Abstract
Endosulfan is an endocrine disruptor that negatively affects the human central nervous system. Although perennial root vegetable crops have high risks of endosulfan absorption and transfer in soil, safety management studies addressing this problem are lacking. We evaluated endosulfan absorption and transition, as well as plant growth in ginseng cultivation soil, and developed a safety management method for field application. Total endosulfan residual concentrations in the soil and biochar 0.1–1.0% treatment groups were 52–73% after 532 d of spraying, and there was no reduction effect owing to biochar treatment. However, the endosulfan sulfate conversion rate decreased by 21.6–47.1% as the biochar amount increased. Further, there was a 47–95% reduction in the absorption and migration of endosulfan into ginseng in the biochar treatment compared to the control, demonstrating a reduction effect (p < 0.05). Ginseng grown in soil treated with 0.1% biochar showed no growth parameter differences compared to the control (p > 0.05); however, germination rates decreased to <59% when the soil was treated with ≥0.3% biochar. Soil treatment with 0.1% biochar can reduce endosulfan absorption and migration without adversely affecting crop growth. This treatment can be used at the cultivation site, depending on soil conditions.
1. Introduction
For agricultural products, substances for which the maximum residue limit has not been set have been collectively applied at 0.01 mg kg−1 since the implementation of the positive list system and zero tolerance system [1,2]. As a result, highly persistent substances that have been banned as pesticides are absorbed and transferred to agricultural products through the soil, acting as a new cause of nonconformity. Monitoring pesticide residues in farmland soil reveals that substances that have been banned as pesticides are still detected in these soils.
Previous studies monitoring organochlorine pesticides in the cultivation soil of facilities growing fruits and vegetables, such as strawberries, peppers, and melons, found that the frequency of detection of β-endosulfan and endosulfan sulfate at the survey sites was 11–35% and 38–51%, whereas the residual concentrations were 2.2–78.7 and 1.8–214.1 μg kg−1, respectively [3,4]. In the cultivation soil of facilities cultivating leafy vegetables, such as perilla leaves and lettuce, the detection frequency of endosulfan sulfate was 11.9–48.1%, and the residual concentration was 22.0–123.8 μg kg−1 [5]. These studies confirm that endosulfan has been continuously detected in farmland soil. In addition, based on the monitoring of residual pesticides in agricultural products conducted by the National Agricultural Products Quality Management Service from 2013 to 2018, the number of detections of endosulfan was 21–156 in Korea, and the residual concentration was 900–9000 μg kg−1. Previous studies have also reported that endosulfan in the soil is absorbed and transferred to agricultural products [6,7,8].
Endosulfan is a mixture of stereoisomers α and β in a ratio of 7:3. In a slightly acidic-neutral soil environment, it is mainly converted into endosulfan sulfate and has a half-life of up to 2–3 years [9]. The Stockholm Convention was adopted in 2001 and enforced in May 2004, banning the manufacture, use, import, and export of persistent organic pollutants because they are bioaccumulated and have long-distance mobility. Endosulfan was classified as an endocrine disruptor at the 5th Stockholm Convention in 2009 in Genoa, Switzerland, owing to its negative effects on the central nervous system of mammals, including humans [10]. In Korea, it has been banned as a pesticide since 2011. It is currently designated as a restricted item for manufacturing, import, and export under the Toxic Chemicals Control Act of the Ministry of Environment [11,12]. Endosulfan’s human toxicity level is classified as Modern Hazardous by the World Health Organization (WHO). Exposure to a certain amount of endosulfan is considered harmful to the human body. The Joint FAO/WHO Meeting on Pesticide Residues (1998) set the non-toxic dose at 0.6 mg/kg bw/day, based on endosulfan-induced neurotoxicity, hepatotoxicity, renal toxicity, and weight loss, and a safety factor of 100 was applied to set the acceptable daily intake to 0–0.006 mg/kg bw/day, as evaluated previously [13].
Chemical substances in the soil are transferred to the human body mainly through ingesting food, which contains the chemical substance transferred from agricultural products. Previous studies have shown that endosulfan in the soil is absorbed and transferred to agricultural products [6,7,8]. Compared to the soil concentration of endosulfan, the crop concentration was reported to be <0.1% in grains, 1.3–4.7% in leafy vegetables, and 0.1–13.6% in fruits and vegetables [14,15,16]. Choi et al. [14] reported that carrot, a root vegetable, showed 28% of soil concentration, and a study by Hwang et al. [17] revealed that potatoes and carrots contained 26% of the initial soil concentration, showing a high level of transfer compared to other crops. The absorption and transfer of residues in the soil is known to increase as contact with the soil and contact time increase [6,18]. Accordingly, it is judged that root vegetables cultivated as perennials will have a high amount of endosulfan transfer in the soil, resulting in negative effects, such as making them unsuitable as raw material for agricultural products. Ginseng is one of the representative root vegetable crops in Korea. Ginseng (Panax ginseng C.A. Meyer) is a plant belonging to the Araliaceae family and has a growing period of 4–6 years, which is longer than that of other crops. Ginsenoside, a useful component found in ginseng, has many functional effects, such as liver protection, blood pressure control, and anticancer effects [19,20]. Therefore, ginseng is used to manufacture a variety of health-promoting functional foods and accounts for the highest production ratio among crops used as raw materials for health-promoting functional foods in Korea [18]. It is also one of the major agricultural items exported, currently being exported to 105 countries, accounting for 3.5% of total agricultural exports in 2021 [21,22]. In 2018, global ginseng production was approximately 86,223 tons, with China producing 50,164 tons, South Korea 23,265 tons, Canada 11,367 tons, the United States 1285 tons, Japan 30 tons, and other countries totaling 112 tons. The global value of ginseng production is estimated to be around $ 5900 million. The ginseng market is expected to expand further in the future due to the growing consumer interest in alternative medicine and health foods worldwide [23].
The residual endosulfan in ginseng cultivation soil is slowly reduced by photolysis and leaching as ginseng cultivation land is mulched and rain screens are installed. Therefore, it is judged that the risk of residual endosulfan in ginseng cultivation soil is high compared to other crop soils. In addition, as ginseng is a perennial root crop, there is a high possibility of endosulfan transfer in the soil. However, studies on the transfer of pollutant residues to crops for perennial root vegetables in the current soil are insufficient, and management plans are also lacking [12].
The safety management method for highly persistent substances such as endosulfan in the soil is to reduce them through physical methods, such as soil improvement, heating, and chemical treatment. However, the above-mentioned physical and chemical treatment methods are difficult to apply to farmland soil where crops are continuously grown because the methods require high treatment costs and do not allow crop cultivation [24,25]. Instead, methods for reducing absorption and transfer to crops using soil conditioners or inactivating soil materials can be applied to agricultural sites. Among soil conditioners, quicklime has an endosulfan-reducing effect. However, it is difficult to apply in the field because it increases the soil pH to over 10 and affects the cultivation of agricultural products [26]. Therefore, adsorbing endosulfan in the soil using biochar is considered the most realistic method for minimizing endosulfan absorption and transfer to ginseng.
Biochar is a carbon-rich by-product produced after the pyrolysis of biomass (agricultural by-products, wood, waste, etc.) heated in an anoxic environment. It does not affect the crop cultivation environment and is used for carbon sequestration, greenhouse gas reduction, and soil fertility improvement [27,28]. In addition, it also has a large surface area per unit volume and a porous surface, resulting in good water retention and high adsorption due to the large surface area of porosity [29]. It mitigates the effects of heat, drought, and salinity stress on crops. It also improves crop growth and productivity [30], increases biological N fixation in legumes [31,32], and facilitates carbon sequestration. However, the above-mentioned results are highly dependent on the type of biochar, the temperature at which the biochar is made, the biochar dose, and the soil type. A study evaluating the adsorption capacity of organic pollutants by treating soil with biochar reported that when the soil was treated with 2% (weight/weight) biochar, the adsorption capacity of the herbicide isoproturon was five times higher than that in untreated soil [30]. Tatarkova et al. [33] reported that treating soil with 1% biochar (weight/weight) increased 4-chloro-2-methylphenoxy acetic acid adsorption 2.53-fold. Yang et al. [34] reported that biochar in the soil increases the adsorption capacity of the soil, increasing the adsorption of diuron up to 80 times, thereby reducing the concentration of diuron in barnyard grass.
