Photosynthetic Physiological Basis of Forage Mass Stability in a Progeny of Rhizome-Rooted ‘Qingshui’ Medicago sativa L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Growth Conditions and Treatments
2.3. Measurement Indexes
2.3.1. Determination of Forage Mass
2.3.2. Determination of Photosynthetic Pigment Content
2.3.3. Determination of Photosynthetic Parameters
2.3.4. Determination of the Photosynthesis Products
2.4. Statistical Analyses
3. Results
3.1. Analysis of Forage Mass in Hybrid Strains and Parents
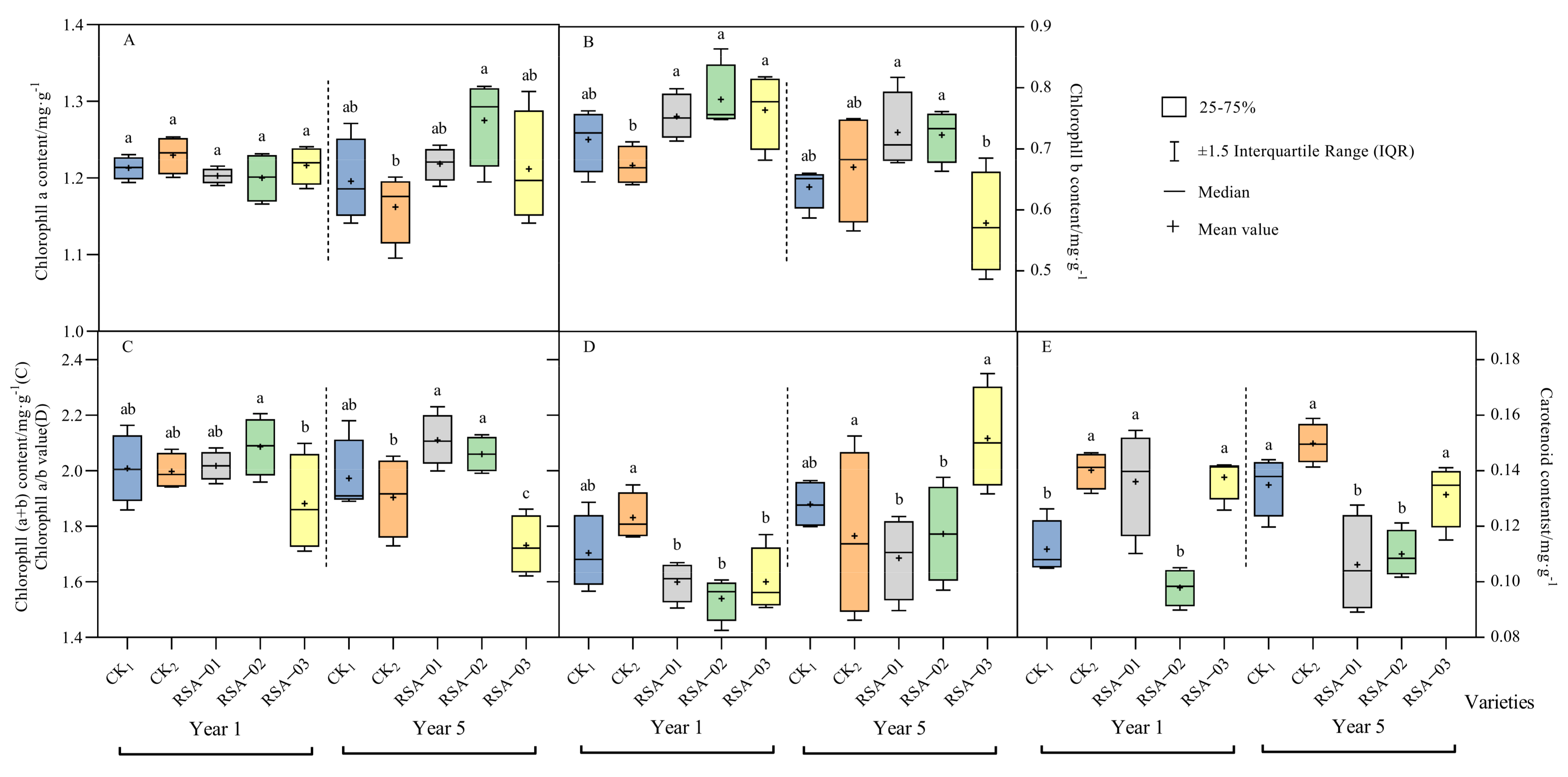
3.2. Analysis of Photosynthetic Pigment Content in Hybrid Strains and Parents
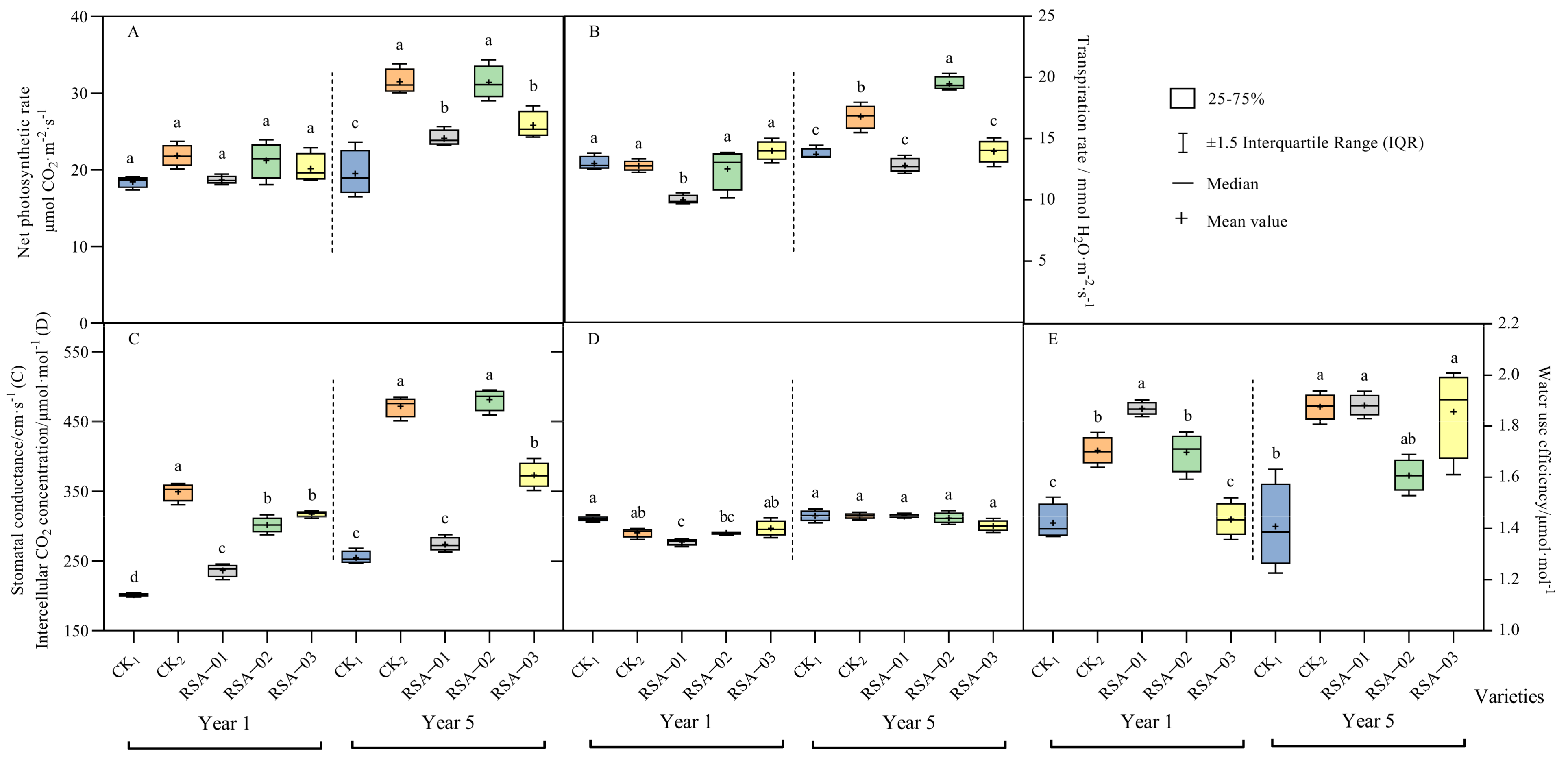
3.3. Analysis of Photosynthetic Parameters in Hybrid Strains and Parents
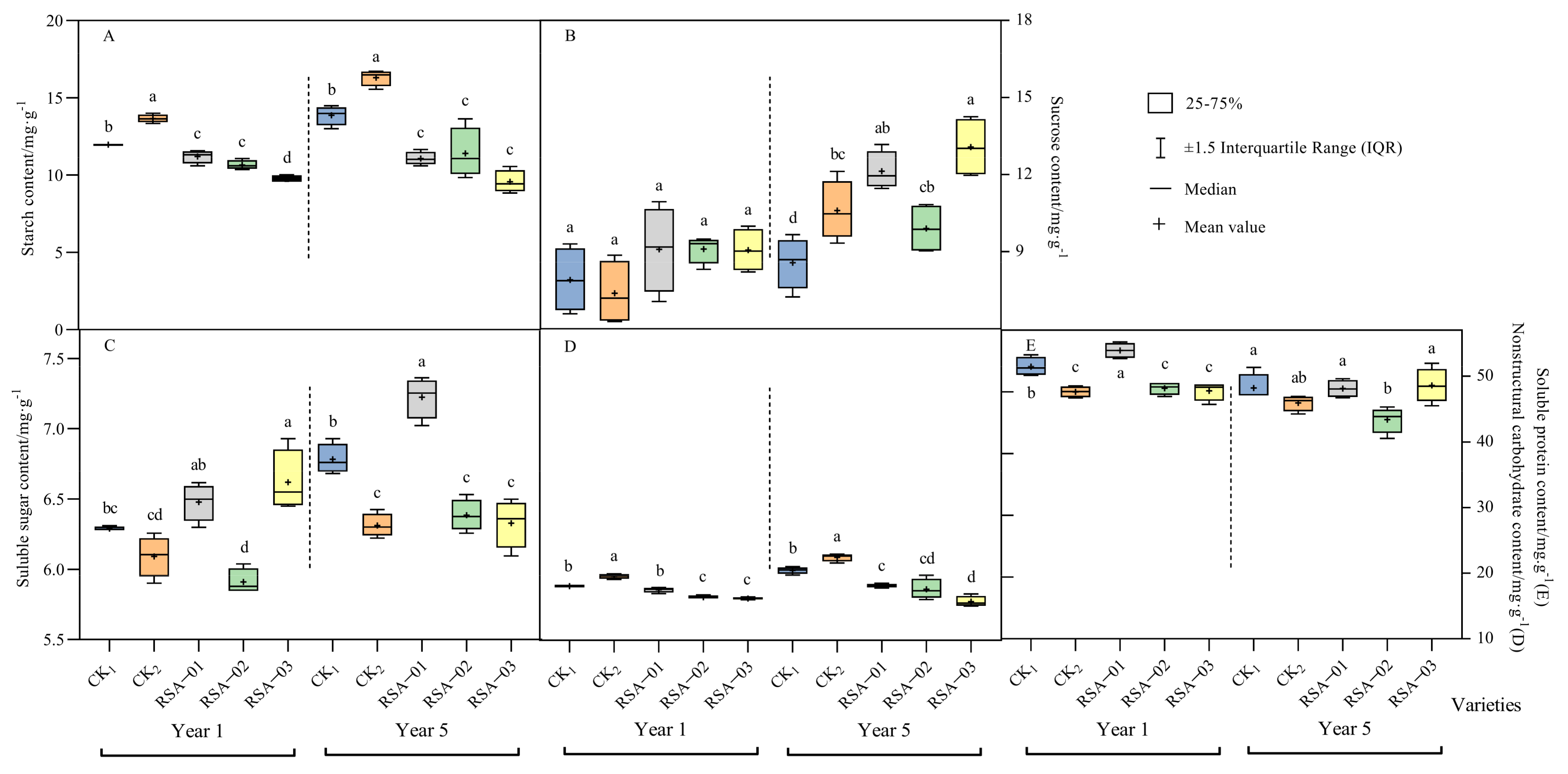
3.4. Analysis of Photosynthetic Products in Hybrid Strains and Parents
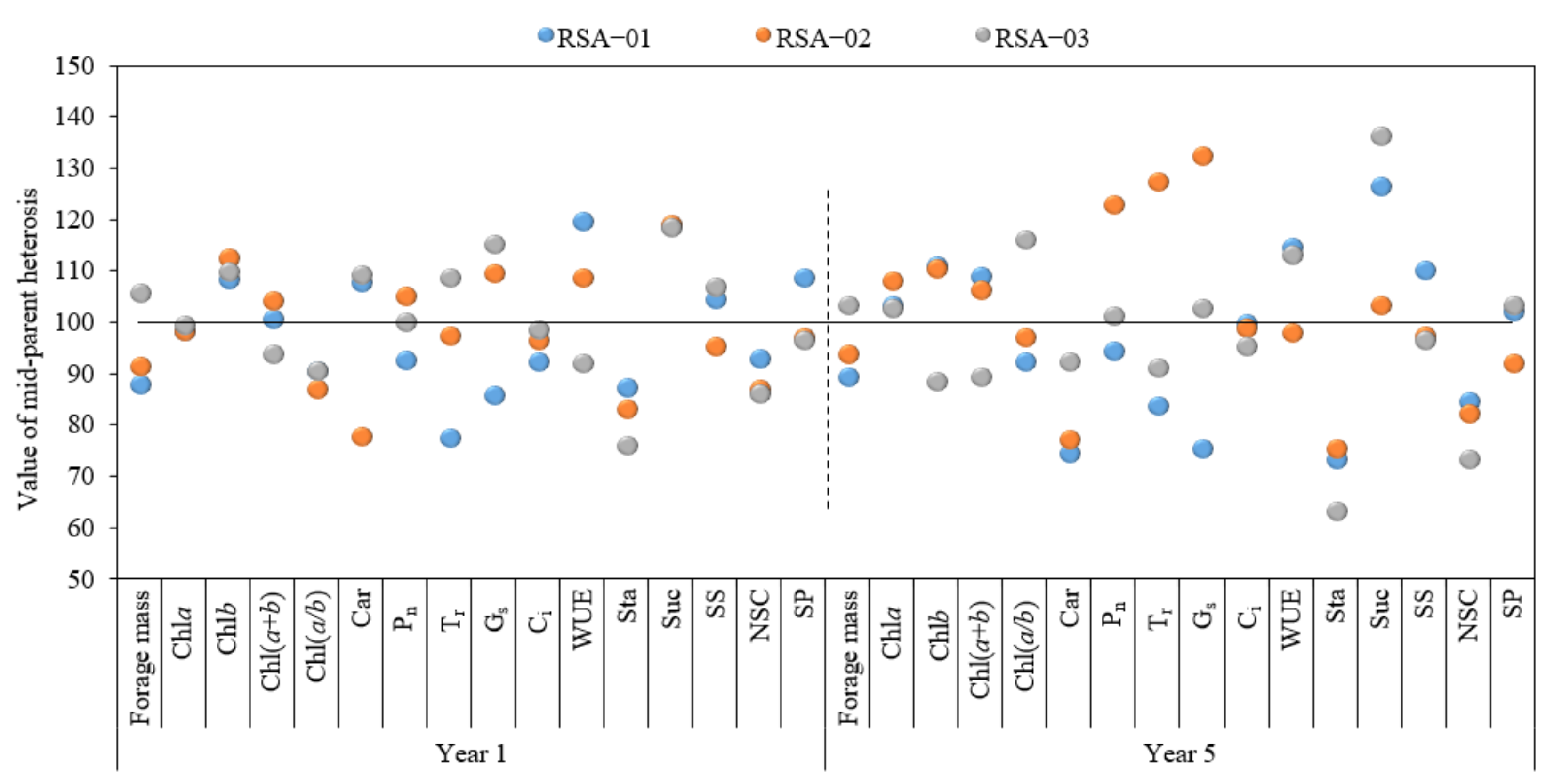
3.5. Analysis of Mid-Parent Heterosis of Photosynthetic Physiological Indices in Hybrid Strains
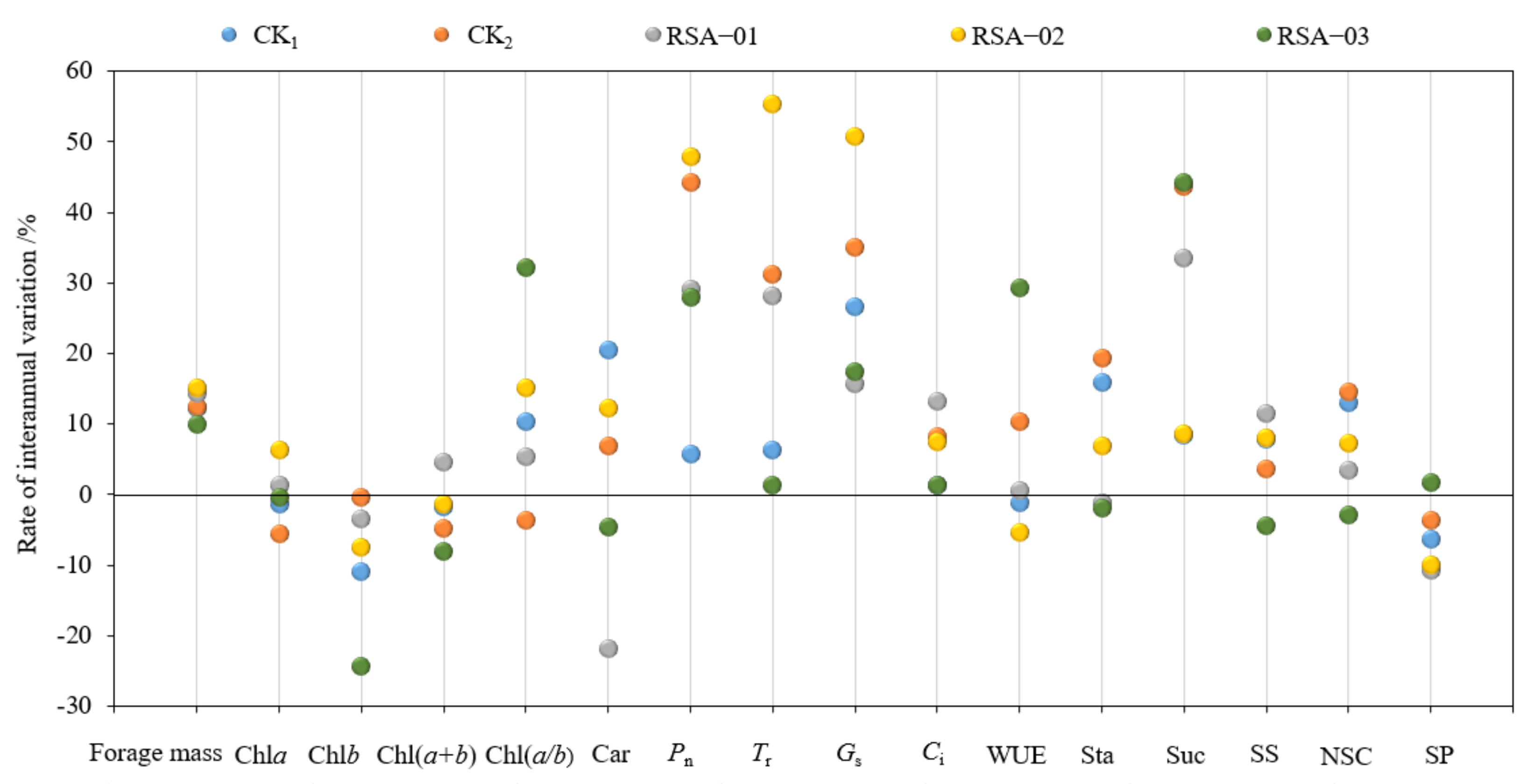
3.6. Interannual Stability of Forage Mass and Photosynthetic Physiological Indices in Alfalfa at One and Five Years of Age
3.7. Path Analysis of Forage Mass Yield and Photosynthetic Physiological Indexes in Alfalfa at 1 and 5 Years of Age
3.8. Effect of Interannual Variation and Genetic Differences in Photosynthetic Physiological Indexes of Alfalfa
4. Discussion
4.1. Effect of Genetic Differences on the Photosynthetic Physiology of Alfalfa
4.2. Effect of Interannual Variation on the Photosynthetic Physiology of Alfalfa
4.3. Relationship between Forage Mass and Photosynthetic Physiological Indicators in Alfalfa
4.4. Comprehensive Analysis of Interannual Variation and Genetic Differences in Photosynthetic Physiological Indicators of Alfalfa
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nan, L.L.; Shi, S.L.; Zhu, X.Q.; Zhao, W.H.; Han, L.; Tian, C.X. Physiological change of different root types of alfalfa under drought stress at seedling stage. Agric. Res. Arid. Areas 2011, 29, 106–110. [Google Scholar]
- A, Y.; Shi, S.L.; Sun, S.J.; Jing, Y.Y.; Li, Z.L.; Zhang, X.Y.; Li, X.L.; Wu, F. Telomerase activity, relative telomere length, and longevity in alfalfa (Medicago sativa L.). Peer J. 2022, 10, e14102. [Google Scholar] [CrossRef]
