Abstract
Goosegrass (Eleusine indica (L.) Gaertn.) is one of the most vicious weeds worldwide, competing with crops and greatly reducing their yields. Glyphosate, a non-selective, broad spectrum, post-emergence herbicide has inevitably induced severe resistance in many weeds owing to its intensive use. Additionally, control strategies rely on the clarity of resistance mechanisms. In this study, we aimed to investigate the resistance levels and potential resistance mechanisms of two goosegrass populations collected from orchards. Results showed that the resistance indexes of LL and SS populations were 3.8 and 1.9, respectively. A single nucleotide change led to a Pro106Leu (P106L) mutation in the LL population and the SS population had a Pro106Ser (P106S) amino acid substitution. The EPSPS expression in both populations was 2.6 times that of the wild-type population. However, the relative copy number and EPSPS protein content in the LL population were higher than those of the SS population, as indicated by immunoblot analysis and enzyme-linked immunosorbent assays. Overall, we confirmed EPSPS amplification with a P106L mutation, resulting in overproduction of this mutated EPSPS protein, which conferred moderate glyphosate resistance. This study details a case of simultaneous evolution of mutation and amplification in EPSPS of glyphosate resistance weeds.
1. Introduction
Glyphosate (N-phosphonomethyl-glycine) is a systemic and broad spectrum herbicide that has been used to effectively control weeds in tea gardens, orchards, and non-crop areas [1]. Glyphosate inhibits the target enzyme, 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS; E.C. 2.5.1.19), complexly affecting the shikimate pathway and disturbing the biosynthesis of phenylalanine, tyrosine, tryptophan, and other secondary aromatic products [2]. Since its commercial introduction in the early 1970s and the promotion of glyphosate-resistant (GR) crops, reliance on glyphosate has become increasingly critical, and 56 weed species, including goosegrass, have evolved resistance [3]. Target-site (TS) and non-target-site (NTS) resistance are reported as the two main mechanisms of glyphosate resistance in weeds [4,5,6]. Single, double, and triple amino acid substitutions in the conserved region of EPSPS (Pro 106, Thr 102, and Gly 101) have been reported to be one of the target site mechanisms [7,8,9]. In addition to EPSPS gene mutations, amplification of this gene is another target site alteration mechanism, which subsequently leads to overproduction of the EPSPS enzyme and varying resistance levels [6]. Since the discovery of EPSPS gene amplification in Palmer amaranth (Amaranthus palmeri S. Watson) [10], several other evolved GR weed species have been reported to possess this resistance mechanism [4,11,12,13]. Some GR weeds with moderate EPSPS expression levels, but not corresponding copies, sometimes show insufficient resistance levels, probably caused by differences in transcript regulation to control gene expression [14]. Non-target site resistance mechanisms are caused by the reduction in glyphosate translocation or absorption, enhanced herbicide metabolism, and the sequestration of herbicides, indicating greater complexity in determining the comprehensive mechanism [15,16,17]. NTSR mechanisms to glyphosate have typically been reported in several different glyphosate-resistance biotypes of Aster squamatus, Conyza bonariensis, and Bidens pilosa L. [18,19,20].
Goosegrass (Eleusine indica (L.) Gaertn.), one of the most problematic weeds worldwide, is mainly distributed in temperate and tropical regions, including Brazil, the United States, Europe, Australia, and China [21,22]. Since the first identification of a resistance-endowing EPSPS point mutation, P106S, several other resistance-endowing single amino acid substitutions at P106 (P106T, P106A, P106S, and P106L) have been reported in GR goosegrass [7,23,24]. In addition, a double mutation identified in EPSPS (Thr102Ile + Pro106Ser) was first found in a goosegrass population in Malaysia [25]. A mechanism of EPSPS amplification was also found in some goosegrass populations [26,27].
In China, goosegrass populations distributed across many provinces have evolved resistance to glyphosate; however, few studies have been conducted to investigate the resistance mechanisms [26,27,28,29]. In this study, two suspected GR goosegrass populations were collected from banana plantations in Guangdong Province which were not well controlled under the recommended dose. The aim of the present study was to clear the resistance level of these two populations, and investigate the potential resistance mechanisms to glyphosate. A new target site resistance mechanism was found, wherein amplification and EPSPS mutation (P106L) coexisted in the same individuals and conferred moderate glyphosate resistance.
2. Materials and Methods
2.1. Plant Materials and Dose–Response Test
Seeds (at least 100 individuals) of two suspected GR goosegrass populations, LL (113°52′23.7″ E, 23°11′15.6″ N) and SS (113°47′50.5″ E, 23°9′31.4″ N), were collected from banana plantations in Guangzhou, Guangdong Province. Additionally, seeds of a glyphosate-susceptible (GS) population WT (113°48′14.3″ E, 23°9′25.8″ N) were also collected in a field without herbicide application history near the GR populations. Seeds of the three populations were soaked in gibberellin (1%) for 24 h to break dormancy, then cultured in plastic pots (9 × 9 cm). All the pots were kept in a greenhouse, watered as needed, and the temperature was controlled at 30 ± 2 °C in the day with a 14 h photoperiod, and at 27 ± 2 °C overnight [30]. Different doses (0, 112.5, 225, 450, 900, and 1800 g ae ha−1 for the GS population; 0, 225, 450, 900, 1800, and 3600 g ae ha−1 for the suspected GR populations) of glyphosate with a recommended rate of 900 g ae ha−1 were designed and applied to individuals with 5- to 7-leaf stage (four replicates for each treatment). Glyphosate (Roundup, 300 g ae L−1 glyphosate, 410 g ae L−1 isopropylamine salt of glyphosate, Bayer Crop Science, 800 North Lindbergh Blvd, Saint Louis, MO 63167, USA) was applied using a moving cabinet sprayer (TeeJet® XR8002) and a compressed air cabinet sprayer (3WPSH-500D) [31]. The fresh weights of the shoots in each pot (8 plants) were measured at 14 days after treatment (DAT). The experiment of whole plant assay was repeated twice in a completely randomized design.