However, some studies have reported adverse effects on crop growth depending on the amount of biochar applied. Copley et al. [35] reported that the occurrence rate of Rhizoctonia solani, which can frequently occur in ginseng, increased by 40% among the experimental plants treated with biochar. Another study reported that biochar treatment could affect soil microbial activity by changing the soil environment [36]. Therefore, it is important to process an appropriate amount of biochar considering the soil characteristics in the cultivation environment.
In this study, we aimed to develop a safety management method for biochar that can be used in ginseng cultivation soil. To develop a safety management method for endosulfan in ginseng cultivation soil using biochar, ① we investigated the endosulfan reduction effect of different biochar treatments in soil; ② we evaluated the absorption, transfer, and reduction effect in ginseng; and ③ we evaluated the growth of above- and below-ground parts of ginseng and conducted germination surveys, according to the biochar treatments of the soil. Based on a comprehensive evaluation of these aspects, we propose a biochar treatment method that can be used in ginseng cultivation soil.
2. Materials and Methods
2.1. Chemicals and Reagents
The α-endosulfan, β-endosulfan, and endosulfan sulfate used in the experiment were purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany), and the dichloromethane and acetone used for pretreatment and the working solution were purchased from J.T. Baker (Phillipsburg, NJ, USA) and Merck (Darmstadt, Germany).
2.2. Properties of Biochar and Soil Used in the Experiment
To prepare biochar of pH 10.2, EC 7.3 dS m−1, bamboo and oak were dried in the shade to lower the moisture content to 15% in 15–20 °C, and it was further lowered to 10% using a dryer for 24 h. Following this, the dried material was placed in a biochar maker (DK1015, STI), maintained at 400 °C for 4 h, and then maintained at 600 °C for 4 h.
The physicochemical properties of the experimental soil were investigated after treatment with endosulfan and biochar according to the standards of agricultural science and technology research and analysis (ISBN: 9788948016499). Soil pH was measured using the shaking method [37], and organic matter content was measured using the Tyurin method [38] (Table 1).

Table 1.
Physicochemical properties of field soil used in the experiment.
2.3. Field Experiment and Sampling
The experiment site was located at the Major Achievement and Prospect of Ginseng in Soi-myeon, Eumseong-gun, Chungcheongbuk-do, Republic of Korea (latitue 36.94321403023995, longitude 127.7545139107935 The average temperature of the experimental field area is 11 °C, with a high of 18 and a low of 5.1, and the average annual precipitation is 1220.8 mm (National Weather Service Weather Data Open Portal of Korea). To measure the soil retention of endosulfan and the degree of transfer to ginseng, endosulfan was applied to the soil at a concentration of 1.0 mg kg−1 on 27 March 2018. After spraying 1.0 kg of endosulfan evenly on soil pre-mixed with topsoil in the treatment quadrat (four treatments with a total area of 48.6 × 8 m), it was homogenized by cultivating it ten times using a rotary cultivator. Biochar treatment was performed 5 d after endosulfan treatment to stabilize endosulfan in the soil. Biochar was applied at 0.1, 0.3, and 1.0% of soil weight.
After 2 d of biochar treatment, 63 ginseng seedlings (Panax ginseng C.A. Meyer, 7 × 9) were transplanted per treatment quadrat (1.8 × 1.8 m; 3.24 m2), and the transplanted ridges were covered with rice straw thatch and mulched with black vinyl to prevent moisture evaporation (Figure 1).
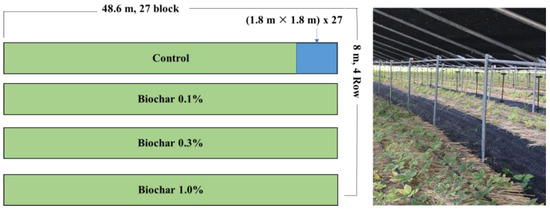
Figure 1.
The coordinates of the experiment with view of the experimental site.
Soil sampling was performed at an average interval of 30 d in the first year (2018) and 60 d in the following year (2019) from the 3rd day after transplanting ginseng seedlings. For sampling the treatment quadrat, 1–2 cm of the topsoil was removed, and the soil was collected to a depth of 15 cm in each of the 27 compartments for each treatment quadrat and then mixed. The collected samples were dried in the shade for approximately 5–7 d and passed through a 2 mm sieve to ensure homogeneity (Agri-Environmental Resource Analysis Method. Jeollabuk-do Agricultural Research & Extension Services. Accessed on 8 August 2017 https://www.jbares.go.kr/board/download.jbares?boardId=BBS_0000001&menuCd=DOM_000000101001001000&paging=ok&startPage=47&dataSid=63772&command=update&fileSid=24466).
2.4. Ginseng Growth Evaluation by Treatment
A cool environment in the shade was created, and the temperature was maintained at around 15 °C in the early stage of growth and 20–25 °C in the late stage of growth, and 6~10 L per compartment was irrigated in the early and late stages of growth, and 15~18 L in midsummer. The quality and growth traits of ginseng were evaluated using the germination rate, weight, and growth length of the above-ground and below-ground parts for each treatment quadrat. The investigated traits were root length, body length, body diameter, weight, stem height, stem diameter, stem length, and leaf length (Figure 2). The germination rate was investigated by randomly selecting five compartments for each treatment quadrat six weeks after transplanting seedlings. At the time of collection, the number of individuals present above the ground surface as the above-ground part grew was measured for each treatment quadrat. Approximately 11 weeks after transplanting ginseng seedlings, the above-ground parts were randomly collected from five compartments for each treatment quadrat. Stem height was measured as the length from the ground part to the highest extended stem, leaf length was measured as the vertical length of the above-ground leaf, and stem diameter was measured as the width of the above-ground stem.
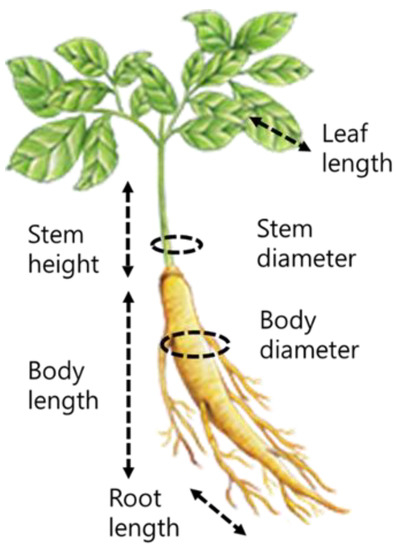
Figure 2.
The growth form of ginseng (names of each part, illustration by the National Agricultural Cooperative Federation Korea).
Growth of the below-ground part was evaluated by collecting crops from three randomly selected compartments for each treatment quadrat approximately six months after transplanting ginseng seedlings. Body diameter was measured as the diameter of the thickest part of the body of the root, body length as the length of the root body, root length as the length of the root under the body, and weight as the weight of the ginseng root body (Figure 2).
2.5. Residue Analysis of Endosulfan in Soil
The liquid–liquid extraction (LLE) method and the Florisil SPE cartridge (1 g 6 mL) method were used for the experiment.
An amount of 100 mL of acetone was added to 50 g of soil, shaking extraction was performed for 1 h, and the mixture was filtered under reduced pressure. The filtrate was concentrated under reduced pressure at 40 °C using a rotary vacuum concentrator (RV 10, IKA, Seoul, Korea) and transferred to a separatory funnel. Subsequently, 50 mL of distilled water, 10 mL of saturated NaCl, and 90 mL of dichloromethane were added, and the mixture was shaken at a constant speed. The dichloromethane layer was passed through sodium sulfate. Then, after nitrogen concentration in a 15-mL tube, 2 mL of acetone was added to the tube to dissolve the compound again. After activating the mixture with 6 mL of hexane using a Florisil cartridge (SPE, 1 g, 6 mL), 2 mL of the sample was loaded, and elution was performed with 8 mL of acetone/hexane mixture (1:9, v/v) and subjected to nitrogen concentration (EYELA MG-2200, 40 °C.). It was then re-dissolved in 2 mL of acetone and filtered through a syringe filter (0.45 μm), and the sample solution was analyzed using a GC-micro electron capture detector (μECD, Hewlett Packard, Palo Alto, CA, USA, HP5890). Analytical instrument conditions are listed in Table 2.