- Pecetti, L.; Barre, P.; Delaunay, S.; Lambroni, P.; Annicchiarico, P.; Julier, B. QTL analysis for grazing tolerance, autumn dormancy and growth habit offers prospects for marker-assisted selection in lucerne. Euphytica 2021, 217, 171. [Google Scholar] [CrossRef]
- A, Y.; Shi, S.L.; Gong, W.L.; Zhang, J.Q.; Zhang, X.Y.; Zhang, J. Cross-breeding improvement and performance analysis of dominant production traits in grazing-type alfalfa (Medicago sativa L.). Biomed. Res. Int. 2022, 2022, 1252310. [Google Scholar] [CrossRef] [PubMed]
- A, Y.; Shi, S.L.; Li, Z.L.; Zhang, X.Y.; Ou, K.J.; Zhou, W.W. Study on phenotypic and physiological characteristics of progenies of ‘Qingshui’ Alfalfa and ‘WL168’. Acta Agrestia Sin. 2021, 29, 2184–2190. [Google Scholar] [CrossRef]
- Chen, L.Q.; Shi, S.L. Genetic diversity of 42 alfalfa accessions revealed by SSR markers. Pratacultural Sci. 2015, 32, 372–381. [Google Scholar]
- Li, Z.L.; A, Y.; Zhang, H.H.; Li, X.L. Identification and physiological study of variation in reproductive fertility in clonal lines og Medicago sativa cultivar ‘Qingshui’. Acta Prataculturae Sin. 2022, 31, 135. [Google Scholar] [CrossRef]
- Suchowilska, E.; Wiwart, M.; Krska, R.; Kandler, W. Do Triticum aestivum L. and Triticum spelta L. hybrids constitute a promising source material for quality breeding of new wheat varieties? Agronomy 2020, 10, 43. [Google Scholar] [CrossRef]
- Acharya, J.P.; Lopez, Y.; Gouveia, B.T.; de Bem Oliveira, I.; Resende, M.F.R.; Muñoz, P.R.; Rios, E.F. Breeding alfalfa (Medicago sativa L.) adapted to subtropical agroecosystems. Agronomy 2020, 10, 742. [Google Scholar] [CrossRef]
- Fujimoto, R.; Taylor, J.M.; Shirasawa, S.; Peacock, W.J.; Dennis, E.S. Heterosis of Arabidopsis hybrids between C24 and Col is associated with increased photosynthesis capacity. Proc. Natl. Acad. Sci. USA 2012, 109, 7109–7114. [Google Scholar] [CrossRef]
- Hu, G.X.; Peng, R.; Cui, X.N.; Liu, Y.J.; Li, Y.S.; Zeng, W.F. Effect of phosphorus application on carbohydrate distribution in roots, stems and leaves of alfalfa and resistance to thrips (Thysanoptera: Thripidae). Chin. J. Eco-Agric. 2020, 28, 969–978. [Google Scholar] [CrossRef]
