Effect of Presowing Magnetic Field Stimulation on the Seed Germination and Growth of Phaseolus vulgaris L. Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Agrotechnical Conditions
2.2. Conditions of Irradiation with an FM
2.3. Biometric Assessment
2.4. Statistical Analysis
2.5. Soil Conditions
2.6. Meteorological Conditions
3. Results
3.1. Germination Rate
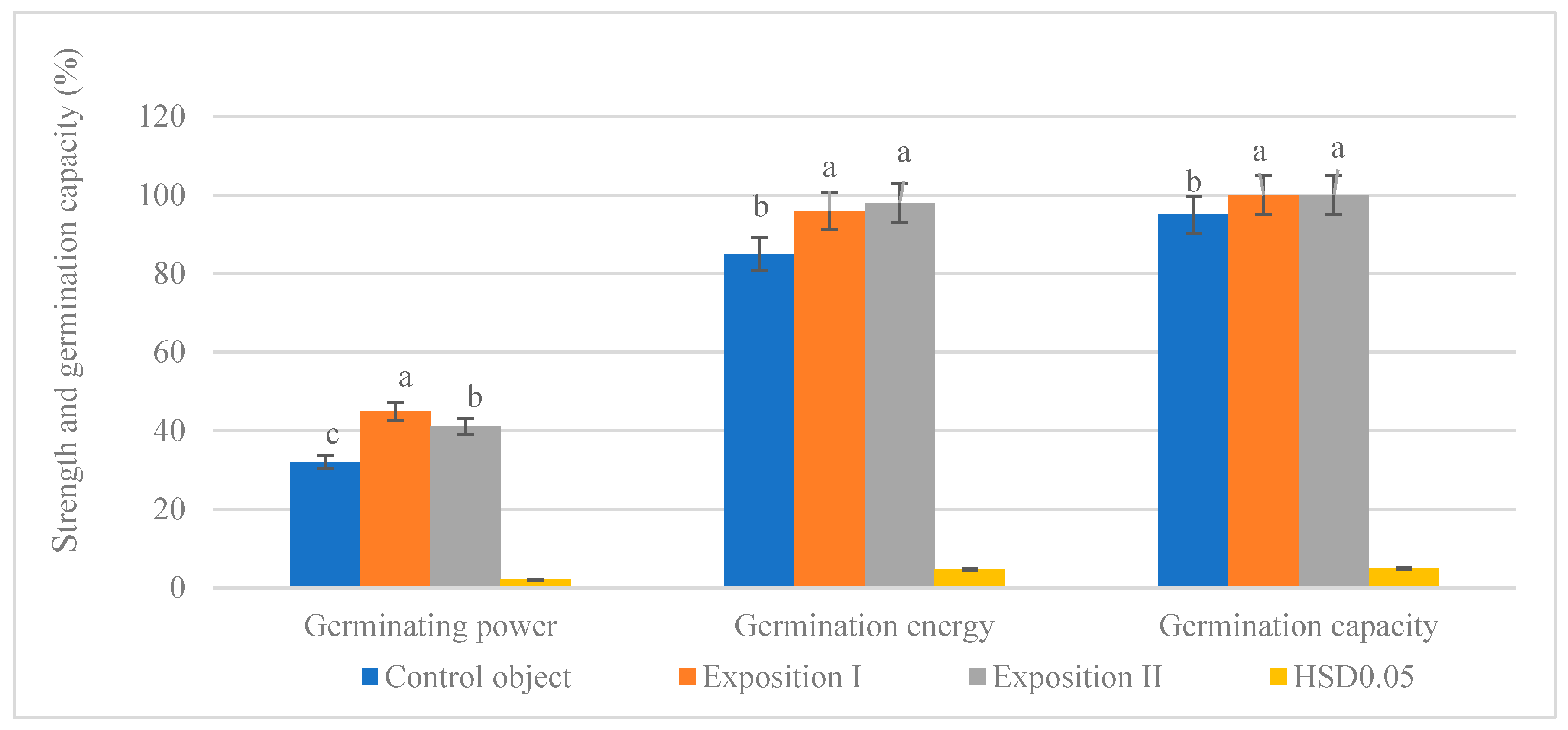
3.2. Germination Energy, Strength, and Capacity of Seeds
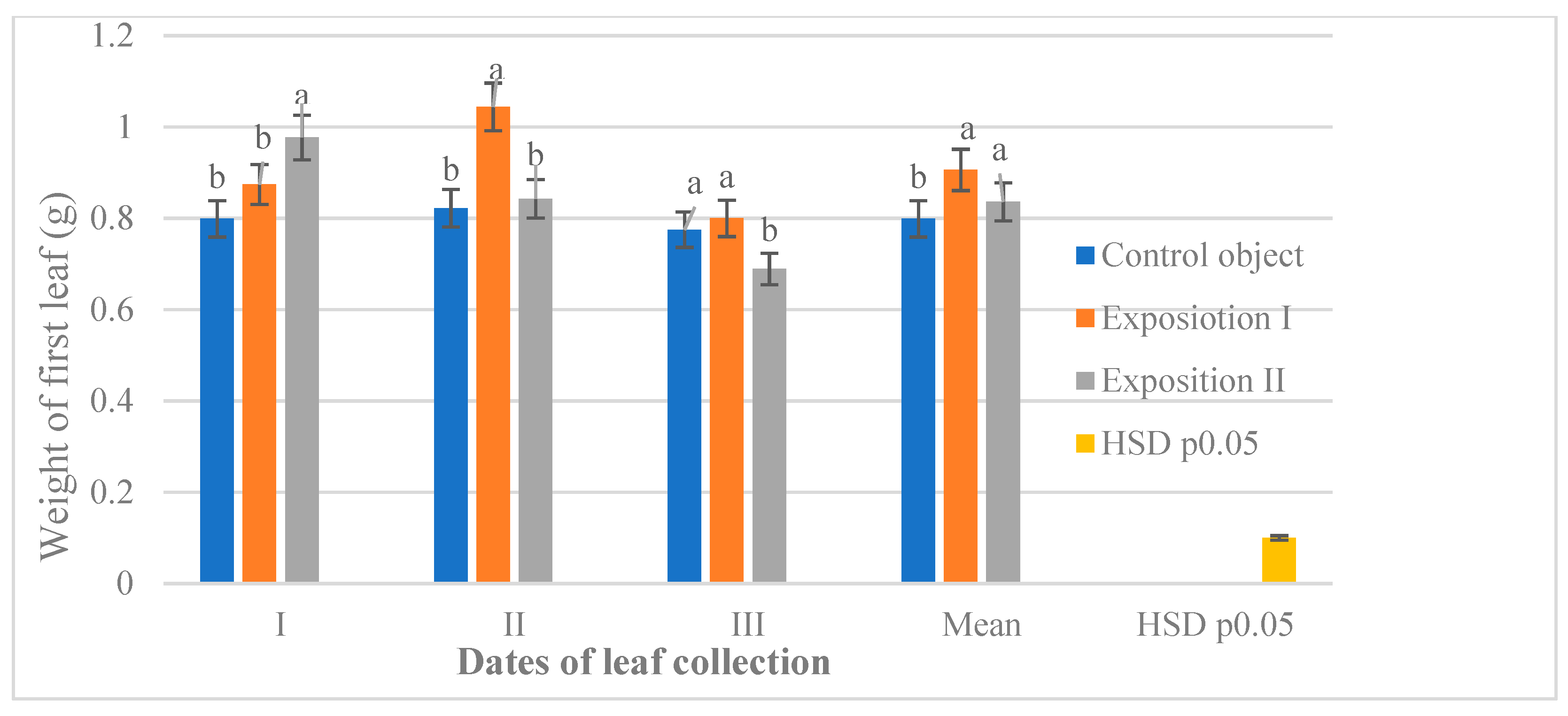
3.3. Weight of the Leaf
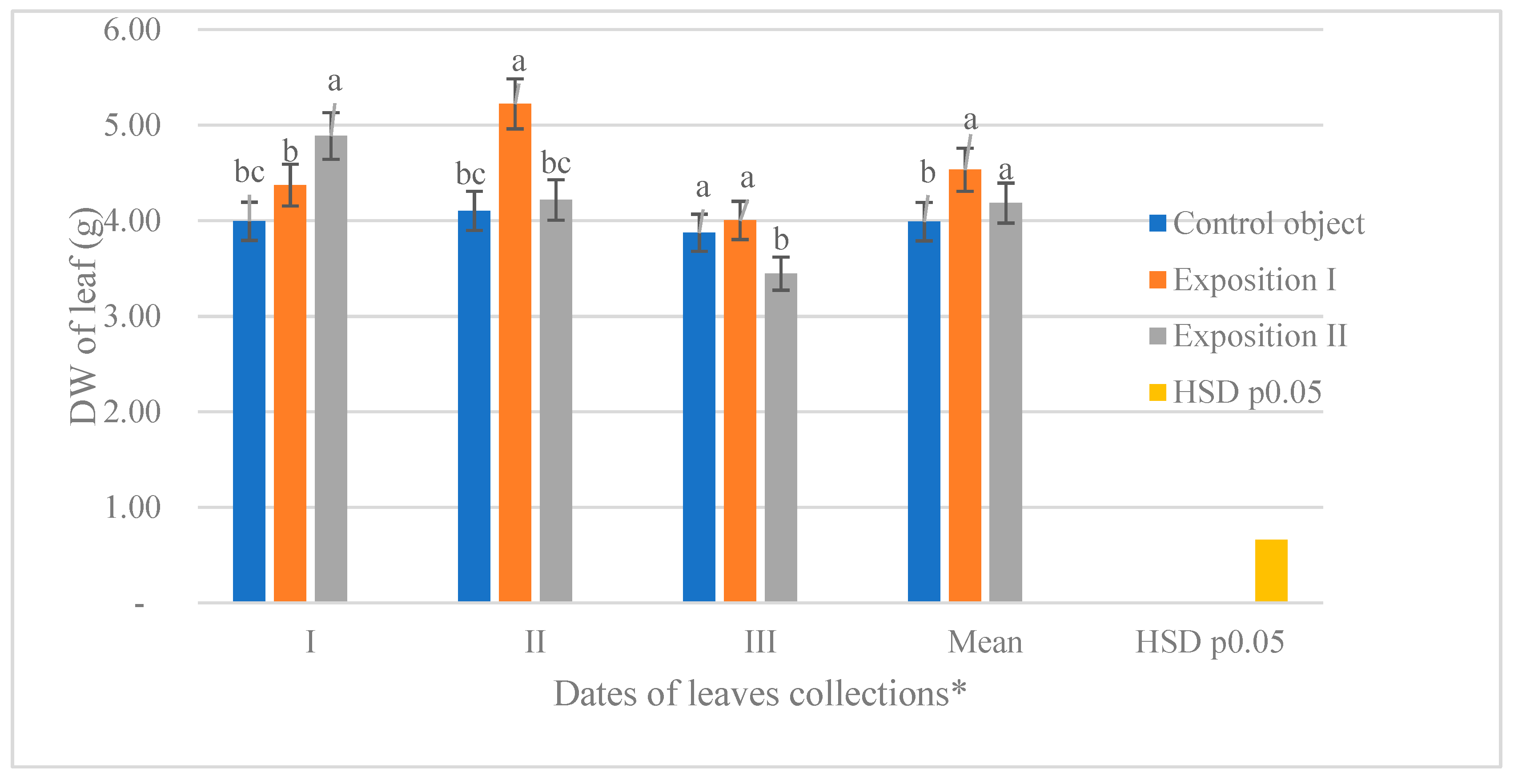
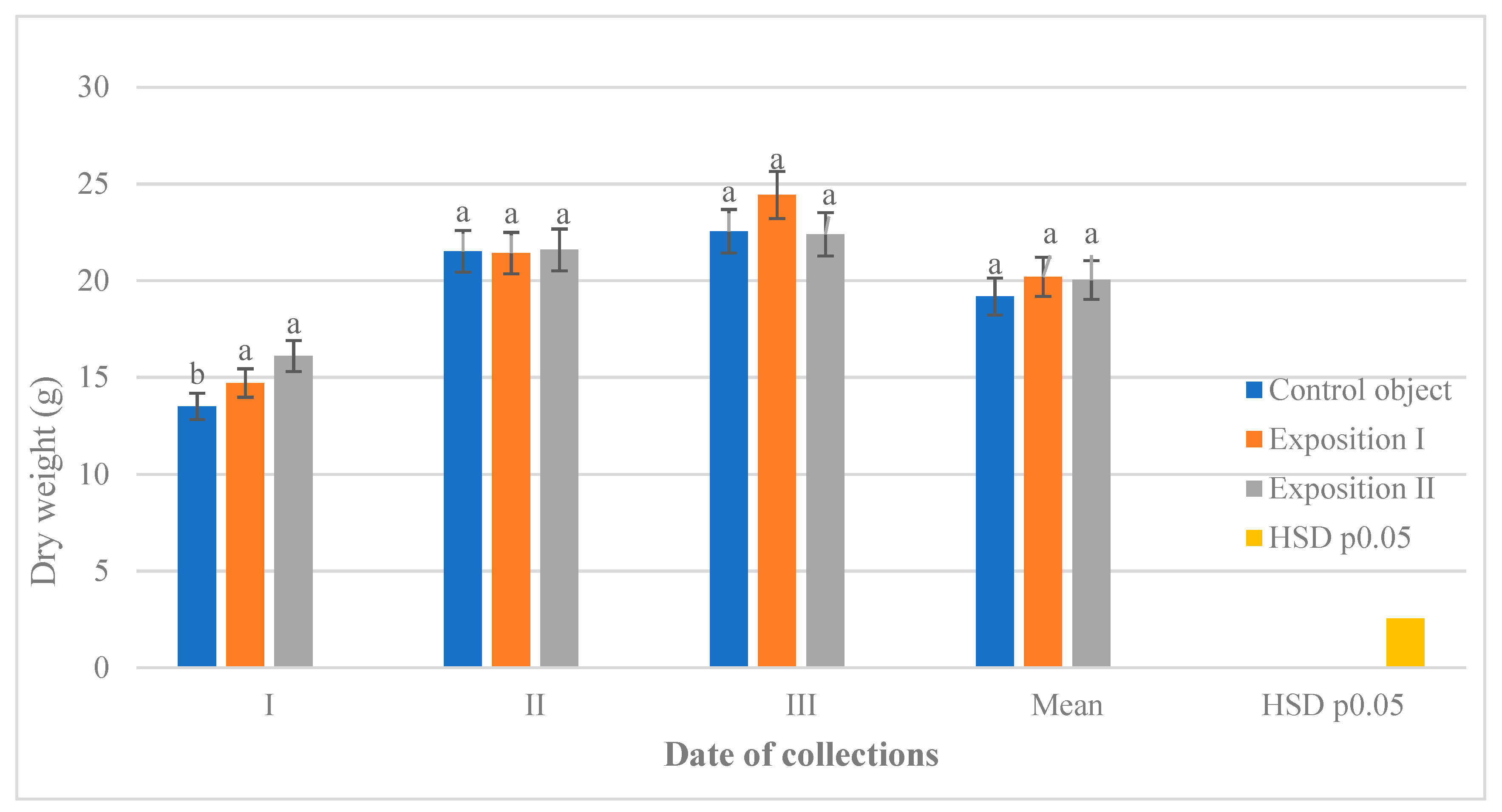
3.4. The Effect of Stimulation of Seeds and Deadlines of Collection on the Dry Weight of Leaves
3.5. Significant Differences in Plant Growth Were Observed Only between the Plant Measurement Terms