2.2. Shikimate Accumulation Experiment
Plants were cultivated to the 4- to 6-leaf stage (8 plants per pot) and glyphosate was applied in a dose of 1800 g ae ha−1. Treated leaf tissues were collected at 2, 4, 6, 8, 10, and 12 DAT and stored at −80 °C until used for shikimate detection. Shikimate accumulation was determined according to the method previously described [29]. In brief, leaf samples (0.5 g) were finely ground in liquid nitrogen and added to 1.0 mL of 0.25 mol L−1 HCl and centrifuged at 12,000 rpm for 30 min. The supernatant (0.2 mL) was mixed with 2 mL periodic acid (1%). After 3 h, the mixtures were supplemented with 2 mL of 1 mol L−1 NaOH and 1.2 mL of 0.1 mol L−1 glycine. Subsequently, 0.2 mL of each mixture were added to 96-well plate to analyze the shikimate accumulation. Sample absorbance was measured at 380 nm using a Tecan Infinite 200 Pro plate reader (Tecan Group Ltd., Männedorf, Switzerland). This experiment was repeated twice.
2.3. EPSPS Sequencing
The leaf tissues of twenty plants without glyphosate treatment from each population were sampled and stored at −80 °C when the plants reached the 5- to 7-leaf stage. Genomic DNA was extracted using a plant DNA extraction kit (Tiangen Biotech Beijing Co., Ltd., Beijing, China). Primers were used to amplify a fragment of 310 bp in length which included the conserved region of EPSPS (Table S1). PCR was performed as described previously, with the annealing temperature set at 60 °C [27]. The PCR product was then loaded onto a 1% agarose gel and sequenced using Sanger sequencing by Sangon Biotech (Sangon Biotech Shanghai Co. Ltd., Beijing, China). The EPSPS sequences of all populations were aligned using DNAMAN software (Lynnon BioSoft, San Ramon, CA, USA) to detect mutations in the target gene.
2.4. EPSPS Gene Expression and Copy Number Detection
The leaf tissues of twenty individuals were randomly collected from each population without glyphosate treatment, and RNA was extracted using a plant RNA extraction kit (Tiangen Biotech Beijing Co., Ltd., Beijing, China). First-strand complementary DNA (cDNA) synthesis was performed using the EasyScript All-in-One First-Strand cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China) with 1000 ng RNA in a total volume of 20 μL, according to the manufacturer’s instructions. Primer pairs described in a previous study were used to detect the relative expression and copy number of the EPSPS gene and acetolactate synthase (ALS) was used as the reference gene which has already been screened in our previous study (Table S1) [30]. The relative expression of EPSPS was analyzed using the 2−∆∆Ct method. The relative copy number was calculated using the 2∆Ct method [10,32,33]. This experiment was repeated twice.
2.5. Protein Extraction and Immunoblot Analysis
Protein extraction and immunoblotting analysis were performed as described previously [13,28,34]. In brief, leaf tissues (0.2 g) were collected for each population and were cultured under the conditions described above. Protein extraction for the leaf tissue was performed using the MinuteTM Total Protein Extraction Kit for Plant Tissues (Invent Biotechnologies, Inc., Eden Prairie, MN, USA) following the manufacturer’s protocol. The protein concentration was determined, followed by separation using 10% SDS-PAGE and then transferred to nitrocellulose membranes (0.45 μm). Additionally, the proteins were probed with the primary anti-EPSPS antibody (Shanghai Youlong Biotech Co., Ltd., Shanghai, China) at a dilution of 1:5000 overnight at 4 °C. The membranes were then incubated with the rabbit secondary antibodies diluted 1:5000 at room temperature for 1 h (ABclonal Biotech Co., Ltd., Cambridge, MA, USA). Finally, immunoreactive bands were visualized using ECL (enhanced chemiluminescence).