- Carillo, P.; Arena, C.; Modarelli, G.C.; Pascale, S.D.; Paradiso, R. Photosynthesis in Ranunculus asiaticus L.: The Influence of the Hybrid and the Preparation Procedure of Tuberous Roots. Front. Plant Sci. 2019, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.X.; Tian, F.P.; Hu, Y.; Wei, X.X.; Zhu, X.Q.; Wang, X.L.; Wang, C.M.; Li, J.H.; Degen, A.A.; Duan, H.R. Photosynthesis, fluorescence, and nutrition of Zhonglan No. 2, a new alfalfa cultivar. J. Sci. Food. Agric. 2021, 101, 6434–6442. [Google Scholar] [CrossRef] [PubMed]
- Leister, D. Enhancing the light reactions of photosynthesis: Strategies, controversies and perspectives. Mol. Plant. 2022, 15, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Zhao, X.G.; Guan, Z.B.; Wang, X.F.; Hou, J.L.; Tian, J.H.; Li, D.R.; Lu, C.M. A review on photosynthetic physiological Research and high photosynthetic Efficiency breeding of Brassica napus. Chin. Agric. Sci. Bull. 2017, 33, 44–51. [Google Scholar] [CrossRef]
- Hao, P.; Ning, Y.; Gao, Q.; Liu, F.; Li, Y.; Zhang, S. A study of crown physiological mechanisms for cold tolerance of different alfalfa varieties in Horqin sandy land. Acta Prataculturae Sin. 2019, 28, 87–95. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts: Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim. Feed Sci. Technol. 1997, 67, 319–338. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; do Amaral Júnior, A.T.; Vergara-Diaz, O.; Gracia-Romero, A.; Fernandez-Gallego, J.A.; Chang-Espino, M.C.; Buchaillot, M.L.; Rezzouk, F.Z.; Lima, V.J.D.; Serret, M.D. Heterosis and reciprocal effects for physiological and morphological traits of popcorn plants under different water conditions. Agric. Water Manag. 2022, 261, 107371. [Google Scholar] [CrossRef]
- Hu, S.L.; Wan, S.M.; Jia, Z.K.; Wang, Y. A study on photosynthetic characteristics of alfalfas grown for different lengths of time in the semi-humid region of the Loess Plateau. Acta Prataculturae Sin. 2008, 5, 60–67. [Google Scholar]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Deng, Y.P.; Wang, X.F.; Ni, Y.Y.; Wang, Q.; Xiao, W.F.; Lei, J.P.; Jiang, Z.P.; Li, M.H. The concentration of non-structural carbohydrates, N, and P in Quercus variabilis does not decline toward its northernmost distribution range along a 1500 km transect in China. Front. Plant Sci. 2018, 9, 1444. