Morphological Characterization of Metamorphosis in Stamens of Anemone barbulata Turcz. (Ranunculaceae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Environmental Characteristics of Collection Sites
2.2. Morphological Observations of Floral Organs
2.3. Scanning Electron Microscope Observations of Floral Organs
2.4. Histological Examinations of Floral Organs
3. Results
3.1. Morphological Characteristics of Normal Flowers and Stamens in Anemone barbulata
3.2. Morphological Characteristics of Abnormal Stamens in Anemone barbulata
3.2.1. Variations in Number, Size, and Color of Abnormal Stamens
3.2.2. Variations in Shape of Abnormal Stamens
3.2.3. Variations in Venations, Stomata, Trichomes, and Persistence Patterns of Abnormal Stamens
3.3. Histological Analysis of Abnormal Stamens
4. Discussion
4.1. Metamorphic Variations of Stamens in Anemone barbulata
4.1.1. Characterization and Variation Patterns of Metamorphic Stamens
4.1.2. Metamorphic Characterization of Anthers
4.1.3. Homeosis in Floral Organs
4.2. Possible Mechanisms of Metamorphosis in Stamens of Anemone barbulata
4.3. Persistence Patterns of Stamens in Anemone barbulata
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolff, C.F. Theoria Generationis Editio Nova; I.C. Hendel: Halle, Germany, 1774. [Google Scholar]
- von Goethe, J.W. Versuch die Metamorphose der pflanzen zu erklären. An Attempt to Interpret the Metamorphosis of Plants Gotha: Ettinger, C.W. Transl. Arber A. 1946. Goethe’s botany. Chron. Bot. 1790, 10, 63–126. [Google Scholar]
- de Candolle, A.P. Organographie Végétale; Deterville: Paris, French, 1827. [Google Scholar]
- Brongniart, A. Examen de quelques cas de monstruosités végétales propres àéclairer la structure du pistil et l’origine des ovules. Ann. Sci. Nat. Bot. Sér. 1844, 3, 20–32. [Google Scholar]
- Masters, M.T. Vegetable Teratology, and Account of the Principal Deviations from the Usual Construction of Plants; Ray Society: London, UK, 1869. [Google Scholar]
- Reynolds, J.; Tampion, J. Double Flowers: A Scientific Study; Van Nostrand Reinhold: New York, NY, USA, 1983. [Google Scholar]
- Leavitt, R.G. A vegetative mutant, and the principle of homoeosis in plants. Bot. Gaz. 1909, 47, 30–68. [Google Scholar] [CrossRef]
