Crop Nitrogen Fertilization Schedule in Bread Wheat Affects the Mechanical Performances of Thermoplastic Films Obtained by Plasticization of Flours
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin of the Source Grains: The Field Trial and Crop N Fertilization Treatments
2.2. Grain Morphology and Microstructure of Flours
2.3. Grain Milling and Determination of Flour Alveographic Parameters
2.4. Analysis of Gluten Content and Gluten Fractions
2.5. Thermal Analysis of Wheat Flours
2.6. Plasticization, Filming, and Measurements on Films
2.7. Tensile Properties of Produced Films
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leblanc, N.; Saiah, R.; Beucher, E.; Gattin, R.; Castandet, M.; Saiter, J.-M. Structural investigation and thermal stability of new extruded wheat flour based polymeric materials. Carbohydr. Polym. 2008, 73, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, P.; Dominici, F.; Bocci, L.; Governatori, C.; Panfili, I.; Tosti, G.; Torre, L.; Puglia, D. Relationships between wheat flour baking properties and tensile characteristics of derived thermoplastic films. Ind. Crops Prod. 2017, 100, 138–145. [Google Scholar] [CrossRef]
- Dominici, F.; Luzi, F.; Benincasa, P.; Torre, L.; Puglia, D. Biocomposites based on plasticized wheat flours: Effect of bran content on thermomechanical behavior. Polymers 2020, 12, 2248. [Google Scholar] [CrossRef]
- Puglia, D.; Dominici, F.; Kenny, J.M.; Santulli, C.; Governatori, C.; Tosti, G.; Benincasa, P. Tensile behavior of thermoplastic films from wheat flours as function of raw material baking properties. J. Polym. Environ. 2015, 24, 37–47. [Google Scholar] [CrossRef]
- Day, L. 10-Wheat gluten: Production, properties and application. In Handbook of Food Proteins, Woodhead Publishing Series in Food Science, Technology and Nutrition; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Sawston, UK, 2011; pp. 267–288. [Google Scholar] [CrossRef]