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.K.; Reddy, K.S.; Gautam, R.; Maddela, S.; Reddy, A.R.; Gudipalli, P. Improved photosynthetic characteristics correlated with enhanced biomass in a heterotic F1 hybrid of maize (Zea mays L.). Photosynth. Res. 2021, 147, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Zhou, X.Y.; Liu, J.F.; Qiu, Y.B.; Fan, F.F.; Xu, Q.G. Analysis of combining ability of photosynthetic characteristics in indica hybrid rice. J. Plant Genet. Resour. 2014, 15, 699–705. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Dong, W.Q.; Xu, X.M.; Tang, C.M. Heterosis effects on photosynthesis of upland cotton (Gossypium hirsutum) hybrid cultivars. Photosynthetica 2021, 59, 106–115. [Google Scholar] [CrossRef]
- Holá, D.; Benešová, M.; Fischer, L.; Haisel, D.; Hnilička, F.; Hniličková, H. The disadvantages of being a hybrid during drought: A combined analysis of plant morphology, physiology and leaf proteome in maize. PLoS ONE 2017, 12, e0176121. [Google Scholar] [CrossRef]
- Wu, Y.T.; Shi, F.L.; Xue, X.L.; Shi, F.L.; Cui, N. Photosynthetic physiological characteristics and heterosis of hybrids of alfalfa male sterile lines. Acta Agrestia Sin. 2018, 26, 741–747. [Google Scholar] [CrossRef]
- Liao, S.M.; Deng, Y.D.; Zhai, S.H.; Zhang, H.Q.; Shi, S.B. Correlations of the characteristics of photosynthates between female parent and F1 inter-subspecific hybrids of rice. Agric. Sci. Technol. 2016, 17, 1513–1518. [Google Scholar] [CrossRef]
- Li, D.W.; Lu, X.Y.; Zhu, Y.H.; Pan, J.; Zhou, S.Q.; Zhang, X.Y.; Zhu, G.T.; Shang, Y.; Huang, S.W.; Zhang, C.Z. The multi-omics basis of potato heterosis. J. Integr. Plant Biol. 2022, 64, 671–687. [Google Scholar] [CrossRef]
- Liu, X.D.; Wu, J.W.; Qasim, S. Development of neo-tetraploid rice and research progress on its heterosis mechanism. Biotechnol. Bull. 2022, 38, 44–50. [Google Scholar] [CrossRef]
- Li, Y.B.; Tao, F.L.; Hao, Y.F.; Tong, J.Y.; Xiao, Y.G.; He, Z.H.; Reynolds, M. Wheat traits and the associated loci conferring radiation use efficiency. Plant J. 2022, 112, 565–582. [Google Scholar] [CrossRef]