- Meyer, V.G. Flower abnormalities. Bot. Rev. 1966, 32, 165–218. [Google Scholar] [CrossRef]
- Bateson, W.; Bateson, A. On variations in floral symmetry in certain plants having irregular corollas. Linn. J. Bot. 1891, 28, 386–424. [Google Scholar] [CrossRef]
- Coen, E.S.; Meyerowitz, E.M. The war of the whorls: Genetic interactions controlling flower development. Nature 1991, 353, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Mabberley, D.J.; Hay, A. Homoeosis, canalization, decanalization, ‘characters’ and angiosperm origins. Edinb. J. Bot. 1994, 51, 117–126. [Google Scholar] [CrossRef]
- Lehmann, N.L.; Sattler, R. Floral development and homeosis in Actaea rubra (Ranunculaceae). Int. J. Plant Sci. 1994, 155, 658–671. [Google Scholar] [CrossRef]
- De Craene, L.P.R.; Soltis, P.S.; Soltis, D.E. Evolution of floral structures in basal angiosperms. Int. J. Plant Sci. 2003, 164, S329–S363. [Google Scholar] [CrossRef]
- Pelaz, S.; Ditta, G.S.; Baumann, E.; Wisman, E.; Yanofsky, M.F. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 2000, 405, 200–203. [Google Scholar] [CrossRef]
- Glover, B. Historical interpretations of flower induction and flower development. In Understanding Flowers and Flowering, 2nd ed.; Oxford Academic: Oxford, UK, 2014. [Google Scholar]
- Jabbour, F.; Nadot, S.; Espinosa, F.; Damerval, C. Ranunculacean flower terata: Records, a classification, and some clues about floral developmental genetics and evolution. Flora 2016, 221, 54–64. [Google Scholar] [CrossRef]
- Bowman, J.L.; Smyth, D.R.; Meyerowitz, E.M. Genes directing flower development in Arabidopsis. Plant Cell 1989, 1, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.; Coen, E.S. Floral homeotic mutations produced by transposon- mutagenesis in Antirrhinum majus. Genes Dev. 1990, 4, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Weigel, D.; Meyerowitz, E.M. The ABC’s of floral homeotic genes. Cell 1994, 78, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Theissen, G.; Saedler, H. Plant biology—Floral quartets. Nature 2001, 409, 469–471. [Google Scholar] [CrossRef]
- De Craene, L.P.R. The evolutionary significance of homeosis in flowers: A morphological perspective. Int. J. Plant Sci. 2003, 164, S225–S235. [Google Scholar] [CrossRef]
