Extracellular DNA of Fusarium oxysporum f. sp. cubense as a Priming Agent for Inducing the Resistance of Banana Plantlets
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Foc TR4 eDNA and Banana Plantlets
2.2. Self-Inhibition Test
2.3. eDNA Treatment and Plantlet Resistance Test towards Foc TR4
2.4. Morphological Observation and Disease Index Determination
2.5. Detection of Superoxide and Hydrogen Peroxide in Plantlets
2.6. Gene Expression Analysis
2.7. Statistical Analysis
3. Results
3.1. Exposure to Fragmented Self-DNA Limited Growth of Fusarium oxysporum (Foc) TR4
3.2. The Application of Foc TR4 eDNA Increases Resistance in Banana Plantlets
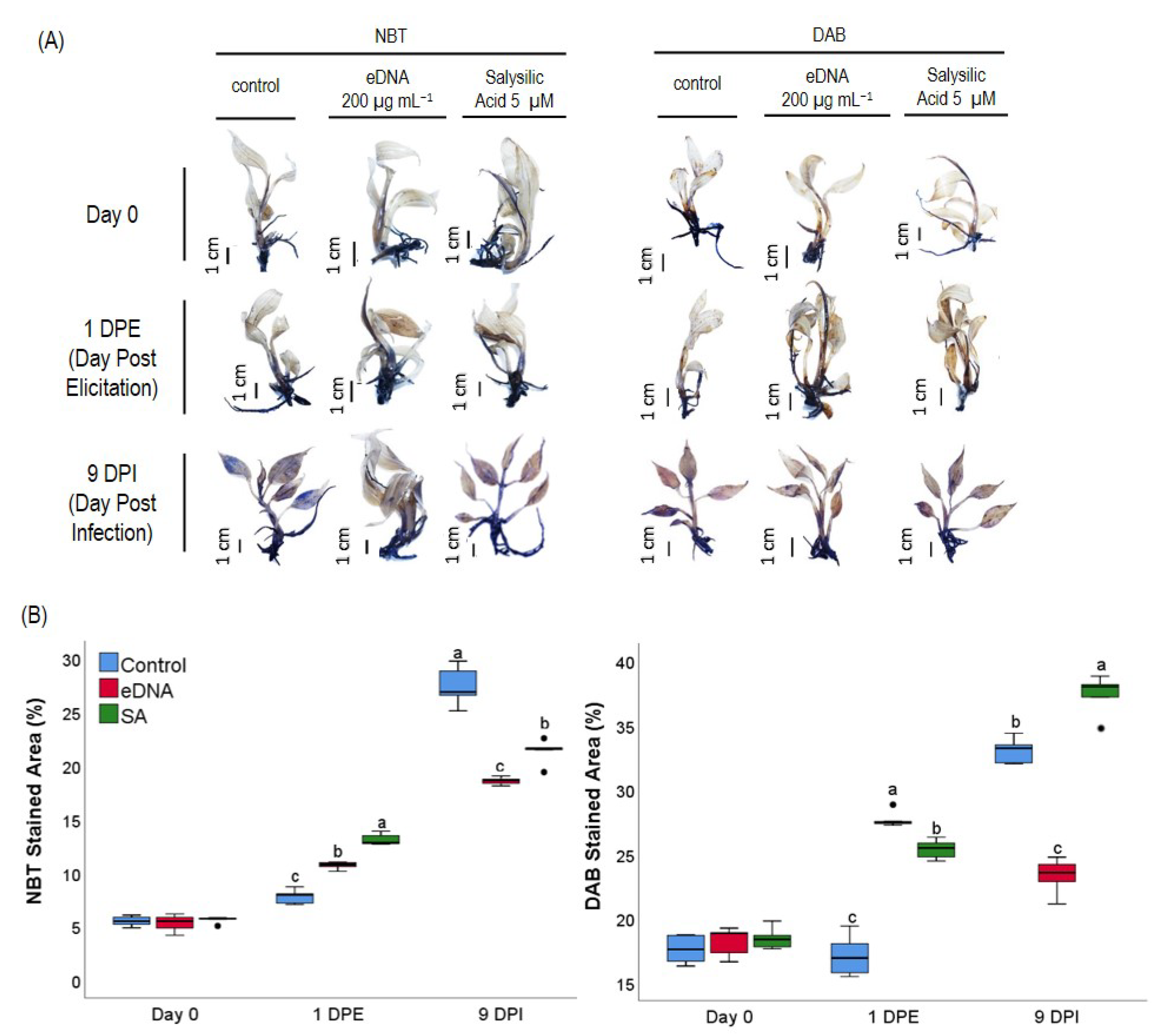
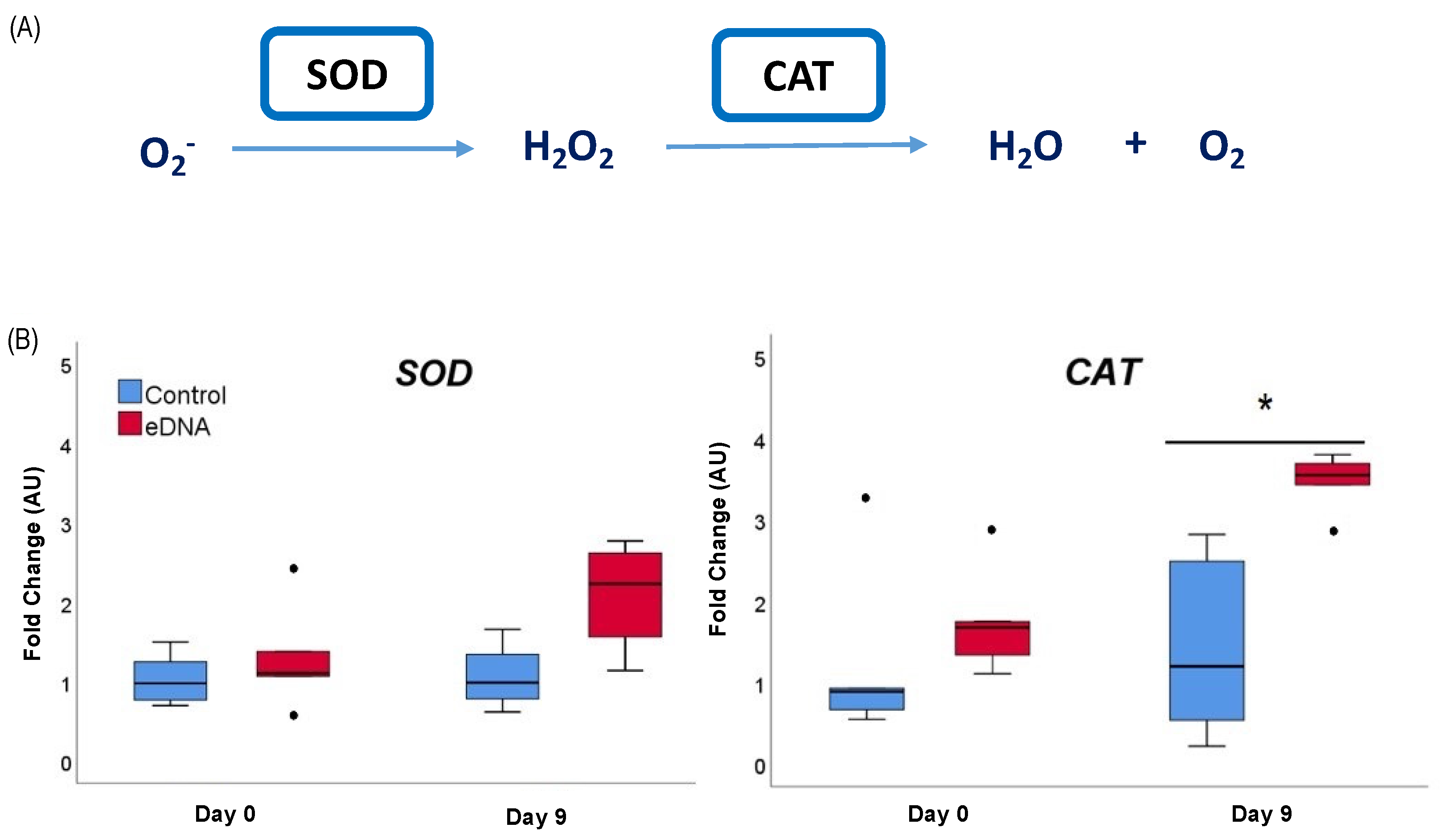
3.3. eDNA of Foc TR4 Modulated ROS (O2− and H2O2) Production and Expression of the Gene Coding for Antioxidant Enzymes in Banana Plantlets
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No | Accession Number | Gene Name | Score | E-Value | Ident. |
|---|---|---|---|---|---|