2.6. Detection of EPSPS Protein Content Using an Enzyme-Linked Immunosorbent Assay
The leaf tissues were collected for each population and thoroughly ground in liquid nitrogen. Ground leaves (0.1 g) were added to 1.0 mL sample extract and centrifuged at 12,000 rpm for 10 min. EPSPS content was detected using an enzyme-linked immunosorbent assay (ELISA) kit (Shanghai Youlong Biotech Co., Ltd., China) according to the manufacturer’s protocol. Briefly, 0.1 mL supernatants were protected from light for 45 min in an antibody-coated plate. After washing the plate, 0.1 mL monoclonal and multi-antibodies as reagents were added to the ELISA plate to react for 30 min under strict exclusion of light. Washing the plate again, 0.1 mL chromogenic agent was added to the ELISA plate to react for 15 min under strict exclusion of light. Sample absorbance was measured using a Tecan Infinite 200 Pro plate reader (Tecan Group Ltd., Männedorf, Switzerland) at 450 nm after adding the stop solution. A standard curve was constructed based on the OD values of the EPSPS standards. The EPSPS concentration was calculated by extrapolating the OD value of the test sample to the standard curve. This experiment was repeated twice.
2.7. Statistical Analysis
Dose–response data were subjected to nonlinear regression analysis using a log-logistic equation: Y = y0 + a/[1 + (X/X0)b]. In this model, y is the inhibition rate, X is the glyphosate dose (g ae ha−1), b is the curve slope around X0, y0 is the lower limit, a is the difference between the upper and lower limits, and X0 is the herbicide dose required for 50% plant growth reduction (GR50) [35]. The GR50 was used to calculate the resistance index (RI) as the ratio of the GR50 of the resistant population to that of the WT population.
EPSPS expression, shikimate accumulation, relative copy numbers, and EPSPS protein content data were subjected to ANOVA and analyzed using a non-parametric Kruskal–Wallis H test with SPSS (version 13.0; SPSS, Chicago, IL, USA).
3. Results
3.1. Dose–Response Assays
The biomass of the three goosegrass populations decreased with increasing glyphosate doses (Figure 1). At 14 DAT, all plants from the WT population were in a state of death with a dose of 900 g ae ha−1, whereas all plants of the GR populations (LL and SS) were killed by 3600 g ae ha−1 glyphosate (Figure 1). The GR50 values, indicating the herbicide doses required for 50% growth reduction in the LL and SS populations, were 878.3 and 442.6 g ae ha−1, respectively (Table 1). Compared with the WT population (GR50 = 233.1 g ae ha−1), the RIs of the LL and SS populations were 3.8 and 1.9, suggesting a moderate and low level of resistance, respectively.
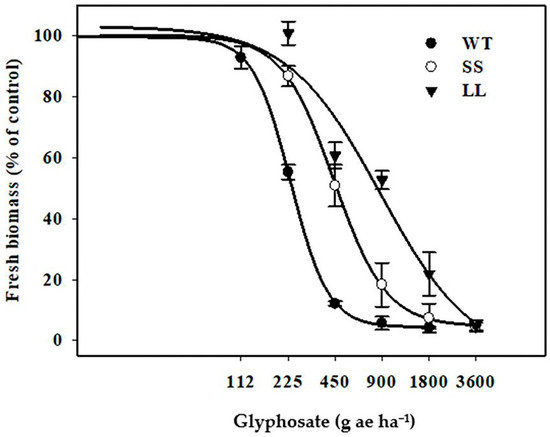
Figure 1.
Response of three populations to different doses of glyphosate at 14 days after treatment (DAT). The dose–response curve of these three populations calculated by nonlinear regression analysis, vertical bars represent the standard errors of the means (SEM).

Table 1.
Parameter estimates (SE) by nonlinear regression for the dose–response assay using fresh shoot weights, and four samples were designed and selected for each treatment.
3.2. Shikimate Accumulation
After spraying the herbicide at 1800 g ae ha−1, slight shikimate accumulation was observed in the LL and SS populations, whereas the WT population showed significant shikimate accumulation, reaching 3381.0 μg g−1 at 12 DAT, which was 39.3 times higher than that at the first detection (2 DAT) with fatal damage to the plants (Table 2). The LL and SS populations showed similar changes in shikimate accumulation, with moderate increases after glyphosate treatment. However, the final shikimate accumulation remained at approximately 200–300 μg g−1 in the LL and SS populations. These results suggested that shikimate accumulation provided evidence of evolved resistance in both of the two populations.

Table 2.
Shikimate accumulation in the leaf tissues of three populations at different days after glyphosate treatment under a dose of 1800 g ae ha−1. Letters indicates differences among values according to Student’s t-test at p < 0.05. Data are means ± standard error of four replicates samples.
3.3. EPSPS Gene Sequence Analysis
To determine the existence of a target site resistance mechanism in plants, we examined a conserved region of the EPSPS gene. Designed primer pairs were used to obtain an EPSPS DNA fragment of approximately 310 bp covering the Gly101, Thr102, and Pro106 positions (Figure 2). The PCR products of 20 individuals were sequenced for each population. After sequence alignment, all LL and SS populations exhibited a codon change at position 106, resulting in an amino acid change. In the LL population, a single nucleotide polymorphism (CCA to CTA) resulted in a proline to leucine substitution (P106L), and another nucleotide substitution from CCA to TCA at codon 106 in all SS individuals resulted in an amino acid substitution from proline to serine (P106S).