- Foca, G.; Ulrici, A.; Corbellini, M.; Pagani, M.A.; Lucisano, M.; Franchini, G.C.; Tassi, L. Reproducibility of the Italian ISQ method for quality classification of bread wheats: An evaluation by expert assessors. J. Sci. Food Agric. 2007, 87, 839–846. [Google Scholar] [CrossRef]
- Khatkar, B.S.; Barak, S.; Mudgil, D. Effects of gliadin addition on the rheological, microscopic and thermal characteristics of wheat gluten. Int. J. Biol. Macromol. 2013, 53, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Bonciarelli, U.; Onofri, A.; Benincasa, P.; Farneselli, M.; Guiducci, M.; Pannacci, E.; Tosti, G.; Tei, F. Long-term evaluation of productivity, stability and sustainability for cropping systems in Mediterranean rainfed conditions. Eur. J. Agron. 2016, 77, 14655. [Google Scholar] [CrossRef]
- Slafer, G.A.; Savin, R.; Sadras, V.O. Coarse and fine regulation of wheat yield components in response to genotype and environment. Field Crops Res. 2014, 157, 71–83. [Google Scholar] [CrossRef]
- Dupont, F.M.; Altenbach, S.B. Molecular and biochemical impacts of environmental factors on wheat grain development and protein synthesis. J. Cereal Sci. 2003, 38, 133–146. [Google Scholar] [CrossRef]
- De Santis, M.A.; Giuliani, M.M.; Flagella, Z.; Reyneri, A.; Blandino, M. Impact of nitrogen fertilisation strategies on the protein content, gluten composition and rheological properties of wheat for biscuit production. Field Crops Res. 2020, 254, 107829. [Google Scholar] [CrossRef]
- Blandino, M.; Visioli, G.; Marando, S.; Marti, A.; Reyneri, A. Impact of late-season N fertilisation strategies on the gluten content and composition of high protein wheat grown under humid Mediterranean conditions. J. Cereal Sci. 2020, 94, 102995. [Google Scholar] [CrossRef]
- Guiducci, M.; Tosti, G.; Falcinelli, B.; Benincasa, P. Sustainable management of nitrogen nutrition in winter wheat through temporary intercropping with legumes. Agron. Sustain. Dev. 2018, 38, 31. [Google Scholar] [CrossRef]
