1. Introduction
Abiotic stress in agriculture is the impact of non-living pressures imparted on plants, such as heat, cold/frost, drought, excess moisture, salinity, etc., all of which can lead to significant crop and yield losses [
1]. Arguably, abiotic stress is the most critical factor impacting the growth and productivity of crops worldwide [
2], particularly when various stresses occur together in combination. Climate change can exacerbate overall abiotic stress [
3], and worldwide, the percentage of the planet affected by drought has more than doubled in the last 40 years [
4], with agriculture bearing 80% of the direct impact [
5]. According to the American Meteorologic Society, recent droughts in Texas, California, East Africa, Kenya, and Cape Town have been more severe or likely to occur due to climate change [
6]. Rainfall patterns are shifting worldwide, and such changes could intensify in the coming years. This likely means more intense periods of heavy rain and lengthier dry periods, even within the same regions [
7]. Worsening or extreme changes (regarding temperatures, rainfall, and water quality) that increase abiotic stress will occur when the global population demand for food is expanding faster than increases in arable land [
8,
9]. Therefore, it is critical that agriculture adopts new technologies and implements practices that not only compensate for the increase in abiotic stresses, but does so while decreasing the practices that are contributing to climate change.
The 2018 Farm Bill (for the purposes of a report to the US Congress) considers a “plant biostimulant” to be “a substance or micro-organism that, when applied to seeds, plants, or the rhizosphere, stimulates natural processes to enhance or benefit nutrient uptake, nutrient efficiency, tolerance to abiotic stress, or crop quality and yield” [
10]. Biostimulant is a large umbrella term that encompasses a number of diverse categories, such as organic acids including humic and fulvic acids, seaweed extracts, microbial inoculants, beneficial fungi, and amino acids and protein hydrolysates, and numerous reviews on their use have been recently published [
11,
12,
13,
14]. Natural organic matter (NOM), which contains humic and fulvic acids [
15,
16,
17] and is ubiquitous in soils, sediments, aquatic sources and aerosols, is formed primarily by the microbial degradation of plant matter and contains thousands of individual components [
18,
19]. The chemical nature of this complex mixture can be analytically characterized using infrared spectroscopy, ultraviolet visible absorption spectroscopy, fluorescence spectroscopy, nuclear magnetic resonance spectroscopy, and ultrahigh resolution mass spectrometry [
20,
21,
22,
23,
24,
25,
26,
27]. The understanding of the exact mechanisms for how and why NOM-based biostimulants improve plant health is still in its infancy. Still, many studies have shown increases in nutrient use efficiency (NUE), abiotic stress mitigation, and quenching of reactive oxygen species (ROS) [
9,
28,
29,
30,
31,
32,
33]. The NOM-based biostimulant used here for all trials is obtained from a terrestrial source in Northern Europe that has been concentrated and refined by FBSciences, Inc. and has been extensively chemically characterized previously [
34]. This complex mixture of thousands of organic compounds contains a wide variety of functional groups, and it can be fractionated based on solubility under acidic vs. alkaline conditions and according to polarity using a hydrophobic resin. The components within this diverse mixture belong to various biomolecular compound classes, such as lignin, tannin, and condensed aromatic compounds. For the purpose of biostimulant applications, the treatments described here are on a total organic carbon (TOC) basis, where TOC concentrations are measured using high temperature catalytic combustion on a Shimadzu TOC
CSN total organic carbon analyzer calibrated with potassium hydrogen phthalate [
34].
Because abiotic stresses are naturally occurring events, it is challenging to conduct replicated field trials, as predictions cannot accurately be made as to what will occur in nature during the course of a trial. However, it is possible to simulate the stress conditions that may potentially ensue under certain circumstances in the field by conducting stress trials under controlled conditions in a greenhouse or nursery. Here, the results of 21 trials on corn, wheat, soybean, and various high-value crops are discussed in the context of the abiotic stress encountered, where the stress was experimentally induced or naturally occurring. Six trials in California executed on tomatoes, peppers, strawberries, and avocados simulated drought and salt stress and examined treatments that included stressed and non-stressed controls and applications of grower standard (GS) fertilizers and the NOM-based biostimulant each alone and together. Eleven field trials on corn in Nebraska using the GS treatments with and without the NOM-based biostimulant are shown for a variety of conditions that occurred over the course of three years, including little abiotic stress, cold and wet soils due to excess rain and high water tables, and high temperatures and drought. Two field trials conducted in Australia on wheat and lentils experienced cold and severe frost events, while two field trials on soybeans and sugarcane in Brazil suffered heat and drought stress, and each of these trials utilized GS treatments with and without the NOM-based biostimulant. The results of the 21 trials described here, taken together, highlight the positive impact on improved plant health and yields under various abiotic stress circumstances using a biostimulant that can be easily incorporated into a grower’s current program.