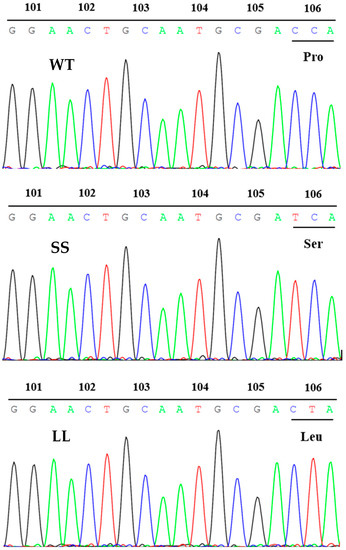
Figure 2.
Partial nucleotide sequence results of EPSPS gene for the WT, SS, and LL populations, respectively. A homozygous mutation of Pro106Ser was detected in the SS population and a homozygous mutation of Pro106Leu was identified in the LL population by sequencing chromatograms.
3.4. EPSPS Expression and Relative Copy Number
EPSPS gene expression and copy number were evaluated in 20 random individuals without glyphosate treatment from each population after nucleic acid extraction (Figure 3). The reference gene, ALS, was used to normalize and guarantee accuracy. EPSPS expression in the LL and SS populations was 2.6 times higher than that in the WT population. The average copy number of EPSPS was 3.0 and 1.3 in the LL and WT individuals, respectively, according to the relative expression of EPSPS. Although the expression of EPSPS in the SS population was the same as that in the LL population, the average copy number of EPSPS in this population was only 1.6, showing no significant difference to the WT population.
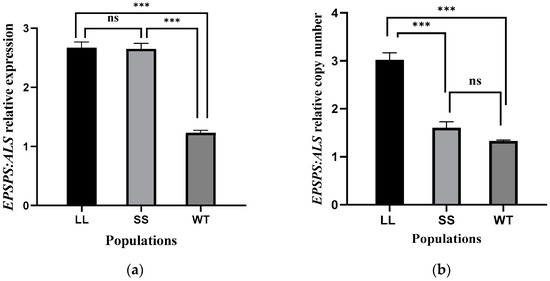
Figure 3.
EPSPS gene expression (a) and copy number (b) relative to acetolactate synthase (ALS) in the leaf tissue of three goosegrass populations (LL, SS, WT) without glyphosate treatment. Twenty individuals were selected from each population and vertical bars show the SEM for the replicates. The non-parametric Kruskal-Wallis H test (p < 0.05) was selected to analyze the data. Asterisks indicate significant differences based on data of expression or copy number among goosegrass populations, and ns indicates no significant differences.
3.5. EPSPS Protein Abundance by Immunoblot Analysis and Enzyme-Linked Immunosorbent Assay
Anti-EPSPS immunoblotting was used to detect EPSPS protein content in the three goosegrass populations. EPSPS protein can be probed with signals from specific binding with antibodies. The results showed that signals from the LL population with a higher copy number and expression were stronger than those from the SS population with a lower copy number, and the signals from the sensitive population were weaker than those from the GR populations (Figure S1). In addition, an enzyme-linked immunosorbent assay was used for the quantitative detection of EPSPS protein. Unlike anti-EPSPS immunoblotting, this method allows quantitative analysis of EPSPS proteins by measuring the OD450 nm of the EPSPS protein standard to obtain a regression equation: y = 10.441x − 2.1484, R2 = 0.9913 > 0.99, where x is the OD450 nm value and y is the EPSPS protein content. Accordingly, the average EPSPS protein content in LL and WT populations was 1.7 and 0.6 μg g−1, respectively (Figure 4). The EPSPS protein content in the SS population was only 0.4 μg g−1, which was significantly lower than that in the LL population and inconsistent with the gene expression results. Compared to the glyphosate sensitive population (WT), the protein content in the SS population had no obvious difference. These results indicate that other biochemical reactions, such as translation, might affect EPSPS protein content.
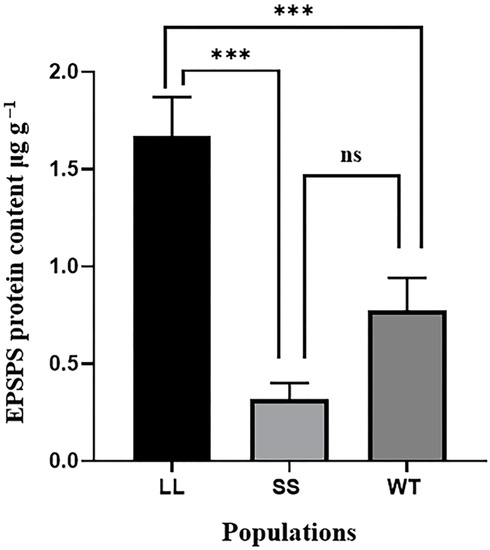
Figure 4.
EPSPS protein content in leaf tissues of three goosegrass populations using enzyme-linked immunosorbent assay. Ten individuals were selected from each population. Vertical bars show the standard error of the mean for the replicates. Asterisks indicate significant differences in protein content when analyzed using the non-parametric Kruskal–Wallis H test (p < 0.05) and ns indicates no significant differences.
