Table 2).
2.3. COI Sequence Comparison and Haplotype Network Reconstruction
The mitochondrial COI gene diversity among different H. obsoletus haplotypes was determined by comparing the nucleotide variability and substitutions at each aligned position (Figure 1). Mean genetic distances and diversities (overall, within, and between groups) were assessed based on best-fit substitution model analyses as determined in MEGA 7 (following the lowest BIC scores, i.e., Bayesian information criterion, considered to best describe the substitution pattern) [67] among haplotypes of the H. obsoletus sensu lato grouped according to their associated host plant: C. arvensis–U. dioica (Ca–Ud), V. agnus-castus (Vac), or C. foetida (Cf). The haplotype originating from C. cantabrica was grouped within the Ca–Ud haplogroup based on Convolvulus being its host plant and initial haplogroup determination using concatenated COI-COII and 16S-ND1 gene regions according to published protocols [54,60]. TCS version 1.21 [68] was used to infer haplotype networks using statistical parsimony [69] with a confidence limit of 91% (connection limit = 22) to assess the evolutionary relatedness and genealogy of the H. obsoletus host plant-associated haplotypes. In addition, connections between haplotypes were validated by performing TCS network and median-joining (MJ) calculation [70], keeping the parameter e = 0 using the software PopART version 1.7 (http://popart.otago.ac.nz) (accessed on 6 February 2023). Finally, the H. obsoletus COI gene haplotypes were compared to a previously published reference sequence to establish their relationship [10,38,48,62,71,72].
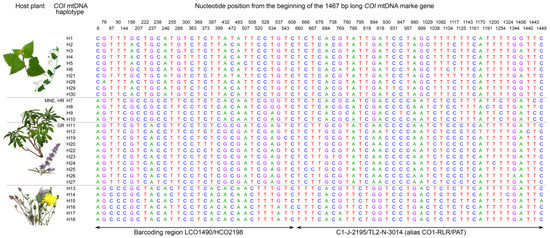
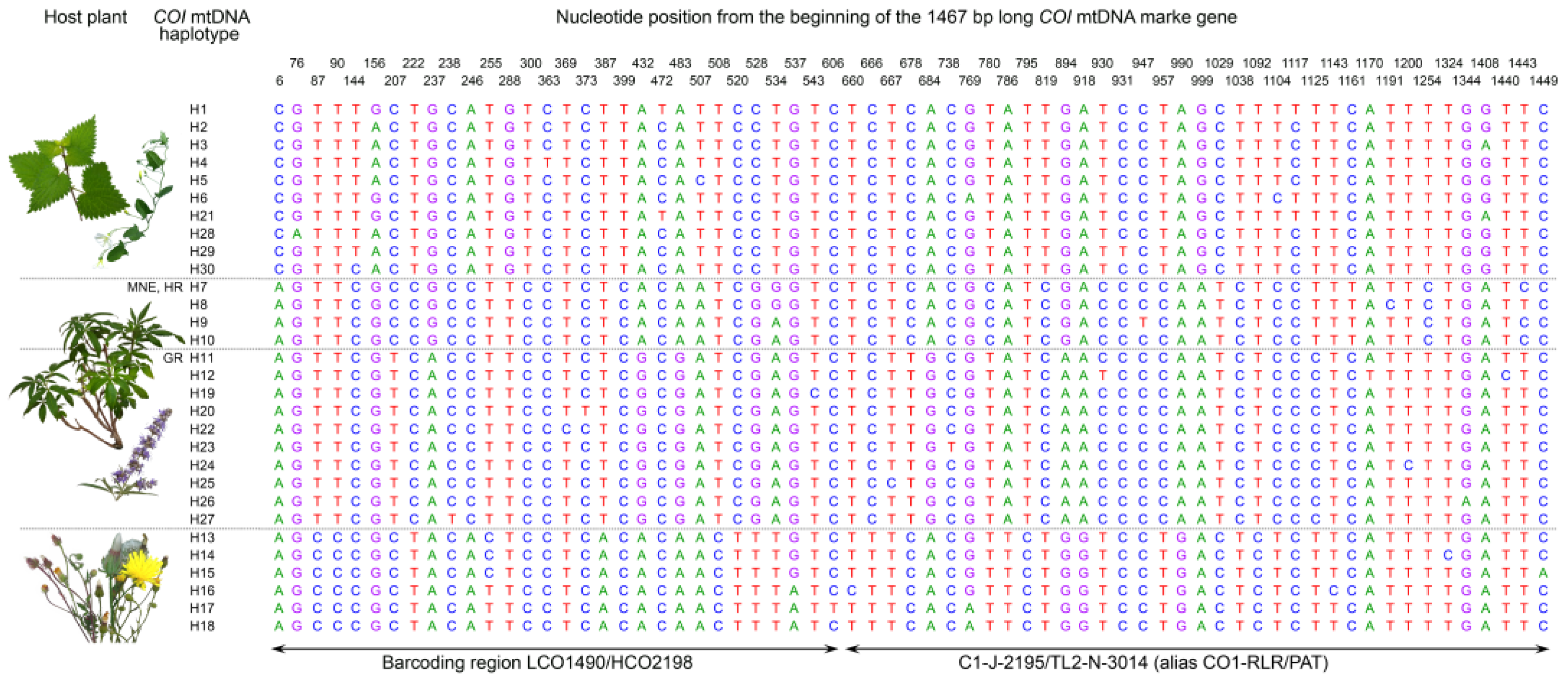
Figure 1.
Positions of 67 variable sites within the 1467-bp long COI mtDNA marker gene fragment of the 30 Hyalesthes obsoletus haplotypes associated with C. arvensis–U. dioica (H1–H6, H21, and H28–H30), V. agnus-castus (H7–H12, H19, H20, and H22–27), and C. foetida (H13–H18). Due to the additional geographic divisions within V. agnus-castus associated haplotypes [56], these are also designated according to their country of origin: “MNE” for Montenegro, “HR” for Croatia, and “GR” for Greece. Bases are denoted according to the beginning of the COI sequence reads. Variable nucleotide positions within the range of the COI barcoding region delineated by LCO1490 and HCO2198 primer annealing positions [65] and a 3′ segment of the COI region originally employed for identification of Hyalesthes spp. [10] delineated by C1-J-2195 and TL2-N-3014 primers [64] are indicated by arrows.
2.4. Design of Multiplex PCR Tool for Specific Identification of Hyalesthes obsoletus Host Plant Associations
Based on the mtCOI sequence variability (Figure 1), three forward primers were designed with binding positions in distinct regions of the gene, in accordance with variable nucleotide positions identified as host plant-specific for each H. obsoletus haplogroup. In addition, a single reverse primer containing nucleotide sequences corresponding to all three host-associated haplogroups was designed at the 3′-end of the gene. Primer 3 software v. 0.4.0 (https://bioinfo.ut.ee/primer3-0.4.0/) (accessed on 6 February 2023) was used to evaluate the characteristics (melting temperature, stability, self-complementarity, etc.) of the primers [73,74].
Primer specificity and efficiency were initially tested and evaluated in a separate single-primer set (singleplex) reaction before being incorporated into a multiplex PCR assay as a mixture of primers. Conditions of multiplex amplification protocol were adjusted to enable the specific, selective, and reliable amplification of targeted host plant associations of H. obsoletus. More than a thousand H. obsoletus samples from the three host plant associations (Ca–Ud, Vac, and Cf), originating from our earlier research and surveys in Serbia, North Macedonia, Montenegro, Bulgaria, Romania, Greece, and Turkey [21,30,38,40,56], as well as newly collected specimens, were utilized to assess the specificity of the primers and amplification protocol.
2.5. Evaluation of the Strain Diversity of ‘Ca. Phytoplasma solani’ in Hyalesthes obsoletus and Shared Host Plants
Epidemiological data on the association of ‘Ca. P. solani’ stamp strain(s) with the specific host plant association of H. obsoletus were retrieved from our previous studies [21,23,24,30,38,40] and combined with the geographical origin of insect and plant samples to provide an overview of the utility value of the presented method of vector identification to be used for the epidemiological discrimination of ‘Ca. P. solani’ routes of transmission. The host-specific origin of these H. obsoletus specimens carrying ‘Ca. P. solani’ stamp strains was determined using the multiplex PCR identification protocol. To supplement the data on ‘Ca. P. solani’ occurrence in plant-specialized H. obsoletus, specimens collected on V. agnus-castus in Greece were tested for ‘Ca. P. solani’ presence and strain genotype using ‘Ca. P. solani’-specific stamp gene amplification [59].