2. Materials and Methods
2.1. Trial #1: Nursery Drought Stress Trial on Tomatoes Using Soil Applications
A simulated drought stress trial was conducted in Oxnard, CA to examine the effects of the natural organic matter (NOM)-based biostimulant for stress mitigation using soil applications. This randomized complete block design (RCBD) trial utilized tomatoes (Lycopersicon esculentum) grown from seed and transplanted as single plants in 3 × 3 inch pots in sandy-loam soil. There were 25 replicates for each of the 3 treatments: (1) untreated check (UTC) with no induced drought stress; (2) UTC with induced drought stress; and (3) NOM biostimulant with induced drought stress, so that there were enough plants to sacrifice in order to have 8 replicates per treatment at 3 assessment time points. Applications started after the plants had at least 2–4 true leaves (at transplant), were made 14 days apart, and were made by applying 25 mL per pot as a soil drench of the biostimulant diluted in water to give a final total organic carbon (TOC) content of 0.03 mg/L TOC. All watering was stopped 8 days after the second application to induce drought stress for 8 days, after which normal watering resumed. Plant assessments (plant height and weight and root and shoot weights) were taken pre-stress (i.e., before watering was stopped), during stress (after 8 days of drought stress), and during recovery (after watering was resumed). One-way analysis of variance (ANOVA) was utilized to determine if measurements from 8 replicates across the 3 treatments were statistically different (p = 0.10). If p < 0.10 from ANOVA, then a post hoc multiple comparison test was performed using least significant difference (LSD) to ascertain which averages were different. Statistical analysis was performed in MATLAB.
2.2. Trials #2–3: Nursery Salt Stress Trials on Tomatoes Using Soil and Foliar Applications
Two simulated salt stress trials were conducted in Oxnard, CA to examine the effects of the NOM-based biostimulant for stress mitigation using soil and foliar applications. These RCBD trials utilized tomatoes (L. esculentum) grown from seed and transplanted as single plants in 3 × 3 inch pots in sandy-loam soil. There were 4 treatments for each trial: (1) untreated check (UTC) with no induced salt stress; (2) induced salt stress using NaCl at 200 mg/L for soil applications and NaCl at 5000 mg/L for foliar applications; (3) NOM biostimulant alone without induced salt stress; and (4) NOM biostimulant with NaCl to induce salt stress (at the same concentrations as treatment 2), and there were 8 replicates per treatment.
For the trial using soil applications, two NOM applications (at 0.13 mg/L TOC) started 13 days after the plants had at least 2–4 true leaves (at transplant), were made 12 days apart, and were made by applying 25 mL per pot as a soil drench. The salt treatment applications (25 mL per pot as a soil drench) were made 7 days after the second NOM application. For the trial using foliar applications, applications were made at transplant (when the plants had 2–4 true leaves) to provide a final spray volume equivalent to 208 L/ha, with the NaCl applied at a rate equivalent to 2.6 g NaCl per hectare and the NOM at 585 mg TOC per hectare.
For both trials, plant assessments (plant height and weight and root and shoot weights) were made 10–11 days after the salt treatment. One-way ANOVA was utilized to determine if measurements from 8 replicates across each of the 4 treatments per trial were statistically different (p = 0.10). If p < 0.10, then LSD as a post hoc multiple comparison test was executed.
2.3. Trial #4: Nursery Salt Stress Trial on Strawberries
Because strawberries are very sensitive to salt, a nursery trial was conducted in Camarillo, CA to evaluate the impact of the NOM-based biostimulant for the mitigation of salt stress. This RCBD trial utilized strawberries (Fragaria x ananassa) grown in 2 L containers in a commercial potting soilless media (Agromin Premium Blend, Oxnard, CA, USA). There were 3 treatments: (1) grower standard (GS) fertilizer; (2) GS with NaCl added to the irrigation water at 200 mg/L to induce salt stress; (3) GS plus NOM biostimulant with NaCl at 200 mg/L to induce salt stress, and there were 6 replicates per treatment. Four NOM applications, equivalent to 585 mg TOC per hectare, were made as a soil drench approximately 3 weeks apart from mid-July to the end of September (7/17, 8/11, 9/2, 9/22). Salt was included in the irrigation water 3 times about 3 weeks apart from mid-August to the end of September (8/16, 9/3, 9/22). The cumulative fruit yield was counted throughout the season.
2.4. Trial #5: Field Drought Stress Trial on Peppers
A simulated drought stress trial was conducted in Oxnard, CA to examine the effects of the NOM-based biostimulant for stress mitigation, where drought was induced using two different conditions. Drought A was induced shortly after the plants were established (on 6/3), while Drought B was induced at bloom (6/27). In both cases, watering was stopped for two weeks. This RCBD trial utilized peppers (Capsicum annuum), and there were 6 treatments with 6 replicates each: (1) grower standard (GS) fertilizer with no drought stress; (2) GS plus NOM biostimulant with no drought stress; (3) GS under Drought A; (4) GS plus NOM biostimulant under Drought A; (5) GS under Drought B; and (6) GS plus NOM biostimulant under Drought B. Three NOM applications, equivalent to 585 mg TOC per hectare, were made via the drip irrigation system approximately 3–4 weeks apart from mid-April to June (4/17, 5/14, and 6/3). Marketable yields of red, green, and the total number of peppers were taken at the end of August. One-way ANOVA was utilized to determine if measurements from 6 replicates across the 6 treatments were statistically different (p = 0.10). If p < 0.10, then LSD as a post hoc multiple comparison test was executed.
