Mineralization and Fixed Stable Carbon Isotopic Characteristics of Organic Carbon in Cotton Fields with Different Continuous Cropping Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Overview
2.2. Field Sampling
2.3. Measurement Method
2.4. Carbon Isotope Calculation Method
2.5. Calculation of Change from SOC to SIC
2.6. Data Analysis
3. Results
3.1. Characteristics of Soil SOC and SIC Distributions in Cotton Fields with Different Continuous Cropping Years
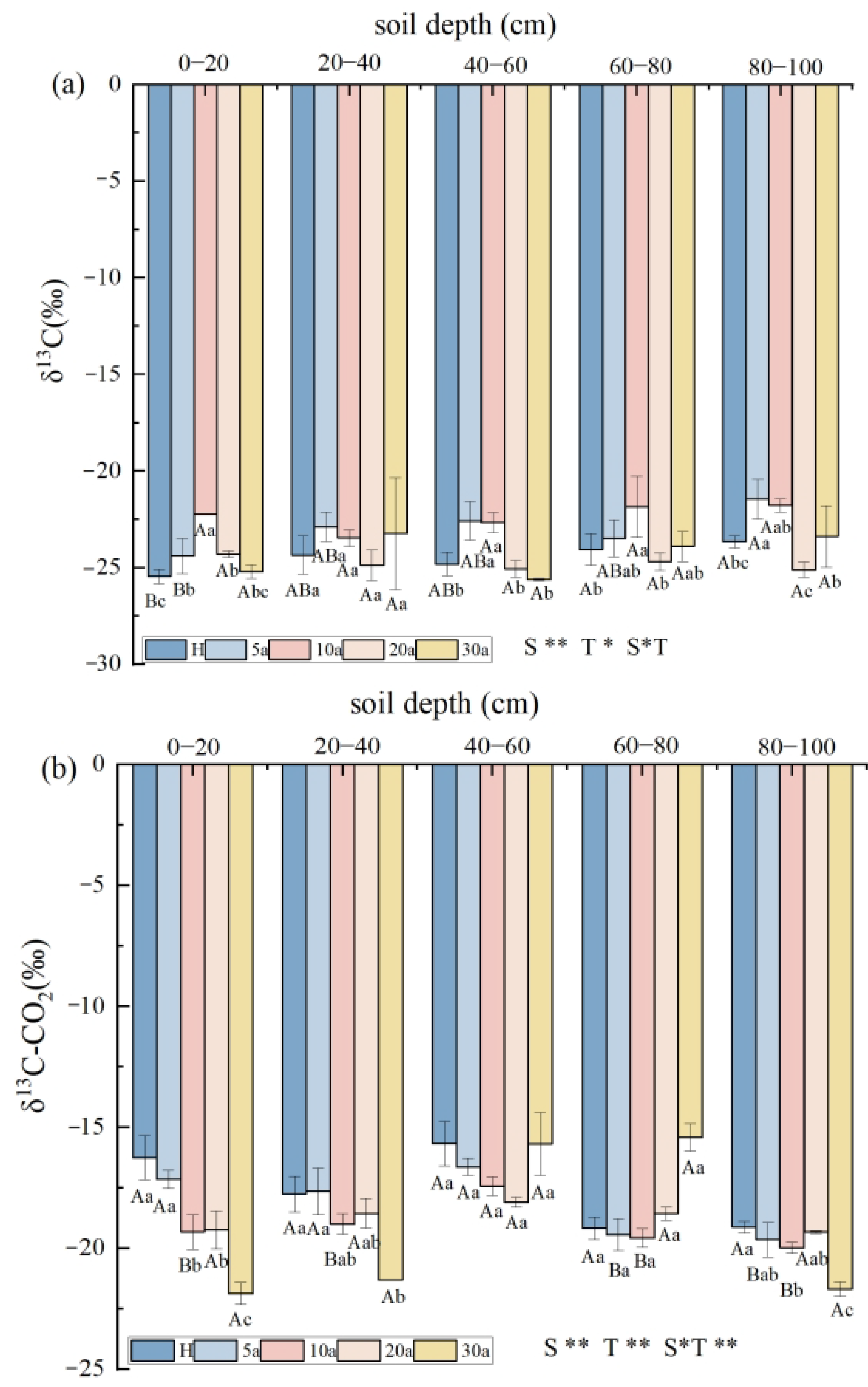
3.2. δ13C Values of Soil SIC and Soil CO2 in Cotton Fields with Different Continuous Crop Years
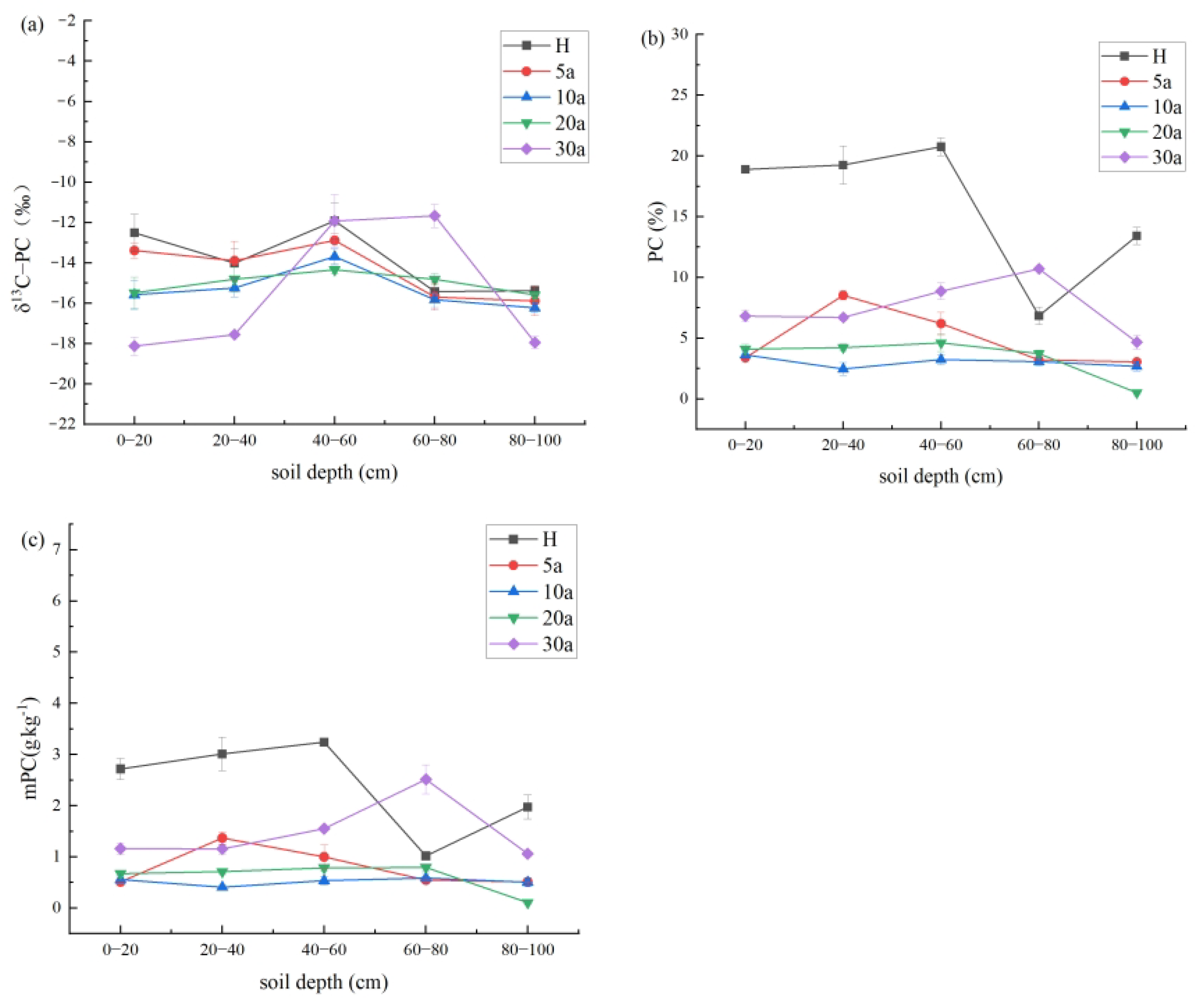
3.3. Soil PC Content of Cotton Fields with Different Continuous Crop Years
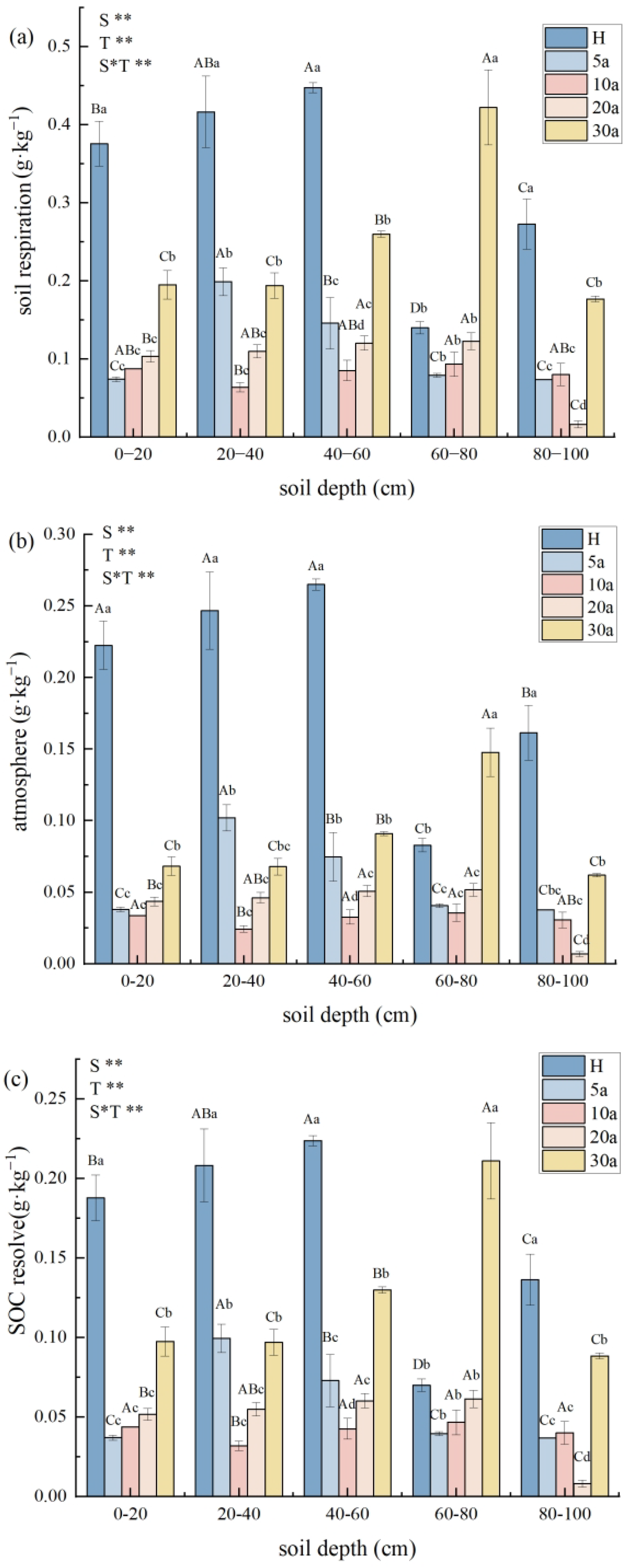
3.4. Change from Soil SOC to SIC in Cotton Fields with Different Continuous Crop Years
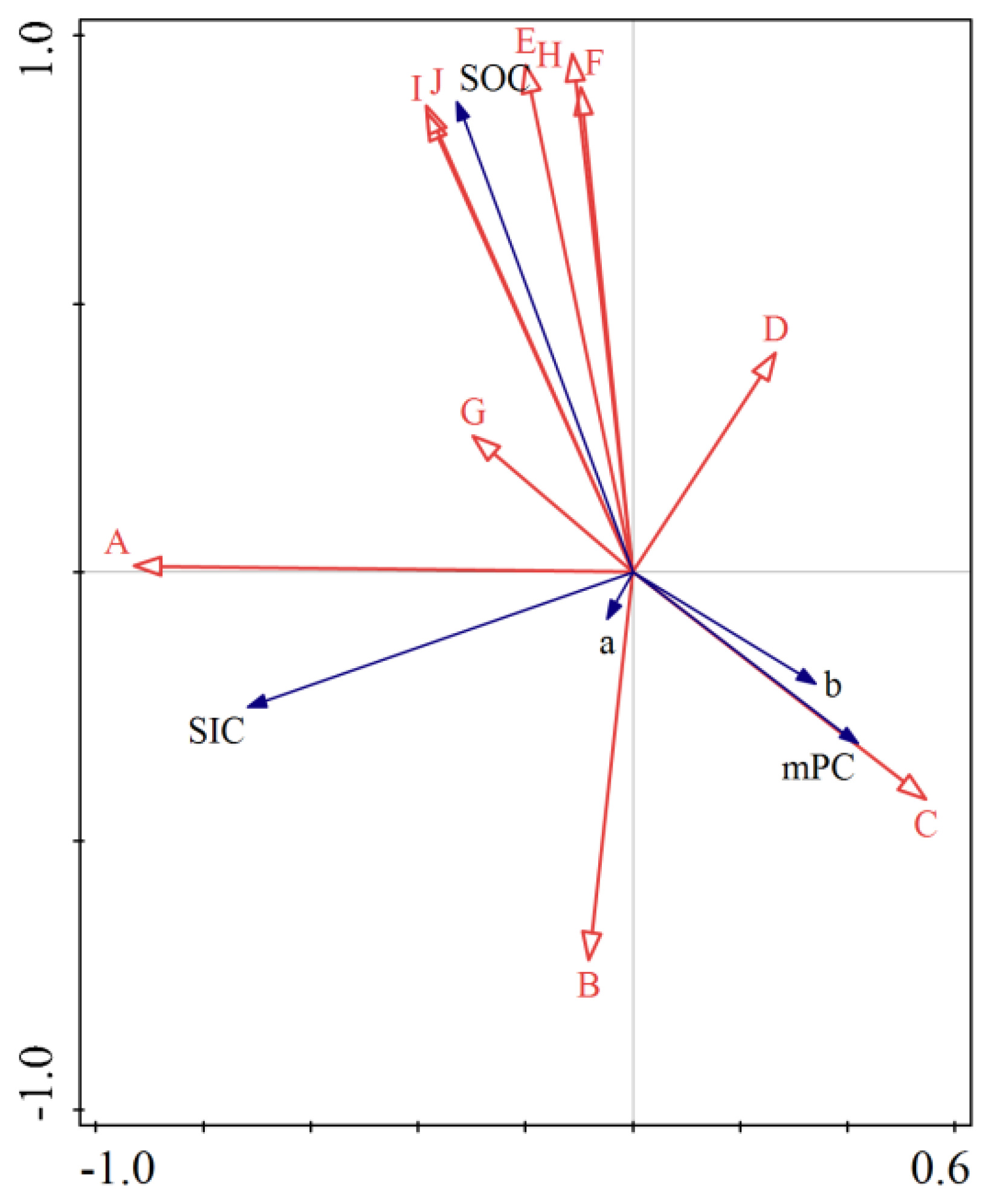
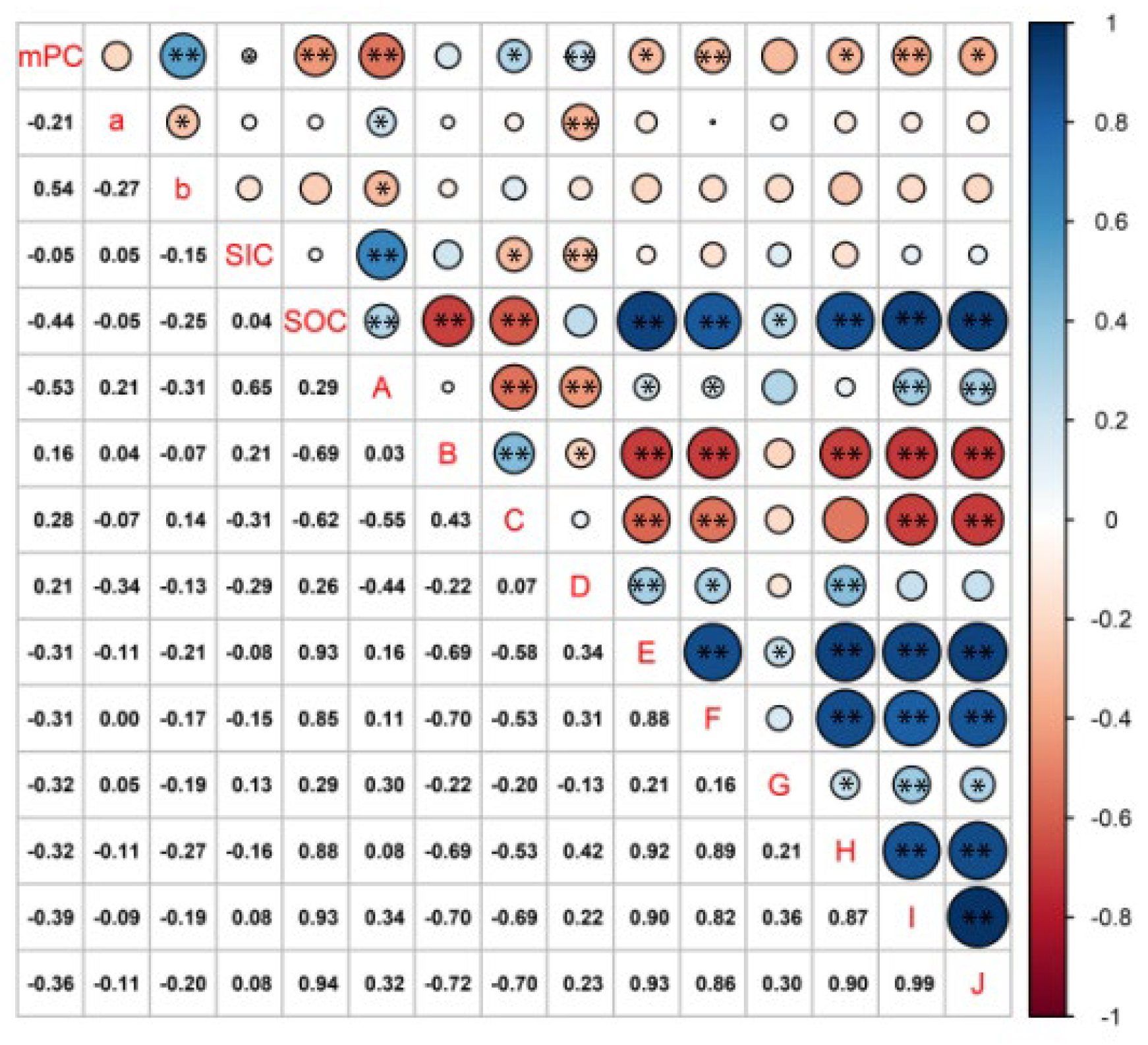
3.5. Factors Influencing the Change from SOC to SIC in Soils of Cotton Fields with Different Continuous Crop Years
4. Discussion
4.1. Effects of Different Cropping Years on Organic and Inorganic Carbon in Cotton Field Soils
4.2. Change from Organic Carbon to Inorganic Carbon in Soils with Different Continuous Crop Years
4.3. Correlation between the Change from Organic Carbon to Inorganic Carbon and Physicochemical Factors in Soils with Different Continuous Cropping Years
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.L.; Zhao, C.Y.; Ma, Q.L.; Li, Y.K.; Jing, H.J.; Sun, T.; Milne, E.; Easter, M.; Keith, P.; Au, Y.H.W.; et al. Carbon benefitsof wolfberry plantation on secondary saline land in Jingtai oasis, Gansu-a case study on application of the CBP model. J. Environ. Manag. 2015, 157, 303–310. [Google Scholar] [CrossRef]