In addition, we conducted a case study in 2018 and 2019 to evaluate the multiplex PCR protocol for H. obsoletus host plant haplogroup identification in combination with stamp genotyping of the associated ‘Ca. P. solani’ strain they harbor. We assessed the use of this information for identifying the ‘Ca. P. solani’ epidemiological cycle, i.e., the underlying epidemiology, by analyzing H. obsoletus specimens within an agroecosystem. For the case study, we chose a vineyard in the Podunavlje district of Central Serbia that was infected with BN (Krnjevo, GPS: N44 25.722 E21 02.907). Each year, sampling occurred at the beginning and middle of July. Using sweep-nets and mouth aspirators, H. obsoletus specimens were collected from the groundcover bordering the vineyard and the first two rows of the vineyard. Collections were also made from patches of U. dioica some 300 m outside the vineyard. Specimens were preserved in 96% ethanol, and DNA was extracted as previously described. All isolates were analyzed by multiplex PCR for host plant-specific H. obsoletus identification and by ‘Ca. P. solani’-specific stamp gene amplification [59] for pathogen identification and strain genotyping.
All of the ‘Ca. P. solani’ stamp gene sequences obtained in this study were compared to the available genotypes in NCBI GenBank using the BLASTn algorithm (https://blast.ncbi.nlm.nih.gov) (accessed on 6 February 2023), as well as by haplotype network reconstruction with reference strains [32,33,34,59,75,76,77] using the median-joining (MJ) calculation [70], with the parameter e = 0 in the software PopART. The new stamp genotype identified in our study, GR-Ho-Vac, originating from a H. obsoletus specimen associated with V. agnus-castus in Greece, was submitted to NCBI GenBank under accession number OQ377809.
3. Results
3.1. Haplotype Diversity and Differentiation of Hyalesthes obsoletus Host Plant Associations
The nearly complete COI gene sequences of 1476 bp were obtained for 75 H. obsoletus individuals associated with one of the three host-plant associations: Ca–Ud (including C. cantabrica), Vac, and Cf. A total of 30 haplotypes that originated from a wide geographic area and all host plant associations were identified. Among previously reported H. obsoletus specimens [56], eighteen haplotypes were identified [78], six per host plant association (Table 1). The analysis of newly collected specimens revealed the existence of 12 additional haplotypes (Table 2). In total, nine haplotypes were identified in specimens associated with C. arvensis-U. dioica; a single haplotype was collected on C. cantabrica; four haplotypes were found in association with V. agnus-castus in Montenegro and Croatia; ten with V. agnus-castus in Greece; and six haplotypes were identified in association with C. foetida.
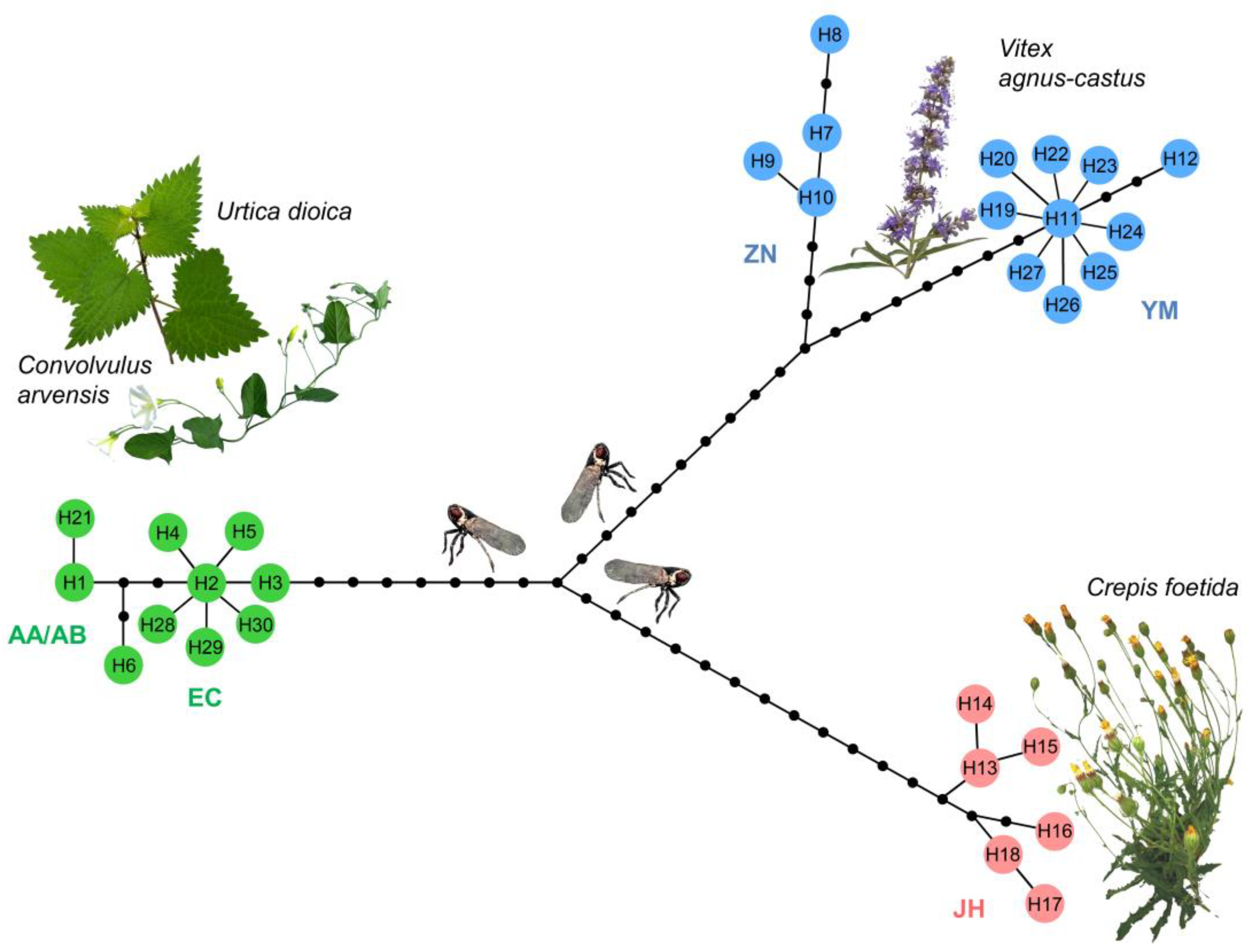
Nucleotide variability analyses confirmed the genetic segregation according to host-plant specialization in each of the 30 COI haplotypes, as revealed by haplotype network reconstruction (Figure 2). Three distinct clusters of H. obsoletus host plant-associated haplotypes, as well as two sub-clusters of geographically separated haplotypes associated with V. agnus-castus from Montenegro and Croatia, and from Greece, were identified. The topology of the COI phylogenetic network matched that which was previously obtained using concatenated COI-COII and 16S-ND1 gene regions [56], but it also revealed further geographic variation in all clusters. For example, Cf-associated H. obsoletus haplotypes from Turkey (H16, H17, and H18) formed a separate haplogroup from those originating in Serbia, Romania, and Bulgaria (Figure 2). On the COI gene, the geographic separation of Ca–Ud-associated AA/AB and EC haplogroups was also confirmed, with the former group being affiliated to H1 and its deriving haplotypes and the latter group being affiliated with H2 and its deriving haplotypes. The occurrence of the CB haplotype (COI haplotype H21) of H. obsoletus belonging to the AB haplogroup in association with C. cantabrica in Southeastern Serbia was a new discovery. This haplotype was affiliated to the group of H1 derived haplotypes in accordance with the compatible phylogeographic informativeness of the COI gene with concatenated COI-COII and 16S-ND1 gene regions (Figure 2). The AB group of H. obsoletus haplotypes had not previously been identified in Serbia, despite extensive surveys [56]. Therefore, the occurrence of this haplotype could be the result of insect vector’s further host plant adaptation or geographic range expansion, although neither is currently supported by evidence and therefore will require more comprehensive research in the future.
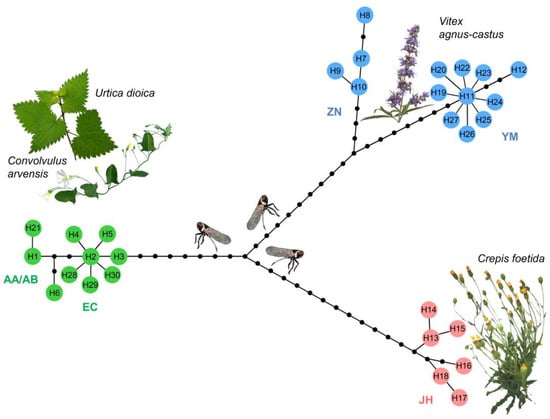
Figure 2.