2.5. Trial #6: Field Salt and Drought Stress Trial on Avocados
A drought and salt stress trial was conducted in Somis, CA to examine the effects of the NOM-based biostimulant for mitigation of multiple simultaneous stress factors. This RCBD trial utilized Hass avocados (Persea americana) that were already a well-established mature crop. There were 4 treatments: (1) grower standard (GS) fertilizer with no induced stress; (2) GS with NaCl at 200 mg/L and 80% of the regular watering volume to induce both salt and drought stress; (3) GS plus NOM biostimulant without induced stress; and (4) GS plus NOM biostimulant with NaCl at 200 mg/L and 80% of the regular watering volume to induce salt and drought stress, and there were 6 replicates per treatment. Salt was applied as soil drench 6 times from May through August (5/11, 5/26, 6/16, 8/13, 8/25, and 8/31). Four NOM applications, equivalent to 878 mg TOC per hectare, were made as a soil application from May through August (5/11, 6/9, 8/13, and 9/1). Fruit set occurred in May, with a typical fruit drop post-set occurring in June. Sizing of the set fruit was taken on 8/10 and 12/7. Fruit counts were extrapolated to estimate yields based on an average fruit weight of 8 wet oz per fruit. One-way ANOVA was utilized to determine if measurements from 6 replicates across the 4 treatments were statistically different (p = 0.10). If p < 0.10, then LSD as a post hoc multiple comparison test was executed.
2.6. Trials #7–17: Three-Year Corn Trials in Nebraska
Between 2010 and 2012, a series of 11 corn trials were conducted near Lexington, Nebraska, where fields are irrigated and strip-till farming was utilized. More specific details of the 11 trials are given in
the Supplementary Information. In general, there were two treatments, where a liquid starter NPK fertilizer was applied 10–15 days prior to planting either in two bands below (at 4–5 and 9–10 inches) and to the side of the seedbed or in only 1 band at 9–10 inches below the soil surface, with and without the NOM-based biostimulant that was applied at 730–1170 mg TOC per hectare. Root pits were dug using a backhoe, allowing for the excavation of the entire root system, which was done at 25 and 60 days after emergence (DAE). Once the root systems were isolated, the dirt was carefully removed and the roots were washed, so that detailed measurements (root profile widths at various depths, roots count per node) could be made. Yield data was collected at harvest.
2.7. Trials #18–19: Field Trials on Winter Wheat and Lentils in Australia
Two field trials on winter wheat and lentils were conducted near the Horsham area of Victoria, Australia. Similar to the corn trials, there were two treatments for the wheat trial, where a grower standard of urea ammonium nitrate was foliarly applied (at 35 L/ha) alone and with the NOM-based biostimulant (at 500 mg TOC per hectare) at tillering. This RCBD trial was conducted on plots of 0.52 ha with 3 replicates per treatment. Sap Brix was measured by refractometry at 1, 17, 39, and 52 days after applications, and yields were taken at harvest.
Similarly, for the lentil trial, there were two treatments where a GS was applied alone and with the NOM-based biostimulant (at 500 mg TOC per hectare) between V5 and Vn (approximately 1 week prior to flowering). This RCBD trial was conducted on plots of 0.42 ha with 4 replicates per treatment. Sap Brix was measured by refractometry the day before applications were made (shown as day −1) and at 4, 12, and 31 days after applications, and yields were taken at harvest. One-way ANOVA was utilized to determine if measurements from replicates were statistically different (p = 0.10), and if p < 0.10, then LSD as a post hoc multiple comparison test was executed.
2.8. Trials #20–21: Field Trials on Soybean and Sugarcane in Brazil
A soybean (
Glycine max L.) field trial was conducted in the Paranapanema Valley of São Paulo State, Brazil in a medium texture sandy clay soil. There were two treatments, where a GS of monoammonium phosphate (11-30-0, at 200 kg/ha) and potassium chloride (50 kg/ha) was applied alone and with the NOM-based biostimulant. In this trial, the NOM-based biostimulant was applied 3 times during the season: as a seed treatment at 25 mL of 5000 mg/L TOC per 100 kg of seed and as a foliar spray at 500 mg TOC per ha at both R1 and R3. This RCBD trial was conducted on plots of 40 m
2 with 4 replicates per treatment. A sugarcane (
Saccharum officinarum) field trial was conducted in the Vitoriana District of São Paulo State, Brazil. There were two treatments, where a grower standard was applied alone and with the NOM-based biostimulant (580 mg TOC per ha). This RCBD trial was conducted on plots of 42 m
2 with 4 replicates per treatment. For both trials, stomatal conductance (measured using a porometer), enzyme (superoxide dismutase, catalase, and peroxidase) activity levels (as described in
the Supplementary Information), plant heights, and yields were assessed.