- Bhattacharya, S.S.; Kim, K.H.; Das, S.; Uchimiya, M.; Jeon, B.H.; Kwon, E.; Szulejko, J.E. A review on the role of organic inputs in maintaining the soilcarbon pool of the terrestrial ecosystem. J. Environ. Manag. 2016, 167, 214–227. [Google Scholar] [CrossRef]
- Liao, Y.; Wu, W.L.; Meng, F.Q.; Smith, P.; Lal, R. Increase in soil organic carbon by agricultural intensification in northern China. Biogeosciences 2015, 12, 1403–1413. [Google Scholar] [CrossRef]
- Tong, J.H.; Hu, J.H.; Lu, Z.; Sun, H.R.; Yang, X.F. The impact of land use and cover change on soil organic carbon and total nitrogen storage in the Heihe River Basin: A meta-analysis. J. Geogr. Sci. 2019, 29, 1578–1594. [Google Scholar] [CrossRef]
- Nyachoti, S.; Jin, L.; Tweedie, C.E.; Ma, L. Insight into factors controlling formation rates of pedogenic carbonates: A combined geochemical and isotopic approach in dryland soils of the US Southwest. Chem. Geol. 2019, 527, 118503. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, J.P.; Wang, Y.; Zhang, X.C.; Zhen, Q.; Li, P.F. Spatial patterns of nitrogen and phosphorus in soil andtheir influencing factors in a typical agro-pastoral ecotonel. Environ. Sci. 2021, 42, 3010–3017. [Google Scholar]
- Srivastava, R.; Mohapatra, M.; Latare, A. Impact of land use changes on soil quality and species diversity in theVindhyan dry tropical region of India. J. Trop. Ecol. 2020, 36, 72–79. [Google Scholar] [CrossRef]
- Wang, J.P.; Wang, X.J.; Zhang, J.; Zhao, C.Y. Soil organic and inorganiccarbon and stable carbon isotopes in the Yanqi Basin of northwestern China. Eur. J. Soil Sci. 2015, 66, 95–103. [Google Scholar] [CrossRef]
- Su, Y.Z.; Wang, X.F.; Yang, R.; Jaehoon, L. Effects of sandy desertified land rehabilitation on soil carbon sequestration and aggregation in an arid region in China. J. Environ. Manag. 2010, 91, 2109–2116. [Google Scholar] [CrossRef]