The phylogenetic haplotype network obtained using statistical parsimony algorithm [69] in TCS program [68] on 1467 bp of mitochondrial COI gene of the 30 Hyalesthes obsoletus haplotypes identified in this study. Haplotype colors correspond to the host plant associations. Black dot vertices represent missing or unsampled haplotypes. Haplotypes designation (H1–H30) corresponds to those in Table 1 and Table 2. Below each group of haplotypes, their affiliation to previously established nomenclature of concatenated COI-COII and 16S-ND1 haplogroups (AA/AB, EC, ZN, YM, JH) [54,56,60] is denoted.
Comparing the COI haplotype diversity identified in our study with data on previously (partially) COI-genotyped H. obsoletus specimens [10,48,62,71,72] allowed us to identify geographic ranges for some of them that were distinct from our own. The H1 haplotype, which was found in Montenegro and Greece (Table 1), is also present in Northern Italy (FN179291 and GU552999), Southeastern France (isolate Ho-Ss5, LT841328), and Germany (ON210854), whereas the H2 haplotype from Serbia, Romania, North Macedonia, and Montenegro is also found in South Russia, Rostov Oblast (GU553002), and confirmed for Romania (GU553001). The Vac-associated haplotypes of the Montenegrin phylogeographic sub-group were also confirmed to occur in the coastal area of Herzegovina (Bosnia and Herzegovina), where H. obsoletus specimens were collected on V. agnus-castus [72] and affiliated to either the H7 or H10 haplotype based on the available 3′-end of the COI gene sequence (KY320569).
The overall mean genetic distance among 30 COI haplotypes was 1.4%, while within Ca–Ud-associated and Cf-associated haplogroups was 0.2% for each group, and 0.5% for the group of Vac-associated haplotypes. The mean genetic distance between groups ranged from 1.7% for the Ca–Ud and Cf associations to 2.2% for the Vac and Cf associations (Table 3). Comparing the 1476 bp-long COI gene sequence of all identified haplotypes within H. obsoletus sensu lato revealed 67 variable, 20 singleton, and 47 parsimony-informative sites. Out of these, 23 were haplogroup-specific and thus informative for discriminating Ca–Ud, Vac, and Cf host plant associations of H. obsoletus (Figure 1).

Table 3.
Mean mitochondrial COI gene distances and diversities based on best-fit substitution model analyses (Tamura 3-parameter + G) among 30 haplotypes of the Hyalesthes obsoletus sensu lato grouped according to their associated host plant: C. arvensis–U. dioica (Ca–Ud), V. agnus-castus (Vac), or C. foetida (Cf).
3.2. Multiplex PCR for Discriminating Host Plant Associations of Hyalesthes obsoletus
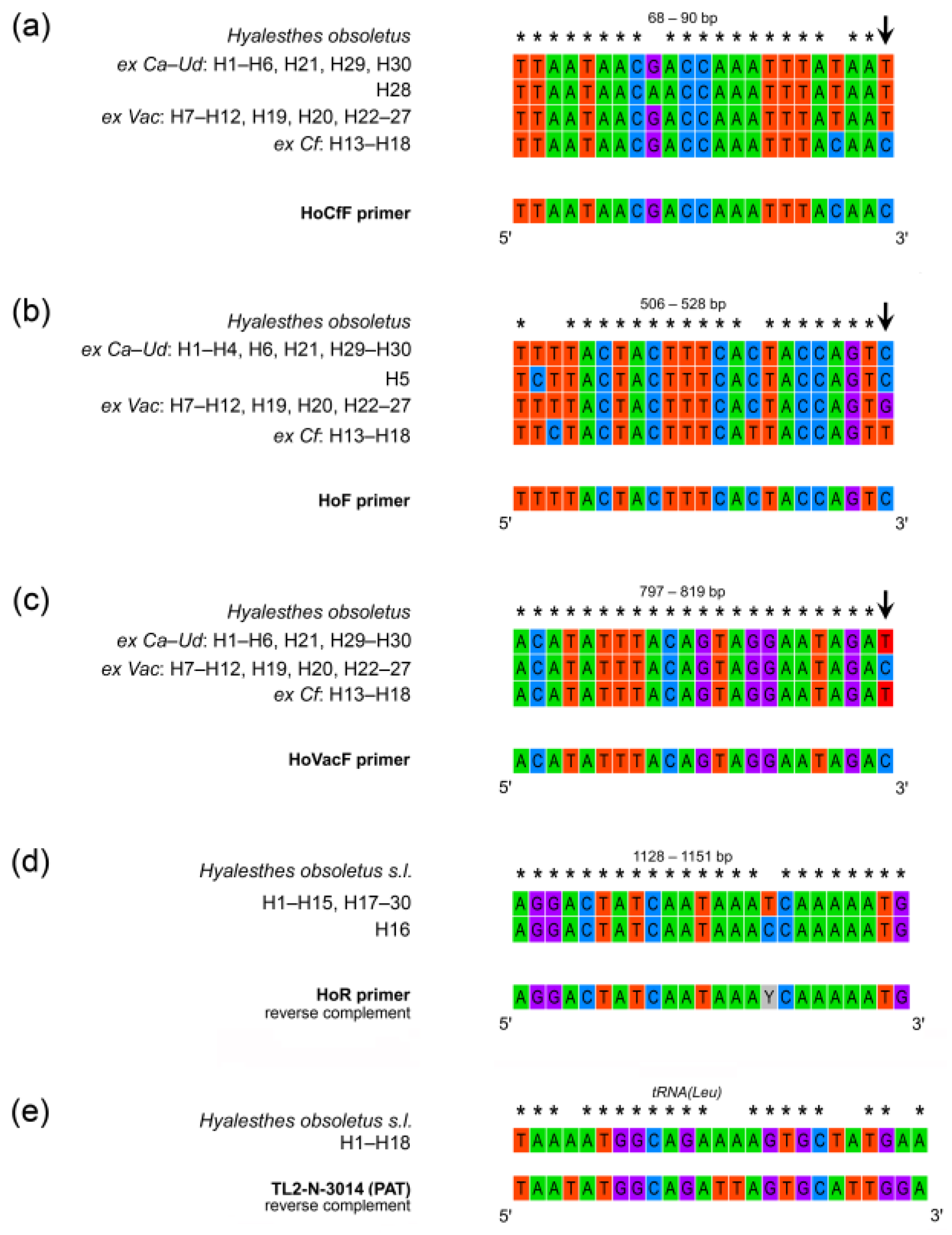
The position of primer sequences for specific amplification of plant-specialized H. obsoletus was determined by intra- and inter-group variability (Figure 1), i.e., the position of host plant group-specific nucleotide substitutions. The primers were designed to match the substitutions specific to each host plant group of H. obsoletus haplotypes at the 3′-end of the primer while positioning them in distinct regions of the COI gene (Table 4). The forward primers were designed for specific amplification of Cf, Ca–Ud, and Vac-associated H. obsoletus haplogroups; their binding sites corresponded to 68 bp, 506 bp, and 797 bp positions counting from the beginning of sequence reads, respectively. For universal amplification of all H. obsoletus haplotypes among the three host plant associations, the HoR reverse primer was designed to match all sequences at 1128-bp position (Table 4, Figure 3).

Table 4.
Primer sequences and conditions for specific and selective multiplex PCR amplification of Hyalesthes obsoletus host plant associations.
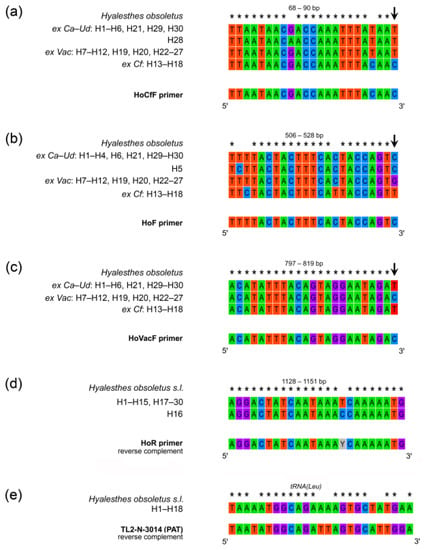
Figure 3.