- Tosti, G.; Farneselli, M.; Benincasa, P.; Guiducci, M. Nitrogen fertilization strategies for organic wheat production: Crop yield and nitrate leaching. Agron. J. 2016, 108, 770–781. [Google Scholar] [CrossRef]
- Benincasa, P.; Reale, L.; Cerri, M.; Tedeschini, E.; Tosti, G.; Falcinelli, B.; Rosati, A. Nitrogen fertilization levels and timing affect the plasticity of yield components in bread wheat (Triticum aestivum L.). Field Crops Res. 2022, 289, 108734. [Google Scholar] [CrossRef]
- Roman-Gutierrez, A.D.; Guilbert, S.; Cuq, B. Description of Microstructural Changes in Wheat Flour and Flour Components during Hydration by using Environmental Scanning Electron Microscopy. LWT 2002, 35, 730–740. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists. 38-12, Wet Gluten, Dry Gluten, Water-Binding Capacity, and Gluten Index. In Approved Methods of Analysis, 11th ed.; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- Pogna, N.E.; Autran, J.C.; Mellini, F.; Lafiandra, D.; Feillet, P. Chromosome 1B-encoded gliadins and glutenin subunits in durum wheat: Genetics and relationship to gluten strength. J. Cereal Sci. 1990, 11, 15–34. [Google Scholar] [CrossRef]
- Payne, P.I.; Lawrence, G.J. Catalogue of alleles for the complex gene loci, Glu-A1, Glu-B1, and Glu-D1 which code for high-molecular-weight subunits of glutenin in exaploidy wheat. Cereal Res. Commun. 1983, 11, 29–35. [Google Scholar]
- Bushuk, W.; Zillman, R.R. Wheat cultivar identification by gliadin electrophoregrams, I. Apparatus, method and nomenclature. Can. J. Plant Sci. 1978, 58, 505–515. [Google Scholar] [CrossRef]
- Ku, H.H. Notes on the use of propagation of error formulas. J. Res. Natl. Bur. Stand. 1966, 70, 263. [Google Scholar] [CrossRef]
- Altenbach, S.B.; Chang, H.-C.; Yu, X.B.; Seabourn, B.W.; Green, P.H.; Alaedini, A. Elimination of Omega-1,2 gliadins from bread wheat (Triticum aestivum) flour: Effects on immunogenic potential and end-use quality. Front. Plant Sci. 2019, 10, 580. [Google Scholar] [CrossRef]