- Bai, P.; Bai, R.Q.; Jin, Y.X. Characteristics and coordination of source-sink relationships in super hybrid rice. Open Life Sci. 2016, 11, 470–475. [Google Scholar] [CrossRef]
- Wu, Z. Research on Physiological and Biochemical, Carbon and Nitrogen Metabolism Characteristics of Elymus sibiricus L. with Different Growth Years; Gansu Agricultural University: Lanzhou, China, 2020; pp. 38–50. [Google Scholar] [CrossRef]
- Huang, L.H.; Liu, H.L.; Chen, Y.; Li, X.H.; Zhang, H.; Zhao, Y. Comparative study of starch content in ginseng from different regions, years and growth periods. Liaoning J. Tradit. Chin. Med. 2013, 40, 1207–1209. [Google Scholar] [CrossRef]
- Pourtau, N.; Marès, M.; Purdy, S.; Quentin, N.; Ruël, A.; Wingler, A. Interactions of abscisic acid and sugar signalling in the regulation of leaf senescence. Planta 2004, 219, 765–772. [Google Scholar] [CrossRef]
- Mandić, V.; Bijelić, Z.; Krnjaja, V.; Petricevic, M.; Simic, A.; Krga, I. Trend analysis of harvested area, total production and yield of alfalfa in Vojvodina. Biotechnol. Anim. Husb. 2019, 35, 409–416. [Google Scholar] [CrossRef]
- Annicchiarico, P. Alfalfa forage yield and leaf/stem ratio: Narrow-sense heritability, genetic correlation, and parent selection procedures. Euphytica 2015, 205, 409–420. [Google Scholar] [CrossRef]
- Zhou, Z.B.; Zhang, Y.J.; Zhang, F.G. Community assembly correlates with alfalfa production by mediating rhizosphere soil microbial community composition in different planting years and regimes. Plant Soil. 2022, 479, 355–370. [Google Scholar] [CrossRef]
- Cai, L.; Luo, Z.; Wang, L.; Niu, Y.; Cai, L.; Xie, J. Meta-analysis of alfalfa yield and WUE response to growing ages in China. Acta Prataculturae Sin. 2020, 29, 27–38. [Google Scholar] [CrossRef]
- Woo, H.R.; Kim, H.J.; Nam, H.G.; Lim, P.O. Plant leaf senescence and death—Regulation by multiple layers of control and implications for aging in general. J. Cell Sci. 2013, 126, 4823–4833. [Google Scholar] [CrossRef]
- Zhao, Y.; Yi, Q.; Wei, X.; Xin, X.; Han, T.; Yue, K.; Wang, F.L. Role of NO-mediated Ca2+ signaling in regulation of photosynthesis and resistance to osmotic stress in alfalfa seedlings. Acta Prataculturae Sin. 2018, 27, 130–140. [Google Scholar] [CrossRef]