- Zhang, L.H.; Xie, Z.K.; Wang, Y.J.; Guo, Z.H. The impact of land use changeon soil organic carbon and inorganic carbon contents at Loess Plateauin Longzhong. Chin. J. Soil Sci. 2013, 44, 119–125. [Google Scholar]
- Jin, X.X.; Wang, J.K.; Sun, L.J.; Yu, S.; Pei, J.B.; An, T.T.; Ding, F.; Gao, X.D.; Xu, Y.D. Progress of carbon cycle in farmlanoand sequestration in soil aggregates revealed by stable 13C isotope. Soils 2017, 49, 217–224. [Google Scholar]
- Zhao, Y.F.; Wang, X.; Ou, Y.S.; Jia, H.X.; Li, J.; Shi, C.M.; Liu, Y. Variations in soil δ13C with alpine meadow degradation on the eastern Qinghai-Tibet Plateau. Geoderma 2019, 338, 178–186. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Cui, L.J.; Zhang, M.Y.; Liu, W.W.; Wang, D.A.; Yang, S.; Xiao, H.Y. Effect of exogenous carbon input on organic carbon mineralization and its temperature sensitivity in farmland and wetland soil in the North China Plain. Chin. J. Ecol. Agric. 2019, 27, 1463–1471. [Google Scholar]
- Zhang, Y.H.; Liu, F.; Zhong, S. Progress on soil inorganic carbon. Hubei Agric. Sci. 2021, 60, 5–9, 14. [Google Scholar]
- Magaritz, M.; Amiel, A. Influence ofintense cultivation and irrigation on soilpropertiesin the Jordon Valley, Israel: Recrystalli-zation ofcarbonate minerals. Soil Sci. 1986, 45, 201–205. [Google Scholar]
- An, S.; Mentler, A.; Mayer, H.; Blum, W.E. Soil aggregation, aggregate stability, organic carbon and nitrogen in different soil aggregate fractions under forest and shrub vegetation on the Loess Plateau, China. Catena 2010, 81, 226–233. [Google Scholar] [CrossRef]
- Rovira, P.; Aranjuelo, I.; Nowak, R.S.; Nogués, S. Limited carbon inputs from plants into soils in arid ecosystems: A study of changes in the δ13C in the soil-root interface. Plant Soil 2019, 443, 307–322. [Google Scholar] [CrossRef]