- Pogna, N.E.; Mellini, F.; Beretta, A.; Dal Belin Peruffo, A. The high-molecular-weight glutenin subunits of common wheat cultivars grown in Italy. J. Genet Breed. 1989, 43, 17–24. [Google Scholar]
- Uthumporn, U.; Karim, A.A.; Fazilah, A. Defatting improves the hydrolysis of granular starch using a mixture of fungal amylolytic enzymes. Ind. Crops Prod. 2013, 43, 441–449. [Google Scholar] [CrossRef]
- Kumar, R.; Khatkar, B.S. Thermal, pasting and morphological properties of starch granules of wheat (Triticum aestivum L.) varieties. J. Food Sci. Technol. 2017, 54, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Yu, L.; Tong, Z.; Chen, L.; Liu, H.; Li, X. Thermal degradation and stability of starch under different processing conditions. Starch-Stärke 2013, 65, 48–60. [Google Scholar] [CrossRef]
- Li, M.; Yue, Q.; Liu, C.; Zheng, X.; Hong, J.; Li, L.; Bian, K. Effect of gliadin/glutenin ratio on pasting, thermal, and structural properties of wheat starch. J. Cereal Sci. 2020, 93, 102973. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, S.; Sun, B.; Wang, F.; Huang, J.; Wang, X.; Bao, Q. Effects of thermal properties and behavior of wheat starch and gluten on their interaction: A review. Int. J. Biol. Macromol. 2021, 177, 474–484. [Google Scholar] [CrossRef]
- Lagrain, B.; Thewissen, B.G.; Brijs, K.; Delcour, J.A. Mechanism of gliadin–glutenin cross-linking during hydrothermal treatment. Food Chem. 2008, 107, 753–760. [Google Scholar] [CrossRef]
- Ma, S.; Han, W.; Li, L.; Zheng, X.; Wang, X. The thermal stability, structural changeability, and aggregability of glutenin and gliadin proteins induced by wheat bran dietary fiber. Food Funct. 2019, 10, 172–179. [Google Scholar] [CrossRef]
- Khatkar, B.S.; Bell, A.E.; Schofield, J.D. The dynamic rheological properties of glutens and gluten sub-fractions from wheats of good and poor bread making quality. J. Cereal Sci. 1995, 22, 29–44. [Google Scholar] [CrossRef]