- Rudall, P.J.; Bateman, R.M. Evolutionary change in flowers and inflorescences: Evidence from naturally occurring terata. Trends Plant Sci. 2003, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.L.; Ren, Y.; Feng, L.T. Morphological observations on metamorphosed sepals in Anemone rivularis var. flore-minore (Ranunculaceae). Acta Phytotax. Sin. 2005, 43, 225–232. [Google Scholar]
- Bateman, R.M.; Rudall, P.J. The good, the bad and the ugly: Using naturally occurring terata to distinguish the possible from the impossible in the orchid floral evolution. Aliso 2006, 22, 481–496. [Google Scholar] [CrossRef]
- Nutt, P.; Ziermann, J.; Hintz, M.; Neuffer, B.; Theißen, G. Capsella as a model system to study the evolutionary relevance of floral homeotic mutants. Plant Syst. Evol. 2006, 259, 217–235. [Google Scholar] [CrossRef]
- Hameister, S.; Nutt, P.; Theißen, G.; Neuffer, B. Mapping a floral trait in Shepherds purse—‘Stamenoid petals’ in natural populations of Capsella bursa-pastoris (L.) Medik. Flora 2013, 208, 641–647. [Google Scholar] [CrossRef]
- Salamah, A.; Prihatiningsih, R.; Rostina, I.; Dwiranti, A. Comparative morphology of single and double flowers in Hibiscus rosa-sinensis L. (Malvaceae): A homeosis study. AIP Conf. Proc. 2018, 2023, 020136. [Google Scholar] [CrossRef]
- Hintz, M.; Bartholmes, C.; Nutt, P.; Ziermann, J.; Hameister, S.; Neuffer, B.; Theißen, G. Catching a “hopeful monster”: Shepherd’s purse (Capsella bursa-pastoris) as a model system to study the evolution of flower development. J. Exp. Bot. 2006, 57, 3531–3542. [Google Scholar] [CrossRef]
- Hameister, S.; Neuffer, B.; Bleeker, W. Genetic differentiation and reproductive isolation of a naturally occurring floral homeotic mutant within a wild-type population of Capsella bursa-pastoris (Brassicaceae). Mol. Ecol. 2009, 18, 2659–2667. [Google Scholar] [CrossRef]
- Hameister, S. Ecological and Molecular Characterisation of a Naturally Occurring Floral Homeotic Variant of Capsella bursa-pastoris (L.) Medik. Ph.D. Dissertation, Osnabrück University, Lower Saxony, Germany, 2009. [Google Scholar]
- Hameister, S.; Neuffer, B. Establishment of a natural floral variant of Shepherd’s purse in the wild: Analysis of life-history traits in ‘Capsella apetala’ (Brassicaceae). Plant Ecol. Evol. 2017, 150, 67–75. [Google Scholar] [CrossRef]