- An, S.Q.; Gong, L.; Li, Y.M.; Chen, X.; Sun, L. Soil organic carbon components and their correlation with soil physicochemical factors in four different land use types of the northern TarimBasin. Environ. Sci. 2018, 39, 3382–3390. [Google Scholar]
- Lv, Y.Z.; Li, B.G. Soil Science Experiment; China Agricultural Press: Beijing, China, 2010. [Google Scholar]
- Bucha, M.; Jidrysek, M.O.; Kufka, D.; Pleśniak, L.; Marynowski, L.; Kubiak, K.; Błaszczyk, M. Methanogenic fermentation of lignite with carbon- bearing additivesinferred from stable carbon and hydrogen isotopes. Int. J. Coal Geol. 2018, 186, 65–79. [Google Scholar] [CrossRef]
- Breecker, D.O.; Bergel, S.; Nadel, M.; Tremblay, M.M.; Orozco, R.O.; Larson, T.E.; Sharp, Z.D. Minor stablecarbon isotope fractionation between respired carbon dioxide and bulk soil organic matter during laboratory incubation of topsoil. Biogeochemistry 2015, 123, 83–98. [Google Scholar] [CrossRef]
- Bughio, M.A.; Wang, P.L.; Meng, F.Q.; Qing, C.; Kuzyakov, Y.; Wang, X.J.; Junejo, S.A. Neoformation of pedogenic carbonates by irrigation and fertilizationand their contribution to carbon sequestration in soil. Geoderma 2016, 262, 12–19. [Google Scholar] [CrossRef]
- Huang, Q.B.; Qin, X.Q.; Liu, P.Y.; Zhang, L.K.; Su, C.T. Proportion of pedogenic carbonates and the impact on carbonsink calculation in karst area with semiarid environment. Carsologica Sin. 2016, 35, 164–172. [Google Scholar]
- Zhang, L.; Sun, X.Y.; Cao, J.; Gao, C.D.; Bao YH, X.G. Transfer of soil organic carbon to soil inorganic carbon in carbonate rocksoil of desert grassland. Arid. Land Geogr. 2010, 33, 732–739. [Google Scholar]
- Li, X.G.; Li, F.M.; Rengel, Z.; Bhupinderpal, S.; Wang, Z.F. Cultivation effects on temporal Changes of organic carbon and aggregate stability in desert soils of Hexi Corridor region in China. Soil Tillage Res. 2006, 91, 22–29. [Google Scholar] [CrossRef]