- Muneer, F.; Andersson, M.; Koch, K.; Hedenqvist, M.S.; Gällstedt, M.; Plivelic, T.S.; Menzel, C.; Rhazi, L.; Kuktaite, R. Innovative gliadin/glutenin and modified potato starch green composites: Chemistry, structure, and functionality induced by processing. ACS Sustain. Chem. Eng. 2016, 4, 6332–6343. [Google Scholar] [CrossRef]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, X.; Wang, L.; Yang, Y.; Zhu, X.; Shao, S.; Cui, W.; Xiong, F. Novel insights into the effect of nitrogen on storage protein biosynthesis and protein body development in wheat caryopsis. J. Exp. Bot. 2017, 68, 2259–2274. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Wang, Z.; Yin, Y.; Li, W.; Yan, S.; Cai, T. Starch granule size distribution in wheat grain in relation to phosphorus fertilization. J. Agric. Sci. 2012, 150, 45–52. [Google Scholar] [CrossRef]
- Ottenhof, M.A.; Farhat, I.A. Starch retrogradation. Biotechnol. Genet. Eng. Rev. 2004, 21, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Tanetrungroj, Y.; Prachayawarakorn, J. Effect of starch types on properties of biodegradable polymer based on thermoplastic starch process by injection molding technique. Songklanakarin J. Sci. Technol. 2015, 37, 193–199. [Google Scholar]
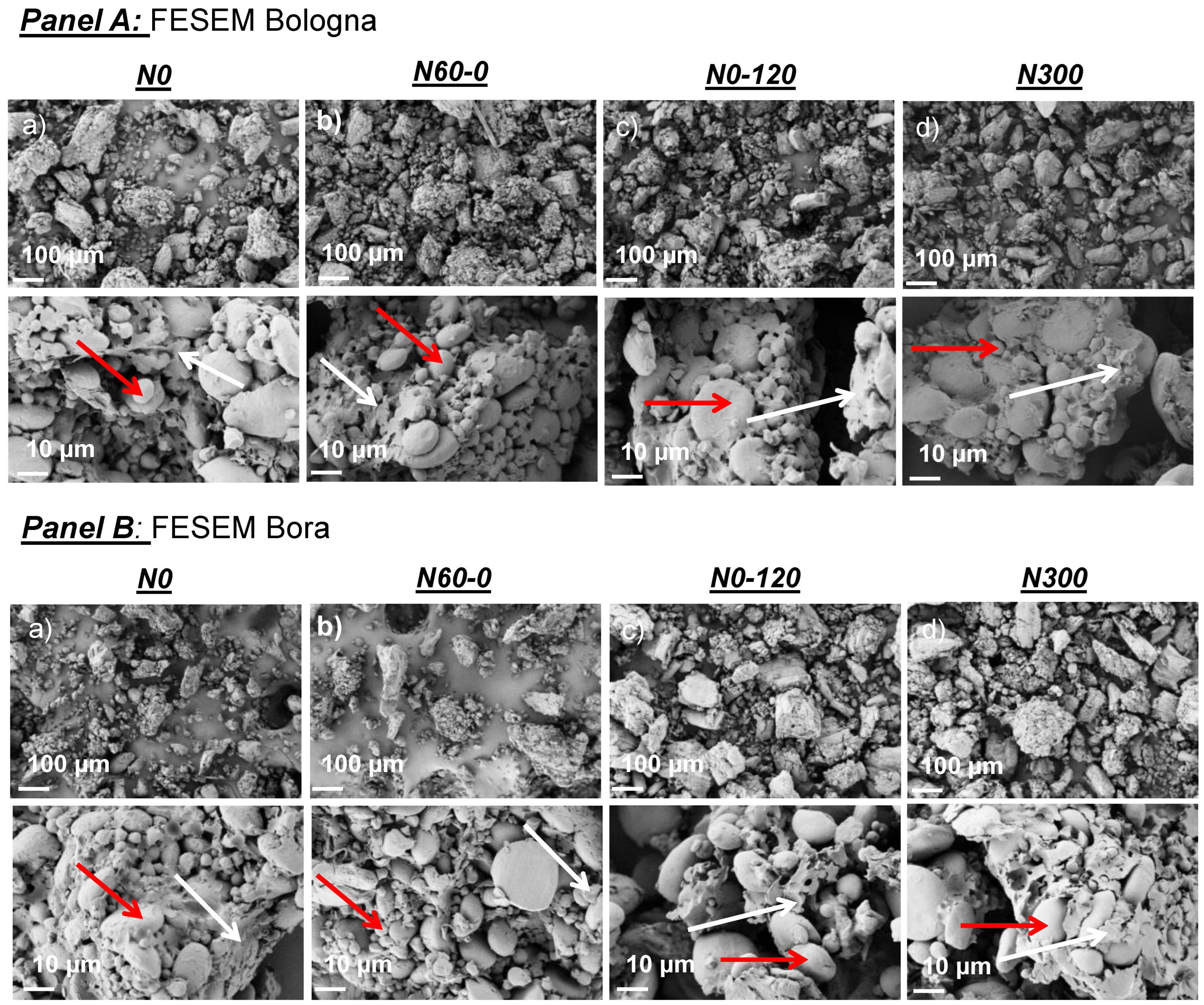
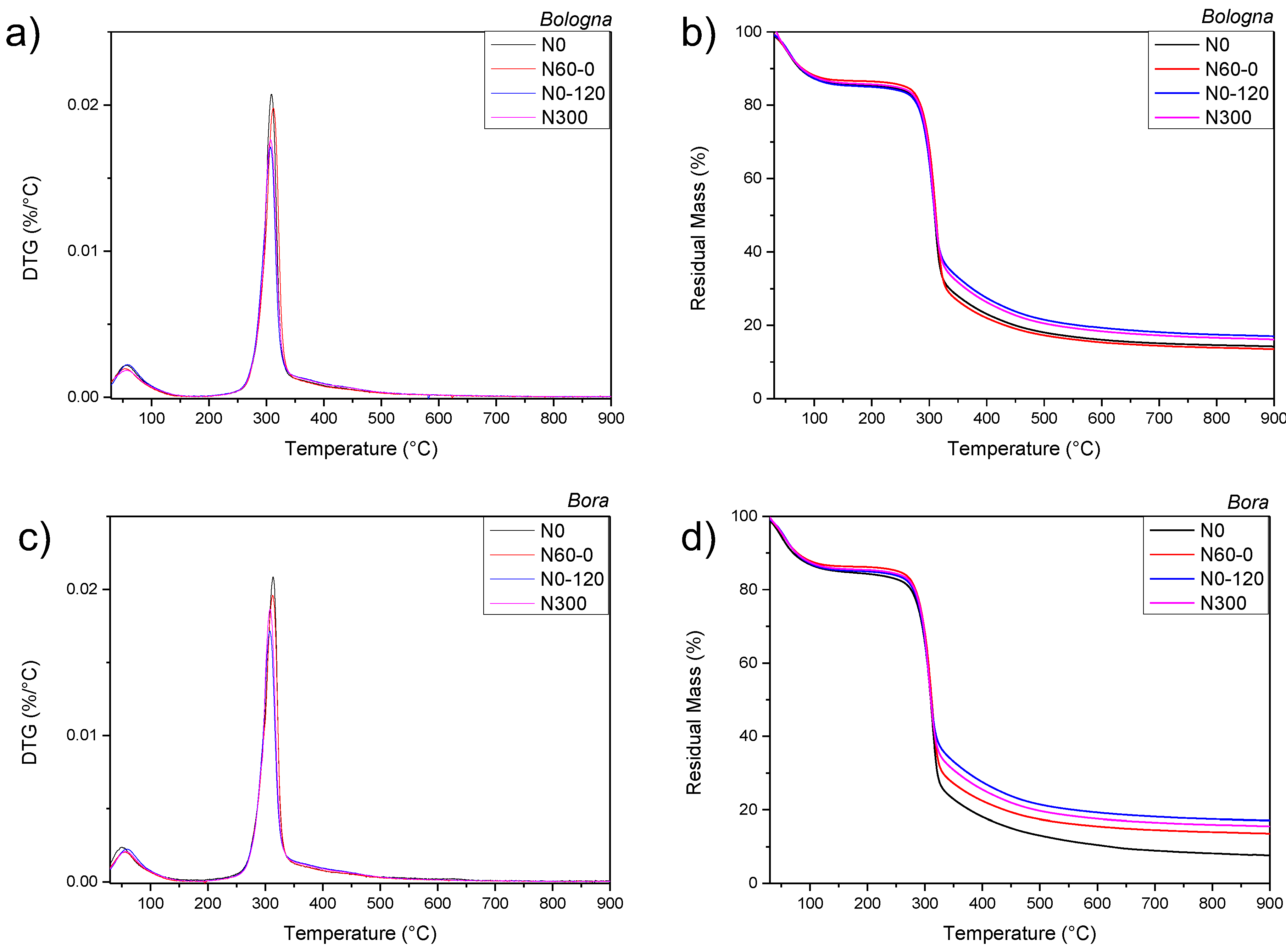

| Cultivar | N Treatment | Humidity (%) | Gluten Content (% d.m.) | P | L | W | P/L |
|---|---|---|---|---|---|---|---|
| Bologna | 0 | 14.2 | 6.02 (0.03) | 72 | 78 | 215 | 0.92 |
| 60-0 | 14.5 | 4.54 (0.04) | 88 | 43 | 166 | 2.05 | |
| 0-120 | 15.6 | 12.75 (0.01) | 74 | 202 | 539 | 0.37 | |
| 300 | 15.8 | 10.00 (0.11) | 96 | 95 | 380 | 1.01 | |
| Bora | 0 | 14.7 | 6.31 (0.01) | 83 | 59 | 200 | 1.41 |
| 60-0 | 14.3 | 5.30 (0.04) | 105 | 32 | 145 | 3.28 | |
| 0-120 | 15.6 | 13.14 (0.9) | 84 | 140 | 361 | 0.60 | |
| 300 | 15.5 | 9.93 (0.06) | 79 | 114 | 277 | 0.69 |
| Fractions | Bands | N0 | N60-0 | N0-120 | N300 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
| Bologna | |||||||||
| HMW-GS | 2 * | 11.5 | 2.3 | 9.0 | 0.9 | 18.2 | 2.0 | 17.3 | 1.5 |