4. Discussion
Glyphosate inhibits EPSPS activity in the shikimate pathway, an important biological metabolic pathway that links carbohydrate metabolism and aromatic compound biosynthesis [36]. This leads to reduced feedback inhibition in this pathway, resulting in high levels of shikimate accumulation in tissues [2]. In this study, shikimate accumulation in the GS population was more than 11.3 times those of the LL and SS populations, which had RI values of 3.8 and 1.9, respectively, indicating the evolution of resistance. Generally, the GR populations accumulated lower levels of shikimate compared to the GS populations, which changed with time under the effect of glyphosate [13,37,38].
As often observed in studies on GR goosegrass, glyphosate resistance in this study was attributed to target site resistance [25,27,39,40]. EPSPS gene sequencing demonstrated that the LL and SS populations possessed a mutation at the Pro106 codon, resulting in amino acid substitutions at this genomic EPSPS position. Mutations at the Pro106 position have also been found in other weeds, such as Lolium rigidum, Lolium perenne ssp. multiflorum, and Amaranthus tuberculatus [41,42,43,44]. Substitutions of proline at position 106 cause a slight structural change in the EPSPS active site, which has different effects on catalytic activity and glyphosate resistance [45,46]. Many studies have demonstrated that a single mutation at position 106 results in decreased glyphosate resistance levels (approximately 2-fold) than those obtained with double (more than 180-fold) or triple mutations (84-fold) because of changes in the EPSPS enzyme [25,39,40,47]. In our previous study, individuals with the P106L mutation showed 2.1–3.0-fold resistance relative to that of the sensitive biotype, and showed GR50 values of approximately 700 g ae ha−1 [23].
Gene amplifications or duplications of the EPSPS gene have been associated with the glyphosate resistance mechanism since the first report on Amaranthus palmeri in 2010 [10]. Others studies have reported the increase in EPSPS gene expression, and overproduction of the EPSPS protein might correspond positively to the level of glyphosate resistance in plants of other weed species (Lolium perenne ssp. multiflorum, Amaranthus palmeri, and Kochia scoparia), with EPSPS gene amplification ranging from 2- to 100-fold [48,49,50,51]. Gene amplification can produce extra EPSPS enzyme products that are inhibited by glyphosate, allowing increased synthesis of amino acid. In this study, the average relative expression of EPSPS in individuals was similar between the LL and SS populations, which were collected from the same area. However, the copy numbers and EPSPS protein content in the LL population were both higher than those in the SS population, and the GR50 of LL was 2.0 times than that of the SS biotype. The slight increase observed in EPSPS gene expression in the SS population did not affect its resistance level. This phenomenon also occurred in other GR goosegrass populations; the expression and copy numbers of EPSPS were reported to be 5.7 and 15.4 times higher, respectively, than those in the susceptible population; however, this resulted in a relatively low resistance level, with a resistance index of 3.4 [26]. In some species of Lolium perenne ssp. multiflorum and Amaranthus palmeri, > 10 and 30–50 EPSPS copies enabled the conferral of different levels of glyphosate resistance [10,45]. Furthermore, the number of EPSPS copies is not always positively correlated with expression and transcript abundance [37,52]. EPSPS overexpression is influenced by gene duplication as well as by efficient transcriptional regulation of the promoter or increased transcription factor activity [53]. Zhang et al. reported differences in EPSPS promoter activity among sensitive and resistant goosegrass, attributed to transcriptional regulation [54].
In recent years, some studies have revealed that resistance in GR weeds is conferred by more than one resistance mechanism. In this study, we found that EPSPS P106L mutation and overexpression coexisted within the same individual. Reduced glyphosate binding but enhanced PEP binding depends on the three-dimensional conformation of the enzyme, as well as the availability of excess EPSPS protein to combine with glyphosate to induce higher resistance levels. Target site mechanisms involving P106S mutation and overexpression were first reported in goosegrass [55]. Subsequently, we have found that the EPSPS amplification mechanism also existed in mutated (Pro106Ala) GR goosegrass [28]. A deeper understanding of resistance alleles and amplification mechanisms within these plant populations is thus critical for predicting weed occurrence and combating weeds efficiently.
5. Conclusions
In conclusion, two goosegrass populations (LL and SS) from banana plantations in Guangdong Province were confirmed to have evolved resistance to glyphosate in our study. A single nucleotide change at position 302 (C to T) and 301 (C to T) led to a P106L and P106S amino acid substitution in LL and SS populations, respectively. In addition, we found that EPSPS amplification and mutation (P106L) coexisted in the same individuals, resulting in overproduction of the mutated EPSPS protein, which conferred moderate glyphosate resistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13030699/s1, Figure S1: Immunoblot analysis of EPSPS protein in leaf tissue of plants from the three goosegrass populations. The primary anti-EPSPS antibody at a dilution of 1:5000. The molecular weight of EPSPS protein in goosegrass was 50 kD.; Table S1: The designed primers for EPSPS gene clone, qPCR, and the reference genes for E. indica.