| 1 | EF055881.2 | Musa acuminata pathogenesis-related protein 1 (PR1) cds | 185 | 2 × 10−43 | 97% |
 | |||||
| 2 | FJ040801.1 | Musa acuminata AAA Group chitinase I mRNA | 127 | 6 × 10−29 | 98% |
 | |||||
| 3 | AF510071.1 | Musa acuminata superoxide dismutase mRNA. Partial cds | 383 | 10−107 | 96% |
 | |||||
| 4 | HM061079.1 | Musa acuminata AAA Group catalase (CAT) mRNA partial cds | 104 | 2 × 10−18 | 98% |
 | |||||
| 5 | AY821550.1 | Musa acuminata glyceraldehyde-3-phosphate dehydrogenase mRNA, partial cds | 147 | 10−31 | 100% |
 | |||||
| Treatment | Colony Count |
|---|---|
| Control | 635 ± 111.25 c |
| eDNA 80 µg mL−1 | 557 ± 85 bc |
| eDNA 400 µg mL−1 | 552 ± 48 b |
| eDNA 800 µg mL−1 | 337 ± 47 a |
References
- Nelson, S.C.; Ploetz, R.C.; Kepler, A.K.; Daniells, J. Species profiles for pacific island agroforestry Musa species (banana and plantain). Species Profiles Pac. Isl. Agrofor 2006, 2, 1–27. Available online: http://www.traditionaltree.org (accessed on 15 November 2019).
- FAO. Banana Market Review 2021; FAO: Rome, Italy, 2022; Volume 5. [Google Scholar]
- FAO. Food Outlook—Biannual Report on Global Food Markets; FAO: Rome, Italy, 2019; pp. 5–7. [Google Scholar]
- Aquino, A.P.; Bandoles, G.G.; Lim, V.A.A. R&D and Policy Directions for Effective Control of Fusarium Wilt Disease of Cavendish Banana in the Asia-Pacific Region. FFTC Agricultural Policy Platform (FFTC-AP). 2013. Available online: https://ap.fftc.org.tw/article/598 (accessed on 15 November 2019).
- Swarupa, V.; Ravishankar, K.V.; Rekha, A. Plant defense response against Fusarium oxysporum and strategies to develop tolerant genotypes in banana. Planta 2014, 239, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.G.; Staver, C.P. Fusarium wilt of banana: Current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci. 2018, 871, 1468. [Google Scholar] [CrossRef] [PubMed]
- Dinas Pertanian Kabupaten Buleleng. Gejala dan Cara Pengendalian Penyakit Layu Fusarium Pada Tanaman Pisang. 2019. Available online: https://distan.bulelengkab.go.id/informasi/detail/artikel/gejala-dan-cara-pengendalian-penyakit-layu-fusarium-pada-tanaman-pisang-36 (accessed on 15 November 2019).