- Mustafa, A.; Hu, X.; Abrar, M.M.; Shah SA, A.; Nan, S.; Saeed, Q.; Kamran, M.; Naveed, M.; Cid, M.C.; Gao, H.J.; et al. Long-term fertilization enhanced carbon mineralization and maize biomassthrough physical protection of organic carbon in fractions under continuous maize cropping. Appl. Soil Ecol. 2021, 165, 103971. [Google Scholar] [CrossRef]
- Majhi, P.; Rahman, F.H.; Bhattacharya, R. Continuous rice cropping system with integrated use of inorganic andorganic sources of nutrients for soil quality improvement. Int. J. Environ. Clim. Chang. 2021, 11, 109–123. [Google Scholar] [CrossRef]
- Du, M.Y.; Feng, H.Y.; Fan, S.H.; Su, W.H.; Mao, C.; Tang, X.L.; Liu, G.L. Effects of fertilization on vertical distribution and seasonal dynamics of soil organic carbon in Phyllostachys edulis forests, western Fujian province. Sci. Silvae Sin. 2017, 53, 12–20. [Google Scholar]
- Wang, Y.Q.; Shao, M.A.; Zhang, C.C.; Liu, Z.P.; Zou, J.L.; Xiao, J.F. Soil organic carbon in deep profiles under Chinese continental monsoon climate and its relations with land uses. Ecol. Eng. 2015, 82, 361–367. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Tan, W.F.; Zhang, R.; Cao, H.; Huang, C.Q.; Yang, Q.K.; Wang, M.K.; Koopal, L.K. Soil inorganic carbon stock under different soil types and land uses on the Loess Plateau region of China. Catena 2014, 121, 22–30. [Google Scholar] [CrossRef]
- Edwards, K.R.; Čížková, H.; Kateřina, M.Z.; Alžbětá, S. Nutrient addition effects on carbon fluxes in wet grasslands with either organic or mineral soil. Wetlands 2015, 35, 55–68. [Google Scholar] [CrossRef]
- Song, X.D.; Yang, F.; Wu, H.Y.; Zhang, J.; Li, D.C.; Liu, F.; Zhao, Y.G.; Yang, J.L.; Ju, B.; Cai, C.F.; et al. Significant loss of soil inorganic carbon at the continental scale. Natl. Sci. Rev. 2022, 9, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.R.; Li, C.H.; Wang, Y.G.; Tang, L.S.; Chen, X.M. Effects of long-term fertilization on organic and inorganic carbon in oasis farmland. Study Arid. Areas 2012, 29, 593–597. [Google Scholar]