3. Results
3.1. Trial #1: Nursery Drought Stress Trial on Tomatoes using Soil Applications
This trial simulated drought stress on tomatoes, where an untreated check (UTC) with no induced drought stress was compared to a UTC with induced drought stress and drought-stressed plants that were treated with the natural organic matter (NOM)-based biostimulant using soil applications. Plants were assessed for plant height and plant, root, and shoot weights pre-stress (before watering was stopped), during stress (after eight days with no water), and after stress (eight days after watering resumed). The summary data is shown in
Supplementary Table S1, and
Figure 1 shows the plant weights during the three assessment times. Prior to the induced stress, there were no statistically significant differences between the treatments, except for plant height, which showed the plants with the NOM-based biostimulant were the tallest. During the drought stress, it was clear that all of the stressed plants were significantly affected by the lack of water, with 99% confidence. The drought-stressed UTC group plants were less than half the size of those in the unstressed UTC group, but the stressed plants with the NOM-based biostimulant were larger than the untreated plants under stress by 36%, 27%, and 41% for the plant, root, and shoot weights, respectively (statistically significant for the plant and shoot weights). The NOM-based biostimulant treated plants were also able to recover more quickly than the untreated stressed plants. About eight days after regular watering was resumed, the NOM-based biostimulant treated plants had almost caught up to the non-stressed plants, only being smaller by about 20%. In contrast, the untreated stressed plants were still significantly smaller (at a 99% confidence interval), remaining at about half the size of the non-stressed plants.
3.2. Trials #2–3: Nursery Salt Stress Trials on Tomatoes Using Soil and Foliar Applications
These trials simulated salt stress on tomatoes, where a UTC with no induced salt stress was compared to a UTC with induced salt stress and to two treatment groups where plants were treated with the NOM-based biostimulant each with and without the induced salt stress. Plants were assessed for plant height and plant, root, and shoot weights 10–11 days after the salt-induced stress occurred. The summary data is shown in
Supplementary Tables S2 and S3, and
Figure 2 and
Figure 3 shows the plant, root, and shoot weights.
For the trial where treatments were applied as soil drenches, there were no significant differences between any of the treatments for plant height. However, the UTC plants with salt stress were significantly smaller than the non-stressed UTC plants, and the plant weights for the treatments including the NOM-based biostimulant were significantly larger than those without. When the biostimulant was utilized for the non-stressed plants, the plant, root, and shoot weights increased by 39%, 74%, and 13%, respectively, which was statistically significant at the 99% confidence interval for the plant and root weights (only numerically higher for the shoots). It was hypothesized that the biostimulant would mitigate the salt stress to some degree, but the NOM-based biostimulant had a much greater impact when salt stress was applied than when the plants were unstressed. The percent increases were larger for the salt-stressed plants treated with the biostimulant versus the untreated stressed plants; in this case, by 87%, 188%, and 22% for the plant, root, and shoot weights, respectively, which was statistically significant for all parameters. It is clear that there is a synergistic effect between the NOM-based biostimulant and the salt, because the plant and root weights for stressed plants treated with the biostimulant were significantly larger than those treated with the biostimulant but not under salt stress (by 15% and 30%, respectively). When the salt was applied in the presence of the biostimulant, it acted as a growth enhancer rather than a suppressant.
For the trial where treatments were applied as foliar sprays, it should be noted that the plants were treated using a singular foliar application at an earlier growth stage, and thus, were smaller at the time of assessment than those in the previous trial where multiple soil applications were made 12–14 days apart. The plants treated with the NOM-based biostimulant were significantly taller than those that were not treated (
Supplementary Table S3). In this case, there were no statistically significant differences for the plant, root, and shoot weights for the non-stressed plants, regardless of the use of the biostimulant (
Figure 3). However, the percent increases for the plant, root, and shoot weights of the salt-stressed plants were larger by 67%, 113%, and 45%, respectively, for the plants treated with the NOM-based biostimulant (all of which were statistically significant at the 95% confidence level). Results for this trial using foliar applications are consistent with those described above for the soil applications, where the plant and root weights for stressed plants treated with the biostimulant were larger than those treated with the biostimulant but not under salt stress (by 13% and 55%, respectively), which was statistically significant for the root weights. This evidenced synergistic effect between the NOM-based biostimulant and the salt was validated in this trial for tomatoes.