Author Contributions
Conceptualization, J.C.; methodology, X.L., J.C. and Z.L.; investigation, J.C. and Z.L.; resources, J.C., X.L., H.C. and H.Y.; supervision, J.C., X.L. and H.C.; data curation, J.C., Z.L.; writing—original draft, J.C., Z.L.; writing—review and editing, J.C., X.L.; funding acquisition, X.L., J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Beijing Natural Science Foundation (6222051); the Nanfan special project, CAAS (SWAQ03); and the China Agriculture Research System (CARS-25).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bradshaw, L.D.; Padgette, S.R.; Kimball, S.L.; Wells, B.H. Perspectives on glyphosate resistance. Weed Technol. 1997, 11, 189–198. [Google Scholar] [CrossRef]
- Steinrücken, H.C.; Schulz, A.; Amrhein, N.; Porter, C.A.; Fraley, R.T. Overproduction of 5-enolpyruvylshikimate-3-phosphate synthase in a glyphosate-tolerant Petunia hybrida cell line. Arch. Biochem. Biophys. 1986, 244, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Heap, I. The International Survey of Herbicide Resistant Weeds. Available online: www.weedscience.org (accessed on 30 November 2022).
- Sammons, R.D.; Gaines, T.A. Glyphosate resistance: State of knowledge. Pest. Manag. Sci. 2014, 70, 1367–1377. [Google Scholar] [CrossRef]
- Heap, I.; Duke, S.O. Overview of glyphosate-resistant weeds worldwide. Pest. Manag. Sci. 2018, 74, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Gaines, T.A.; Patterson, E.L.; Neve, P. Molecular mechanisms of adaptive evolution revealed by global selection for glyphosate resistance. New Phytol. 2019, 223, 1770–1775. [Google Scholar] [CrossRef]
- Baerson, S.R.; Rodriguez, D.J.; Tran, M.; Feng, Y.; Biest, N.A.; Dill, G.M. Glyphosate-resistant goosegrass. Identification of a mutation in the target enzyme 5-enolpyruvylshikimate-3-phosphate synthase. Plant Physiol. 2002, 129, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, Q.; Han, H.; Nyporko, A.; Kulynych, T.; Yu, Q.; Powles, S. Glyphosate Resistance in Tridax procumbens via a Novel EPSPS Thr-102-Ser Substitution. J. Agric. Food Chem. 2018, 66, 7880–7888. [Google Scholar] [CrossRef]
- Perotti, V.E.; Larran, A.S.; Palmieri, V.E.; Martinatto, A.K.; Alvarez, C.E.; Tuesca, D.; Permingeat, H.R. A novel triple amino acid substitution in the EPSPS found in a high-level glyphosate resistant Amaranthus hybridus population from Argentina. Pest Manag. Sci. 2019, 75, 1242–1251. [Google Scholar] [CrossRef]
- Gaines, T.A.; Zhang, W.; Wang, D.; Bukun, B.; Chisholm, S.T.; Shaner, D.L.; Nissen, S.J.; Patzoldt, W.L.; Tranel, P.J.; Culpepper, A.S. Gene amplification confers glyphosate resistance in Amaranthus palmeri. Proc. Natl. Acad. Sci. USA 2010, 107, 1029–1034. [Google Scholar] [CrossRef]
- Malone, J.M.; Morran, S.; Shirley, N.; Boutsalis, P.; Preston, C. EPSPS gene amplification in glyphosate-resistant Bromus diandrus. Pest Manag. Sci. 2015, 72, 81–88. [Google Scholar] [CrossRef]
- Ngo, T.D.; Malone, J.M.; Boutsalis, P.; Gill, G.; Preston, C. EPSPS gene amplification conferring resistance to glyphosate in windmill grass (Chloris truncata) in Australia. Pest Manag. Sci. 2018, 74, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Wiersma, A.T.; Gaines, T.A.; Preston, C.; Hamilton, J.P.; Giacomini, D.; Buell, C.R.; Leach, J.E.; Westra, P. Gene amplification of 5-enol-pyruvylshikimate-3-phosphate synthase in glyphosate-resistant Kochia scoparia. Planta 2014, 241, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, G.; Huang, H.; Wei, S.; Zhou, X.; Chen, J.; Chen, J.; Zhang, C. Isolation and functional analysis of Convolvulus arvensis EPSPS promoter. Plant Mol. Biol. Rep. 2015, 33, 1650–1658. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M.; Balbi, M.C.; Distéfano, A.J.; Fernández, L.; Hopp, E.; Yu, Q.; Powles, S.B. Glyphosate resistance in perennial Sorghum halepense (Johnsongrass), endowed by reduced glyphosate translocation and leaf uptake. Pest Manag. Sci. 2012, 68, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. Glyphosate degradation in glyphosate-resistant and-susceptible crops and weeds. J. Agric. Food Chem. 2010, 59, 5835–5841. [Google Scholar] [CrossRef]
- Brunharo, C.A.; Patterson, E.L.; Carrijo, D.R.; de Melo, M.S.; Nicolai, M.; Gaines, T.A.; Nissen, S.J.; Christoffoleti, P.J. Confirmation and mechanism of glyphosate resistance in tall windmill grass (Chloris elata) from Brazil. Pest Manag. Sci. 2016, 72, 1758–1764. [Google Scholar] [CrossRef]