- Moinardeau, C.; Mesléard, F.; Ramone, H.; Dutoit, T. Extensive horse grazing improves grassland vegetation diversity, seed bank and forage quality of artificial embankments (Rhône River-southern France). J. Nat. Conserv. 2020, 56, 125865. [Google Scholar] [CrossRef]


| Categories | Characteristics | |
|---|---|---|
| Parental varieties | CK1 | Horizontal or oblique rhizomatous roots and strong extension habit; horizontal and sloping stems, slender and stiff stalks, lax plants and short plant height (62 cm); resistance to drought, cold temperatures, and trampling; hay yield (21,000 kg·h−1); germination rate (87%). |
| CK2 | Horizontal roots and strong extension habit; erect stems, thick stalks, high absolute plant height (71 cm); high adaptation, and high resistance to stress [16]; hay yield (27,000 kg·h−1); germination rate (95%). | |
| Hybrid strains | RSA−01 | Erect stems, with an angle of 70–80° between stem and ground; high absolute plant height (64 cm); germination rate (86%). |
| RSA−02 | Semi-horizontal stems, with an angle of 30–69° between stem and ground; high absolute plant height (64 cm); germination rate (87%). | |
| RSA−03 | Horizontal stems, with an angle of <30° between stem and ground; high absolute plant height (69 cm); germination rate (87%). | |
| Index | R | D.E. | I. E. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chla | Chlb | Chl(a+b) | Chl(a/b) | Car | Pn | Gs | Ci | WUE | Suc | SS | NSC | SP | |||
| Chla | 0.465 | −0.069 | 0.003 | 0.018 | −0.020 | −0.032 | −0.010 | −0.023 | −0.013 | 0.011 | 0.011 | −0.001 | −0.028 | 0.024 | |
| Chlb | −0.276 | 0.022 | −0.001 | −0.006 | 0.006 | 0.010 | 0.003 | 0.007 | 0.004 | −0.004 | −0.003 | 0.001 | 0.009 | −0.008 | |
| Chl(a+b) | −0.298 | 0.064 | −0.017 | 0.007 | −0.012 | −0.018 | 0.002 | −0.008 | −0.004 | 0.038 | 0.007 | −0.026 | 0.012 | 0.021 | |
| Chl(a/b) | 0.524 * | −0.179 | −0.051 | 0.171 | 0.033 | −0.040 | 0.025 | −0.010 | −0.022 | 0.0360 | 0.130 | 0.006 | −0.128 | 0.011 | |
| Car | 0.440 | −0.203 | −0.095 | 0.034 | 0.058 | −0.045 | −0.009 | −0.069 | 0.044 | −0.031 | −0.030 | −0.095 | −0.067 | −0.003 | |