- Kasianov, A.S.; Klepikova, A.V.; Kulakovskiy, I.V.; Gerasimov, E.S.; Fedotova, A.V.; Besedina, E.G.; Kondrashov, A.S.; Logacheva, M.D.; Penin, A.A. High-quality genome assembly of Capsella bursa-pastoris reveals asymmetry of regulatory elements at early stages of polyploid genome evolution. Plant J. 2017, 91, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Ziermann, J.; Ritz, M.S.; Hameister, S.; Abel, C.; Hoffmann, M.H.; Neuffer, B.; Theissen, G. Floral visitation and reproductive traits of Stamenoid petals, a naturally occurring floral homeotic variant of Capsella bursa-pastoris (Brassicaceae). Planta 2009, 230, 1239–1249. [Google Scholar] [CrossRef]
- Maximowicz, C.J. Flora Tangutica; 1889; p. 6. [Google Scholar]
- Liu, H.Q.; Di, W.Z. Morphological studies on the metamorphosed flowers of Anemone rivularis var. flore-minore Maxim. I. Morphological observation on the metamorphosed flowers. Acta Bot. Boreali-Occident. Sin. 1991, 11, 292–298. [Google Scholar]
- Decraene, L.P.R.; Smets, E.F. Evolution of the androecium in the Ranunculiflorae. Plant Syst. Evol. 1995, 9, 63–70. [Google Scholar] [CrossRef]
- Pi, M.; Hu, S.; Cheng, L.; Zhong, R.; Cai, Z.; Liu, Z.; Yao, J.-L.; Kang, C. The MADS-box gene FveSEP3 plays essential roles in flower organogenesis and fruit development in woodland strawberry. Hortic. Res. 2021, 8, 247. [Google Scholar] [CrossRef]
- Lin, Z.; Damaris, R.N.; Shi, T.; Li, J.; Yang, P. Transcriptomic analysis identifies the key genes involved in stamen petaloid in lotus (Nelumbo nucifera). BMC Genom. 2018, 19, 554. [Google Scholar] [CrossRef] [PubMed]
- Turczaninow, N.S. Naturalistes Moscou. Bull. Soc. Imp. 1837, 10, 149. [Google Scholar]
- Govaerts, R. World Checklist of Seed Plants; MIM: Antwerp, Belgium, 1995; Volume 1, pp. 1–483, 529. [Google Scholar]
- Wang, W.T. A new classification of Anemone (Ranunculaceae) of China. Guihaia 2021, 41, 1–118. [Google Scholar]
- Chang, H.L.; Ren, Y.; Lu, A.M. Floral morphogenesis of Anemone rivularis var. flore-minore (Ranunculaceae) with special emphasis on androecium development sequence. J. Integr. Plant Biol. 2005, 47, 257–263. [Google Scholar] [CrossRef]