Primer positions and nucleotide variation on mtDNA COI gene and flanking tRNA(Leu) in Hyalesthes obsoletus haplotypes associated with C. arvensis–U. dioica (ex Ca–Ud: H1–H6, H21, H28–H30), V. agnus-castus (ex Vac: H7–H12, H19, H20, and H22–27), and C. foetida (ex Cf: H13–H18). Primer annealing sites and sequence variability exploited for primer design are presented as follows: (a) HoCfF, forward primer specific for selective amplification of H. obsoletus ex Cf; (b) HoF, forward primer specific for selective amplification of H. obsoletus ex Ca–Ud; (c) HoVacF, forward primer specific for selective amplification of H. obsoletus ex Vac; (d) HoR, reverse primer universal for amplification of all host plant associations of H. obsoletus; (e) sequence variability of tRNA(Leu) in H. obsoletus haplotypes associated with all three host plant groups compared with the sequence of TL2-N-3014 primer [64] formerly used for Hyalesthes spp. identification [10]. Asterisks (*) indicate identical nucleotides. The arrows indicate nucleotide substitutions at the 3′ end of the primer binding site which allow for specificity in amplification. Data on tRNA(Leu) sequence variability are taken from our previous work [56].
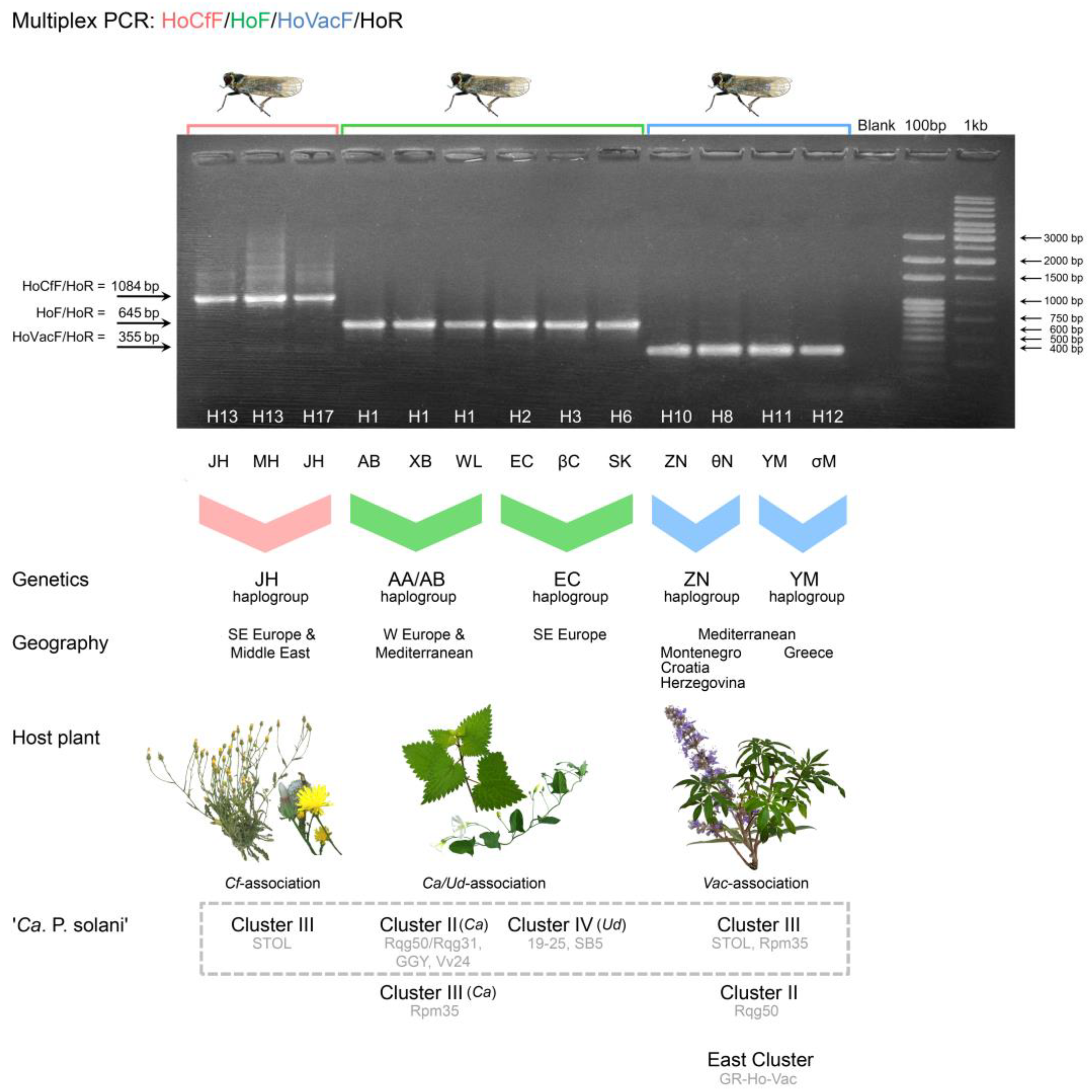
The reaction conditions for multiplex PCR were similar to those described previously for COI amplification, with the exception that the annealing temperature was increased to 56 °C to enable specificity and that the elongation step was 120 s for a total of 30 cycles (Table 4). The host plant association of H. obsoletus was determined by the size of the amplified fragments: 1084 bp for the Cf association, 645 bp for the Ca–Ud association, and 355 bp for the Vac association (Figure 4). The multiplex PCR method enabled the precise identification of all H. obsoletus specimens retrieved from our previous studies and subsequently analyzed [21,30,38,40,56]. No cross-amplifications between the haplogroups of diverse host plant associations were recorded. In particular, among over 700 specimens from our previous study on the host plant association of H. obsoletus [56], all of them produced selective amplification products that corresponded to their original host plant of collection. All 57 H. obsoletus specimens harboring ‘Ca. P. solani’ from BN-affected vineyards in North Macedonia [30] and all 38 ‘Ca. P. solani’-infected specimens from potato fields in Serbia [40] produced a 645 bp fragment. Among twenty-six H. obsoletus individuals collected from BN-affected vineyards in Montenegro [21], twenty-two produced 645 bp fragments and four produced 355 bp fragments, indicating the co-occurrence of Ca–Ud-association and Vac-association. Similarly, among 54 individuals from vineyards under BN infection in Serbia [38], 38 produced fragments of 1084 bp and the remaining specimens yielded fragments of 645 bp, confirming both Ca–Ud and Cf-association.
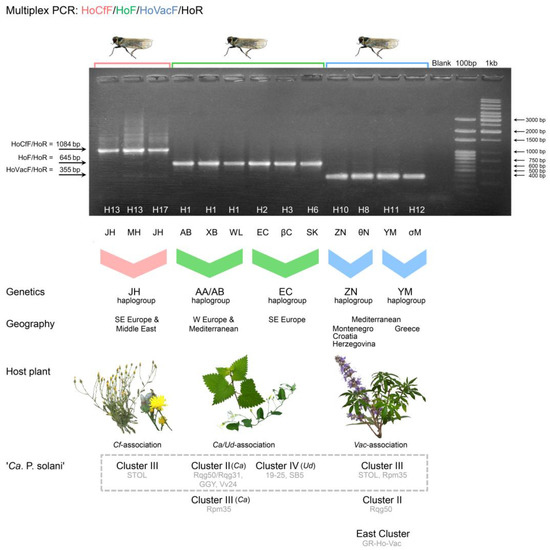
Figure 4.
A multiplex PCR assay for the specific identification of Hyalesthes obsoletus host plant associations using the HoCfF/HoF/HoVac/HoR primer mix and specific protocol. The size of the amplified fragment determines the host plant association: 1084 bp for Cf-association, 645 bp for Ca/Ud-association, and 355 bp for Vac-association. Molecular weight markers: 100-bp DNA ladder (Solis BioDyne, Tartu, Estonia) and GeneRuler 1 kb DNA ladder (Thermo Fisher Scientific, Waltham, MA, US). The COI and concatenated COI-COII and 16S-ND1 haplotypes of presented individuals are designated on and below the agarose gel. The genetics of H. obsoletus (i.e., haplogroups according to [54,56,60]), geography, host plants, and ‘Ca. Phytoplasma solani’ stamp strains are given below the gel. Data on the ‘Ca. Phytoplasma solani’ strains are retrieved from our previous works [21,23,24,30,38,40] and from the case study presented herein. Stamp Clusters II, III, and IV are designated as explained in previous studies [32,33,59], while “East Cluster” refers to ‘Ca. Phytoplasma solani’ strains from Azerbaijan [32] and Thailand [77] that formed a joint cluster with the new strain from H. obsoletus associated with V. agnus-castus collected in Greece in this study. The cluster of most frequently found ‘Ca. Phytoplasma solani’ genotypes associated with each H. obsoletus haplogroup and host plant are denoted within the grey hashed square.