- Alcántara-de la Cruz, R.; Fernández-Moreno, P.T.; Ozuna, C.V.; Rojano-Delgado, A.M.; Cruz-Hipolito, H.E.; Domínguez-Valenzuela, J.A.; Barro, F.; De Prado, R. Target and non-target site mechanisms developed by glyphosate-resistant hairy beggarticks (Bidens pilosa L.) populations from Mexico. Front. Plant Sci. 2016, 7, 1492. [Google Scholar] [CrossRef]
- Palma-Bautista, C.; Vázquez-García, J.G.; Domínguez-Valenzuela, J.A.; Ferreira Mendes, K.; Alcantara De la Cruz, R.; Torra, J.; De Prado, R. Non-target-site resistance mechanisms endow multiple herbicide resistance to five mechanisms of action in Conyza bonariensis. J. Agric. Food Chem. 2021, 69, 14792–14801. [Google Scholar] [CrossRef]
- Domínguez-Valenzuela, J.A.; Alcántara-de la Cruz, R.; Palma-Bautista, C.; Vázquez-García, J.G.; Cruz-Hipolito, H.E.; De Prado, R. Non-target site mechanisms endow resistance to glyphosate in Saltmarsh Aster (Aster squamatus). Plants (Basel) 2021, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds: Distribution and Biology; University Press of Hawaii: Honolulu, HI, USA, 1977; pp. 47–53. [Google Scholar]
- Takano, H.K.; Mendes, R.R.; Scoz, L.B.; Ovejero, R.F.L.; Constantin, J.; Gaines, T.A.; Westra, P.; Dayan, F.E.; Oliveira, R.S. Proline-106 EPSPS mutation imparting glyphosate resistance in goosegrass (Eleusine indica) emerges in South America. Weed Sci. 2018, 67, 48–56. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, C.; Huang, H.; Wei, S.; Huang, Z.; Wang, H.; Zhao, D.; Zhang, C. Characterization of Eleusine indica with gene mutation or amplification in EPSPS to glyphosate. Pest Biochem. Physiol. 2017, 143, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Franci, J.; Lam, K.W.; Chuah, T.S.; San Cha, T. Genetic diversity and in silico evidence of target-site mutation in the EPSPS gene in endowing glyphosate resistance in Eleusine indica (L.) from Malaysia. Pest Biochem. Physiol. 2020, 165, 104556. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Jalaludin, A.; Han, H.; Chen, M.; Sammons, R.D.; Powles, S.B. Evolution of a double amino acid substitution in the EPSP Synthase in Eleusine indica conferring high level glyphosate resistance. Plant Physiol. 2015, 167, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cui, H.; Ma, X.; Ma, Y.; Li, X. Distribution differences in the EPSPS gene in chromosomes between glyphosate-resistant and glyphosate-susceptible goosegrass (Eleusine indica). Weed Sci. 2020, 68, 33–40. [Google Scholar] [CrossRef]
- Chen, J.; Huang, H.; Zhang, C.; Wei, S.; Huang, Z.; Chen, J.; Wang, X. Mutations and amplification of EPSPS gene confer resistance to glyphosate in goosegrass (Eleusine indica). Planta 2015, 242, 859–868. [Google Scholar] [CrossRef]
- Chen, J.; Huang, H.; Wei, S.; Cui, H.; Li, X.; Zhang, C. Glyphosate resistance in Eleusine indica: EPSPS overexpression and P106A mutation evolved in the same individuals. Pest Biochem. Physiol. 2020, 164, 203–208. [Google Scholar] [CrossRef]
- Chen, J.; Huang, H.; Wei, S.; Zhang, C.; Huang, Z. Characterization of glyphosate-resistant goosegrass (Eleusine indica) populations in China. J. Integr. Agric. 2015, 14, 919–925. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Huang, H.; Wei, S.; Yan, L.; Jiang, C.; Jie, Z.; Zhang, C. Selection of relatively exact reference genes for gene expression studies in goosegrass (Eleusine indica) under herbicide stress. Sci. Rep. 2017, 7, 46494. [Google Scholar] [CrossRef]
- Wang, J.; LI, X.; Li, D.; Han, Y.; Zheng, L.; Yu, H.; Cui, H. Non-target-site and target-site resistance to AHAS inhibitors in American sloughgrass (Beckmannia syzigachne). J. Integr. Agric. 2018, 17, 2714–2723. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huuggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Van Horn, C.R.; Moretti, M.L.; Robertson, R.R.; Segobye, K.; Weller, S.C.; Young, B.G.; Johnson, W.G.; Schulz, B.; Green, A.C.; Jeffery, T. Glyphosate resistance in Ambrosia trifida: Part 1. Novel rapid cell death response to glyphosate. Pest Manag. Sci. 2018, 74, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Seefeldt, S.S.; Fuerst, E.P. Log-logistic analysis of herbicide dose-response relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- Herrmann, K.M.; Weaver, L.M. The shikimate pathway. Annu. Rev. Plant Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef] [PubMed]
- Dinelli, G.; Marotti, I.; Bonetti, A.; Catizone, P.; Urbano, J.; Barnes, J. Physiological and molecular bases of glyphosate resistance in Conyza bonariensis biotypes from Spain. Weed Res. 2008, 48, 257–265. [Google Scholar] [CrossRef]