- Malik, N.A.A.; Kumar, I.S.; Nadarajah, K. Elicitor and Receptor Molecules: Orchestrators of Plant Defense and Immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef] [PubMed]
- Ranf, S. Sensing of molecular patterns through cell surface immune receptors. Curr. Opin. Plant Biol. 2017, 38, 68–77. [Google Scholar] [CrossRef]
- Ferrusquía-Jiménez, N.I.; Chandrakasan, G.; Torres-Pacheco, I.; Rico-Garcia, E.; Feregrino-Perez, A.A.; Guevara-González, R.G. Extracellular DNA: A Relevant Plant Damage-Associated Molecular Pattern (DAMP) for Crop Protection Against Pests—A Review. J. Plant Growth Regul. 2020, 40, 451–463. [Google Scholar] [CrossRef]
- Serrano-Jamaica, L.M.; Villordo-Pineda, E.; González-Chavira, M.M.; Guevara-González, R.G.; Medina-Ramos, G. Effect of Fragmented DNA From Plant Pathogens on the Protection Against Wilt and Root Rot of Capsicum annuum L. Plants. Front. Plant Sci. 2021, 11, 2043. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Cartenì, F.; Bonanomi, G.; Senatore, M.; Termolino, P.; Giannino, F.; Incerti, G.; Rietkerk, M.; Lanzotti, V.; Chiusano, M.L. Inhibitory effects of extracellular self-DNA: A general biological process? New Phytol. 2015, 206, 127–132. [Google Scholar] [CrossRef]
- Nagler, M.; Insam, H.; Pietramellara, G.; Ascher-Jenull, J. Extracellular DNA in natural environments: Features, relevance and applications. Appl. Microbiol. Biotechnol. 2018, 102, 6343–6356. [Google Scholar] [CrossRef]
- Yakushiji, S.; Ishiga, Y.; Inagaki, Y.; Toyoda, K.; Shiraishi, T.; Ichinose, Y. Bacterial DNA activates immunity in Arabidopsis thaliana. J. Gen. Plant Pathol. 2009, 75, 227–234. [Google Scholar] [CrossRef]
- Duran-Flores, D.; Heil, M. Extracellular self-DNA as a damage-associated molecular pattern (DAMP) that triggers self-specific immunity induction in plants. Brain. Behav. Immun. 2018, 72, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Vega-Muñoz, I. Nucleic Acid Sensing in Mammals and Plants: Facts and Caveats. In International Review of Cell and Molecular Biology; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 345, pp. 225–285. [Google Scholar] [CrossRef]
- Quintana-Rodriguez, E.; Duran-Flores, D.; Heil, M.; Camacho-Coronel, X. Damage-associated molecular patterns (DAMPs) as future plant vaccines that protect crops from pests. Sci. Hortic. 2018, 237, 207–220. [Google Scholar] [CrossRef]
- Chiusano, M.L.; Incerti, G.; Colantuono, C.; Termolino, P.; Palomba, E.; Monticolo, F.; Benvenuto, G.; Foscari, A.; Esposito, A.; Marti, L.; et al. Arabidopsis thaliana Response to Extracellular DNA: Self Versus Nonself Exposure. Plants 2021, 10, 1744. [Google Scholar] [CrossRef]
- Maryani, N.; Lombard, L.; Poerba, Y.S.; Subandiyah, S.; Crous, P.W.; Kema, G.H.J. Phylogeny and genetic diversity of the banana Fusarium wilt pathogen Fusarium oxysporum f. sp. cubense in the Indonesian centre of origin. Stud. Mycol. 2019, 92, 155–194. [Google Scholar] [CrossRef] [PubMed]
- Rizanti, M. Expression of PR1 and PR3 Resistance Genes in In Vitro Assay of Banana Plantlets Cultivars Kepok and Mas Infected with Fusarium oxysporum f.sp cubense Tropical Race 4 (Foc TR4); Bandung Institute of Technology: Bandung, Indonesia, 2018. [Google Scholar]