3.3. Trial #4: Nursery Salt Stress Trial on Strawberries
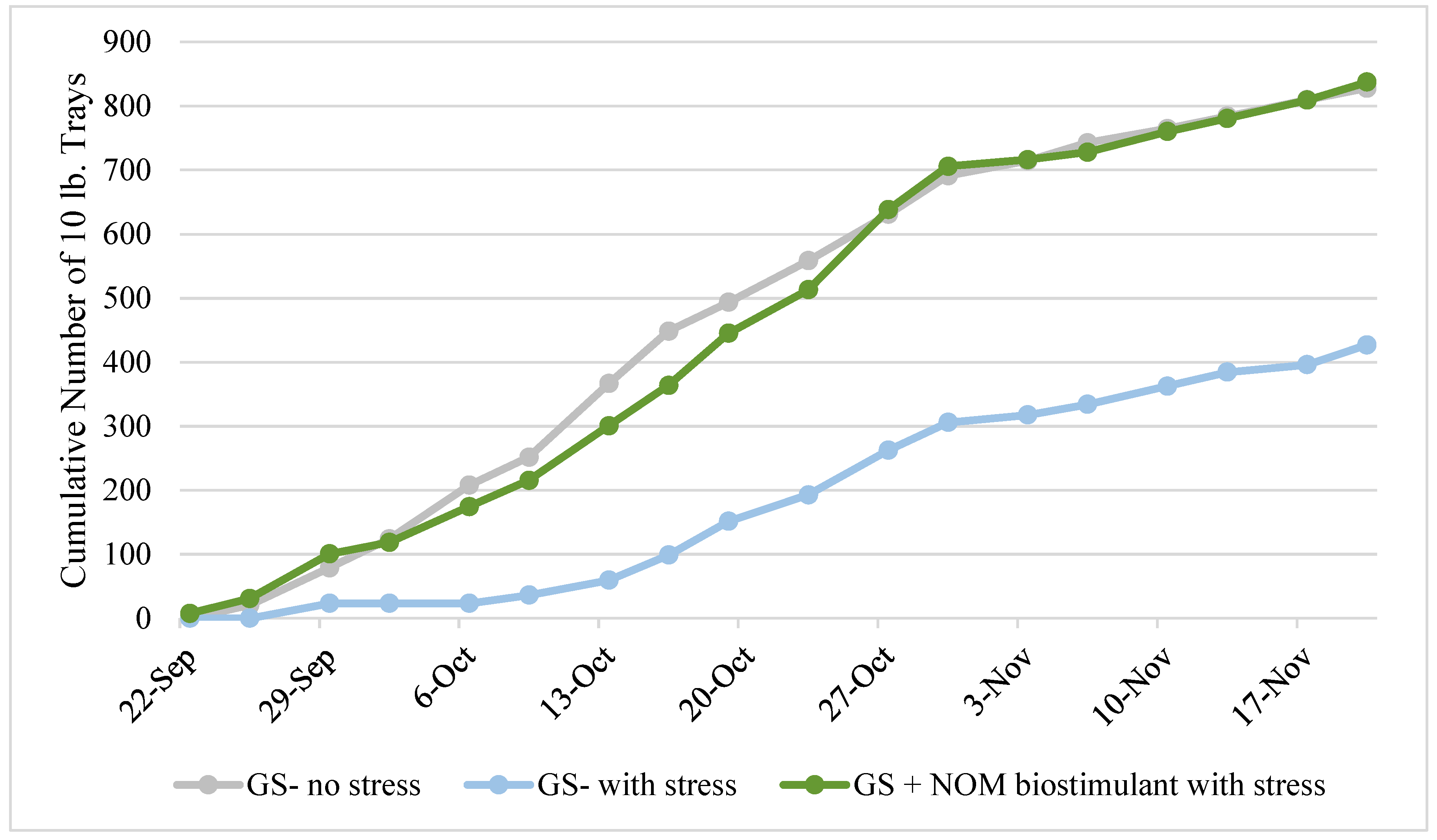
This trial simulated salt stress on strawberries, where a grower standard (GS) fertilizer program with no induced salt stress was compared with the GS program with induced salt stress and the GS program incorporating the NOM-based biostimulant with the induced salt stress. In this trial, treatments were applied as soil drenches. The last of the three salt inclusions in the irrigation water and the last of the four NOM applications done as a soil drench both occurred on 9/22, when the first of the strawberries were picked. As shown in
Figure 4, including salt in the irrigation water caused substantial stress to the strawberry plants, as shown by the decrease in yield, but the inclusion of the NOM-based biostimulant in the GS program completely mitigated this stress. For the first month of picking, the salt-stressed GS plants (no biostimulant applied) yielded only 20% on average of those that were not stressed. For the second month of picking, the salt-stressed GS plants began to recover, showing cumulative yields that were about half of those that were not stressed. From 10/30 until the end of the trial, the yields per pick for all three treatments were approximately the same. This indicates that most of the salt had been leached out of the root zone, and the level of stress was similar for all plants regardless of treatment. At the end of the trial, salt without the NOM-based biostimulant caused the yield to be reduced from 828 trays to 427 trays, a reduction of 48%. For the stressed treatment with the NOM-based biostimulant, the yields were consistent with the non-stressed plants, and the final count was actually 10 trays more (838 trays). When comparing the salt-stressed treatments, the use of the biostimulant with the GS showed a 96% increase in yield over the stressed plants that received the GS alone.