- Cross, R.B.; McCarty, L.B.; Tharayil, N.; McElroy, J.S.; Chen, S.; McCullough, P.E.; Powell, B.A.; Bridges, W.C. A Pro106 to Ala substitution is associated with resistance to glyphosate in annual bluegrass (Poa annua). Weed Sci. 2015, 63, 613–622. [Google Scholar] [CrossRef]
- Kaundun, S.S.; Zelaya, I.A.; Dale, R.P.; Lycett, A.J.; Carter, P.; Sharples, K.R.; McIndoe, E. Importance of the P106S target-site mutation in conferring resistance to glyphosate in a goosegrass (Eleusine indica) population from the Philippines. Weed Sci. 2008, 56, 637–646. [Google Scholar] [CrossRef]
- Janel, L.H.; Riggins, C.W.; Steckel, L.E.; Tranel, P.J. The EPSPS Pro106Ser substitution solely accounts for glyphosate resistance in a goosegrass (Eleusine indica) population from Tennessee, United States. J. Integr. Agric. 2016, 15, 1304–1312. [Google Scholar] [CrossRef]
- Yu, Q.; Cairns, A.; Powles, S. Glyphosate, paraquat and ACCase multiple herbicide resistance evolved in a Lolium rigidum biotype. Planta 2007, 225, 499–513. [Google Scholar] [CrossRef]
- Liu, M.; Hulting, A.G.; Mallory-Smith, C.A. Characterization of multiple-herbicide-resistant Italian ryegrass (Lolium perenne spp. multiflorum). Pest Manag. Sci. 2014, 70, 1145–1150. [Google Scholar] [CrossRef]
- Patzoldt, W.L.; Tranel, P.J.; Hager, A.G. A waterhemp (Amaranthus tuberculatus) biotype with multiple resistance across three herbicide sites of action. Weed Sci. 2005, 53, 30–36. [Google Scholar] [CrossRef]
- Perez-Jones, A.; Park, K.-W.; Polge, N.; Colquhoun, J.; Mallory-Smith, C.A. Investigating the mechanisms of glyphosate resistance in Lolium multiflorum. Planta 2007, 226, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ng, E.; Lu, J.; Fenwick, T.; Tao, Y.; Bertain, S.; Sandoval, M.; Bermudez, E.; Hou, Z.; Patten, P. Desensitizing plant EPSP synthase to glyphosate: Optimized global sequence context accommodates a glycine-to-alanine change in the active site. J. Biol. Chem. 2019, 294, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Healy-Fried, M.L.; Funke, T.; Priestman, M.A.; Han, H.; Schönbrunn, E. Structural basis of glyphosate tolerance resulting from mutations of Pro101 in Escherichia coli 5-enolpyruvylshikimate-3-phosphate synthase. J. Biol. Chem. 2007, 282, 32949–32955. [Google Scholar] [CrossRef] [PubMed]
- García, M.J.; Palma-Bautista, C.; Rojano-Delgado, A.M.; Bracamonte, E.; Portugal, J.; Alcántara-de la Cruz, R.; De Prado, R. The triple amino acid substitution TAP-IVS in the EPSPS gene confers high glyphosate resistance to the superweed Amaranthus hybridus. Int. J. Mol. Sci. 2019, 20, 2396. [Google Scholar] [CrossRef] [PubMed]
- Koo, D.H.; Sathishraj, R.; Friebe, B.; Gill, B.S. Deciphering the mechanism of glyphosate resistance in Amaranthus palmeri by Cytogenomics. Cytogenet. Genome Res. 2021, 161, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Jha, P. Growth and Reproduction of Glyphosate-resistant and susceptible populations of Kochia scoparia. PLoS ONE 2015, 10, e0142675. [Google Scholar] [CrossRef]
- Ribeiro, D.N.; Pan, Z.; Duke, S.O.; Nandula, V.K.; Baldwin, B.S.; Shaw, D.R.; Dayan, F.E. Involvement of facultative apomixis in inheritance of EPSPS gene amplification in glyphosate-resistant Amaranthus palmeri. Planta 2014, 239, 199–212. [Google Scholar] [CrossRef]
- Salas, R.A.; Scott, R.C.; Dayan, F.E.; Burgos, N.R. EPSPS gene amplification in glyphosate-resistant Italian ryegrass (Lolium perenne ssp. multiflorum) populations from Arkansas (United States). J. Agric. Food. Chem. 2015, 63, 5885–5893. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.D.; Krishnan, M.; Boutsalis, P.; Gill, G.; Preston, C. Target-site mutations conferring resistance to glyphosate in feathertop Rhodes grass (Chloris virgata) populations in Australia. Pest Manag. Sci. 2018, 74, 1094–1100. [Google Scholar] [CrossRef]
- Martinez, E. Multi-protein complexes in eukaryotic gene transcription. Plant Mol. Biol. 2002, 50, 925–947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Feng, L.; Tian, X. Alterations in the 5′untranslated region of the EPSPS gene influence EPSPS overexpression in glyphosate-resistant Eleusine indica. Pest Manag. Sci. 2019, 74, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Gherekhloo, J.; Fernándezmoreno, P.T.; Cruz, R.A.L.; Sánchezgonzález, E.; Cruzhipolito, H.E.; Prado, R.D. Pro-106-Ser mutation and EPSPS overexpression acting together simultaneously in glyphosate-resistant goosegrass (Eleusine indica). Sci. Rep. 2017, 7, 6702. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).