Table 2.
Analytical conditions for endosulfan in soils (GC-μECD, Hewlett Packard, Palo Alto, CA, USA, HP5890).
2.6. Analysis of Endosulfan Residue in Ginseng
As with the soil experiments, the LLE method and Florisil SPE cartridge were used. The residual amounts of α-endosulfan, β-endosulfan, and endosulfan sulfate in the ginseng samples were purified using LLE and a Florisil cartridge (1 g, 6 mL) and then analyzed. An amount of 20 mL of ethyl acetate was added to 5 g of ginseng, followed by sonication for 10 min and shaking extraction for 1 h, followed by sonication for 10 min. After centrifugation (3000 rpm, 10 min, 4 °C), it was transferred to a separatory funnel and 10 mL of supernatant, 20 mL of distilled water, and 60 (20 × 3) mL of ethyl acetate were added to it, and the sample was shaken at a constant speed. The ethyl acetate layer was removed, concentrated under reduced pressure, and dissolved again by adding 1 mL of acetone/hexane 1:9 (v/v) solution. After activating the Florisil cartridge by adding 5 mL of hexane, 1 mL of the sample was loaded and eluted with 20 mL of acetone/hexane mixture (1:9, v/v), followed by nitrogen concentration (30 °C rotary evaporators). An amount of 1 mL of acetone was added to the dry matter to redissolve it, and this was then filtered through a syringe filter (0.22 μm), and the filtrate was analyzed using gas chromatography–mass spectrometry (GC-MSD; GCMS-QP2020, Shimadzu, Japan). Analytical instrument conditions are listed in Table 3.

Table 3.
Analytical conditions for endosulfan in ginseng (GC-MSD, Shimadzu GCMS-QP2020).
2.7. Statistical Analysis: Repeated Measures ANOVA
Statistical analysis was conducted using the free and open-source statistical software, R project (version 4.0.0, R Core Team, 2020) and Jamovi (version 1.2.22.0, Stats Open Now, https://www.jamovi.org, accessed on 31 December 2020). ANOVA and repeated measures ANOVA TEST were used. ANOVA was used to evaluate the quality evaluation for each biochar treatment. Repeated measures ANOVA was used to compare the temporal change in residual soil endosulfan after biochar treatment. We used 3 replicates of data for soil and 5 replicates for crops. The repeated measures ANOVA compares means across one or more variables based on repeated observations. According to repeated measures ANOVA, the residual concentration of endosulfan, artificially treated in a ginseng-cultivated area, was decomposed over time.
2.8. Calculating the Half-Life of Endosulfan
Sigmaplot (version 14.0, Systat Software, Erkrath, Germany) and first-order kinetics (Equation (1)) were used to determine the half-life of endosulfan in the soil for each treatment. Ct indicates the endosulfan concentration at time t (mg kg−1), CO indicates the initial concentration (mg kg−1), t is the day number, and k is the reduction rate constant [39]. The half-life of endosulfan was calculated as a value of ln 2/k.
3. Results
3.1. Limit of Quantitation and Recovery Rate Analysis
The linearity of the calibration curves of α-endosulfan, β-endosulfan, and endosulfan sulfate, which were the components tested for in this study, were analyzed. For this purpose, each standard was dissolved in acetone to prepare a standard solution with 0.002 to 1.0 mg L−1 concentration and then analyzed using GC-ECD and GC-MSD. Linearity was confirmed by preparing a calibration curve for each concentration from the peak area of each component. Based on this, calibration curves and regression equations for each component were prepared. The R2 values of α-endosulfan, β-endosulfan, and endosulfan sulfate were higher than 0.9994, indicating good linearity of the calibration curve for each component. The validity of the test method for the quantitative analysis of α-endosulfan, β-endosulfan, and endosulfan sulfate was evaluated using the linearity of the calibration curve, limits of quantitation (LOQ), recovery rate, and relative standard deviation. To obtain the linearity of the calibration curve, α-endosulfan, β-endosulfan, and endosulfan sulfate were each dissolved in 100 mL of acetone, and a 100 mg L−1 stock solution was prepared. This solution was diluted and mixed to prepare mixed standards at concentrations of 0.05, 0.1, 0.5, 1, and 5 mg L−1. The linearity of the calibration curve was obtained as the average value obtained by repeatedly injecting these solutions five times. In addition, we determined the concentration at which the signal-to-noise ratio was 10 as the LOQ of α-endosulfan, β-endosulfan, and endosulfan sulfate. The recovery rate from soil and ginseng was 74.5–107.5, and the coefficient of variation was 4.9–9.5%.
3.2. Comparison of the Residual Dissipation Pattern of Endosulfan for Each Biochar Treatment
The traces of endosulfan for each biochar treatment in soil were investigated. Total endosulfan (sum of endosulfan-α, -β, sulfate) in soil not treated with biochar decreased from the initial level of 1.3 mg kg−1 to 0.94 mg kg−1 (73.1% of the initial residual amount) after 533 d of treatment. In the 0.1, 0.3, and 1.0% biochar treatment quadrats, total endosulfan was 0.79 mg kg−1 (53.9%), 0.87 mg kg−1 (73.6%), and 0.64 mg kg−1 (52.11%), respectively (Figure 3). The eta squared (η2) value was calculated using the repeated ANOVA test to identify the main cause of decrease in the residual amount of total endosulfan in the soil considering time, biochar treatment, and the interaction between the two factors as potential causes (Table 4). The reduction effect of biochar was 0.15, and that of the interaction between the two factors was 0.15. The reduction effect over time was the largest at 0.59. Therefore, the decrease in the residual amount of total endosulfan in the soil was most affected by time.
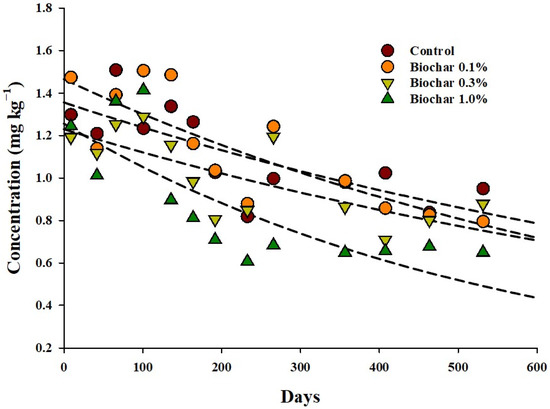
Figure 3.
Comparison of the residual dissipation pattern of total endosulfan for different biochar treatments.

Table 4.
Repeated ANOVA, Test of Normality between treatments (endosulfan in soil).
The initial soil residues of α-endosulfan and β-endosulfan in all test treatment quadrats were 0.65–0.82, and 0.48–0.62 mg kg−1, respectively. After 532 d of endosulfan treatment, the residual amount of α-endosulfan decreased to 0.16~0.26 mg kg−1 and the residual amount of β-endosulfan decreased to 0.24~0.30 mg kg−1 per treatment quadrat (Figure 4). After 532 d of endosulfan treatment, the reduction rates of α-endosulfan in the soil were 98, 84, 64, and 63%, whereas those of β-endosulfan were 50, 57, 37, and 50%, respectively, in the control and treatment quadrats treated with 0.1, 0.3, and 1.0% biochar.
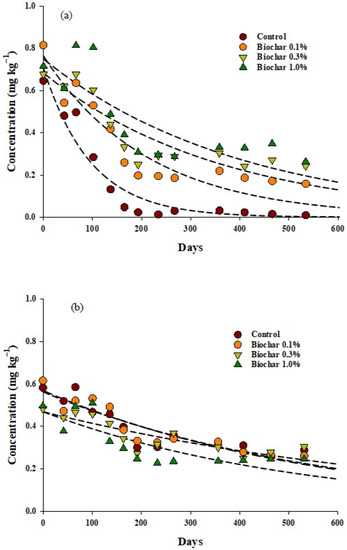
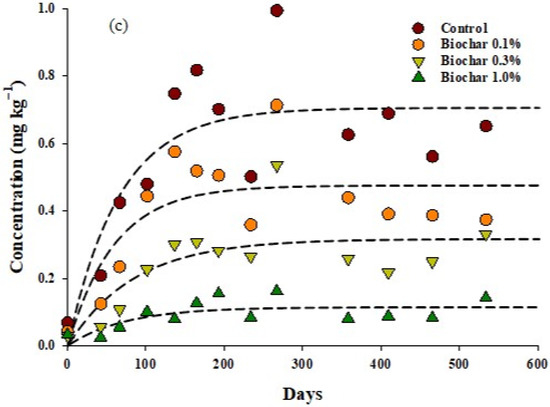
Figure 4.