For the case study, we collected 175 H. obsoletus specimens from a BN-infected vineyard in Central Serbia, 140 specimens from the vineyard’s border, and 35 from patches of U. dioica outside the vineyard. All specimens were tested with multiplex PCR for host plant association identification. In 87 individuals, the Ca–Ud-associated 645 bp fragment was amplified, while in 88 individuals, the Cf-associated 1084 bp long fragment was amplified.
3.3. Linking ‘Ca. Phytoplasma solani’ Epidemiology with Host Plant-Specialized Hyalesthes obsoletus
We were able to make plausible links between host plant association, genetics of the vector, geographic area of origin, and pathogen strain by summarizing the data from previous studies on the occurrence of ‘Ca. P. solani’ in H. obsoletus [21,30,38,40] (Figure 4). The H. obsoletus specimens from Serbia in which a 645 bp fragment was amplified by multiplex PCR for host association identification harbored the ‘Ca. P. solani’ stamp strains of either Cluster II (genotypes Rqg31/Rqg50 and Vv24) or Cluster III (Rpm35), whereas specimens from North Macedonia with the same product length harbored either strains of Cluster IV (SB5 and 19–25 and their deriving genotypes M1–M3) or of Cluster II (Rqg50 and GGY). This indicated that in Serbia, there is a ‘Ca. P. solani’ transmission route originating from C. arvensis, whereas in North Macedonia, there are transmission routes originating from both C. arvensis and U. dioica. In contrast, specimens of H. obsoletus from Serbia that yielded an amplification of a 1084 bp fragment via multiplex PCR harbored only the STOL stamp genotype of the Cluster III, indicating the C. foetida transmission route. Furthermore, among H. obsoletus specimens from Montenegrin vineyards, three types of epidemiological associations were linked: (i) a 645 bp fragment of multiplex PCR amplification and ‘Ca. P. solani’ stamp strains of Cluster II (Rqg31/Rqg50) or Cluster III (Rpm35), indicating a C. arvensis source of transmission, (ii) a 645 bp multiplex PCR fragment and stamp strains of Cluster IV (SB5 and 19–25), indicating a U. dioica transmission route, and (iii) a 355 bp fragment amplification and infection with ‘Ca. P. solani’ strains of Cluster III (STOL) or Cluster II (Rqg50), indicating a V. agnus-castus transmission route.
As a case study, the presence of ‘Ca. P. solani’ was identified in 54 of the 175 (31%) H. obsoletus specimens collected from the BN-affected vineyard. The stamp gene Cluster III genotype STOL was found in 37 individuals, all of whom amplified the Cf-associated 1084 bp-long fragment in multiplex PCR. Cluster II (Rqg31/Rqg50) and Cluster IV (19–25) genotypes were identified in fourteen and three H. obsoletus individuals, all of which amplified the Ca–Ud-associated 645 bp fragment. Consequently, these results indicated the presence of three transmission cycles and two host plant-group associations of the vector (Ca–Ud and Cf) within and beyond the studied vineyard. The results agreed with the stamp genotyping of symptomatic grapevine isolates from the same vineyard that revealed a majority of grapevines infected with the STOL genotype and a smaller proportion with the Rqg50 genotype (data not shown).
Concerning the experimentally demonstrated V. agnus-castus-sourced route of transmission in Montenegrin vineyards [21], indications include H. obsoletus specimens producing a 355 bp fragment in specific multiplex PCR and the STOL or Rpm35 genotypes of the stamp Cluster III being the most frequently found in association with either the vector or the reservoir plant, or being transmitted by the Vac-associated H. obsoletus. Additionally, among plant-specialized H. obsoletus collected on V. agnus-castus in Greece, we found a single specimen harboring ‘Ca. P. solani’. The GR-Ho-Vac strain from this specimen showed a maximum identity of 97.5% with the available sequences in the GenBank database (12/474 nucleotide substitutions). The strain was affiliated through haplotype network reconstruction to the stamp cluster, herein designated as “East” (Figure 4), which gathered the strains from Azerbaijan (isolates AZ13-H40 and AZ13-H26, accession numbers LT899749 and LT899744) [32] and Thailand (isolate TH-NKT-P26, accession number MW464312) [77] next to the strain found in H. obsoletus collected on V. agnus-castus in Greece (data not shown), thus indicating their common origin or adaptation to similar host(s). Given that 16 species of Vitex are naturally occurring in Thailand [79] and V. agnus-castus is present along the Caspian Sea coast in Azerbaijan and has been discussed as a plausible source of new diversified stamp genotypes [32], the natural reservoir plant may be the link between the isolates in this cluster and an indication of a specific transmission route.
4. Discussion
The planthopper Hyalesthes obsoletus is a major, widespread vector and driver of the ‘Ca. P. solani’ epidemiology. This claim is supported by numerous evidences of its ecological specialization (e.g., [20,25,55]), followed by genetic differentiation of host plant-associated populations [54,55,56,60] toward plants that serve as common reservoirs of ‘Ca. P. solani’ [20,21,38]. Evidences of ongoing processes of host plant specialization [33,61,62,80] and speciation [56] leading to the formation of host races or cryptic species within the vector’s populations in sympatry are simultaneously evidences of host plant-specialized routes of ‘Ca. P. solani’ transmission at the level that host races or cryptic taxa are driving the diversification of the pathogen, the emergence of new strains, and the separate epidemiological cycles (e.g., [33,61]). Adults of H. obsoletus are frequently found on diverse natural and cultivated plants, leading to the assumption that it is a polyphagous species (reviewed in [56]). However, it is the strict host plant preferences of nymphs and adults during mating and oviposition that drive the specialization processes of both vector and the pathogen, as well as the resulting transmission route and epidemiological cycle. The prevailing perception of H. obsoletus as a polyphagous species, with a few exceptions (e.g., [33,38,54,56,60,80]), highlights the importance of our findings and the need to treat the vector according to its true role, i.e., as an ecologically adapted host plant-specialized key vector and driver of ‘Ca. P. solani’ epidemics, and as a species complex with the potential for further shifts in host plant use and adaptations to new environmental conditions.
Molecular tools and genetic data are now available for the accurate, cost-effective, and rapid identification of host plant associations of H. obsoletus (i.e., cryptic species) using multiplex PCR, as well as for comparison and identification of further diversification, differentiation, and specialization by phylogenetic analyses of the most widely used barcoding fragment of the COI gene [1,7,15,63]. This study’s analysis of the COI gene encompassed a 630 bp (without primers) of the barcoding region and the entire second portion of the gene at its 3′-end, facilitating future comparisons of H. obsoletus genetic diversity regardless of the primers used, length, or position of the amplified fragment. As intended, the multiplex PCR method for discriminating host plant associations of H. obsoletus should enable accurate identification of the vector’s phylogenetic lineage, natural host plant preference and epidemiological transmission routes of ‘Ca. P. solani’. When applied to specimens collected on cultivated plants within an agroecosystem and combined with ‘Ca. P. solani’ genotyping, the method should provide valuable information on disease epidemiology and source(s) of emergence and transmission.
In addition to defining the intended applications and expectations of the presented multiplex PCR method, we consider it essential to highlight its limitations as well. The limitations are defined by the biological and genetic characteristics of H. obsoletus as a complex of cryptic, recently evolved species [56]. Due to shallow genetic differentiation in host-associated haplogroups or species caused by ecological adaptation, the number of segregating nucleotide positions that can be used for discrimination is limited (e.g., [12]). This is especially true for host races or morphologically indistinguishable (cryptic) species (e.g., [5,55]). Hence, the method of multiplex PCR identification of H. obsoletus host plant associations presented here could not be used to discriminate H. obsoletus sensu lato from its morphologically distinct congeners (a total of 32 species excluding H. obsoletus [81]). In contrast to methods previously developed for molecular identification and differentiation of morphologically distinct cixiid planthoppers, including H. obsoletus [10,11,48], the current method could not be used for the differentiation of H. obsoletus sensu lato, i.e., host plant associations, from other Hyalesthes species that may co-occur together, such as H. luteipes. However, the latter species has distinctive morphological features compared to H. obsoletus sensu lato, and thus this is not a true limitation but rather a notion that specimens should be examined through a stereo microscope and identified by external morphological characteristics to the genus level prior to the molecular identification of host associations. Likely, H. obsoletus has distinct external morphological features of shiny black body coloration and a white collar-like pronotum [10], while other Hyalesthes species commonly found in or surrounding agroecosystems in Europe are of brownish body coloration and yellowish pronotum (H. luteipes, H. scotti, and H. philesakis Hoch, 1985). Notably, the method described for the molecular identification of three Hyalesthes species [10] is inapplicable to the identification and differentiation of H. obsoletus sensu lato from its congeners, which is to be expected given the limited knowledge of H. obsoletus’ genetic variability at the time the method was developed.