- González-Mendoza, D.; Argumedo-Delira, R.; Morales-Trejo, A.; Pulido-Herrera, A.; Cervantes-Díaz, L.; Grimaldo-Juarez, O.; Alarcón, A. A rapid method for isolation of total DNA from pathogenic filamentous plant fungi Isolation of total DNA from pathogenic filamentous plant fungi. Genet. Mol. Res. 2010, 9, 162–166. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Bonanomi, G.; Incerti, G.; Chiusano, M.L.; Termolino, P.; Mingo, A.; Senatore, M.; Giannino, F.; Carten, F.; Rietkerk, M.; et al. Inhibitory and toxic effects of extracellular self-DNA in litter : A mechanism for negative plant—Soil feedbacks? New Phytol. 2015, 205, 1195–1210. [Google Scholar] [CrossRef]
- Al-Amin, M.D.; Karim, M.R.; Amin, M.R.; Rahman, S.M.A.N. In Vitro Micropropagation of Banana (Musa spp.). Bangladesh J. Agril. Res. 2009, 13, 645–659, ISSN 0258-7122. [Google Scholar]
- Emilda, D.; Sutanto, A.; Sukartini; Jumjunidang. Application of salicylic acid to induce disease resistance against fusarium wilt on banana. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Changchun, China, 21–23 August 2020; Institute of Physics Publishing: Bristol, UK, 2020; Volume 468. [Google Scholar] [CrossRef]
- Purwati, R.D.; Harran, S. In Vitro Selection of Abaca for Resistance to Fusarium oxysporum f.sp cubense. HAYATI J. Biosci. 2007, 14, 65–70. [Google Scholar] [CrossRef]
- Subramaniam, S.; Maziah, M.; Sariah, M.; Puad, M.P.; Xavier, R. Bioassay method for testing Fusarium wilt disease tolerance in transgenic banana. Sci. Hortic. 2006, 108, 378–389. [Google Scholar] [CrossRef]
- Mak, C.; Mohamed, A.A.; Liew, K.W.; Ho, Y.W. Early screening technique for Fusarium wilt resistance in banana micropropagated plants. In Banana Improvement: Cellular, Molecular Biology, and Induced Mutations, Proceedings of The Bananas and Crop Improvement Congresses, Leuven, Belgium, 24–28 September 2001; Science Publishers, Inc.: Hauppauge, NY, USA, 2004; Volume 17, pp. 219–227. ISBN 1578083400. [Google Scholar]
- Liu, X.; Williams, C.E.; Nemacheck, J.A.; Wang, H.; Subramanyam, S.; Zheng, C.; Chen, M.S. Reactive oxygen species are involved in plant defense against a gall midge. Plant Physiol. 2010, 152, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.M.; Ogawa, D.; Taguchi-shiobara, F.; Ishimoto, M.; Matsunaga, S.; Habu, Y. Direct quantitative evaluation of disease symptoms on living plant leaves growing under natural light. Breed. Sci. 2017, 67, 316–319. [Google Scholar] [CrossRef]
- Cordeiro, M.C.M.; Silva, M.S.; de Oliveira-Filho, E.C.; Miranda, Z.; Aquino, F.; Fragoso, R.; Almeida, J.; Andrade, L. Optimization of A Method of Total RNA Extraction From Brazilian Native Plants Rich in Polyphenols and Polysaccharides. In Proceedings of the Simposio Nacional Cerrado, ParlaMundi, Brazil, 12–17 October 2008; Volume 1, pp. 1–6. [Google Scholar] [CrossRef]
- Haimes, J.; Kelley, M. Demonstration of a ΔΔCq Calculation Method to Compute Relative Gene Expression from qPCR Data. Horiz. Tech Note 2018, 1, 1–4. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- IBM Corp. IBM SPSS Statistics for Windows, Version 20.0; IBM Corp.: Armonk, NY, USA, 2011. [Google Scholar]
- Tang, W.; Zhu, S.; Li, L.; Liu, D.; Irving, D.E. Differential expressions of PR1 and chitinase genes in harvested bananas during ripening, and in response to ethephon, benzothiadizole and methyl jasmonate. Postharvest Biol. Technol. 2010, 57, 86–91. [Google Scholar] [CrossRef]