- Espinosa, F.; Damerval, C.; Guilloux, M.L.; Deroin, T.; Wang, W.; Pinedo-Castro, M.; Nadot, S.; Jabbour, F. Homeosis and delayed floral meristem termination could account for abnormal flowers in cultivars of Delphinium and Aquilegia (Ranunculaceae). Bot. J. Linn. Soc. 2021, 195, 485–500. [Google Scholar] [CrossRef]
- Endress, P.K. The Chloranthaceae: Reproductive structures and phylogenetic position. Bot. Jahrb. Syst. 1987, 109, 153–226. [Google Scholar]
- Endress, P.K. The Flowers in Extant Basal Angiosperms and Inferences on Ancestral Flowers. Int. J. Plant Sci. 2001, 162, 1111–1140. [Google Scholar] [CrossRef]
- Kong, H.Z.; Lu, A.M.; Endress, P.K. Floral organogenesis of Chloranthus sessilifolius, with special emphasis on the morphological nature of the androecium of Chloranthus (Chloranthaceae). Plant Syst. Evol. 2002, 232, 181–188. [Google Scholar] [CrossRef]
- Kong, H.Z.; Chen, Z.D.; Lu, A.M. Phylogeny of Chloranthus (Chloranthaceae) based on nuclear ribosomal ITS and plastid trnLF sequence data. Am. J. Bot. 2002, 89, 940–946. [Google Scholar] [CrossRef]
- Doyle, J.A.; Eklund, H.; Herendeen, P.S. Floral evolution in Chloranthaceae: Implications of a morphological phylogenetic analysis. Int. J. Plant Sci. 2003, 164, S365–S382. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group. An ordinal classification for the families of flowering plants. Ann. Mo. Bot. Gard. 1998, 85, 531–553. [Google Scholar] [CrossRef]
- Lehmann, N.L.; Sattler, R. Homeosis in floral development of Sanguinaria canadensis and S. canadensis “Multiplex” (Papaveraceae). Am. J. Bot. 1993, 80, 1323–1335. [Google Scholar] [CrossRef]
- Tian, X.H.; Zhao, L.; Ren, Y.; Zhang, X.H. Number of floral organs in Circaeaster agrestis (Circaeasteraceae) and possible homeosis among floral organs. Plant Syst. Evol. 2007, 265, 259–265. [Google Scholar] [CrossRef]
- Fisher, J.E. The Transformation of Stamens to Ovaries and of Ovaries to Inflorescences in Triticum aestivum L. Under Short-Day Treatment. Bot. Gaz. 1972, 133, 78–85. [Google Scholar] [CrossRef]
- Sattler, R. Homeosis in plants. Am. J. Bot. 1988, 75, 1606–1617. [Google Scholar] [CrossRef]
- Meyerowitz, E.M.; Smyth, D.R.; Bowman, J.L. Abnormal flowers and pattern formation in floral development. Development 1989, 106, 209–217. [Google Scholar] [CrossRef]