- Guo, Y.; Li, X.L.; Wang, X.J.; Wang, J.P.; Wang, X.F.; Ma, M.G.; Xie, X.H.; Sun, M.M. Study on the vertical profile of soil in arid and semi-arid areas. J. Soil 2016, 53, 1433–1443. [Google Scholar]
- Bai, S.G. Characteristics of Inorganic Carbon Profile Distribution and Carbon Sequestration Potential in Soil with Different Salinity Levels; Inner Mongolia Normal University: Hohhot, China, 2018. [Google Scholar]
- Andrew, T.N.; Benjamin, L.T.; Andrew, W.S.; Edmund VJ, T. Nitrogen and phosphorus constrain labile and stable carbonturnover in lowland tropical forest soils. Soil Biol. Biochem. 2015, 80, 826–839. [Google Scholar]
- Su, Y.Z.; Zhang, K.; Liu, T.N.; Fan, G.P.; Wang, T. Changes in soil properties and accumulation of soilcarbon after cultivation of desert sandy land in a marginal oasis in Hexi corridor reqion Northwest China. Sci. Agric. Sin. 2017, 50, 1646–1654. [Google Scholar]
- Zhang, L.; Sun, X.Y.; Gao, C.D.; Qiao, Y.; Li, S.Y. lisuyan Study on CO2 sequestration during the formationand turnover of soil secondary carbonate in desert steppe. J. Soil 2011, 48, 578–586. [Google Scholar]
- Yan, M.F.; Fan, L.N.; Wang, L. Restoration of soil carbonwith different tree species in a post-mining land in eastern Loess Plateau, China. Ecol. Eng. 2020, 158, 106025. [Google Scholar] [CrossRef]
- Zhu, S.F.; Liu, C.Q.; Tao, F.X.; Wang, Z.L.; Pu, H.C. Stable carbon isotope geochemical characteristics of soil organic matter in karst areas. Earth Environ. 2006, 34, 51–58. [Google Scholar]
- Lei, W.J.; Gu, G.A. Aridsoil pedogenesis and the main diagnosis layer division basis inChina[C]//GONG Zitong. In The Study of the Soil Classification System B Series: Exploration of Chinese Soil Taxonomy; Science Press: Beijing, China, 1992; pp. 73–98. [Google Scholar]