| Pn | 0.378 | −0.221 | −0.031 | −0.038 | −0.007 | 0.031 | −0.010 | −0.144 | −0.004 | −0.039 | −0.002 | 0.043 | −0.018 | 0.108 | |
| Gs | 0.864 ** | 0.978 | 0.321 | 0.005 | −0.118 | 0.057 | 0.333 | 0.638 | −0.241 | 0.129 | 0.064 | −0.222 | 0.065 | −0.765 | |
| Ci | −0.048 | −0.251 | −0.048 | 0.018 | 0.016 | −0.030 | 0.055 | −0.004 | 0.062 | 0.166 | 0.080 | −0.042 | −0.029 | 0.028 | |
| WUE | −0.083 | −0.382 | 0.060 | −0.050 | −0.226 | 0.077 | −0.058 | −0.067 | −0.050 | 0.252 | −0.136 | 0.077 | −0.084 | −0.149 | |
| Suc | −0.170 | 0.048 | −0.007 | 0.031 | 0.005 | −0.035 | 0.007 | 0.001 | 0.003 | −0.015 | 0.017 | 0.009 | −0.025 | 0.010 | |
| SS | 0.040 | 0.524 | 0.008 | 0.008 | −0.209 | −0.019 | 0.246 | −0.102 | −0.119 | 0.088 | −0.105 | 0.094 | −0.101 | 0.177 | |
| NSC | 0.328 | 0.772 | 0.310 | −0.499 | 0.148 | 0.550 | 0.252 | 0.062 | 0.051 | 0.090 | 0.171 | −0.399 | -0.149 | 0.089 | |
| SP | −0.697 ** | −0.307 | 0.086 | 0.012 | −0.082 | 0.015 | −0.004 | 0.121 | 0.193 | 0.028 | −0.097 | −0.049 | −0.084 | −0.028 | |
| Index | R | D.E. | I. E. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chla | Chlb | Chl(a+b) | Car | Tr | Gs | Ci | WUE | Suc | SS | NSC | SP | |||
| Chla | −0.377 | −0.284 | −0.019 | −0.068 | 0.133 | −0.036 | −0.022 | 0.046 | 0.046 | 0.009 | −0.028 | 0.071 | 0.103 | |
| Chlb | −0.141 | −0.370 | −0.025 | −0.089 | 0.173 | −0.047 | −0.029 | 0.060 | 0.060 | 0.012 | −0.037 | 0.093 | 0.134 | |
| Chl(a+b) | −0.395 | 0.468 | 0.112 | 0.347 | −0.182 | 0.053 | −0.086 | 0.121 | −0.102 | −0.161 | 0.165 | 0.082 | −0.138 | |
| Car | 0.489 | 0.171 | −0.080 | −0.074 | −0.066 | −0.007 | 0.020 | −0.002 | −0.026 | −0.036 | −0.076 | 0.095 | −0.007 | |
| Tr | 0.241 | −0.151 | −0.019 | −0.072 | −0.017 | 0.006 | −0.124 | −0.030 | 0.021 | 0.048 | 0.092 | −0.013 | 0.119 | |
| Gs | 0.660 ** | 1.363 | 0.106 | 0.319 | −0.249 | 0.157 | 1.116 | −0.098 | 0.333 | 0.027 | −0.987 | 0.142 | −0.946 | |
| Ci | −0.193 | −0.112 | 0.018 | −0.043 | −0.029 | 0.001 | −0.022 | 0.008 | 0.025 | 0.052 | −0.017 | −0.062 | 0.004 | |
| WUE | 0.506 | 0.290 | −0.047 | 0.044 | −0.063 | −0.044 | −0.041 | 0.071 | −0.065 | 0.154 | −0.002 | −0.022 | 0.082 | |
| Suc | 0.239 | −0.236 | 0.007 | 0.080 | 0.081 | 0.049 | 0.074 | −0.005 | 0.110 | −0.125 | 0.008 | 0.149 | −0.088 | |