Comparison of the residues of endosulfan over time for the different components tested in this study: (a) α endosulfan, (b) β endosulfan, (c) endosulfan sulfate.
After stabilization for 10 d following biochar treatment, the residual amount of endosulfan sulfate in the soil was 0.07, 0.04, 0.03, and 0.03 mg kg−1 in the control and 0.1, 0.3, and 1.0% biochar-treated quadrats, respectively. To identify the factors affecting the residual amount of endosulfan sulfate in soil, η2 was calculated using a repeated ANOVA test. The results revealed that the effect of biochar was 0.51, which was higher than that of time (0.35) and the interaction of both factors (0.11) (Table 4). In addition, when the pH is higher than 7, a lower amount of endosulfan is converted to endosulfan sulfate [40], and the conversion to endosulfan diol is dominant [41,42]. In this study, when 1.0% biochar was added to the soil, the soil pH increased to 7.4, suggesting that the conversion to endosulfan sulfate was reduced (The biochar 0.3% treatment residue data is same as reference [43]).
At 66 days in each treatment, endosulfan sulfate conversion decreased from 28.2% in the control treatment to 16.8, 8.6, and 3.9% in the 0.1, 0.3, and 1.0% biochar treatments, respectively (Figure 5), and the rate of endosulfan sulfate production decreased over time in each treatment as the biochar content increased (Figure 6). The conversion rate of endosulfan sulfate according to biochar treatment in soil remained at approximately 68.8% of total endosulfan. However, total endosulfan residues were 47.2, 37.8, and 21.7%, respectively, for each quadrat treated with 0.1, 0.3, and 1.0% biochar. Therefore, the conversion rate of endosulfan sulfate decreased as the biochar throughput increased. The formation of endosulfan sulfate in the soil environment over time may have contributed to the lack of significant results according to the treatment quadrat.
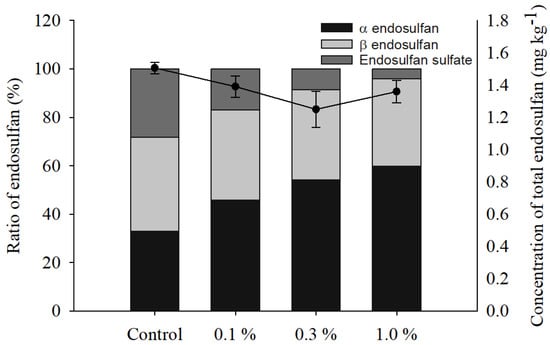
Figure 5.
Comparison of the residues of endosulfan at 66 days by biochar treatment.
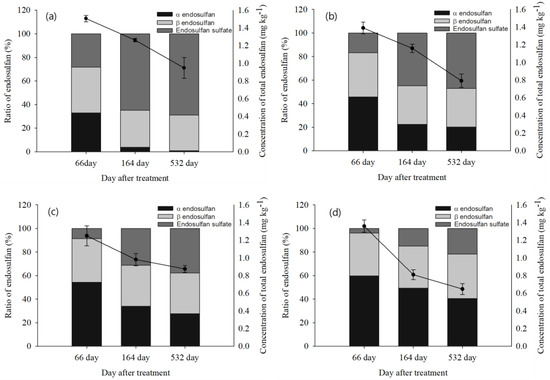
Figure 6.
Comparison of the residues of endosulfan over time for different biochar treatments in soil: (a) control (non-biochar), (b) biochar 0.1%, (c) biochar 0.3%, (d) biochar 1.0%.
α-Endosulfan showed a half-life of 87.4 d in the control treatment and 72.4, 67.0, and 64.6 d in the biochar 0.1, 0.3, and 1.0% treatments, respectively, indicating a reduction effect according to biochar treatment; however, β-endosulfan and total endosulfan showed no reduction effect (63.5–66.9 d and 50.4–56.3 d, respectively).
3.3. Reduction Effect of Different Biochar Treatments on Endosulfan Uptake from Soil to Ginseng
The effect of the reduction in the absorption and transfer of endosulfan to ginseng for different biochar treatments in the soil was investigated. Ginseng was harvested after cultivating two-year-old ginseng seedlings for six months after transplantation. The total endosulfan concentration of ginseng harvested from untreated quadrat soil was 2.30 mg kg−1, and those of quadrats treated with 0.1, 0.3, and 1.0% biochar were 1.31, 0.79, and 0.20 mg kg−1, respectively. Therefore, in ginseng cultivated under different biochar treatments, the reduction effect of endosulfan absorption concentration in ginseng increased by 51.0, 70.4, and 92.5% compared to that of ginseng cultivated in the untreated quadrats (Figure 5). The residual amounts of α-endosulfan in ginseng were 0.50, 0.19, 0.14, and 0.07 mg kg−1, respectively. Treatment with biochar reduced absorption and transfer by 62–86% compared to the control treatment quadrat; this percentage was 55–92% for β-endosulfan with residual amounts of 0.53, 0.24, 0.16, and 0.04 mg kg−1, respectively. The amount of endosulfan sulfate in ginseng was 1.63 mg kg−1 in untreated quadrats. Quadrats treated with 0.1, 0.3, and 1.0% biochar showed residual amounts of 0.87, 0.49, and 0.09 mg kg−1, respectively, showing a reduction effect of approximately 47–95% (Figure 7).
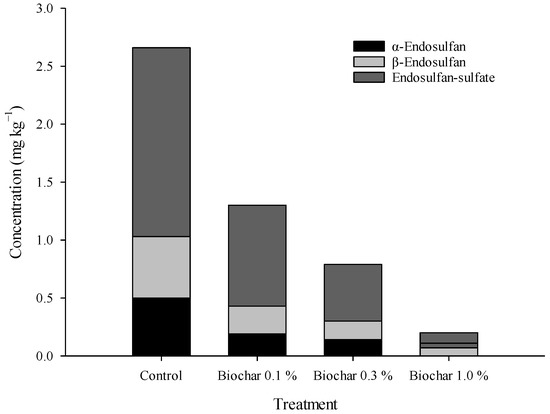
Figure 7.
Endosulfan concentration of two-year-old ginseng under different biochar treatments.
BCF (BCF, concentration in ginseng/concentration in soil) was used to determine the extent of absorption and transfer of endosulfan in the soil. BCF was calculated using the initial residual concentration in soil and the residual concentration in harvested ginseng. The BCF in the control treatment quadrat was 2.01. In the 0.1, 0.3, and 1.0% biochar treatment quadrats, the BCF was 0.89, 0.66, and 0.16, respectively, showing a decrease of 56.9–92.03% compared to the control treatment quadrat (Figure 8, Table 5).
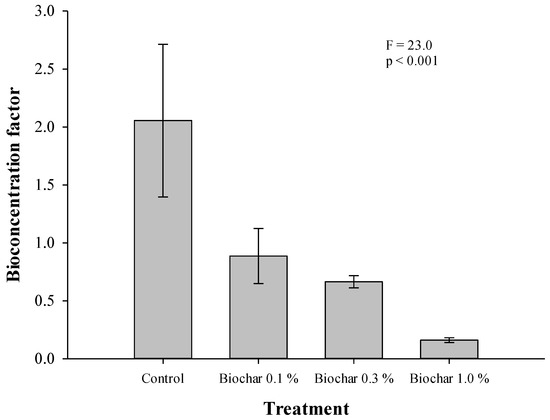
Figure 8.
BCF (bioconcentration factor) of total endosulfan for two-year-old ginseng.

Table 5.
Concentration of endosulfan in ginseng with BCF (bioconcentration factor), SE–Standard Error.