- Zhang, X.N.; Lv, G.H.; Gong, L.; Qin, L.; Li, C.J.; Sun, J.X.; Ren, M.L. Distribution characteristics of inorganic carbon of different soil types in Aibi Lake Wetland Nature Reserve in Xinjiang. J. Desert Res. 2013, 33, 1084–1090. [Google Scholar]
- Li, Z.W.; Liu, C.; Dong, Y.T.; Chang, X.F.; Nie, X.D.; Liu, L.; Xiao, H.B.; Lu, Y.M.; Zeng, G.M. Response of soil organic carbon and nitrogen stocks to soilerosion and land use types in the Loess hilly-gully region of China. Soil Tillage Res. 2017, 166, 1–9. [Google Scholar] [CrossRef]
- Gritsch, C.; Egger, F.; Zehetner, F.; Sophie, Z.B. The effect of temperature and moisture on trace gas emissions from deciduous and coniferous leaf liter. J. Geophys. Res. Biogeosciences 2016, 121, 1339–1351. [Google Scholar] [CrossRef]
- Yu, Z.H.; Lv, G.Y.; Wang, X.Y.; Xu, X.B.; Jia, D.X.; Wang, C.J. Effect of intensity on soil carbon and nitrogen and its stable isotopes in Inner Mongolia. J. Meadow 2022, 30, 544–552. [Google Scholar]
- Cao, Z.P.; Hu, C.; Ye, Z.N.; Wu, W.L. Effect of different soil fertilizer measures on microbial biomass carbon in high-yield farmland in North China. J. Ecol. 2006, 26, 1486–1493. [Google Scholar]
- Basile, D.L.; Balesdent, J.; Pellerin, S. Reviews and syntheses:the mechanisms underlying carbon storage in soil. Biogeosciences 2020, 17, 5223–5242. [Google Scholar] [CrossRef]
- Jagadamma, S.; Mayes, M.A.; Steinweg, J.M.; Schaeffer, S.M. Substrate quality alters microbial mineralization of added substrate and soil organic carbon. Biogeosciences Discuss. 2014, 11, 4451–4482. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, R.; Huang, C.Q.; Huang, C.Q.; Wang, B.Q.; Cao, H.; Koopal, L.K.; Tan, W.F. Effect of different vegetation cover on the vertical distribution of soil organic and inorganic carbon in the Zhifanggou Watershed on the loess plateau. Catena 2016, 139, 191–198. [Google Scholar] [CrossRef]