- Ma, B.C.; Tang, W.L.; Ma, L.Y.; Li, L.L.; Zhang, L.B.; Zhu, S.J.; Zhuang, C.; Irving, D. The Role of Chitinase Gene Expression in the Defense of Harvested Banana Against Anthracnose Disease. J. Am. Soc. Hortic. Sci. 2009, 134, 379–386. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, G.; Peng, X.; Huang, B.; Liu, E.; Zhang, J. Systemic acquired resistance in Cavendish banana induced by infection with an incompatible strain of Fusarium oxysporum f. sp. cubense. J. Plant Physiol. 2013, 170, 1039–1046. [Google Scholar] [CrossRef]
- Van Den Berg, N.; Berger, D.K.; Hein, I.; Birch, P.R.J.; Wingfield, M.J.; Viljoen, A. Tolerance in banana to Fusarium wilt is associated with early up-regulation of cell wall-strengthening genes in the roots. Mol. Plant Pathol. 2007, 8, 333–341. [Google Scholar] [CrossRef]
- Goldfarb, I.T.; Adeli, S.; Berk, T.; Phillippe, M. Fetal and Placental DNA Stimulation of TLR9: A Mechanism Possibly Contributing to the Pro-inflammatory Events During Parturition. Reprod. Sci. 2018, 25, 788–796. [Google Scholar] [CrossRef]
- Tuomela, J.; Sandholm, J.; Kaakinen, M.; Patel, A.; Kauppila, J.H.; Ilvesaro, J.; Chen, D.; Harris, K.W.; Graves, D.; Selander, K.S. DNA From Dead Cancer Cells Induces TLR9-Mediated Invasion and Inflammation In Living Cancer Cells. Breast Cancer Res. Treat. 2013, 142, 477. [Google Scholar] [CrossRef]
- Cartení, F.; Bonanomi, G.; Giannino, F.; Incerti, G.; Vincenot, C.E.; Chiusano, M.L.; Mazzoleni, S. Self-dna inhibitory effects: Underlying mechanisms and ecological implications. Plant Signal. Behav. 2016, 11, e1158381. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Leon-Reyes, A.; Van Der Ent, S.; Van Wees, S.C.M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Linthorst, H.J.M. Pathogenesis-related proteins of plants. Crit. Rev. Plant Sci. 1991, 10, 123–150. [Google Scholar] [CrossRef]
- Newman, M.A.; Sundelin, T.; Nielsen, J.T.; Erbs, G. MAMP (microbe-associated molecular pattern) triggered immunity in plants. Front. Plant Sci. 2013, 4, 139. [Google Scholar] [CrossRef]
- Xing, Y.; Jia, W.; Zhang, J. AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J. 2008, 54, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Jammes, F.; Song, C.; Shin, D.; Munemasa, S.; Takeda, K.; Gu, D.; Cho, D.; Lee, S.; Giordo, R.; Sritubtim, S.; et al. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 20520–20525. [Google Scholar] [CrossRef]
- Rouhier, N. Plant glutaredoxins: Pivotal players in redox biology and iron–sulphur centre assembly. New Phytol. 2010, 186, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Bashandy, T.; Guilleminot, J.; Vernoux, T.; Caparros-Ruiz, D.; Ljung, K.; Meyer, Y.; Reichheld, J.P. Interplay between the NADP-Linked Thioredoxin and Glutathione Systems in Arabidopsis Auxin Signaling. Plant Cell 2010, 22, 376. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Signorelli, S.; Corpas, F.J.; Rodríguez-Ruiz, M.; Valderrama, R.; Barroso, J.B.; Borsani, O.; Monza, J. Drought stress triggers the accumulation of NO and SNOs in cortical cells of Lotus japonicus L. roots and the nitration of proteins with relevant metabolic function. Environ. Exp. Bot. 2019, 161, 228–241. [Google Scholar] [CrossRef]
- Dvořák, P.; Krasylenko, Y.; Zeiner, A.; Šamaj, J.; Takáč, T. Signaling Toward Reactive Oxygen Species-Scavenging Enzymes in Plants. Front. Plant Sci. 2021, 11, 2178. [Google Scholar] [CrossRef] [PubMed]