3.4. Quality Evaluation and Germination of Ginseng by Biochar Treatment
Table 6 shows the post-harvest appearance of two-year-old ginseng (Figure 9) and the quality evaluation results for each trait. In addition, the germination rate observed during the optimal growth period of ginseng was confirmed for each treatment quadrat. Ginseng harvested from soil in the control and 0.1, 0.3, 1.0% biochar-treated quadrats weighed 3.5–5 g, the stem length was 5.4–7.8 cm, stem diameter was 1.8–2.2 mm, leaf length was 6.5–8 cm, root length was 13.7–19.8 cm, fuselage length was 10.4–13.3 cm, and fuselage diameter was 7.7–9.6 mm. No significant difference was observed between treatment quadrats (p > 0.05).

Table 6.
Comparison of ginseng quality evaluation by biochar treatment (average of each treatment with standard error).
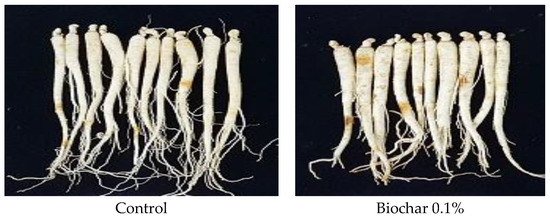
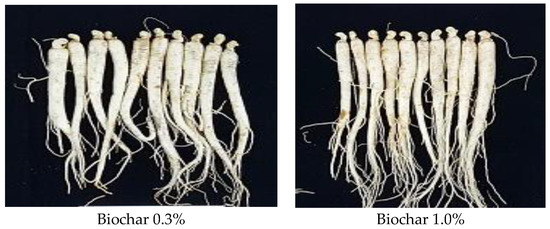
Figure 9.
Comparison of two-year-old ginseng root growth under different biochar treatments.
However, unlike the quality of harvested ginseng, the germination rate tended to decrease in quadrats treated with more than 0.3% biochar (p < 0.05). In the growth evaluation of ginseng, the germination rate of two-year-old ginseng was higher than 80% in the control and 0.1% biochar treatment quadrats, 59.4% in the 0.3% biochar treatment quadrats, and 56% in the 1.0% biochar treatment quadrats. The germination rate was significantly lower in quadrats treated with more than 0.3% biochar. In ginseng-cultivated soil, pH indicates the nutrient supply capacity, and the optimum pH is 5.0–6.5. When the soil was treated with 0.3% and 1.0% biochar, the pH increased to approximately 6.8 and 7.4, which was outside the optimal pH range. In addition, one of the crucial factors that can affect ginseng germination rate is the soil’s organic matter content. In this study, the control and 0.1% biochar-treated quadrats contained 10 to 30 g kg−1 of soil organic matter content, which was an appropriate level.
4. Discussion
In this study, the concentration of endosulfan sulfate increased over time in the treatment area where biochar was not applied, and biochar treatment inhibited the conversion of metabolite endosulfan sulfate by adsorption with biochar in the soil. As a result, a significant reduction in the concentration of endosulfan sulfate was achieved through biochar treatment. Furthermore, the concentration of α-endosulfan decreased over time, along with the concentration of endosulfan sulfate, and soil pH increased from 6.5 to 7.4 when the soil was treated with 1.0% biochar, promoting the decomposition of α-endosulfan, which may have affected the reduction of α- and β-endosulfan. β-Endosulfan in the soil did not depend on pH. However, the decomposition rate of α-endosulfan decreased with increasing pH [23]. An alternative explanation for this may be found in the study by Walse et al. [44], who reported that α-endosulfan is more readily converted to endosulfan sulfate compared to β-endosulfan. Therefore, the differences in degradation rates observed in the current study may have occurred because residual α-endosulfan was more readily converted to endosulfan sulfate in the soil environment compared to β-endosulfan. The distribution coefficient (Kd) of β-endosulfan in the soil is higher than that of α-endosulfan [45], which suggests that β-endosulfan is preferentially adsorbed on clay and organic surfaces and remains there.
Endosulfan sulfate is one of the major metabolites in the agricultural use of endosulfan, which is mainly formed in weakly acidic and neutral soil environments [46] and is more difficult to decompose and more persistent than the parent compounds [47,48,49,50,51]. Further, endosulfan sulfate is associated with high reproductive toxicity, such as a decrease in the hatching rate of zebrafish [52]; thus, further research on endosulfan toxicity and behavior in the environment is required. Endosulfan sulfate affects the cytochrome P450 hormone in mammals [53,54]. Endosulfan sulfate can cause an increase in MDA levels in living organisms, which can lead to biologically harmful effects of disturbance in organs. [55]. In the present study, the conversion of endosulfan sulfate in soil was suppressed through biochar treatment, and at the same time the endosulfan concentration transferred to ginseng was reduced. This was due to the irreversible adsorption effect in the biochar treatment, which led to a low desorption effect [56], which is believed to have reduced the bio-use rate of pesticides. Such strategies may help create a sustainable agricultural environment. Further, the adsorption capacity of biochar is expected to result in more suitable soil conditions for the subsequent crop in agricultural systems where the organic substances dildrin, heptachlor, and chlordein occur with high Koc values.
Biochar has high adsorption capacity for organic pollutants. Wang et al. [57] report-ed that heat treatment of biochar at 700 °C and 300 °C increased the adsorption coefficient (Kd) of soil by 63-fold and 2.9-fold, respectively. As a result, they reported that the adsorption of terbuthylazine could be increased by 2.9 times through biochar heat-treated at 700 °C. When a pesticide is adsorbed on biochar, its bioavailability is reduced because it is not a bioavailable fraction, thus inhibiting the uptake and transfer of the pesticide to plants [34,58,59]. In addition, biochar is porous and has strong adsorption properties. In addition, pesticides in the soil can be adsorbed through mechanisms such as covalent bonding of soil organic matter and entrapment in micropores [60]. As a result, it is likely that the effect of reducing absorption and transfer increased as the amount of biochar applied increased.
Bioaccumulation data are expressed using a bioconcentration factor (BCF, concentration in ginseng/concentration in soil), translocation factor (TF, transfer from roots to edible parts), and bioaccumulation factor (BAF, coefficient considering all bioconcentration, surface absorption, and feeding) [61,62]. In this study, as the root in contact with the soil is the main edible part of ginseng, BCF was used to determine the extent of absorption and transfer of endosulfan in the soil.
There was no significant difference in the quality effect of two-year-old ginseng, according to biochar treatment in soil in all treatment groups (p > 0.05). The results suggest that treatment of ginseng cultivation soil with 1% biochar does not affect ginseng growth. As a soil conditioner, biochar has the effect of improving water retention and nutrition [63]; it improves the physicochemical properties of soil treated with biochar [41] and increases fertilizer application efficiency and crop production by effectively maintaining the level of soil organic matter [64,65,66,67]. In addition, biochar has an excellent ability to adsorb organic and inorganic contaminants owing to its high surface area carbon structure, which is believed to inhibit conversion to organochlorine pesticides [36,56]. However, in this study, biochar treatment did not significantly improve the quality of ginseng, as measured through different growth parameters. Because crop quality is affected by climate and soil physicochemical properties, the results of this study differ from those of previous studies [68]. However, when the soil was treated with 0.3% biochar or more, it exceeded the appropriate range and increased to a maximum of approximately 59 g kg−1. Optimum organic matter content can improve soil and increase productivity. However, excessive organic matter content in ginseng-cultivated soil can cause physiological disorders owing to an imbalance in the soil environment [69]. As a soil conditioner, biochar may contain small amounts of phenolic compounds and heavy metals harmful to plants. These compounds can affect seed germination and seedling growth [70]. Some studies have shown that when the amount of biochar applied to the soil is more than 3%, it can negatively affect plant growth and accelerate plant disease [71,72,73]. Therefore, it is necessary to maintain an appropriate level of biochar. In this study, as the amount of biochar was increased to 0.1, 0.3, and 1.0%, the soil pH increased by 3, 4, and 14%, and the organic matter content increased by 0.8, 1.9, and 4.9 times, respectively, compared to the control. The average pH of agricultural soil in Korea is 6.2, and the organic matter content is 23.3 g kg−1 [74]. Considering this, 0.1% biochar application, which does not affect the growth environment, is appropriate. However, the physicochemical characteristics of the soil environment differ depending on the region, cultivation period, and type [75]. Therefore, it is necessary to determine how much biochar should be added after checking the physical and chemical properties of the soil used for ginseng cultivation.