- Canright, J.E. The comparative morphology and relationships of the Magnoliaceae. I. Thends of specialization in the stamens. Am. J. Bot. 1952, 39, 484–497. [Google Scholar] [CrossRef]
- Eames, A.J. The flower. In Morphology of the Angiosperms; McGraw-Hill Publishing Company, Inc.: New York, NY, USA, 1961; pp. 87–95. [Google Scholar]
- Savidge, J.P. The angiosperm flower and its structure. In Plant Structure, Function and Adaptation; Hall, M.A., Ed.; MacMillan Press: New York, NY, USA, 1976; pp. 221–248. [Google Scholar]
- Dornelas, M.C.; Dornelas, O.A.A. From leaf to flower: Revisiting Goethe’s concepts on the “metamorphosis” of plants. Braz. J. Plant Physiol. 2005, 17, 335–344. [Google Scholar] [CrossRef]
- Moschin, S.; Nigris, S.; Ezquer, I.; Masiero, S.; Cagnin, S.; Cortese, E.; Colombo, L.; Casadoro, G.; Baldan, B. Expression and Functional Analyses of Nymphaea caerulea MADS-Box Genes Contribute to Clarify the Complex Flower Patterning of Water Lilies. Front. Plant Sci. 2021, 12, 730270. [Google Scholar] [CrossRef]
- Martinez, E.; Ramos, C.H. Lacandoniaceae (Triuridales): Una nueva familia de Mexico. Ann. Mo. Bot. Gard. 1989, 76, 128–135. [Google Scholar] [CrossRef]
- Smith-Huerta, N.L. A comparison of floral development in wild type and a homeotic sepaloid petal mutant of Clarkia tembloriensis (Onagraceae). Am. J. Bot. 1992, 79, 1423–1430. [Google Scholar] [CrossRef]
- Hill, J.P.; Lord, E.M. Floral development in Arabidopsis thaliana: A comparison of the wild type and the homeotic pistillata mutant. Can. J. Bot. 1989, 67, 2922–2936. [Google Scholar] [CrossRef]
- Zhang, T.; Xing, N.; Wang, C.; Liu, H.Q. Cloning and sequence analysis of AP3-3 gene in normal plant and natural variant from Anemone rivularis var. floral-minore. Acta Bot. Boreali-Occident. Sin. 2016, 36, 0231–0240. [Google Scholar]
- Rong, L.Q.; Li, X.D.; Liu, H.Q. Transcriptome analysis of Anemone rivularis var. floral-minore based on High-throughput sequencing technology and flower development gene screening. Acta Bot. Boreali-Occident. Sin. 2018, 38, 437–442. [Google Scholar]
- Bull-Hereñu, K.; dos Santos, P.; Toni, J.F.G.; El Ottra, J.H.L.; Thaowetsuwan, P.; Jeiter, J.; Ronse De Craene, L.P.; Iwamoto, A. Mechanical Forces in Floral Development. Plants 2022, 11, 661. [Google Scholar] [CrossRef] [PubMed]