3.6. Variability of Bean Features and Their Interdependencies
4. Discussion
The Stimulant Effect on Plants in the Early Stages of Their Growth
- -
- -
- the movement of a living organism in relation to the FM, which may be the reason for inducing currents;
- -
- the magnetomechanical effects occurring inside organisms, consisting in the orientation of structures with magnetic anisotropy in homogeneous fields and the shifts of ferromagnetic and paramagnetic substances in fields with nonzero gradients;
- -
- there may also be an impact on the uncompensated magnetic spins of paramagnetic elements and free radicals;
- -
- the Zeeman effect is possible, i.e., the splitting of the spectral lines of atoms placed in a static FM in components that differ slightly in wave numbers;
- -
- there may also be the Dorfman effect, consisting in the reorientation of proteins in the magnetostatic field due to the anisotropy of these molecules;
- -
- the external FM changes the properties of liquid crystals and, therefore, affects the properties of membranes; cell organelles; and, consequently, more complex systems;
- -
- some components of living organisms exhibit magnetostrictive properties. It is therefore possible to influence such components;
- -
5. Conclusions
- The biostimulation of the seeds with an FM contributed to a higher germination capacity, energy, and strength of the common bean seeds. The biological optimum of the seed germination of the common bean determined the regression curves of the second and third degree.