3.4. Trial #5: Field Drought Stress Trial on Peppers
In a season-long trial with peppers, drought stress was simulated by stopping irrigation for two weeks at two different times during the season to induce drought dress. For drought A, water was withheld prior to plants blooming (shortly after plant establishment), and for drought B, the water was withheld starting at bloom. Treatments from a GS fertilizer program with and without the NOM-based biostimulant were each compared to those treatments without the induced drought stress, under drought A conditions, and under drought B conditions, giving six treatment groups in total. The treatments (via the drip irrigation system) of the NOM-based biostimulant occurred three times during April-June, and marketable yields of red and green peppers were taken at the end of August (
Supplementary Table S4,
Figure 5). It is clear that the drought-induced stress hurt the overall pepper yields, as the total yields of the drought A and B treatments decreased by about 35% compared to the non-stressed GS plants (all without the NOM-based biostimulant). Without stress, the NOM-based biostimulant included with the GS improved marketable yields by 45%, which was statistically significant with 99% confidence. When under drought conditions, the yield increases for the treatments including the NOM biostimulant, compared to the stressed GS treatments without the biostimulant, were more than double. There was no statistical difference between the three treatments that included the NOM-based biostimulant with the GS, regardless of the stress (or lack thereof). The ability of the biostimulant to completely mitigate the drought stress is remarkable, and the extrapolation of the yields shown here for two plants would lead to outstanding returns for growers.
3.5. Trial #6: Field Salt and Drought Stress Trial on Avocados
In this season-long trial on mature avocado trees, salt and drought stress were simulated by reducing the amount of irrigation water by 20% for the entire season and adding salt to the irrigation water four times throughout the season. Treatments from a GS fertilizer program with and without the NOM-based biostimulant were each compared to those treatments with and without the combined induced salt and drought stress conditions, giving four treatment groups in total. In this trial, treatments were made as soil applications. At the end of the season, trees receiving only the GS treatment (no biostimulant) that were stressed yielded 19% fewer avocados than the non-stressed trees (
Supplementary Table S5,
Figure 6). Absent stress, the NOM-based biostimulant included with the GS improved yields by 24%. When under drought and salt stress, the yield increased by 45% for the treatment including the NOM biostimulant, but there was little difference between treatments including the biostimulant with and without induced stress.
3.6. Trials #7–17: Three-Year Corn Trials in Nebraska
This data set of 11 field trials (four in 2010, four in 2011, and three in 2012) compared GS fertilizer programs alone and including the NOM-based biostimulant. While the initial purpose of these trials was to evaluate the ability of the biostimulant to enhance nutrient use efficiencies of GS fertilizers, high stress conditions in two of the three years clearly demonstrated the biostimulant’s stress mitigating effect. The Platte River Valley of Nebraska in Dawson County often struggles with early season loss of pre-plant nitrogen applications due to a highly fluctuating water table. In 2010, the major stressors were too much rain and extremely high water tables that resulted in water-saturated soil and extremely low soil temperatures, both of which tend to cause significant yield reductions in corn. Excessive rain leads to fertilizer losses and leaching, and cold and wet soils slow root development. In mid-July, the water table was only 24 inches below the soil surface, and soil temperatures were still around 50 °F (10 °C) for two of the trials. Corn yields in this region were well below the 5-year average (about 26% below normal), but the NOM-based biostimulant mitigated this stress and led to enhanced yields. In these 4 trials, yield results for treated plots were 10–21% greater than for the untreated plots (
Table 1). The largest yield increases were observed for the strip-till applications in two bands (Trials 2010-1 and 2010-2). These high increases were also the test site where the stress was the greatest. The in-furrow trial (2010-3) and the single band strip-till trial (2010-4) gave yield increases of 11% and 10%, respectively.
In 2011, the environmental conditions were more like those typically encountered in that part of Nebraska, and in three of the four separate trials, the yield increases were 5–8%. At the fourth site, the yield increase was 16%. While these sites suffered from higher than average rainfall and a high water table, conditions were not as severe as in the 2010 testing, as can be observed based on the yields for the GS-only treatments. In 2010, the four evaluated sites, all with very high stress, had an average yield of about 150 bu/ac, which is about 27% lower than the 2011 average for the four sites (191 bu/ac). Even with the more favorable conditions, the NOM-based biostimulant still results in excellent yield improvements.
In 2012, the stress conditions were the opposite of the conditions in 2010. There was an extreme drought across most of the Midwest with very high sustained temperatures at critical times. Even with irrigation, it was not possible to get enough water on the crops to make up for the lack of rainfall. In one of the trials (2012-2), the control yielded close to the 5-year average for that field, likely because the water table was higher at this particular location (allowing the roots another source of water). In this case, the NOM-based biostimulant treated plots had a yield increase of 6%. However, in another trial (2012-1), the yield for the control was significantly lower than the 5-year average, but the yield for the biostimulant treated plots was 18% greater than for the GS alone. For both of these trials, as well as all of the 2010 and 2011 trials, Hoegemeyer corn hybrids were utilized. For trial 2012-3, a Mycogen corn hybrid was used, and this variety did not handle the heat well (temperatures in excess of 100 °F (38 °C) for 8 days), leading to the only negative yield result for the 11 trials when the NOM-based biostimulant was included in the treatment along with the grower standard.