The need for the reliable and precise identification of H. obsoletus host associations as vectors driving ‘Ca. P. solani’ transmission and epidemiology is becoming more apparent as the number of ‘Ca. P. solani’ epidemic outbreaks grows [33,53,61], as does the number of cultivated plants affected in a wider geographical area [32,36,77,82], and disease management and control strategies become more demanding [83]. As with the pathogen’s molecular identification and genotyping, the molecular identification of H. obsoletus host associations should be the method of choice in studies elaborating the ‘Ca. P. solani’ transmission routes or epidemiology. We provided an overview of the epidemiological utility of combining data on the genetic characteristics of the vector and the pathogen based on the diversity information currently available. This will hopefully encourage and persuade more researchers to apply the molecular methods for identification of H. obsoletus host plant associations, which will undoubtedly result in a more comprehensive understanding of the epidemiology and origin of ‘Ca. P. solani’.
Author Contributions
Conceptualization, J.J. and I.T.; methodology, J.J. and I.T.; validation, J.J.; formal analysis, J.J. and I.T.; investigation, J.J. and I.T.; data curation, J.J.; visualization, J.J.; writing—original draft preparation, J.J.; writing—review and editing, I.T. and J.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia, Contract No. 451-03-47/2023-01/200010.
Data Availability Statement
Acknowledgments
We are grateful to all of the colleges that have contributed to our research on ‘Candidatus Phytoplasma solani’ epidemiology and insect vectors over the years. We are especially grateful for the fruitful discussions and invaluable assistance provided by members of our research team at the Department of Plant Pests in Zemun.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. B 2003, 270, 313–321. [Google Scholar]
- Hebert, P.D.; Gregory, T.R. The promise of DNA barcoding for taxonomy. Syst. Biol. 2005, 54, 852–859. [Google Scholar] [CrossRef]
- Toševski, I.; Caldara, R.; Jović, J.; Baviera, C.; Hernández-Vera, G.; Gassmann, A.; Emerson, B.C. Revision of Mecinus heydenii species complex (Curculionidae): Integrative taxonomy reveals multiple species exhibiting host specialization. Zool. Scr. 2014, 43, 34–51. [Google Scholar]
- Toševski, I.; Caldara, R.; Jović, J.; Hernańdez-Vera, G.; Baviera, C.; Gassmann, A.; Emerson, B.C. Host-associated divergence and taxonomy in the Rhinusa pilosa Gyllenhal species complex: An integrative approach. Syst. Entomol. 2015, 40, 268–287. [Google Scholar]
- Toševski, I.; Caldara, R.; Jović, J.; Hernańdez-Vera, G.; Baviera, C.; Gassmann, A.; Emerson, B.C. Morphological, molecular and biological evidence reveal two cryptic species in Mecinus janthinus Germar (Coleoptera, Curculionidae), a successful biological control agent of Dalmatian toadflax, Linaria dalmatica (Lamiales, Plantaginaceae). Syst. Entomol. 2011, 36, 741–753. [Google Scholar]
- Tay, W.T.; Evans, G.A.; Boykin, L.M.; De Barro, P.J. Will the real Bemisia tabaci please stand up? PLoS ONE 2012, 7, e50550. [Google Scholar]
- Antil, S.; Abraham, J.S.; Sripoorna, S.; Maurya, S.; Dagar, J.; Makhija, S.; Bhagat, P.; Gupta, R.; Sood, U.; Lal, R.; et al. DNA barcoding, an effective tool for species identification: A review. Mol. Biol. Rep. 2023, 50, 761–775. [Google Scholar]
- Guo, M.; Yuan, C.; Tao, L.; Cai, Y.; Zhang, W. Life barcoded by DNA barcodes. Conserv. Genet. Res. 2022, 14, 351–365. [Google Scholar]
- Di Camillo, C.G.; Gravili, C.; De Vito, D.; Pica, D.; Piraino, S.; Puce, S.; Cerrano, C. The importance of applying Standardised Integrative Taxonomy when describing marine benthic organisms and collecting ecological data. Invertebr. Syst. 2018, 32, 794–802. [Google Scholar]
- Bertin, S.; Picciau, L.; Ács, Z.; Alma, A.; Bosco, D. Molecular identification of the Hyalesthes species (Hemiptera: Cixiidae) occurring in vineyard agroecosystems. Ann. Appl. Biol. 2010, 157, 435–445. [Google Scholar]
- Bertin, S.; Picciau, L.; Ács, Z.; Alma, A.; Bosco, D. Molecular differentiation of four Reptalus species (Hemiptera: Cixiidae). Bull. Entomol. Res. 2010, 100, 551–558. [Google Scholar] [PubMed]
- Toševski, I.; Jović, J.; Krstić, O.; Gassmann, A. PCR-RFLP-based method for reliable discrimination of cryptic species within Mecinus janthinus species complex (Mecinini, Curculionidae) introduced in North America for biological control of invasive toadflaxes. Biol. Control 2013, 58, 563–573. [Google Scholar]
- Andrews, K.J.; Bester, R.; Manrakhan, A.; Maree, H.J. A multiplex PCR assay for the identification of fruit flies (Diptera: Tephritidae) of economic importance in South Africa. Sci. Rep. 2022, 12, 13089. [Google Scholar]
- Gostel, M.R.; Kress, W.J. The Expanding Role of DNA Barcodes: Indispensable Tools for Ecology, Evolution, and Conservation. Diversity 2022, 14, 213. [Google Scholar]
- Hebert, P.D.; Ratnasingham, S.; De Waard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. Lond. B 2003, 270, S96–S99. [Google Scholar]
- Maixner, M. Transmission of German grapevine yellows (Vergilbungskrankheit) by the planthopper Hyalesthes obsoletus (Auchenorrhyncha: Cixiidae). Vitis 1994, 33, 103–104. [Google Scholar]
- Sforza, R.; Clair, D.; Daire, X.; Larrue, J.; Boudon-Padieu, E. The role of Hyalesthes obsoletus (Hemiptera: Cixiidae) in the occurrence of bois noir of grapevines in France. J. Phytopathol. 1998, 146, 549–556. [Google Scholar]
- Quaglino, F.; Zhao, Y.; Casati, P.; Bulgari, D.; Bianco, P.A.; Wei, W.; Davis, R.E. ‘Candidatus Phytoplasma solani’, a novel taxon associated with stolbur- and bois noir-related diseases of plants. Int. J. Syst. Evol. Microbiol. 2013, 63, 2879–2894. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health). Scientific Opinion on the pest categorisation of Candidatus Phytoplasma solani. EFSA J. 2014, 12, 3924–3927. [Google Scholar]
- Langer, M.; Maixner, M. Molecular characterisation of grapevine yellows associated phytoplasmas of the stolbur group based on RFLP analysis of non-ribosomal DNA. Vitis 2004, 43, 191–199. [Google Scholar]
- Kosovac, A.; Radonjić, S.; Hrnčić, S.; Krstić, O.; Toševski, I.; Jović, J. Molecular tracing of the transmission routes of bois noir in Mediterranean vineyards of Montenegro and experimental evidence for the epidemiological role of Vitex agnus-castus (Lamiaceae) and associated Hyalesthes obsoletus (Cixiidae). Plant Pathol. 2016, 65, 285–298. [Google Scholar]
- Pierro, R.; Panattoni, A.; Passera, A.; Materazzi, A.; Luvisi, A.; Loni, A.; Ginanni, M.; Lucchi, A.; Bianco, P.A.; Quaglino, F. Proposal of a new Bois noir epidemiological pattern related to ‘Candidatus Phytoplasma solani’ strains characterized by a possiblemoderate virulence in Tuscany. Pathogens 2020, 9, 268. [Google Scholar] [PubMed]