| 5 | 45.3 | 4.2 | 46.4 | 0.7 | 59.1 | 12.5 | 67.8 | 5.6 | |
| 7 | 81.4 | 8.5 | 82.8 | 1.8 | 82.1 | 9.4 | 92.1 | 6.4 | |
| 8 | 9.8 | 2.9 | 7.2 | 0.6 | 35.0 | 3.9 | 20.9 | 4.5 | |
| 10 | 33.7 | 5.4 | 34.3 | 0.9 | 55.9 | 5.4 | 56.4 | 8.1 | |
| Tot | 181.7 | 23.3 | 179.7 | 0.4 | 250.4 | 29.2 | 254.6 | 26.2 | |
| LMW-GS | 1 | 23.0 | 0.3 | 24.0 | 0.6 | 100.0 | 6.4 | 71.6 | 15.3 |
| 2 + 3 | 91.9 | 15.1 | 97.5 | 5.5 | 174.9 | 12.5 | 150.7 | 6.4 | |
| 4 + 5 | 521.1 | 2.8 | 536.2 | 38.0 | 584.1 | 29.7 | 550.8 | 12.3 | |
| 6 | 60.9 | 18.9 | 58.5 | 8.4 | 83.2 | 7.5 | 85.3 | 11.6 | |
| Tot | 696.9 | 30.8 | 716.2 | 23.5 | 942.2 | 56.0 | 858.4 | 45.7 | |
| Gliadins | ω | 158.4 | 19.1 | 144.5 | 9.3 | 366.8 | 16.6 | 320.8 | 25.9 |
| γ | 274.8 | 30.5 | 266.9 | 8.9 | 320.0 | 36.1 | 400.9 | 35.3 | |
| α + β | 317.5 | 42.4 | 290.1 | 32.6 | 660.3 | 170.5 | 693.0 | 78.1 | |
| Tot | 750.6 | 91.9 | 701.6 | 14.5 | 1347.2 | 223.2 | 1414.8 | 139.3 | |
| Bora | |||||||||
| HMW-GS | 1 | 17.9 | 2.6 | 22.7 | 1.5 | 46.4 | 0.0 | 39.1 | 2.9 |
| 5 | 45.7 | 3.7 | 57.3 | 3.1 | 84.6 | 1.4 | 79.2 | 3.6 | |
| 7 | 49.5 | 2.9 | 62.1 | 3.9 | 86.0 | 1.3 | 83.5 | 5.1 | |
| 8 | 10.7 | 0.3 | 15.3 | 1.4 | 39.0 | 0.5 | 31.0 | 0.7 | |
| 10 | 34.9 | 0.2 | 46.2 | 2.4 | 80.6 | 2.2 | 67.1 | 4.5 | |
| Tot | 158.8 | 9.3 | 203.6 | 12.3 | 336.6 | 4.5 | 300.0 | 9.6 | |
| LMW-GS | 1 | 18.8 | 2.8 | 31.2 | 6.3 | 97.2 | 15.0 | 44.1 | 3.7 |
| 2 + 3 | 79.8 | 7.3 | 118.3 | 15.2 | 281.2 | 45.1 | 182.5 | 11.2 | |
| 4 + 5 | 254.0 | 21.5 | 317.4 | 26.4 | 411.2 | 58.5 | 311.9 | 27.2 | |
| 6 | 195.5 | 42.6 | 242.0 | 60.6 | 269.5 | 10.0 | 187.6 | 13.5 | |
| Tot | 548.2 | 74.1 | 708.8 | 108.5 | 1059.0 | 108.6 | 726.1 | 55.6 | |
| Gliadins | ω | 152.3 | 27.8 | 118.7 | 9.2 | 382.2 | 69.9 | 179.8 | 2.8 |
| γ | 241.9 | 27.7 | 210.3 | 13.2 | 258.2 | 18.5 | 215.5 | 6.3 | |
| α + β | 419.0 | 90.4 | 342.5 | 50.5 | 815.0 | 53.4 | 577.4 | 3.6 | |
| Tot | 813.2 | 145.8 | 671.5 | 72.8 | 1455.4 | 141.8 | 972.8 | 12.7 | |
| MEANS OF TOTALS (CV confounded) | |||||||||