- Green, P.B. Expression of pattern in plants: Combining molecular and calculus-based biophysical paradigms. Am. J. Bot. 1999, 86, 1059–1076. [Google Scholar] [CrossRef]
- Hamant, O.; Heisler, M.G.; Jönsson, H.; Krupinski, P.; Uyttewaal, M.; Bokov, P.; Corson, F.; Sahlin, P.; Boudaoud, A.; Meyerowitz, E.M. Developmental patterning by mechanical signals in Arabidopsis. Science 2008, 322, 1650–1655. [Google Scholar] [CrossRef]
- Ronse de Craene, L.P. Understanding the role of floral development in the evolution of angiosperm flowers: Clarifications from a historical and physico-dynamic perspective. J. Plant Res. 2018, 131, 367–393. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Ito, Y. Molecular mechanisms controlling plant organ abscission. Plant Biotechnol. 2013, 30, 209–216. [Google Scholar] [CrossRef]
- Santiago, J.; Brandt, B.; Wildhagen, M.; Hohmann, U.; Hothorn, L.A.; Butenko, M.A.; Hothorn, M. Mechanistic insight into a peptide hormone signaling complex mediating floral organ abscission. eLife 2016, 5, e15075. [Google Scholar] [CrossRef]
- Geng, R.; Shan, Y.; Li, L.; Shi, C.-L.; Zhang, W.; Wang, J.; Sarwar, R.; Xue, Y.-X.; Li, Y.-L.; Zhu, K.-M.; et al. CRISPR-mediated BnaIDA editing prevents silique shattering, floral organ abscission, and spreading of Sclerotinia sclerotiorum in Brassica napus. Plant Commun. 2022, 3, 100452. [Google Scholar] [CrossRef] [PubMed]
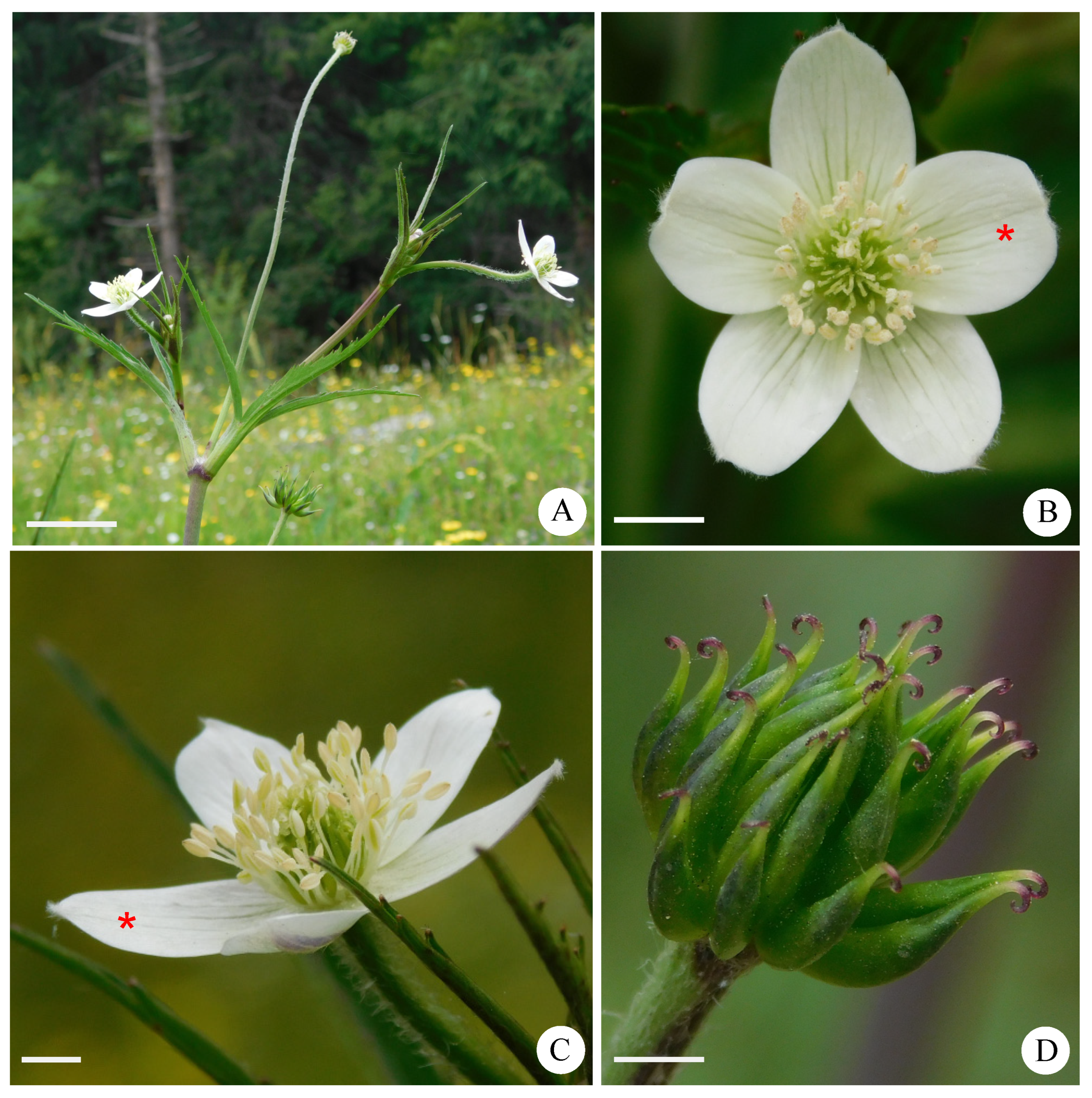
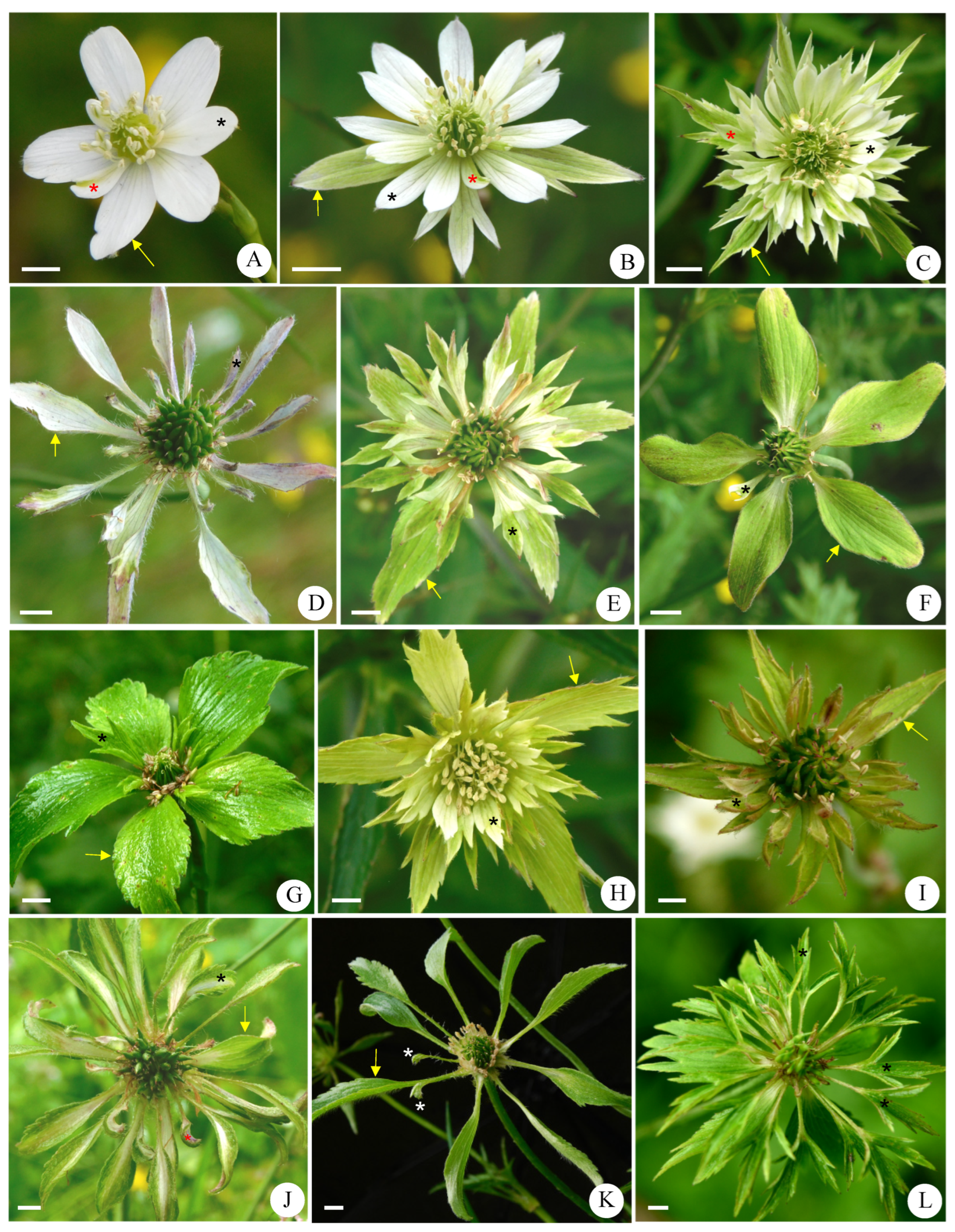
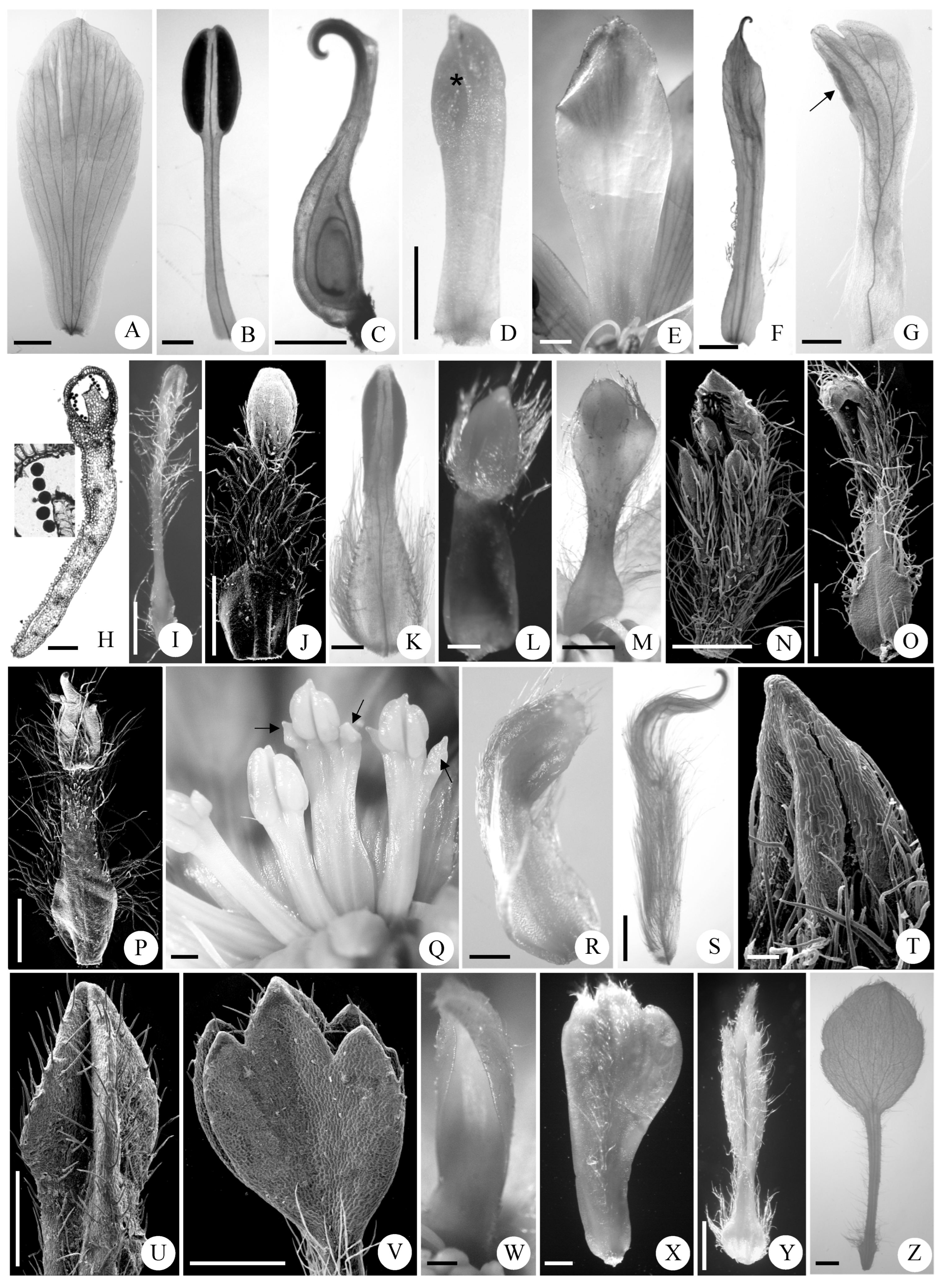
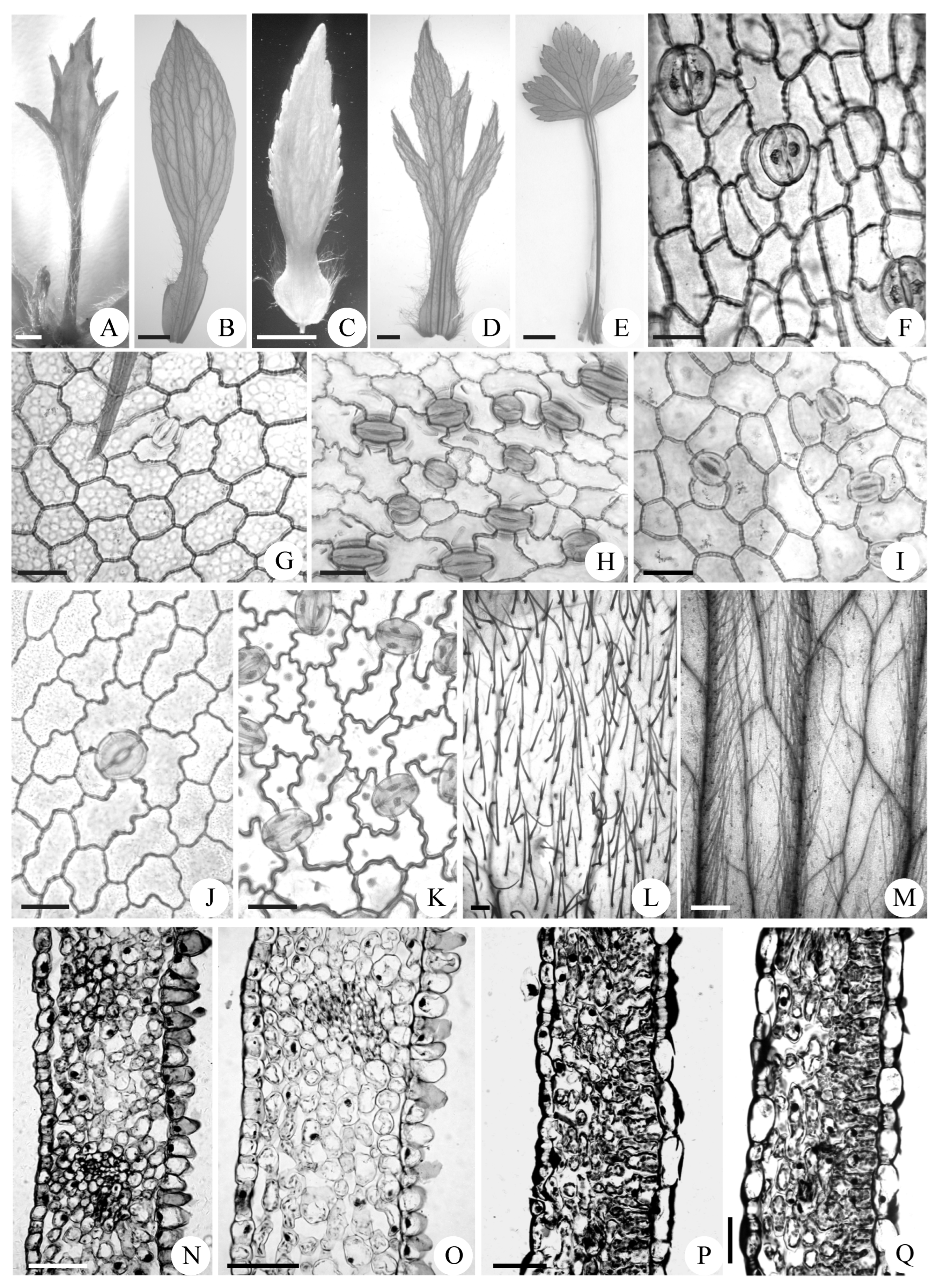
| Characteristics | Mt. Zhegushan | Taibai Mountain | Meixian County | Foping County |
|---|---|---|---|---|
| Environmetnal condition | ||||
| Area (m2) ** | 300 | 150 | 100 | 50 |
| Altitude (m) ** | 3100–3200 | 1800–2100 | 1900–2000 | 1900–2000 |
| Average annual temperature (°C) ** | 6.1 | 6.2 | 14.6 | 11.6 |
| Average annual rainfall (mm) ** | 770 | 875 | 610 | 940 |
| Drought season | May to June | November to February | November to February | November to February |
| Average annual humidity (%) ** | 50 | 75 | 65 | 55 |
| Floral characteristics | ||||
| Number of plants examined | 125 | 48 | 34 | 16 |
| Number of plants with only normal flower * | 19 | 10 | 6 | 3 |