- The presowing FM stimulation of the common bean seeds favourably affected the fresh weight of the first and fifth leaves.
- The highest fresh weight of the leaves was obtained in the full flowering phase of the plants, and their dry weight was obtained in the pod setting phase.
- The biostimulation with an FM led to a significant increase in dry matter accumulation in the plants and leaves. The more remarkable ability of the roots, stems, and leaves of the plants obtained from the seeds treated with a variable FM to accumulate dry matter suggests a beneficial effect on the plants’ development and health.
- The physical method of treating seeds with an FM applied before sowing is a noninvasive method and is environmentally friendly and useful, especially in the seed production not only of legumes but also of other crops and vegetables in ecological systems.
- The obtained results may be a premise to continue research in this direction, taking into account not only other solenoid operating parameters but also the features of the physical raw materials and semifinished products obtained from the bean seeds subjected to an FM.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGTC | average germination time coefficient |
| EF | electromagnetic fields |
| FM | magnetic fields |
| MGR | Maguire’s germination rate |
References
- Capistrán-Carabarin, A.; Aquino-Bolaños, E.A.; García-Díaz, Y.D.; Chávez-Servia, J.L.; Vera-Guzmán, A.M.; Carrillo-Rodríguez, J.C. Complementarity in Phenolic Compounds and the Antioxidant Activities of Phaseolus coccineus L. and P. vulgaris L. Landraces. Foods 2019, 8, 295. [Google Scholar] [CrossRef]
- Łabuda, H.; Baran, A.; Papliński, R. Flowering and setting of pods of seven varieties of string beans (Phaseolus vulgaris L.) in various growing conditions. Acta Agrobot. 2006, 59, 439–446. (In Polish) [Google Scholar] [CrossRef]
- Rochalska, M. The influence of low frequency magnetic field upon cultivable plant physiology. Nukleonika 2008, 53, 17–20. [Google Scholar]
- Shine, M.; Guruprasad, K.; Anand, A. Enhancement of germination, growth, and photosynthesis in soybean by pre-treatment of seeds with magnetic field. Bioelectromagnetics 2011, 32, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Kumari, R.B. Influence of pulsed magnetic field on soybean (Glycine max L.) seed germination, seedling growth and soil microbial population. Indian J. Biochem. Biophys. 2013, 50, 312–317. [Google Scholar] [PubMed]
- Pietruszewski, S. Impact of magnetic and electric fields on seed germination of selected crops. Tech. Agrar. 2002, 1, 75–81. (In Polish) [Google Scholar]
- Podleśny, J. The effect of magnetic stimulation of seeds on the growth, development and yielding of crops. Acta Agrophy. 2002, 4, 459–473. [Google Scholar]
- Podleśny, J.; Pietruszewski, S.; Podleśna, A. Influence of magnetic stimulation of seeds on the formation of morphological features and yielding of the pea. Int. Agrophys. 2005, 19, 61–68. [Google Scholar]
- Podleśny, J.; Pietruszewski, S. The influence of magnetic stimulation of seeds on the growth and yielding of peas grown at different soil moisture. Inżynieria Rol. 2007, 8, 207–212. (In Polish) [Google Scholar]
- Kornarzyński, K.; Pietruszewski, S. Impact of magnetic and electric fields on seed germination of selected crops. Acta Agrophys. 2008, 11, 429–435. (In Polish) [Google Scholar]
- Romankiewicz, B. The Effect of Pre-Sowing Seed Stimulation with a Variable Magnetic Field on the Growth, Development and Yielding of Pea. Doctoral Dissertation, University of Rzeszów, Rzeszów, Poland, 2018; p. 132. (In Polish). [Google Scholar]
- Cieśla, A.; Kraszewski, W.; Skowron, M.; Syrek, P. The influence of the magnetic field on seed germination. Electrotech. Rev. 2015, 91, 125–128. [Google Scholar] [CrossRef]