| SS | −0.673 ** | −0.240 | −0.024 | −0.041 | −0.085 | 0.107 | 0.146 | 0.174 | −0.037 | 0.001 | 0.008 | 0.007 | −0.091 | |
| NSC | 0.257 | −0.148 | 0.037 | −0.032 | −0.026 | −0.082 | −0.013 | −0.015 | −0.081 | 0.011 | 0.094 | 0.004 | 0.028 | |
| SP | −0.117 | 0.188 | −0.068 | −0.084 | −0.056 | −0.008 | −0.149 | −0.131 | −0.007 | 0.053 | 0.070 | 0.071 | −0.036 | |
| Index | Genetic Differences | Interannual Variation | Genetic Differences × Interannual Variation |
|---|---|---|---|
| Forage mass | 1.241 | 7.748 * | 0.100 |
| Chla | 2.290 | 0.260 | 6.840 * |
| Chlb | 4.568 * | 8.713 * | 1.271 |
| Chl (a+b) | 0.049 | 0.902 | 0.143 |
| Chl(a/b) | 1.668 | 8.050 * | 3.628 |
| Pn | 0.240 | 16.836 * | 0.323 |
| Car | 6.141 * | 0.523 | 4.476 * |
| Tr | 0.114 | 10.969 * | 0.233 |
| Gs | 0.133 | 8.161 * | 0.002 |
| WUE | 2.470 | 1.530 | 0.054 |
| Ci | 5.607 * | 20.063 * | 0.710 |
| Sta | 59.064 * | 7.756 * | 6.027 * |
| Suc | 16.419 * | 26.973 * | 0.587 |
| SS | 0.854 | 6.359 * | 0.033 |
| NSC | 56.350 * | 12.975 * | 6.478 * |
| SP | 0.001 | 10.164 * | 0.184 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
A, Y.; Shi, S.; Zhang, J.; Li, X.; Jing, F.; Zhang, H.; Ma, R. Photosynthetic Physiological Basis of Forage Mass Stability in a Progeny of Rhizome-Rooted ‘Qingshui’ Medicago sativa L. Agronomy 2023, 13, 685. https://doi.org/10.3390/agronomy13030685
A Y, Shi S, Zhang J, Li X, Jing F, Zhang H, Ma R. Photosynthetic Physiological Basis of Forage Mass Stability in a Progeny of Rhizome-Rooted ‘Qingshui’ Medicago sativa L. Agronomy. 2023; 13(3):685. https://doi.org/10.3390/agronomy13030685
Chicago/Turabian StyleA, Yun, Shangli Shi, Jinqing Zhang, Xiaolong Li, Fang Jing, Huihui Zhang, and Ruihong Ma. 2023. "Photosynthetic Physiological Basis of Forage Mass Stability in a Progeny of Rhizome-Rooted ‘Qingshui’ Medicago sativa L." Agronomy 13, no. 3: 685. https://doi.org/10.3390/agronomy13030685
APA StyleA, Y., Shi, S., Zhang, J., Li, X., Jing, F., Zhang, H., & Ma, R. (2023). Photosynthetic Physiological Basis of Forage Mass Stability in a Progeny of Rhizome-Rooted ‘Qingshui’ Medicago sativa L. Agronomy, 13(3), 685. https://doi.org/10.3390/agronomy13030685