In addition to enhanced yields, root profiles were examined with depth and the number of roots per node was counted (
Figure 7). With depth, there was a 7–88% increase in the root profile width, with the largest increases observed at the deepest parts of the soil profile. At 25 days after emergence (DAE), there was a 14–104% increase in the average number of roots observed per node. This increase early in the season gives the NOM-treated plants an early advantage that provides benefits that last the entire growing season, as indicated by the measurements at 60 DAE (8–21% increase in roots per node). With the strip-tilling methodology, the product placed at 4–5 inches has the biggest effect on primary and lower-ordered nodal roots, which are those most associated with the uptake of NPK and other nutrients [
35]. The product placed at 9–10 inches has the biggest effect on higher-ordered nodal roots, which are those that grow the largest number of lateral roots, spreading out and away from the plant, and are associated with the uptake of water from deeper parts of the soil profile [
35]. Roots extending further down into the soil profile take advantage of nutrients and deep sub-soil moisture, giving the plant a better chance to manage heat and drought stress that can commonly occur in the late summer, which was particularly noteworthy in the 2012 trials.
3.7. Trials #18–19: Field Trials on Winter Wheat and Lentils in Australia
These two trials, conducted in the Horsham area of Victoria, Australia, where conditions for winter crops can be challenging due to drought, cold and frost events, compared GS fertilizer programs alone and including the NOM-based biostimulant. During both the wheat and lentil trials, severe frost events occurred during critical growth periods of the crops. In addition, there were several periods without any rainfall for the wheat trial, leading to drought conditions that compounded the cold stress. The impact of these stressors is evident from the low harvested yield of the wheat plants only receiving the GS fertilizer, 1052 kg/ha (
Table 2). The plants receiving the GS plus the NOM-based biostimulant had a yield of 1411 kg/ha, a 34% increase over the GS-only treatment, which was statistically significant at the 99% confidence interval. In addition, the sap Brix measurements assessed throughout the season showed consistently elevated Brix concentrations for the plants receiving the biostimulant, by 19–53% from 1–52 days after treatment, all of which were statistically significant at the 99% confidence interval. Increased sap Brix levels are indicative of stronger, more sturdy plants that are better able to resist frost and other abiotic stressors.
Increases in sap Brix and yield were also observed for the lentil trial, where rainfall during the season was sufficient but frost events occurred in the months leading up to harvest. Yield was increased by 64% for the lentils receiving the GS plus biostimulant treatment over the GS only (2025 vs. 1233 kg/ha,
Table 2). One day prior to treatment, the sap Brix was not different for the two treatments, but at 4–31 days after application (DAA), sap Brix was 47–74% higher for the plants receiving the GS plus biostimulant. The increased sap Brix after treatments and the final increased yields for the plants receiving the NOM-based biostimulant were all statistically significant at the 99% confidence interval.
3.8. Trials #20–21: Field Trials on Soybean and Sugarcane in Brazil
These two trials conducted in Brazil, comparing GS fertilizer programs alone and including the NOM-based biostimulant, suffered from water stress associated with high temperatures that occurred mainly in the vegetative phase of development. Stomatal conductance, which is related to the greater capacity of plants to fix CO
2, enhance photosynthetic efficiency, and utilize water more resourcefully, was measured at V4 and R3 and showed statistically significant (at the 95% confidence interval) increases of 7% and 31%, respectively, at these two growth stages for plants that received the NOM-based biostimulant over the GS fertilizer program alone (
Table 3). The increase was the largest at R3, where measurements were taken shortly after the final foliar biostimulant application. Measurements for antioxidative enzymes (superoxide dismutase, catalase, and peroxidase) were also higher for plants receiving the biostimulant, by 39–92%. These enzymes are important scavengers of reactive oxygen species that can flourish and cause severe damage during abiotic stress events. Plants receiving the biostimulant applications were numerically taller and had more pods on both the main stem and lateral branches, leading to a statistically significant (at the 95% confidence interval) increase in yield of 18% (4943 vs. 5847 kg/ha for the GS only and GS plus biostimulant treatments, respectively).
Similar results were obtained during the sugarcane trial, where stomatal conductance was increased by 13% and 66% at 35 and 97 days after application (DAA), respectively, and enzymatic activity was higher by 2–16% at 35 DAA for the plants receiving the biostimulant over the GS only treatment (
Table 3), all of which were statistically significant at the 95% confidence interval. The sugarcane plants receiving the biostimulant applications were taller at harvest, had larger stem diameters, and had more canes per linear meter than the plants receiving only the GS, which led to a statistically significant increase in yield (by 30% for 72,700 vs. 94,583 kg/ha for GS vs. GS plus biostimulant treatments, respectively).
4. Discussion
Here, the results from 21 trials on high-value crops (tomatoes, peppers, strawberries, avocados, sugarcane) and corn, soybean, lentils, and wheat (that underwent a simulated or natural abiotic stress) highlight that stress mitigation due to the addition of a NOM-based biostimulant is repeatable and reproducible, as plant health improvements and increased yields were consistently observed. Whole plant, root, and shoot weights in the tomato trials were larger and harvested yields in the pepper, strawberry, avocado, sugarcane, corn, soybean, and wheat trials were higher (many with statistically significant increases) when the stressed plants were treated with the NOM-based biostimulant. These results are consistent with other studies using a variety of other types of biostimulants (seaweed extracts, protein hydrolysates, and combinations of polysaccharides, amino acids, humic acids, and vitamins) on tomatoes, peppers, grapes, strawberries, and corn, showing increased biomass and yields, as well as improved quality and recovery from abiotic stress events [
36,
37,
38,
39,
40,
41,
42,
43,
44]. Biostimulants play several roles within plants and have numerous modes of action, but many studies have found that the greatest advantages of biostimulant usage occur when plants undergo stress during critical growth stages [
45].