- Maguire, J.D. Germination rate—Aid in selection and assessment of seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Koronacki, J. Statistics, for Students of Technical and Natural Sciences; WNT: Warsaw, Poland, 2009; p. 491. ISBN 83-204-2994-3. [Google Scholar]
- Kornarzyński, K.; Pietruszewski, S.; Segit, Z.; Szwed-Urbaś, K.; Łacek, R. Preliminary investigation of the effect of static and alternating magnetic fields on growth speed of wheat germs. Acta Agrophy. 2004, 3, 521–528. [Google Scholar]
- Podleśny, J.; Pietruszewski, S. Impact of magnetic field treatment of seeds on growth, development and dynamics of mass storage of white lupine (Lupinus albus L.). Inżynieria Rol. 2006, 6, 169–176. (In Polish) [Google Scholar]
- Pietruszewski, S. Improving the quality of seed of plants cultivated by physical methods. In New Trends in Agrophysics; Dobrzański, J., Grudas, S., Nawrocki, S., Rybczyński, R., Eds.; Scientific Publisher FRNA: Lublin, Poland, 2008; pp. 71–72. (In Polish) [Google Scholar]
- Pietruszewski, S.; Kania, K. Effect of magnetic field on germination and yield of wheat. Int. Agrophy. 2010, 24, 297–302. [Google Scholar]
- Bujak, K.; Frant, M. Influence of pre-sowing seed stimulation using magnetic field on winter wheat yielding and technological quality of grain. Acta Agrophys. 2010, 15, 233–245. (In Polish) [Google Scholar]
- Mroczek-Zdyrska, M.; Tryniecki, Ł.; Kornarzyński, K.; Pietruszewski, S.; Gagoś, M. Influence of magnetic field stimulation on the growth and biochemical parameters in Phaseolus vulgaris L. J. Microbiol. Biotech. Food Sci. 2016, 5, 548–551. [Google Scholar] [CrossRef]
- Mghaiouini, R.; Elaouad, A.; Taimoury, H.; Sabir, I.; Chibi, F.; Hozayn, M.; Garmim, T.; Nmila, R.; Rchid, H.; Monkade, M. Influence of the Electromagnetic Device Aqua 4D on Water Quality and Germination of Lettuce (Lactuca sativa L.). Int. J. Curr. Eng. Technol. 2020, 10, 19–24. [Google Scholar] [CrossRef]
- Atak, Ç.; Çelik, Ö.; Olgun, A.; Alikamanoğlu, S. Effect of magnetic field on peroxidase activities of soybean tissue culture. Biotechnol. Biotechnol. Equip. 2007, 21, 166–171. [Google Scholar] [CrossRef]
- Akinyele, B.J.; Akinkunmi, O.C.; Adedayo, K.D. Effect of electromagnetic field on the spoilage fungi of some selected edible fruits in southwestern, Nigeria. J. Microbiol. Biotechnol. Food Sci. 2012, 2, 701–712. [Google Scholar]
- Kataria, S.; Jain, M. Magnetopriming Alleviates Adverse Effects of Abiotic Stresses in Plants. In Plant Tolerance to Environmental Stress, 1st ed.; Role of phytoprotectants; Hasanuzzaman, M., Fujita, M., Oku, H., Islam, T.M., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 427–442. [Google Scholar]
- Kataria, S.; Jain, M.; Tripathi, D.K.; Singh, V.P. Involvement of nitrate reductase-dependent nitric oxide production in magnetopriming—Induced salt tolerance in soybean. Physiol. Plant 2020, 168, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, T.; Dumlupinar, R.; Erdal, S. Acceleration of germination and early growth of wheat and bean seedlings grown under various magnetic field and osmotic conditions. Bioelectromagnetics 2010, 31, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Alkassab, A.T.; Albach, D.C. Response of Mexican aster Cosmos bipinnatus and field mustard Sinapis arvensis to irrigation with magnetically treated water (MTW). Biol. Agric. Hortic. 2014, 30, 62–72. [Google Scholar] [CrossRef]
- Belyavskaya, N.A. Ultrastructure and calcium balance in meristem cells of pea roots exposed to extremely low magnetic fields. Adv. Space Res. 2001, 28, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Belyavskaya, N.A. Biological effects due to weak magnetic field on plants. Adv. Space Res. 2004, 34, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Jouni, F.J.; Abdolmaleki, P.; Ghanati, F. Oxidative stress in broad bean (Vicia faba L.) induced by static magnetic field under natural radioactivity. Mutat. Res. 2012, 741, 116–121. [Google Scholar] [CrossRef]