- González-Bosch, C. Priming plant resistance by activation of redox-sensitive genes. Free Radic. Biol. Med. 2018, 122, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lal, N.K.; Lin, Z.J.D.; Ma, S.; Liu, J.; Castro, B.; Toruño, T.; Dinesh-Kumar, S.P.; Coaker, G. Regulation of reactive oxygen species during plant immunity through phosphorylation and ubiquitination of RBOHD. Nat. Commun. 2020, 11, 1838. [Google Scholar] [CrossRef] [PubMed]
- Considine, M.J.; Foyer, C.H. Stress effects on the reactive oxygen species-dependent regulation of plant growth and development. J. Exp. Bot. 2021, 72, 5795–5806. [Google Scholar] [CrossRef]
- Kim, D.K. Superoxide Quenching Activity of Phenolic Compounds from the Whole Plant of Galium verum var. asiaticum. Nat. Prod. Sci. 2011, 17, 261–266. [Google Scholar]
- Rehman, A.U.; Bashir, F.; Ayaydin, F.; Kóta, Z.; Páli, T.; Vass, I. Proline is a quencher of singlet oxygen and superoxide both in in vitro systems and isolated thylakoids. Physiol. Plant. 2021, 172, 7–18. [Google Scholar] [CrossRef]
- Grant, M.R.; Jones, J.D.G. Hormone (Dis)harmony Moulds Plant Health and Disease. Science 2009, 324, 750–752. [Google Scholar] [CrossRef]
- Caarls, L.; Pieterse, C.M.J.; Van Wees, S.C.M. How salicylic acid takes transcriptional control over jasmonic acid signaling. Front. Plant Sci. 2015, 6, 170. [Google Scholar] [CrossRef]


| No. | Primer Name | Primer Sequence (5′ → 3′) | Amplicon Length (bp) |
|---|---|---|---|
| 1 | Pathogen Related Gene 1–F Pathogen Related Gene 1–R | GCAGTACTACGACTACAACA GTTGCAGATGATGAAGATGG | 142 |
| 2 | Chitinase 1–F Chitinase 1–R | CAAGAAGAAGAGGGAGATCG GTTCTGTTCCTGGACGAAG | 121 |
| 3 | Superoxide dismutase–F Superoxide dismutase–R | ATCAACCACTCGATCTTCTG GCCTCCAAAGAACAAAAGTC | 113 |
| 4 | Catalase–F Catalase–R | AGCAAACATCTGATACGGAG GCGGACTTGACATGGTATAT | 107 |
| 5 | Glyceraldehyde-3-Phosphate Dehydrogenase–F Glyceraldehyde-3-Phosphate Dehydrogenase–R | TGTGGAGGAGGACTTGGTCT CGTGAGCTGTAACCCCACTC | 127 |
| Treatment | Leaf Discoloration Index | Rhizome Discoloration Index | Category |
|---|---|---|---|
| Control | 3.80 ± 0.84 a | 7.00 ± 1.41 A | Highly Susceptible |
| Salicylic Acid 5 µM | 2.20 ± 0.84 bc | 3.40 ± 0.55B C | Susceptible |
| eDNA 40 µg mL−1 | 2.80 ± 0.45 b | 4.60 ± 1.52 B | Susceptible |
| eDNA 80 µg mL−1 | 1.80 ± 0.84 bc | 3.80 ± 1.30 BC | Moderately Susceptible |
| eDNA 200 µg mL−1 | 1.60 ± 0.89 c | 2.60 ± 0.89 C | Tolerant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meitha, K.; Hanisia, R.H.; Signorelli, S.; Fauziah, T.; Iriawati; Esyanti, R.R. Extracellular DNA of Fusarium oxysporum f. sp. cubense as a Priming Agent for Inducing the Resistance of Banana Plantlets. Agronomy 2023, 13, 441. https://doi.org/10.3390/agronomy13020441
Meitha K, Hanisia RH, Signorelli S, Fauziah T, Iriawati, Esyanti RR. Extracellular DNA of Fusarium oxysporum f. sp. cubense as a Priming Agent for Inducing the Resistance of Banana Plantlets. Agronomy. 2023; 13(2):441. https://doi.org/10.3390/agronomy13020441
Chicago/Turabian StyleMeitha, Karlia, Ristag Hamida Hanisia, Santiago Signorelli, Tessa Fauziah, Iriawati, and Rizkita Rachmi Esyanti. 2023. "Extracellular DNA of Fusarium oxysporum f. sp. cubense as a Priming Agent for Inducing the Resistance of Banana Plantlets" Agronomy 13, no. 2: 441. https://doi.org/10.3390/agronomy13020441
APA StyleMeitha, K., Hanisia, R. H., Signorelli, S., Fauziah, T., Iriawati, & Esyanti, R. R. (2023). Extracellular DNA of Fusarium oxysporum f. sp. cubense as a Priming Agent for Inducing the Resistance of Banana Plantlets. Agronomy, 13(2), 441. https://doi.org/10.3390/agronomy13020441