| HMW-GS | Tot | 170.2 | 12.2 | 191.7 | 8.6 | 293.5 | 27.7 | 277.3 | 17.4 |
| LMW-GS | Tot | 622.6 | 54.0 | 712.5 | 45.4 | 1000.6 | 60.2 | 792.3 | 48.2 |
| Gliadins | Tot | 781.9 | 72.7 | 686.6 | 31.5 | 1401.3 | 112.4 | 1193.8 | 139.8 |
| Gluten Fractions | N0 | N60-0 | N0-120 | N300 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Bologna | ||||||||
| Total GLUTENINS/GLIADINS | 1.17 | 0.153 | 1.28 | 0.061 | 0.89 | 0.210 | 0.79 | 0.128 |
| Total HMW-GS/GLIADINS | 0.24 | 0.061 | 0.26 | 0.006 | 0.19 | 0.052 | 0.18 | 0.036 |
| Total LMW-GS/GLIADINS | 0.93 | 0.155 | 1.02 | 0.055 | 0.70 | 0.157 | 0.61 | 0.092 |
| Total HMW-GS/total LMW-GS | 0.26 | 0.045 | 0.25 | 0.009 | 0.27 | 0.047 | 0.30 | 0.046 |
| Total HMW-GS/LMW-GS (1+2+3) | 1.58 | 0.406 | 1.48 | 0.078 | 0.91 | 0.169 | 1.15 | 0.230 |
| HMW-GS (2*+5+7)/HMW-GS (8+10) | 3.18 | 0.951 | 3.33 | 0.170 | 1.75 | 0.399 | 2.29 | 0.549 |
| LMW-GS (1+2+3)/LMW-GS (4+5+6) | 0.20 | 0.031 | 0.20 | 0.020 | 0.41 | 0.051 | 0.35 | 0.047 |
| GLIADINS ω/(α + β + γ) | 0.27 | 0.065 | 0.26 | 0.028 | 0.37 | 0.096 | 0.29 | 0.054 |
| GLIADINS ω/γ | 0.58 | 0.133 | 0.54 | 0.053 | 1.15 | 0.181 | 0.80 | 0.135 |
| Bora | ||||||||
| Total GLUTENINS/GLIADINS | 0.87 | 0.236 | 1.36 | 0.327 | 0.96 | 0.171 | 1.05 | 0.081 |
| Total HMW-GS/GLIADINS | 0.20 | 0.046 | 0.30 | 0.051 | 0.23 | 0.026 | 0.31 | 0.014 |
| Total LMW-GS/GLIADINS | 0.67 | 0.212 | 1.06 | 0.276 | 0.73 | 0.146 | 0.75 | 0.067 |
| Total HMW-GS/total LMW-GS | 0.29 | 0.056 | 0.29 | 0.061 | 0.32 | 0.037 | 0.41 | 0.045 |
| Total HMW-GS/LMW-GS (1+2+3) | 1.61 | 0.257 | 1.36 | 0.278 | 0.89 | 0.153 | 1.32 | 0.130 |
| HMW-GS (1+5+7)/HMW-GS (8+10) | 2.48 | 0.207 | 2.31 | 0.281 | 1.81 | 0.049 | 2.06 | 0.139 |
| LMW-GS (1+2+3)/LMW-GS (4+5+6) | 0.22 | 0.054 | 0.27 | 0.080 | 0.56 | 0.128 | 0.45 | 0.067 |
| GLIADINS ω/(α + β + γ) | 0.23 | 0.083 | 0.21 | 0.041 | 0.36 | 0.089 | 0.23 | 0.006 |
| GLIADINS ω/γ | 0.63 | 0.187 | 0.56 | 0.079 | 1.48 | 0.377 | 0.83 | 0.038 |