| Physical and Chemical Factors | The Importance of Ranking | Interpretation of Quantity/% | F | P |
|---|---|---|---|---|
| Soil water content | 1 | 44.3 | 18.5 | 0.002 |
| Readily available carbon | 2 | 31.5 | 16.8 | 0.002 |
| Microbial biomass carbon | 3 | 8.8 | 5.0 | 0.006 |
| Total salt | 4 | 4.5 | 2.7 | 0.082 |
| Water-soluble carbon | 5 | 2.9 | 1.7 | 0.184 |
| Effective phosphorus | 6 | 3.3 | 2.0 | 0.200 |
| Soil bulk weight | 7 | 1.9 | 1.2 | 0.348 |
| Fast-acting potassium | 8 | 1.1 | 0.7 | 0.556 |
| Total nitrogen | 9 | 0.9 | 0.5 | 0.720 |
| pH | 10 | 0.8 | 0.5 | 0.856 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Gong, L.; Yang, Y.; Ding, Z.; Li, X. Mineralization and Fixed Stable Carbon Isotopic Characteristics of Organic Carbon in Cotton Fields with Different Continuous Cropping Years. Agronomy 2023, 13, 804. https://doi.org/10.3390/agronomy13030804
Ma X, Gong L, Yang Y, Ding Z, Li X. Mineralization and Fixed Stable Carbon Isotopic Characteristics of Organic Carbon in Cotton Fields with Different Continuous Cropping Years. Agronomy. 2023; 13(3):804. https://doi.org/10.3390/agronomy13030804
Chicago/Turabian StyleMa, Xinyu, Lu Gong, Yuxin Yang, Zhaolong Ding, and Xinzhu Li. 2023. "Mineralization and Fixed Stable Carbon Isotopic Characteristics of Organic Carbon in Cotton Fields with Different Continuous Cropping Years" Agronomy 13, no. 3: 804. https://doi.org/10.3390/agronomy13030804
APA StyleMa, X., Gong, L., Yang, Y., Ding, Z., & Li, X. (2023). Mineralization and Fixed Stable Carbon Isotopic Characteristics of Organic Carbon in Cotton Fields with Different Continuous Cropping Years. Agronomy, 13(3), 804. https://doi.org/10.3390/agronomy13030804