5. Conclusions
This study aimed to develop an endosulfan safety management method using biochar that can be applied to ginseng cultivation soil. Specifically, the effect of biochar treatment on endosulfan reduction in soil and absorption/transfer and reduction in ginseng was evaluated after biochar treatment. In addition, we evaluated the growth of the above- and below-ground plant parts before and after treatment with biochar and conducted germination research.
The initial residual amount of total endosulfan (sum of endosulfan-α, β, sulfate) in the soil was 1.3 mg kg−1, which was reduced to 73.1% of the initial residual amount after 533 d of treatment. In the treatment quadrat treated with 0.1, 0.3, and 1.0% biochar, the endosulfan levels were 53.9, 73.6, and 52.1%, respectively. To identify the factor for the decrease in endosulfan in the soil, we calculated the η2 value using two-way ANOVA, and the time factor was the highest at 0.594. However, biochar had the highest η2 value of 0.513 as the main factor that reduced the conversion to endosulfan sulfate. Soil treatment with biochar did not affect total endosulfan reduction, but reduced endosulfan sulfate conversion.
When 1.0% biochar was applied in ginseng cultivation soil, α-endosulfan and β-Endosulfan reduced to 86% and 92% compared to the residual amount in ginseng grown in the control, demonstrating the reduction in the absorption and transfer of endosulfan. In ginseng grown in soil treated with 0.1, 0.3, and 1.0% biochar, the reduction effect of endosulfan sulfate increased from 47 to 95% with increasing treatment amount compared to the control. When soil is treated with biochar, the conversion of endosulfan sulfate in the soil is inhibited; therefore, the residual amount in biochar-treated soil is lower than that in control soil, and the material is strongly adsorbed to biochar and deactivated.
Previous studies have demonstrated that soil treatment with biochar helps crop growth by improving soil fertility; however, excessive use has a negative effect on crop growth. Therefore, optimal conditions that do not affect ginseng cultivation and reduce endosulfan absorption/transfer must be established for field application. Even when the soil was treated with up to 1.0% biochar, there was no significant difference in ginseng weight, root and trunk length, trunk diameter and stem diameter, and length compared to the control (p > 0.05). However, the ginseng germination rate was 59% and 56% in the quadrat treated with 0.3% and 1.0% biochar, respectively, which was lower than the germination rate of over 80% in the control and 0.1% biochar treatments. Optimal soil pH and organic matter content for ginseng cultivation are 5.0–6.6 and 10–30 g kg−1. However, when biochar was applied at concentrations of 0.3% or more, we judged that the two factors increased and exceeded the appropriate range, affecting the germination rate.
It is necessary to consider the absorption/transfer reduction effect and crop growth evaluation to utilize the endosulfan safety management method for ginseng cultivation soil in the field. A proportion of 0.1% biochar is appropriate considering the domestic soil pH and organic matter content and the effect of reducing the absorption and migration of biochar obtained from the results of this study. However, it is necessary to determine the amount of biochar to be added after examining the physical and chemical properties of the soil used for ginseng cultivation.
Ginseng was sown in the first year and transplanted into the cultivation soil in the second year. The germination rate of ginseng was investigated in the second year. The germination rate is an important part of ginseng growth because it is linked to the quality and yield of ginseng in the future. In general, the growth period of ginseng is 4 to 6 years. Ginseng exhibits growth characteristics such as active root development and very rapid nutrient absorption compared to other plants. Therefore, additional studies investigating the effects of various components in the soil on ginseng are required.
Author Contributions
Conceptualization, G.-H.J. and G.-H.C.; methodology, H.-S.L.; software, G.-H.J.; validation, G.-H.J.; formal analysis, G.-H.J. and H.-S.L.; investigation, G.-H.J.; resources, G.-H.C., S.-W.P. and J.-H.K.; data curation, G.-H.J., H.-S.L. and J.-Y.C.; writing—original draft preparation, G.-H.J.; writing—review and editing, H.-S.L. and H.C.; visualization, G.-H.J.; supervision, H.-S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Research Program for Agriculture Science and Technology Development, Establishment of endosulfan residue standards in ginseng and soil of scheduled sites and development of reduction technology (PJ013816) Rural Development Administration, Republic of Korea.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- National Agricultural Products Quality Management Service. Results of Analysis of Pesticide Residues on Agricultural Products; Ministry of Agriculture, Food and Rural Affairs, Yongjeon-ro, Gimcheon-si Gyeongsangbuk-do, Republic of Korea, 2020. Available online: https://www.data.go.kr/data/15047546/fileData.do (accessed on 16 June 2022).
- Kim, K.J. Evaluation on Translocation of Endosulfan Residue in Ginseng Cultivation Soil to Ginseng. Master’s Thesis, Kangwon National University Graduate School, Gangwon-do, Republic of Korea, 2014. Available online: https://www.riss.kr/search/detail/DetailView.do?p_mat_type=be54d9b8bc7cdb09&control_no=5329ef2e638a4497ffe0bdc3ef48d419 (accessed on 1 February 2023).
- Lim, S.J.; Oh, Y.T.; Yang, J.Y.; Ro, J.H.; Choi, G.H.; Ryu, S.H.; Moon, B.C.; Park, B.J. Development of multi-residue analysis and monitoring of persistent organic pollutants (POPs)-Used organochlorine pesticides in Korea. KJPS 2016, 20, 319–325. [Google Scholar] [CrossRef]
- Lim, S.J.; Oh, Y.T.; Jo, Y.S.; Ro, J.H.; Choi, G.H.; Yang, J.Y.; Park, B.J. Persistent organic pollutants (POPs) residues in greenhouse soil and strawberry organochlorine pesticides. Korean J. Environ. Agric. 2016, 35, 6–14. [Google Scholar] [CrossRef]
- Lim, S.J.; Park, J.H.; Ro, J.H.; Lee, M.H.; Yoon, H.I.; Choi, G.H.; Ryu, S.H.; Yu, H.J.; Park, B.J. Investigation of residual organochlorine pesticides in apple and pear orchard soil and fruit. Korean J. Environ. Agric. 2019, 38, 110–118. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Mohan, K.; Ganesan, A.R.; Govarthanan, M.; Yusoff, A.R.; Gu, F.L. Persistence, toxicological effect and ecological issues of endosulfan—A review. J. Hazard. Mater. 2021, 416, 125779. [Google Scholar] [CrossRef]
- Fang, Y.; Nie, Z.; Die, Q.; Tian, Y.; Liu, F.; He, J.; Huang, Q. Organochlorine pesticides in soil, air, and vegetation at and around a contaminated site in southwestern China: Concentration, transmission, and risk evaluation. Chemosphere 2017, 178, 340–349. [Google Scholar] [CrossRef]
- Tomlin, C. The Pesticide Manual, 11th ed.; British Crop Protection Council EU, Membership office 93 Lawrence Weaver RoadCambridge CB3 0LE UK: Cambridge, UK, 1997; p. 456. [Google Scholar]
- Hwang, S.M.; Lee, S.H.; Park, N.J.; Ok, G. Characteristics of Persistent of Hexachlorocyclohexane (HCH) in Ambient Air-Soil-Water-Sediment for a Emerging Persistent Organic Pollutants (POPs). J. Environ. Sci. Int. 2010, 19, 1343–1354. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U.; Jassal, V. Recent strategies for removal and degradation of persistent & toxic organochlorine pesticides using nanoparticles: A review. J. Environ. Manag. 2017, 190, 208–222. [Google Scholar] [CrossRef]
- Gopalan, N.S.; Chenicherry, S. Fate and distribution of organochlorine insecticides (OCIs) in Palakkad soil, India. Sustain. Environ. Res. 2018, 28, 179–185. [Google Scholar] [CrossRef]
- Choi, G.H.; Jeong, D.K.; Lim, S.J.; Ro, J.H.; Ryu, S.H.; Park, B.J.; Moon, B.C.; Kim, J.H. Plant uptake potential of endosulfan from soil by carrot and spinach. J. Appl. Biol. Chem. 2017, 60, 339–342. [Google Scholar] [CrossRef]
- McGregor, D.B. Endosulfan. JMPR 1998, JMPR Evaluations Part II Toxicological, First Draft. Available online: www.inchem.org/documents/jmpr/jmpmono/v098pr08.htm (accessed on 1 February 2023).