- Jović, J.; Marinković, S.; Jakovljević, M.; Krstić, O.; Cvrković, T.; Mitrović, M.; Toševski, I. Symptomatology, (Co)occurrence and Differential Diagnostic PCR Identification of ‘Ca. Phytoplasma solani’ and ‘Ca. Phytoplasma convolvuli’ in Field Bindweed. Pathogens 2021, 10, 160. [Google Scholar] [PubMed]
- Cvrković, T.; Jović, J.; Krstić, O.; Marinković, S.; Jakovljević, M.; Mitrović, M.; Toševski, I. Epidemiological Role of Dictyophara europaea (Hemiptera: Dictyopharidae) in the Transmission of ‘Candidatus Phytoplasma solani’. Horticulturae 2022, 8, 654. [Google Scholar]
- Sforza, R.; Bourgoin, T.; Wilson, S.W.; Boudon-Padieu, E. Field observations, laboratory rearing and descriptions of immatures of the planthopper Hyalesthes obsoletus (Hemiptera: Cixiidae). Eur. J. Entomol. 1999, 96, 409–418. [Google Scholar]
- Jović, J.; Cvrković, T.; Mitrović, M.; Krnjajić, S.; Redinbaugh, M.G.; Pratt, R.C.; Gingery, R.E.; Hogenhout, S.A.; Toševski, I. Roles of stolbur phytoplasma and Reptalus panzeri (Cixiinae, Auchenorrhyncha) in the epidemiology of Maize redness in Serbia. Eur. J. Plant Pathol. 2007, 118, 85–89. [Google Scholar]
- Jović, J.; Cvrković, T.; Mitrović, M.; Krnjanjić, S.; Petrović, A.; Redinbaugh, M.G.; Pratt, R.C.; Hogenhout, S.A.; Toševski, I. Stolbur phytoplasma transmission to maize by Reptalus panzeri and the disease cycle of maize redness in Serbia. Phytopathology 2009, 99, 1053–1061. [Google Scholar]
- Radonjić, S.; Hrnčić, S.; Jović, J.; Cvrković, T.; Krstić, O.; Krnjajić, S.; Toševski, I. Occurrence and distribution of grapevine yellows caused by stolbur phytoplasma in Montenegro. J. Phytopathol. 2009, 157, 682–685. [Google Scholar]
- Cvrković, T.; Jović, J.; Mitrović, M.; Krstić, O.; Toševski, I. Experimental and molecular evidence of Reptalus panzeri as a natural vector of bois noir. Plant Pathol. 2014, 63, 42–53. [Google Scholar]
- Atanasova, B.; Jakovljević, M.; Spasov, D.; Jović, J.; Mitrović, M.; Toševski, I.; Cvrković, T. The molecular epidemiology of bois noir grapevine yellows caused by ‘Candidatus Phytoplasma solani’ in the Republic of Macedonia. Eur. J. Plant Pathol. 2015, 142, 759–770. [Google Scholar]
- Delić, D.; Balech, B.; Radulović, M.; Lolić, B.; Karačić, A.; Vukosavljević, V.; Đurić, G.; Cvetković, T.J. Vmp1 and stamp genes variability of ‘Candidatus phytoplasma solani’ in Bosnian and Herzegovinian grapevine. Eur. J. Plant Pathol. 2016, 145, 221–225. [Google Scholar]
- Balakishiyeva, G.; Bayramova, J.; Mammadov, A.; Salar, P.; Danet, J.L.; Ember, I.; Verdin, E.; Foissac, X.; Huseynova, I. Important genetic diversity of “Candidatus Phytoplasma solani” related strains associated with Bois noir grapevine yellows and planthoppers in Azerbaijan. Eur. J. Plant. Pathol. 2018, 151, 937–946. [Google Scholar]
- Sémétey, O.; Gaudin, J.; Danet, J.L.; Salar, P.; Theil, S.; Fontaine, M.; Krausz, M.; Chaisse, E.; Eveillard, S.; Verdin, E.; et al. Lavender decline in France is associated with chronic infection by lavender-specific strains of “Candidatus Phytoplasma solani”. Appl. Environ. Microbiol. 2018, 84, e01507–e01518. [Google Scholar]
- Quaglino, F.; Passera, A.; Faccincani, M.; Moussa, A.; Pozzebon, A.; Sanna, F.; Casati, P.; Bianco, P.A.; Mori, N. Molecular and spatial analyses reveal new insights on Bois noir epidemiology in Franciacorta vineyards. Ann. Appl. Biol. 2021, 179, 151–168. [Google Scholar]
- Mehle, N.; Kavčič, S.; Mermal, S.; Vidmar, S.; Pompe Novak, M.; Riedle-Bauer, M.; Brader, G.; Kladnik, A.; Dermastia, M. Geographical and Temporal Diversity of ‘Candidatus Phytoplasma solani’ in Wine-Growing Regions in Slovenia and Austria. Front. Plant Sci. 2022, 13, 889675. [Google Scholar]
- Çağlar, B.K.; Şimşek, E. Detection and Multigene Typing of ‘Candidatus Phytoplasma solani’-Related Strains Infecting Tomato and Potato Plants in Different Regions of Turkey. Pathogens 2022, 11, 1031. [Google Scholar] [PubMed]
- Usta, M.; Guller, A.; Sipahioglu, H.M. Detection, in silico analysis and molecular diversity of phytoplasmas from solanaceous crops in Turkey. Plant Prot. Sci. 2022, 58, 31–39. [Google Scholar]
- Kosovac, A.; Jakovljević, M.; Krstić, O.; Cvrković, T.; Mitrović, M.; Toševski, I.; Jović, J. Role of plant-specialized Hyalesthes obsoletus associated with Convolvulus arvensis and Crepis foetida in the transmission of ‘Candidatus Phytoplasma solani’-inflicted bois noir disease of grapevine in Serbia. Eur. J. Plant Pathol. 2019, 153, 183–195. [Google Scholar]
- Bressan, A.; Sémétey, O.; Nusillard, B.; Clair, D.; Boudon-Padieu, E. Insect vectors (Hemiptera: Cixiidae) and pathogens associated with the disease syndrome “basses richesses” of sugar beet in France. Plant Dis. 2008, 92, 113–119. [Google Scholar]
- Mitrović, M.; Jakovljević, M.; Jović, J.; Krstić, O.; Kosovac, A.; Trivellone, V.; Jermini, M.; Toševski, I.; Cvrković, T. ‘Candidatus Phytoplasma solani’ genotypes associated with potato stolbur in Serbia and the role of Hyalesthes obsoletus and Reptalus panzeri (Hemiptera, Cixiidae) as natural vectors. Eur. J. Plant Pathol. 2016, 144, 619–630. [Google Scholar]
- Aleksić, Ž.; Šutić, D.; Aleksić, D. Transmission intensity of stolbur virus by means of Hyalesthes obsoletus Sign. on some host plants. Plant Prot. 1967, 93–95, 67–73. [Google Scholar]
- Emeljanov, A.F. Nomenclatorial changes in the family Cixiidae (Homoptera, Auchenorrhyncha, Fulgoroidea), with fixation of type species of the genus Reptalus Emeljanov, 1971 and description of a new subgenus. Zootaxa 2020, 4780, 197–200. [Google Scholar]
- Gatineau, F.; Larrue, J.; Clair, D.; Lorton, F.; Richard-Molard, M.; Boudon-Padieu, E. A new natural planthopper vector of “stolbur” phytoplasma in the genus Pentastiridius (Hemiptera: Cixiidae). Eur. J. Plant Pathol. 2001, 107, 263–271. [Google Scholar] [CrossRef]
- Trivellone, V.; Pinzauti, F.; Bagnoli, B. Reptalus quinquecostatus (Dufour) (Auchenorrhyncha Cixiidae) as a possible vector of “stolbur”-phytoplasma in a vineyard in Tuscany. Redia 2005, 88, 103–108. [Google Scholar]
- Pinzauti, F.; Trivellone, V.; Bagnoli, B. Ability of Reptalus quinquecostatus (Hemiptera: Cixiidae) to inoculate “stolbur” phytoplasma to artificial feeding medium. Ann. Appl. Biol. 2008, 153, 299–305. [Google Scholar]
- Chuche, J.; Danet, J.L.; Salar, P.; Foissac, X.; Thiéry, D. Transmission of ‘Candidatus Phytoplasma solani’ by Reptalus quinquecostatus (Hemiptera: Cixiidae). Ann. Appl. Biol. 2016, 169, 214–223. [Google Scholar]
- Jović, J.; Riedle-Bauer, M.; Chuche, J. Vector Role of Cixiids and Other Planthopper Species. In Phytoplasmas: Plant Pathogenic Bacteria-II: Transmission and Management of Phytoplasma Associated Diseases; Bertaccini, A., Weintraub, P.G., Rao, G.P., Mori, N., Eds.; Springer: Singapore, 2019; Volume 2, pp. 79–113. [Google Scholar]
- Pfitzer, R.; Varrelmann, M.; Hesse, G.; Eini, O. Molecular Detection of Pentastiridius leporinus, the Main Vector of the Syndrome ‘Basses Richesses’ in Sugar Beet. Insects 2022, 13, 992. [Google Scholar] [PubMed]