- Broszkiewicz, A.; Detyna, J.; Bujak, H. Influence of the magnetic field on the germination process of tosca bean seeds (Phaseolus vulgaris L.). Plant Breed. Seed Sci. 2018, 77, 103–116. [Google Scholar] [CrossRef]
- Pietrzyk, W. Standardization of studies on the impact of electromagnetic fields on materials of biological origin. Acta Agrophys. 2006, 8, 915–921. (In Polish) [Google Scholar]
- Martínez, E.; Carbonell, M.V.; Flórez, M.; Amaya, J.M.; Maqueda, R. Pea and lentil growth stimulation due to exposure to 125 and 250 mT stationary fields. Int. Agrophy. 2009, 18, 657–663. [Google Scholar]
- Odhiambo, J.O.; Ndiritu, F.G.; Wagara, I.N. Effects of static electromagnetic fields at 24 h incubation on the germination of rose coco beans (Phaseolus vulgaris). Romanian J. Biophys. 2009, 19, 135–147. [Google Scholar]
- Li, F.; Tang, Y. The activation mechanism of peroxidase by ultrasound. Ultrason. Sonochem. 2021, 71, 105362. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, T.Q.; Karagić, D.; Liu, X.; Cui, J.; Gui, J.; Gu, M.; Gao, M. Impact of ultrasonication on increased germination and better growth of cuttings of mature grass seeds of high fescue and Russian wildrye. Sci Rep. 2016, 6, 22403. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.T.; Nicolier, M.; Tio, G.; Mouchabac, S.; Haffen, E.; Bennabi, D. Effects of High Frequency Repetitive Transcranial Magnetic Stimulation (HF-rTMS) on Delay Discounting in Major Depressive Disorder: An Open-Label Uncontrolled Pilot Study. Brain Sci. 2019, 9, 230. [Google Scholar] [CrossRef]
- Aladjadjiyan, A. Influence of stationary magnetic field on lentil seeds. Int. Agrophys. 2010, 24, 321–324. [Google Scholar]
- Aghamir, F.; Bahrami, H.; Malakoutia, M.J.; Eshghi, S.; Sharifica, F. Seed germination and seedling growth of bean (Phaseolus vulgaris) as influenced by magnetized saline water. Eurasian J. Soil Sci. 2016, 5, 39–46. Available online: http://www.fesss.org/download/arsiv/YD6R64FX.pdf (accessed on 1 February 2023). [CrossRef]
- WRB. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports 106; Food and Agriculture Organization of The United Nations: Rome, Italy, 2015; ISBN 978-92-5-108369-7. [Google Scholar]
- Rosen, A.D. Studies on the efect of static magnetic fields on biological systems. Piers Online 2010, 6, 133–136. [Google Scholar] [CrossRef]
- Yamashita, M.; Tomita-Yokotani, K.; Hashimoto, H.; Takai, M.; Tsushima, M.; Nakamura, T. Experimental concept for examination of biological effects of magnetic field concealed by gravity. Adv. Space Res. 2004, 34, 1575–1578. [Google Scholar] [CrossRef]
- Maffei, M.E. Magnetic field effects on plant growth, development, and evolution. Front. Plant Sci. 2014, 5, 445. [Google Scholar] [CrossRef]
- Winiarczyk, K.; Skrzypczak, K.; Jaroszuk-Sciseł, J.; Bocianowski, J. Investigations of the capacity and strength of seed germination in Allium victorialis L. Acta Soc. Bot. Pol. 2014, 83, 219–228. [Google Scholar] [CrossRef]
- Çelik, Ö.; Büyükuslu, N.; Atak, Ç.; Rzakoulieva, A. Effects of magnetic field on activity of superoxide dismutase and catalase in Glycine max (L.). Merr. Roots 2009, 18, 175–182. [Google Scholar]
- Kouchebagh, S.B.; Farahvash, F.; Mirshekari, B.; Arbat, H.; Khoei, F. Effects of physical treatments on germination and stand establishment of sunflower (Helianthus annuus L. var. Hyson) under laboratory condition. Int. J. Biosci. 2014, 5, 1–6. [Google Scholar]
- Podleśny, J.; Podleśna, A.; Gładyszewska, B.; Bojarszczuk, J. Effect of Pre-Sowing Magnetic Field Treatment on Enzymes and Phytohormones in Pea (Pisum sativum L.) Seeds and Seedlings. Agronomy 2021, 11, 494. [Google Scholar] [CrossRef]