One key role that biostimulants potentially have in abiotic stress mitigation is the increase in sap Brix of plants. As shown here in the winter wheat and lentils field trials in Australia, where conditions were particularly cold and frost events occurred during the season, plants treated with the NOM-based biostimulant had significantly higher sap Brix than untreated plants, and the increased Brix levels were maintained for seven weeks. Increased Brix levels, which are an indicator of crop quality, have been linked to enhanced photosynthesis and increased root exudates, driving stress mitigation and microbial populations within the soil [
46,
47,
48]. These higher sugar levels observed in the plants receiving the NOM-based biostimulant helped the plant thrive under stressful conditions.
An additional function biostimulants are thought to play during abiotic stress mitigation is related to reactive oxygen species (ROS). While ROS are generated during normal plant metabolism, their production can be increased exponentially under stressful conditions [
49,
50]. Oxidative damage caused by ROS can induce tissue damage, inactivation of certain enzymes, protein degradation and disruption to synthesis, and, ultimately, cellular death, and plants utilize their defense mechanisms to help quench the ROS (both free radicals and non-radicals) that are generated [
51,
52]. A study on water-stressed tomatoes using an enzymatically hydrolyzed animal protein-based biostimulant showed positive effects on antioxidant protection and hormonal balance in treated plants compared to controls [
53]. Another study on salt-stressed tomatoes observed mitigation of decreased root and shoot lengths and weights as well as antioxidant enzyme activity on plants treated with microalgal exopolysaccharides [
54]. A study on water-stressed corn using seaweed extracts led to enhanced plant health and yield responses, which were attributed to a reduced impairment of the plants’ photosystem due to greater protection against ROS [
55]. The use of biostimulants can assist with the activation of the plants’ natural defense system, as well as both ROS quenching and prevention of further ROS generation [
13,
14,
56]. The Brazilian soybean and sugarcane trials discussed here that experienced water stress due to high temperatures highlight the enhanced enzymatic activity of superoxide dismutase, catalase, and peroxidase for plants receiving the NOM-based biostimulant alongside the grower standard program, which also showed statistically significant yield increases. Mitigation appears to be the most effective when the biostimulant application is applied prior to or at the same time as the stress, indicating more of a preventative measure than curative.
Another route to abiotic stress mitigation is increased nutrient use efficiency (NUE), as plants that take up more nutrients are overall stronger and more productive [
11]. In one study, microbial inoculants used in combination with reduced rates of inorganic fertilizers produced tomato plants that had growth and nutrient uptake statistically equivalent to those using the full fertilizer rate without the biostimulants [
57]. Another study on tomatoes found that fruit quality and yield was maintained even when the NPK fertilizer rate was reduced but used alongside a biostimulant [
58]. Using a plant growth promoting bacteria treatment on peppers under salt stress, plant biomass was increased over untreated controls, and stomatal conductance was maintained for treated plants under high stress [
59]. Alongside improved yields and antioxidant enzymatic activity, the water-stressed soybean and sugarcane trials described here also showed increased stomatal conductance. Healthier shoots that are greener and with larger leaves can intercept more sunlight and drive more efficient photosynthesis via enhanced functionality of the plants’ stomatal system [
60,
61,
62,
63]. Greater leaf areas in combination with enhanced stomatal function help increase the size and architecture of the root system, facilitating greater uptake of water and nutrients. Physiology drives phenology, and the results from the Nebraska corn trials, Brazilian soybean and sugarcane trials, and Australian wheat and lentil trials shown here are direct evidence for the benefits of applying biostimulants alongside nutrition, allowing for more of the applied nutrients to be utilized by the plant with less waste. With growing threats of anthropogenic climate change driven partly by agricultural greenhouse gas emissions, such as nitrous oxide (which has a warming potential 298 times that of carbon dioxide), responsible nitrogen use with less waste and leaching is a major global problem [
64] that could be partially solved by incorporation of biostimulants.
Biostimulants have the potential to transform agriculture, but with new terminologies and technologies also comes regulatory challenges [
13,
65,
66]. For instance, the broad concept of a plant nutrient is loosely defined and arguably does not encompass all necessary nutrients. Recently, a new definition has been proposed that more accurately represents nutrients that are essential for plants and beneficial for their quality and productivity [
67]. Also, the 2018 Farm Bill recognized the need to study, develop, and provide a regulatory framework for the use of biostimulants within the USA. While no biostimulant will have a 100% success rate, and even biostimulants extracted from the same original source material can perform differently under the same conditions [
38], the ease of their inclusion into fertilizer and/or crop protection treatments does not require new behavior from growers, which can facilitate widespread adoption. Continuing additional scientific trials covering larger acres will allow for a better understanding of the functions biostimulants play in maximizing crop productivity, along with gaining benefits such as less fertilizer waste and downstream pollution.