- Holzinger, W.E.; Kammerlander, I.; Nickel, H. The Auchenorrhyncha of Central Europe, Fulgoromorpha, Cicadomorpha Excl. Cicadellidae; Brill Academic Publishers: Leiden, The Netherlands, 2003; pp. 1–673. [Google Scholar]
- Emeljanov, A.F. Planthoppers of the Family Cixiidae of Russia and Adjacent Territories; KMK Scientific Press Ltd.: St.-Petersburg, Moscow, Russia, 2015; Volume 177, pp. 1–253. Keys to species of the fauna of Russia published by Zoological Institute of Russian Academy of Sciences. (In Russian) [Google Scholar]
- Sharon, R.; Soroker, V.; Wesley, S.D.; Zahavi, T.; Harari, A.; Weintraub, P.G. Vitex agnus-castus is a preferred host plant for Hyalesthes obsoletus. J. Chem. Ecol. 2005, 31, 1051–1063. [Google Scholar]
- Kessler, S.; Schaerer, S.; Delabays, N.; Turlings, T.C.; Trivellone, V.; Kehrli, P. Host plant preferences of Hyalesthes obsoletus, the vector of the grapevine yellows disease ‘bois noir’, in Switzerland. Entomol. Exp. Appl. 2011, 139, 60–67. [Google Scholar]
- Aryan, A.; Brader, G.; Mörtel, J.; Pastar, M.; Riedle-Bauer, M. An abundant ‘Candidatus Phytoplasma solani’ tuf b strain is associated with grapevine, stinging nettle and Hyalesthes obsoletus. Eur. J. Plant Pathol. 2014, 140, 213–227. [Google Scholar]
- Johannesen, J.; Lux, B.; Michel, K.; Seitz, A.; Maixner, M. Invasion biology and host specificity of the grapevine yellows disease vector Hyalesthes obsoletus in Europe. Entomol. Exp. Appl. 2008, 126, 217–227. [Google Scholar]
- Imo, M.; Maixner, M.; Johannesen, J. Sympatric diversification vs. immigration: Deciphering host-plant specialization in a polyphagous insect, the stolbur phytoplasma vector Hyalesthes obsoletus (Cixiidae). Mol. Ecol. 2013, 22, 2188–2203. [Google Scholar] [PubMed]
- Kosovac, A.; Johannesen, J.; Krstić, O.; Mitrović, M.; Cvrković, T.; Toševski, I.; Jović, J. Widespread plant specialization in the polyphagous planthopper Hyalesthes obsoletus (Cixiidae), a major vector of stolbur phytoplasma: Evidence of cryptic speciation. PLoS ONE 2018, 13, e0196969. [Google Scholar]
- Cimerman, A.; Pacifico, D.; Salar, P.; Marzachi, C.; Foissac, X. Striking diversity of vmp1, a variable gene encoding a putative membrane protein of the stolbur phytoplasma. Appl. Environ. Microbiol. 2009, 75, 2951–2957. [Google Scholar]
- Fialová, R.; Válová, P.; Balakishiyeva, G.; Danet, J.L.; Šafárová, D.; Foissac, X.; Navrátil, M. Genetic variability of stolbur phytoplasma in annual crop and wild plant species in south Moravia. J. Plant Pathol. 2009, 91, 411–416. [Google Scholar]
- Fabre, A.; Danet, J.L.; Foissac, X. The stolbur phytoplasma antigenic membrane protein gene stamp is submitted to diversifying positive selection. Gene 2011, 472, 37–41. [Google Scholar]
- Johannesen, J.; Foissac, X.; Kehrli, P.; Maixner, M. Impact of vector dispersal and host-plant fidelity on the dissemination of an emerging plant pathogen. PLoS ONE 2012, 7, e51809. [Google Scholar]
- Johannesen, J.; Riedle-Bauer, M. Origin of a sudden mass occurrence of the stolbur phytoplasma vector Hyalesthes obsoletus (Cixiidae) in Austria. Ann. Appl. Biol. 2014, 165, 488–495. [Google Scholar]
- Chuche, J.; Danet, J.L.; Rivoal, J.B.; Arricau-Bouvery, N.; Thiéry, D. Minor cultures as hosts for vectors of extensive crop diseases: Does Salvia sclarea act as a pathogen and vector reservoir for lavender decline? J. Pest Sci. 2018, 91, 145–155. [Google Scholar]
- DeSalle, R.; Goldstein, P. Review and interpretation of trends in DNA barcoding. Front. Ecol. Evol. 2019, 7, 302. [Google Scholar]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Marine Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.; Posada, D.; Crandall, K. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1660. [Google Scholar]
- Templeton, A.R.; Crandall, K.A.; Sing, C.F. A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram estimation. Genet 1992, 132, 619–633. [Google Scholar]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar]
- Bressan, A.; Holzinger, W.E.; Nusillard, B.; Sémétey, O.; Gatineau, F.; Simonato, M.; Boudon-Padieu, E. Identification and biological traits of a planthopper from the genus Pentastiridius (Hemiptera: Cixiidae) adapted to an annual cropping rotation. Eur. J. Entomol. 2009, 106, 405–413. [Google Scholar]
- Đurić, Z.; Hrnčić, S.; Delić, D. Morphological and molecular identification of Hyalesthes obsoletus Signoret (Auchenorrhyncha: Cixiidae) in Herzegovina vineyards. Mitt. Klosterneubg. 2017, 67, 177–181. [Google Scholar]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [PubMed]
- Quaglino, F.; Maghradze, D.; Casati, P.; Chkhaidze, N.; Lobjanidze, M.; Ravasio, A.; Passera, A.; Venturini, G.; Failla, O.; Bianco, P.A. Identification and characterization of new ‘Candidatus Phytoplasma solani’ strains associated with bois noir disease in Vitis vinifera L. cultivars showing a range of symptom severity in Georgia, the Caucasus region. Plant Dis. 2016, 100, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Pierro, R.; Passera, A.; Panattoni, A.; Casati, P.; Luvisi, A.; Rizzo, D.; Bianco, P.A.; Quaglino, F.; Materazzi, A. Molecular Typing of Bois Noir Phytoplasma Strains in the Chianti Classico Area (Tuscany, Central Italy) and Their Association with Symptom Severity in Vitis vinifera ‘Sangiovese’. Phytopathology 2018, 108, 362–373. [Google Scholar] [PubMed]
- Donnua, S.; Parkpoom, B.; Thaipong, K. Candidatus phytoplasma solani associated with papaya phytoplasma disease in Thailand. Khon Kaen Agric. J. 2021, 49, 1249–1258. [Google Scholar]
- Jović, J. Use of mitochondrial divergence in plant-specialized populations of Hyalesthes obsoletus for identification of ‘Candidatus Phytoplasma solani’ epidemiology. Phytopathogenic Mollicutes 2019, 9, 91–92. [Google Scholar] [CrossRef]
- Chantaranothai, P. A revision of the genus Vitex (Lamiaceae) in Thailand. Trop. Nat. Hist. 2011, 11, 91–118. [Google Scholar]
- Maixner, M.; Albert, A.; Johannesen, J. Survival relative to new and ancestral host plants, phytoplasma infection, and genetic constitution in host races of a polyphagous insect disease vector. Ecol. Evol. 2014, 4, 3082–3092. [Google Scholar]
- Hoch, H.; Remane, R. Evolution und Speziation der Zikaden-Gattung Hyalesthes SIGNORET, 1865 (Homoptera Auchenorrhyncha Fulgoroidea Cixiidae); Marburger Entomologische Publikationen: Marburg, Germany, 1985; pp. 1–427. [Google Scholar]
- Jamshidi, E.; Murolo, S.; Ravari, S.B.; Salehi, M.; Romanazzi, G. Multilocus Genotyping of ‘Candidatus Phytoplasma Solani’ Associated with Grapevine Bois Noir in Iran. Biology 2022, 11, 835. [Google Scholar]
- Mitrović, M.; Marinković, S.; Cvrković, T.; Jović, J.; Krstić, O.; Jakovljević, M. Framework for risk assessment of ‘Candidatus Phytoplasma solani’ associated diseases outbreaks in agroecosystems in Serbia. J. Plant Pathol. 2022, 104, 537–552. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).