- Choi, G.H.; Lee, D.Y.; Ryu, S.H.; Park, B.J.; Moon, B.C.; Kim, J.H. Investigation of the bioconcentration factor of endosulfan for rice from soil. KJPS 2018, 22, 25–28. [Google Scholar] [CrossRef]
- Choi, G.H.; Lee, D.Y.; Seo, D.C.; Kim, L.S.; Lim, S.J.; Ryu, S.H.; Park, B.J.; Kim, J.H. Endosulfan plant uptake suppression effect on char amendment in oriental radish. Water Air Soil Pollut. 2018, 229, 24. [Google Scholar] [CrossRef]
- Hwang, J.; Zimmerman, A.R.; Kim, J. Bioconcentration factor-based management of soil pesticide residues: Endosulfan uptake by carrot and potato plants. Sci. Total Environ. 2018, 627, 514–522. [Google Scholar] [CrossRef]
- Oh, K.; Choi, G.; Bae, J.; Lee, D.; Lee, S.; Kim, J. Effect of soil organic matter content on plant uptake factor of ginseng for endosulfan. J. Appl. Biol. Chem. 2020, 63, 401–406. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Kawahira, K.; Sakanaka, M. Effects of ginseng saponins isolated from Red Ginseng roots on burn wound healing in mice. Br. J. Pharmacol. 2006, 148, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.D.; Lim, J.D.; Kim, J.B.; Lee, S.D. Review of Red Ginseng in terms of Mechanisms for Pharmacodynamics and Toxicity. J. Korean. Oriental. Med. 2012, 33, 200–230. Available online: https://scienceon.kisti.re.kr/srch/selectPORSrchArticleOrgnl.do?cn=JAKO201203939217719&dbt=NART (accessed on 5 September 2012). [CrossRef]
- Yoon, D.H.; Nam, K.W.; Oh, S.Y.; Kim, G.B. Improvement of certification criteria based on analysis of on-site investigation of good agricultural practice (GAP) for ginseng. J. Food Saf. Hyg. 2019, 34, 40–51. [Google Scholar] [CrossRef]
- MAFRA. Ginseng Statistical Data Book Korea; Ministry of Agriculture, Food and Rural Affairs, 94, Dasom 2-ro, Sejong City, Republic of Korea, 2021. Available online: https://www.mafra.go.kr/bbs/mafra/71/331285/artclView.do (accessed on 6 September 2022).
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Baeg, I.H. The Global Ginseng Market and Korean Ginseng. J. Ginseng Cult. 2022, 4, 1–12. [Google Scholar]
- Kong, L.L.; Liu, W.T.; Zhou, Q.X. Biochar: An effective amendment for remediating contaminated soil. Rev. Environ. Contam. Toxicol. 2014, 228, 83–99. [Google Scholar] [CrossRef]
- Lee, H.S.; Hong, S.M.; Kim, T.K.; Kwon, H.Y.; Kim, D.B.; Moon, B.C.; Moon, J.K. Reduction Effects of Residual Pesticides using the Eco-Friendly Soil Amendments in Agricultural Soil. KJPS 2016, 20, 312–318. [Google Scholar] [CrossRef]
- Spokas, K.A.; Koskinen, W.C.; Baker, J.M.; Reicosky, D.C. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 2009, 77, 574–581. [Google Scholar] [CrossRef]
- Zhang, A.; Cui, L.; Pan, G.; Li, L.; Hussain, Q.; Zhang, X.; Zheng, J.; Crowley, D. Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agric. Ecosyst. Environ. 2010, 139, 469–475. [Google Scholar] [CrossRef]
- Gomez-Eyles, J.L.; Sizmu, T.; Collins, C.D.; Hodson, M.E. Effects of biochar and the earthworm Eisenia fetida on the bioavailability of polycyclic aromatic hydrocarbons and potentially toxic elements. Environ. Pollut. 2011, 159, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Batistas, E.M.; Shultz, J.; Matos, T.T.; Fornar, M.R.; Ferreira, T.M.; Szpoganicz, B.; de Freitas, R.A.; Mangrich, A.S. Effect of surface and porosity of biochar on water holding capacity aiming indirectly at preservation of the Amazon biome. Sci. Rep. 2018, 8, 10677. [Google Scholar] [CrossRef]
- Sopeña, F.; Semple, K.; Sohi, S.; Bending, G. Assessing the chemical and biological accessibility of the herbicide isoproturon in soil amended with biochar. Chemosphere 2012, 88, 77–83. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahmed, Z.; Usman, M.; Tariq, W.; Ullah, Z.; Shareef, M.; Iqbal, H.; Waqas, M.; Tariq, A.; Wu, Y.; et al. Biochar induced modifications in soil properties and its impacts on crop growth and production. J. Plant Nutr. 2021, 44, 1677–1691. [Google Scholar] [CrossRef]
- Osman, A.I.; Fawzy, S.; Farghali, M.; El-Azazy, M.; Elgarahy, A.M.; Fahim, R.A.; Maksoud, M.A.; Ajlan, A.A.; Yousry, M.; Saleem, Y.; et al. Biochar for agronomy, animal farming, anaerobic digestion, composting, water treatment, soil remediation, construction, energy storage, and carbon sequestration: A review. Environ. Chem. Lett. 2022, 20, 2385–2485. [Google Scholar] [CrossRef] [PubMed]
- Tatarková, V.; Hiller, E.; Vaculík, M. Impact of wheat straw biochar addition to soil on the sorption, leaching, dissipation of the herbicide (4-chloro-2-methylphenoxy) acetic acid and the growth of sunflower (Helianthus annuus L.). Ecotoxicol. Environ. Saf. 2013, 92, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sheng, G.; Huang, M. Bioavailability of diuron in soil containing wheat-straw-derived char. Sci. Total Environ. 2006, 354, 170–178. [Google Scholar] [CrossRef]
- Copley, T.R.; Aliferis, K.A.; Jabaji, S. Maple bark biochar affects Rhizoctonia solani metabolism and increases damping-off severity. Phytopathology 2015, 105, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Huang, H.; Li, H.; Li, J.; Zhou, W. Biochar stability assessment methods: A review. Sci. Total. Environ. 2019, 647, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.E. Chemical Analysis of Ecological Materials, 2nd ed.; Blackwell Scientific Publishing Co.: Oxford, UK, 1989; pp. 1–565. [Google Scholar]
- Schollenberger, C.J. A rapid approximate method for determining soil organic matter. Soil Sci. 1927, 24, 65–68. [Google Scholar] [CrossRef]
- Paramasivam, M. Dissipation kinetics, dietary and ecological risk assessment of chlorantraniliprole residue in/on tomato and soil using GC–MS. J. Food Sci. Technol. 2021, 58, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K. Chemical remediation of soils contaminated with endosulfan, organochlorine insecticide. Kor. J. Environ. Agric. 2008. Available online: https://www.riss.kr/link?id=T11499252&outLink=K (accessed on 1 February 2023).
- Parkpian, P.; Anurakpongsatorn, P.; Pakkong, P.; Patrick, W.H., Jr. Adsorption, desorption and degradation of α-endosulfan in tropical soils of Thailand. J. Environ. Sci. Health, 1998; 33, 211–233. Available online: https://www.tandfonline.com/doi/abs/10.1080/03601239809373140 (accessed on 1 February 2023). [CrossRef]
- Kumar, M.; Philip, L. Adsorption and desorption characteristics of hydrophobic pesticide endosulfan in four Indian soils. Chemosphere 2006, 62, 1064–1077. [Google Scholar] [CrossRef]
- Lee, D.Y.; Choi, G.H.; Bae, Y.S.; Lee, S.W.; Kim, S.K.; Bae, J.Y.; Kim, J.H. Fate of endosulfan in ginseng farm and effect of granular biochar treatment on endosulfan accumulation in ginseng. Environ. Geochem. Health 2021, 44, 3953–3965. [Google Scholar] [CrossRef]
- Walse, S.S.; Scott, G.I.; Ferry, J.L. Stereoselective degradation of aqueous endosulfan in modular estuarine mesocosms: Formation of endosulfan gamma-hydroxycarboxylate. J. Environ. Monit. 2003, 5, 373–379. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.C.; Nkedi-Kizza, P.; O’Hair, S.K. Endosulfan losses through runoff and leaching from calcareous gravelly or marl soils. Vadose Zone J. 2003, 2, 231–238. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human services, Public Health Service. Toxicological Profile for Endosulfan; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2015; pp. 221–272. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp41.pdf (accessed on 1 February 2023).