| Number of plants with abnormal flower/Number of plants examined (%) * | 85 | 79 | 82 | 81 |
| Number of abnormal flowers/Number of total flowers per plant (%) * | 72 | 56 | 66 | 60 |
| Number of abnormal flowers/Number of total flowers per collection site (%) * | 80 | 73 | 72 | 75 |
| Number of sepals of 35 flowers (range per flower) * | 189 (5 to 7) | 184 (5 to 7) | 188 (5 to 7) | 189 (5 to 7) |
| Number of sepals per flower ± SD (n = 35) * | 5.4 ± 0.6 | 5.3 ± 0.6 | 5.4 ± 0.6 | 5.4 ± 0.7 |
| Number of stamens of 35 flowers (range per flower) * | 1752 (25 to 90) | 1725 (27 to 88) | 1786 (29 to 83) | 1696 (29 to 95) |
| Number of stamens per flower ± SD (n = 35) * | 50.1 ± 15.8 | 49.3 ± 13.9 | 51.0 ± 12.6 | 48.5 ± 14.3 |
| Number of carpels of 35 flowers (range per flower) * | 1264 (22 to 64) | 1329 (25 to 54) | 1294 (18 to 58) | 1280 (21 to 54) |
| Number of carpels per flower ± SD (n = 35) * | 36.1 ± 9.3 | 38.0 ± 7.6 | 37.0 ± 9.0 | 36.6 ± 8.2 |
| Observation/collection date | 3–9 July 2017; 6–8 July 2019 | 18–20 July 2017 | 26–28 July 2017; 1–4 July 2018 | 10–13 July 2019 |
| Voucher specimen | Chang Hong-Li 201,701 to 201,714 and 201,901 to 201,910 | Chang Hong-Li 201,715 to 201,717 | Chang Hong-Li 201,718 to 201,720 | Chang Hong-Li 201,911 to 201,915 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.; Ji, W.; Xie, Y.; He, S.; Xie, Z.; Sun, F. Morphological Characterization of Metamorphosis in Stamens of Anemone barbulata Turcz. (Ranunculaceae). Agronomy 2023, 13, 554. https://doi.org/10.3390/agronomy13020554
Chang H, Ji W, Xie Y, He S, Xie Z, Sun F. Morphological Characterization of Metamorphosis in Stamens of Anemone barbulata Turcz. (Ranunculaceae). Agronomy. 2023; 13(2):554. https://doi.org/10.3390/agronomy13020554
Chicago/Turabian StyleChang, Hongli, Weihong Ji, Yule Xie, Shujun He, Zhenfeng Xie, and Fengjie Sun. 2023. "Morphological Characterization of Metamorphosis in Stamens of Anemone barbulata Turcz. (Ranunculaceae)" Agronomy 13, no. 2: 554. https://doi.org/10.3390/agronomy13020554
APA StyleChang, H., Ji, W., Xie, Y., He, S., Xie, Z., & Sun, F. (2023). Morphological Characterization of Metamorphosis in Stamens of Anemone barbulata Turcz. (Ranunculaceae). Agronomy, 13(2), 554. https://doi.org/10.3390/agronomy13020554