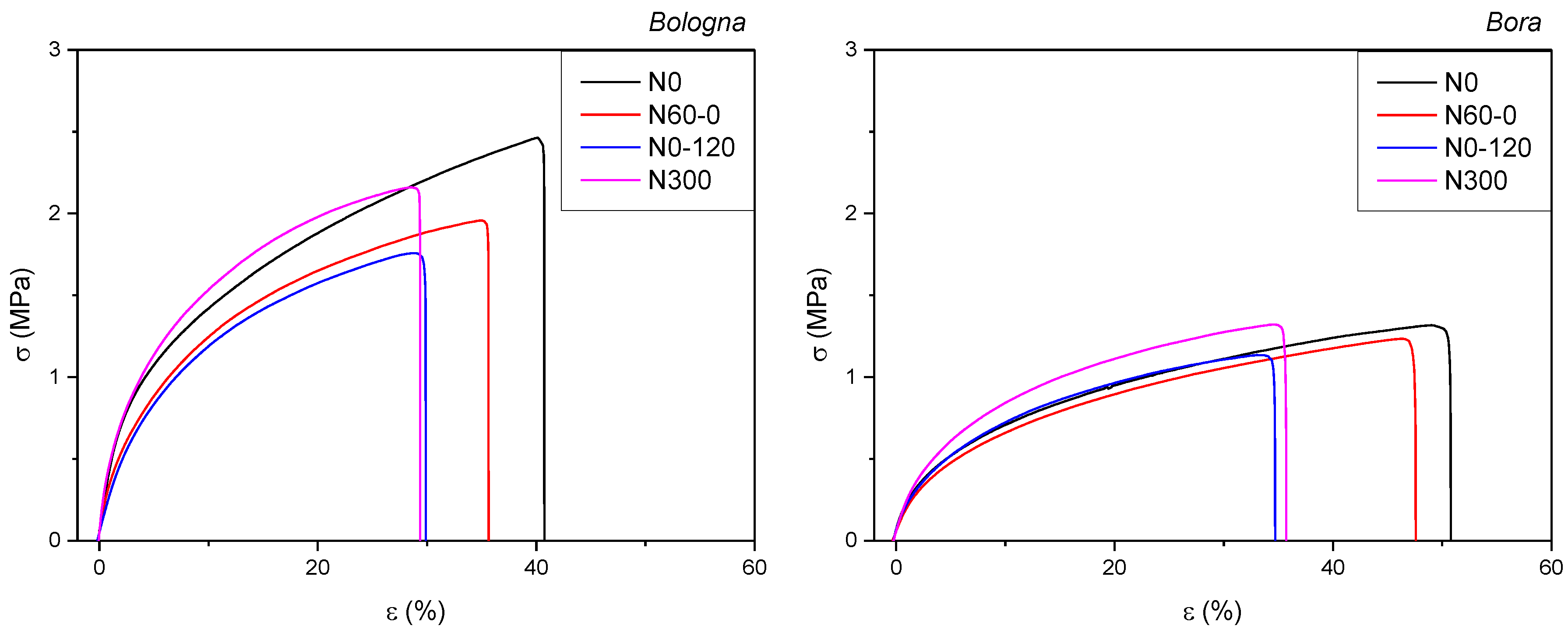
| Source Flours | σb (MPa) | εb (%) | E (MPa) |
|---|---|---|---|
| Bologna | |||
| N0 | 2.31 ± 0.22 c | 40.40 ± 6.89 b | 48.23 ± 2.42 b |
| N60-0 | 1.94 ± 0.05 ab | 36.69 ± 1.52 b | 41.33 ± 5.03 ab |
| N0-120 | 1.73 ± 0.15 a | 33.56 ± 4.03 ab | 33.28 ± 7.96 a |
| N300 | 2.20 ± 0.14 bc | 27.80 ± 2.34 a | 69.56 ± 9.41 c |
| Bora | |||
| N0 | 1.29 ± 0.17 a | 51.75 ± 6.75 c | 19.77 ± 3.53 a |
| N60-0 | 1.27 ± 0.17 a | 45.01 ± 3.87 bc | 22.72 ± 7.32 a |
| N0-120 | 1.16 ± 0.04 a | 35.40 ± 3.13 ab | 20.40 ± 1.83 a |
| N300 | 1.32 ± 0.14 a | 31.64 ± 6.01 a | 23.00 ± 2.99 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benincasa, P.; Dominici, F.; Luzi, F.; Governatori, C.; Gazza, L.; Galassi, E.; Tosti, G.; Puglia, D. Crop Nitrogen Fertilization Schedule in Bread Wheat Affects the Mechanical Performances of Thermoplastic Films Obtained by Plasticization of Flours. Agronomy 2023, 13, 697. https://doi.org/10.3390/agronomy13030697
Benincasa P, Dominici F, Luzi F, Governatori C, Gazza L, Galassi E, Tosti G, Puglia D. Crop Nitrogen Fertilization Schedule in Bread Wheat Affects the Mechanical Performances of Thermoplastic Films Obtained by Plasticization of Flours. Agronomy. 2023; 13(3):697. https://doi.org/10.3390/agronomy13030697
Chicago/Turabian StyleBenincasa, Paolo, Franco Dominici, Francesca Luzi, Catia Governatori, Laura Gazza, Elena Galassi, Giacomo Tosti, and Debora Puglia. 2023. "Crop Nitrogen Fertilization Schedule in Bread Wheat Affects the Mechanical Performances of Thermoplastic Films Obtained by Plasticization of Flours" Agronomy 13, no. 3: 697. https://doi.org/10.3390/agronomy13030697
APA StyleBenincasa, P., Dominici, F., Luzi, F., Governatori, C., Gazza, L., Galassi, E., Tosti, G., & Puglia, D. (2023). Crop Nitrogen Fertilization Schedule in Bread Wheat Affects the Mechanical Performances of Thermoplastic Films Obtained by Plasticization of Flours. Agronomy, 13(3), 697. https://doi.org/10.3390/agronomy13030697