- Rakosy-Tican, L.; Aurori, C.M.; Morariu, V.V. Influence of near null magnetic field on in vitro growth of potato and wild Solanum species. Bioelectromagnetics 2005, 26, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Kataria, M.; Taimourya, H.; Santos, L.O.; Menegatti, R.D.; Jain, M.; Ihtisham, M.; Liu, S. Magnetic Field (FM) Applications in Plants: An Overview. Plants 2020, 9, 1139. [Google Scholar] [CrossRef] [PubMed]
- Sowiński, M. Wpływ Stymulacji Nasion Polem Magnetycznym na Wzrost, Rozwój i Plonowanie Bobiku (Vicia faba L. ssp. minor). Praca Doktorska, IUNG Puławy, Puławy, Poland, 2004. [Google Scholar]
- Carbonell, M.V.; Florez, M.; Martinez, E.; Maqueda, R.; Amaya, J. Study of stationary magnetic fields for the initial growth of peas (Pisum sativum L.) seeds. Seed Sci. Technol. 2011, 39, 673–679. [Google Scholar] [CrossRef]
- Prusiński, J. Yielding of common beans (Phaseolus vulgaris L.) depending on the intensity of cultivation technology. Part I. The amount and quality of seed yield and their agrotechnical conditions. Acta Sci. Pol. Agric. 2006, 5, 65–76. (In Polish) [Google Scholar]
- Da Silva, J.A.T.; Dobránszki, J. Magnetic fields: How is plant growth and development impacted? Protoplasma 2016, 253, 231–248. [Google Scholar] [CrossRef]
- Waqas, M.; Korres, N.E.; Khan, M.D.; Nizami, A.-S.; Deeba, F.; Ali, I.; Hussain, H. Advances in the concept and methods of seed priming. In Priming and Pretreatment of Seeds and Seedlings; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 11–41. [Google Scholar]
- Iderawumi, A.M.; Eneminyene, C. Effect of magnetic field on seedling preparation and germination. J. Agric. Res. 2020, 6, 1–9. [Google Scholar]
- Boix, Y.F.; Dubois, A.F.; Quintero, Y.P.; Alemán, E.I.; Victório, C.P.; Aguilera, J.G.; Betancourt, M.N.; Morales-Aranibar, L. Magnetically. Treated Water in Phaseolus vulgaris L.: An Alternative to Develop Organic Farming in Cuba. Plants 2023, 12, 340. [Google Scholar] [CrossRef]
- Jakubowski, J.; Syrotyuk, S.; Lopushniak, V.; Atilgan, A. Effect of alternating magnetic field stimulation of wheat seeds for various technological purposes. Electrotech. Rev. 2022, 5, 38–42. [Google Scholar] [CrossRef]
- Skowron, M.; Cieśla, A. Biostymulacja ziarniaków silnym stałym polem magnetycznym. Pr. Inst. Elektrotechniki 2008, 236, 143–167. (In Polish) [Google Scholar]
- Cieśla, A.; Skowron, M. The analysis of the static magnetic field in paramagnetic spheroids at the laminar structure on the example grain wheat. In Proceedings of the ISEF’2007 International Symposium on Electromagnetic Fields in Mechatronics, Electrical and Electronic Engineering, Prague, Czech Republic, 13–15 September 2007; pp. 70–71. [Google Scholar]
- Krawczyk, A. Bioelektromagnetyzm; Instytut Naukowo-Badawczy ZTUREK Warszawa: Turek, Poland, 2002. (In Polish) [Google Scholar]
- Pietruszewski, S. Magnetyczna i Elektryczna Biostymulacja Nasion Roślin Uprawnych; Agrolaser: Lubin, Poland, 2003; pp. 63–69. (In Polish) [Google Scholar]
- Pietruszewski, S. Magnetyczna Biostymulacja Materiału Siewnego Pszenicy Jarej. Ph.D. Thesis, Wydawnictwo Akademii Rolniczej w Lublinie, Lublin, Poland, 1999; p. 98. (In Polish). [Google Scholar]

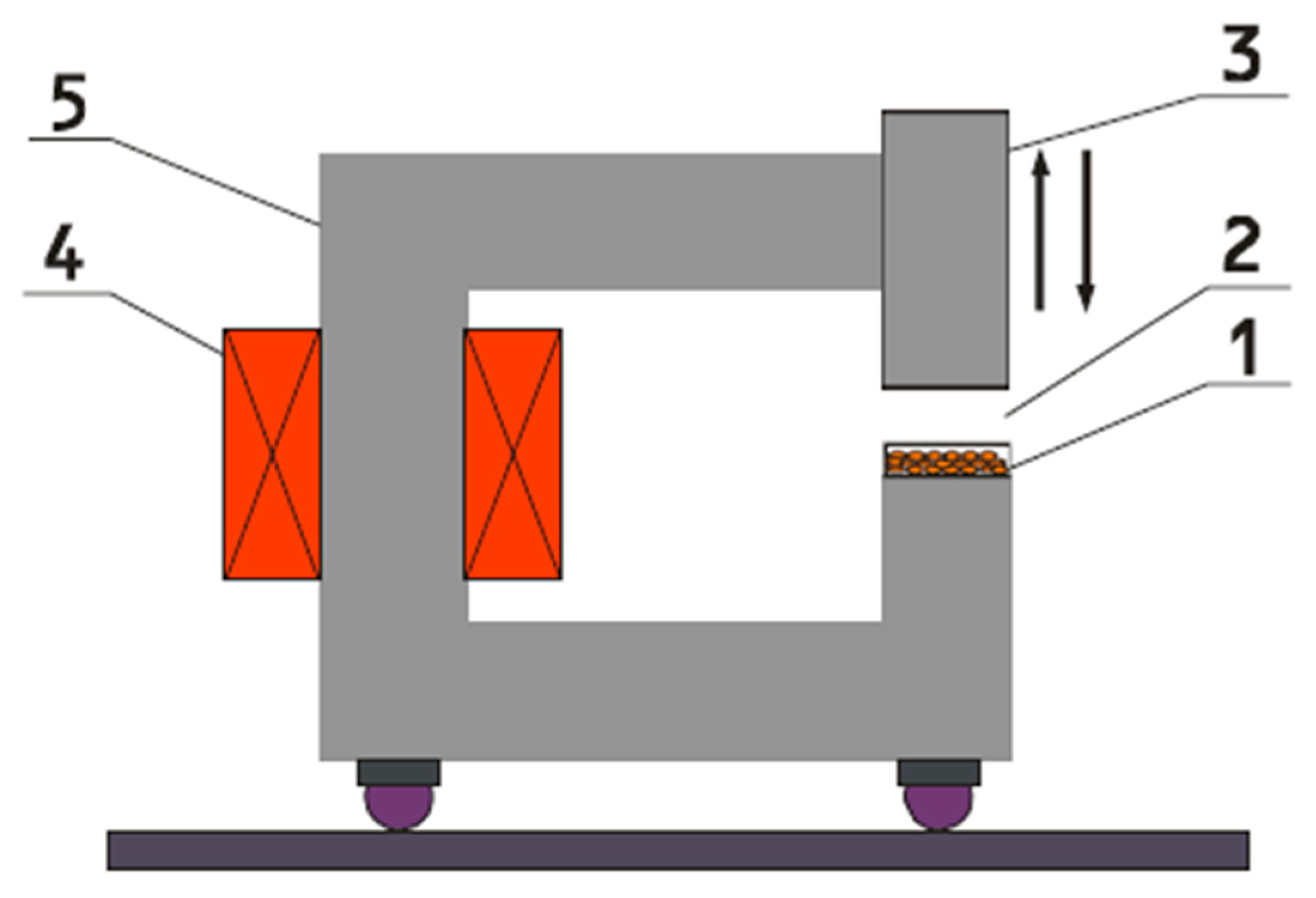


| Years | Months | Mean IV-VIII | ||||
|---|---|---|---|---|---|---|
| IV | V | VI | VII | VIII | ||
| Rainfall (mm) | ||||||
| 2015 | 30.6 | 80.5 | 126.6 | 30.2 | 33.1 | 60.2 |
| 2016 | 63.7 | 119.0 | 52.9 | 164.2 | 52.1 | 90.4 |
| 2017 | 28.2 | 98.2 | 63.1 | 10.6 | 15.8 | 43.2 |
| SCN * | 55.9 | 95.6 | 100.9 | 116.5 | 30.1 | 79.8 |
| Air temperature (°C) | ||||||
| 2015 | 9.5 | 14.5 | 17.6 | 18.7 | 19.1 | 15.9 |
| 2016 | 10.0 | 13.6 | 16.0 | 19.6 | 21.1 | 16.1 |
| 2017 | 8.3 | 12.6 | 15.7 | 20.1 | 18.1 | 15.0 |
| SCN | 9.2 | 13.6 | 16.4 | 19.0 | 19.4 | 15.5 |
| HCS ** | ||||||