- NRA (NRA ECRP). Review of endosulfan. In National Registration Authority for Agricultural and Veterinary Chemicals; Commonwealth of Australia: Kingston, Australia, 1998. [Google Scholar]
- Guerin, T. F The anaerobic degradation of endosulfan by indigenous microorganism from low-oxygen soils and sediments. Environ. Pollut. 1999, 106, 13–21. [Google Scholar] [CrossRef] [PubMed]
- GFEA-U, E. Endosulfan-Draft Dossier Prepared in Support of A Proposal of Endosulfan to be Considered As A Candidate for Inclusion in the UN-ECE LRTAP Protocol on Persistent Organic Pollutants; German Federal Environment Agency-Umweltbundesamt: Berlin, Germany, 2009. [Google Scholar]
- Yan, J.; Wang, D.; Miao, J.; Liu, C.; Wang, Y.; Teng, M.; Zhou, Z.; Zhu, W. Discrepant effects of α-endosulfan, β-endosulfan, and endosulfan sulfate on oxidative stress and energy metabolism in the livers and kidneys of mice. Chemosphere 2018, 205, 223–233. [Google Scholar] [CrossRef]
- Paul, V.; Balasubramaniam, E. Effects of single and repeated administration of endosulfan on behaviour and its interaction with centrally acting drugs in experimental animals: A mini review. Environ. Toxicol. Pharmacol. 1997, 3, 151–157. [Google Scholar] [CrossRef]
- Wan, M.T.; Kuo, J.N.; Buday, C.; Schroeder, G.; Van Aggelen, G.; Pasternak, J. Toxicity of α -, β -,(α + β)–endosulfan and their formulated and degradation products to Daphnia magna, Hyalella azteca, Oncophynchus mykiss, Oncophynchus kisutch, and biological implications in streams. Environ. Toxicol. Chem. 2005, 24, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wang, M.; Dai, H.; Hong, W.; Wang, M.; Wang, J.; Weng, N.; Nie, Y.; Xu, A. Endosulfan isomers and sulfate metabolite induced reproductive toxicity in Caenorhabditis elegans involves genotoxic response genes. Environ. Sci. Technol. 2015, 49, 2460–2468. [Google Scholar] [CrossRef]
- Yan, J.; Wang, D.; Meng, Z.; Yan, S.; Teng, M.; Jia, M.; Li, R.; Tian, S.; Weiss, C.; Zhou, Z.; et al. Effects of incremental endosulfan sulfate exposure and high fat diet on lipid metabolism, glucose homeostasis and gut microbiota in mice. Environ. Pollut. 2021, 268, 115697. [Google Scholar] [CrossRef]
- Lee, H.K.; Kim, K.; Lee, J.; Lee, J.; Lee, J.; Kim, S.; Lee, S.-E.; Kim, J.-H. Targeted toxicometabolomics of endosulfan sulfate in adult zebrafish (Danio rerio) using GC-MS/MS in multiple reaction monitoring mode. J. Hazard. Mater. 2020, 389, 122056. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lonappan, L.; Brar, S.K.; Yang, S. Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: A review. Sci. Total Environ. 2018, 645, 60–70. [Google Scholar] [CrossRef]
- Wang, H.; Lin, K.; Hou, Z.; Richardson, B.; Gan, J. Sorption of the herbicide terbuthylazine in two NewZealand forest soils amended with biosolids and biochars. J. Soils Sediments 2010, 10, 283–289. [Google Scholar] [CrossRef]
- Khorram, M.S.; Wang, Y.; Jin, X.; Fang, H.; Yu, Y. Reduced mobility of fomesafen through enhanced adsorption in biochar-amended soil. Environ. Toxicol. Chem. 2015, 34, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Khorram, M.S.; Zhang, Q.; Lin, D.; Zheng, Y.; Fang, H.; Yu, Y. Biochar: A review of its impact on pesticide behavior in soil environments and its potential applications. J. Environ. Sci. 2016, 44, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Hussen, A.; Megerasa, N.; Jönsson Åke, J. Effect of aging organochlorine pesticides in various soil types on their extractability using selective pressurized liquid extraction. J. Environ. Prot. 2017, 8, 867–883. [Google Scholar] [CrossRef]
- Koh, I.H.; Kim, J.; Kim, G.S.; Ji, W.H. Transfer of arsenic and heavy metals from soils to rice plant under different drainage conditions. J. Soil Groundw. Environ. 2017, 22, 12–21. [Google Scholar] [CrossRef]
- Pascal, P. Guidance on Information Requirements and Chemical Safety Assessment Chapter R.11: PBT/vPvB assessment. 2017. Available online: https://policycommons.net/artifacts/2058778/guidance-on-information-requirements-and-chemical-safety-assessment-chapter-r11/2811869/ (accessed on 22 June 2017).
- Tammeorg, P.; Simojoki, A.; Mäkelä, P.; Stoddard, F.L.; Alakukku, L.; Helenius, J. Short-term effects of biochar on soil properties and wheat yield formation with meat bone meal and inorganic fertiliser on a boreal loamy sand. Agric. Ecosyst. Environ. 2014, 191, 108–116. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of green waste biochar as a soil amendment. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochars as soil amendments. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Deenik, J.L.; Diarra, A.; Uehara, G.; Campbell, S.; Sumiyoshi, Y.; Antal, M.J., Jr. Charcoal ash and volatile matter effects on soil properties and plant growth in an acid Ultisol. Soil Sci. 2011, 176, 336–345. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Toliver, D.K.; Larson, J.A.; Roberts, R.K.; English, B.C.; De La Torre Ugarte, D.G.; West, T.O. Effects of no-till on yields as influenced by crop and environmental factors. Agron. J. 2012, 104, 530–541. [Google Scholar] [CrossRef]
- Chung, H.D.; Choi, Y. Ultrastructural Changes in Leaves of Chinese Cabbage (Brassica Campestris ssp. Pekinensis) and Radicle Tissues of Radish (Raphanus Sativus) Grown in High Soil EC. Hortic. Environ. Biotechnol. 2003, 44, 582–587. Available online: https://www.kci.go.kr/kciportal/ci/sereArticleSearch/ciSereArtiView.kci?sereArticleSearchBean.artiId=ART000890567 (accessed on 14 August 2003).
- Thies, J.E.; Rillig, M.C. Characteristics of biochars: Biological properties. In Biochar for Environmental Management; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 85–105. [Google Scholar]
- Jaiswal, A.K.; Elad, Y.; Graber, E.R.; Frenkel, O. Rhizoctonia solani suppression and plant growth promotion in cucumber as affected by biochar pyrolysis temperature, feedstock and concentration. Soil Biol. Biochem. 2014, 69, 110–118. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Frenkel, O.; Elad, Y.; Lew, B.; Graber, E.R. Non-monotonic influence of biochar dose on bean seedling growth and susceptibility to Rhizoctonia solani: The “Shifted Rmax-Effect. ” Plant Soil 2015, 395, 125–140. [Google Scholar] [CrossRef]
- Huang, W.K.; Ji, H.L.; Gheysen, G.; Debode, J.; Kyndt, T. Biochar-amended potting medium reduces the susceptibility of rice to root-knot nematode infections. BMC Plant Biol. 2015, 15, 267. [Google Scholar] [CrossRef]
- Han, H.J.; Song, C.W.; Lee, J.U. The statistical study on the effects of physicochemical properties of soil on single extraction methods for heavy metals. Econ. Environ. Geol. 2021, 54, 259–269. [Google Scholar] [CrossRef]
- Kilic, K.; Kilic, S.; Kocyigit, R. Assessment of Spatial Variability of Soil Properties in Areas Under Different Land Use. Bulg. J. Agric. Sci. 2012, 18, 722–732. Available online: https://hdl.handle.net/20.500.12513/4202 (accessed on 2 June 2012).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).