| 2015 | 1.1 | 1.8 | 2.4 | 0.5 | 0.6 | 1.3 |
| 2016 | 2.1 | 2.8 | 1.1 | 2.7 | 0.8 | 1.9 |
| 2017 | 1.1 | 2.5 | 1.3 | 0.2 | 0.3 | 1.1 |
| SCN | 2.3 | 2.1 | 2.0 | 0.5 | 2.0 | 1.8 |
| Control Object | |
|---|---|
| Parameter | Value |
| X50 | 68.3117 |
| Equations (show alternative) | |
| Equation | Y = −44.1229 + 106.4187 + 44.1229 × 68.31171 + ()−2.1089 |
| Equation Form | Y = Min + Max − MinXX501 + ()Hill coefficient |
| Exposition I | |
| Parameter | Value |
| X50 | 69.4106 |
| Equations (show alternative) | |
| Equation | Y = −12.1695 + 98.513 + 12.1695 × 69.41061 + ()−4.8941 |
| Equation Form | Y = Min + Max − MinXX501 + ()Hill coefficient |
| Exposition II | |
| Parameter | Value |
| X50 | 75.7738 |
| Equations | |
| Equation | Y = −2.1245 + 99.5213 + 2.1245 × 75.77381 + ()−5.6964 |
| Equation Form | Y = Min + Max − MinXX501 + ()Hill coefficient |
| Experimental Factors | Specification | The Terms of Measurement, Days | HSDp0.05 | |||
|---|---|---|---|---|---|---|
| 20 | 40 | 60 | Mean | |||
| Magnetic field stimulation * | Control object Exposition I Exposition II | 11.37 a 11.97 a 11.70 a | 31.13 a 30.67 a 31.33 a | 36.25 b 38.45 a 36.16 b | 26.25 a 27.03 a 26.40 a | ns ** |
| HSD p0.05 | 1.36 | - | ns | |||
| Years | 2015 | 11.37 a | 31.13 a | 36.25 b | ns | |
| 2016 | 11.97 a | 30.67 a | 38.45 a | |||
| 2017 | 11.70 a | 31.33 a | 36.16 b | |||
| HSDp0.05 | 1.36 | |||||
| Mean | 11.68 c | 31.04 b | 36,9 a | 26.56 | ||
| Specification | Plant Heigh | FW of the First Leaf | FW of Fifth Leaf | DW of the Leaves | Germination Energy | Seed Germination Power | Seed Germination Capacity |
|---|---|---|---|---|---|---|---|
| Mean | 11.68 | 0.88 | 4.42 | 14.77 | 39.33 | 93.00 | 98.11 |
| Standard dev. | 0.67 | 0.09 | 0.43 | 1.10 | 5.60 | 5.88 | 2.33 |
| Kurtosis | −0.61 | 0.98 | 0.96 | −1.54 | −1.48 | −1.49 | −1.14 |
| Skewness | 0.83 | 1.14 | 1.13 | 0.06 | −0.44 | −0.66 | −0.80 |
| V * | 5.71 | 9.84 | 9.83 | 7.45 | 14.24 | 6.33 | 2.37 |
| Specification | Plant Height | FW of the First Leaf | FW of Fifth Leaf | DW of the Leaves | Germination Energy | Seed Germination Power | Seed Germination Capacity |
|---|---|---|---|---|---|---|---|
| Plant height | 1.00 | ||||||
| FW of the first leaf | 0.58 ** | 1.00 | |||||
| FW of fifth leaf | 0.58 ** | 1.00 ** | 1.00 | ||||
| DW of the leaves | 0.95 ** | 0.50 ** | 0.50 ** | 1.00 | |||
| Germination energy | 0.89 ** | 0.75 ** | 0.74 ** | 0.90 ** | 1.00 | ||
| Seed germination power | 0.93 ** | 0.69 ** | 0.69 ** | 0.94 ** | 0.98 ** | 1.00 | |
| Seed germination capacity | 0.65 ** | 0.86 ** | 0.86 ** | 0.62 ** | 0.88 ** | 0.78 ** | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pszczółkowski, P.; Sawicka, B.; Skiba, D.; Barbaś, P.; Krochmal-Marczak, B.; Ahmad, M.A. Effect of Presowing Magnetic Field Stimulation on the Seed Germination and Growth of Phaseolus vulgaris L. Plants. Agronomy 2023, 13, 793. https://doi.org/10.3390/agronomy13030793
Pszczółkowski P, Sawicka B, Skiba D, Barbaś P, Krochmal-Marczak B, Ahmad MA. Effect of Presowing Magnetic Field Stimulation on the Seed Germination and Growth of Phaseolus vulgaris L. Plants. Agronomy. 2023; 13(3):793. https://doi.org/10.3390/agronomy13030793
Chicago/Turabian StylePszczółkowski, Piotr, Barbara Sawicka, Dominika Skiba, Piotr Barbaś, Barbara Krochmal-Marczak, and Mohammad Ayaz Ahmad. 2023. "Effect of Presowing Magnetic Field Stimulation on the Seed Germination and Growth of Phaseolus vulgaris L. Plants" Agronomy 13, no. 3: 793. https://doi.org/10.3390/agronomy13030793
APA StylePszczółkowski, P., Sawicka, B., Skiba, D., Barbaś, P., Krochmal-Marczak, B., & Ahmad, M. A. (2023). Effect of Presowing Magnetic Field Stimulation on the Seed Germination and Growth of Phaseolus vulgaris L. Plants. Agronomy, 13(3), 793. https://doi.org/10.3390/agronomy13030793










