Genome-Wide Identification, Characterization, and Expression Analysis of the U-Box Gene Family in Punica granatum L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Genome and Transcriptome Data Sources
2.2. Identification and Sequence Analysis of U-Box Gene Family
2.3. Phylogenetic Analysis and Protein Motif Prediction
2.4. Chromosomal Distribution and Gene Duplication
2.5. Gene Expression Analysis
3. Results
3.1. Identification and Characterization of U-Box Gene Family
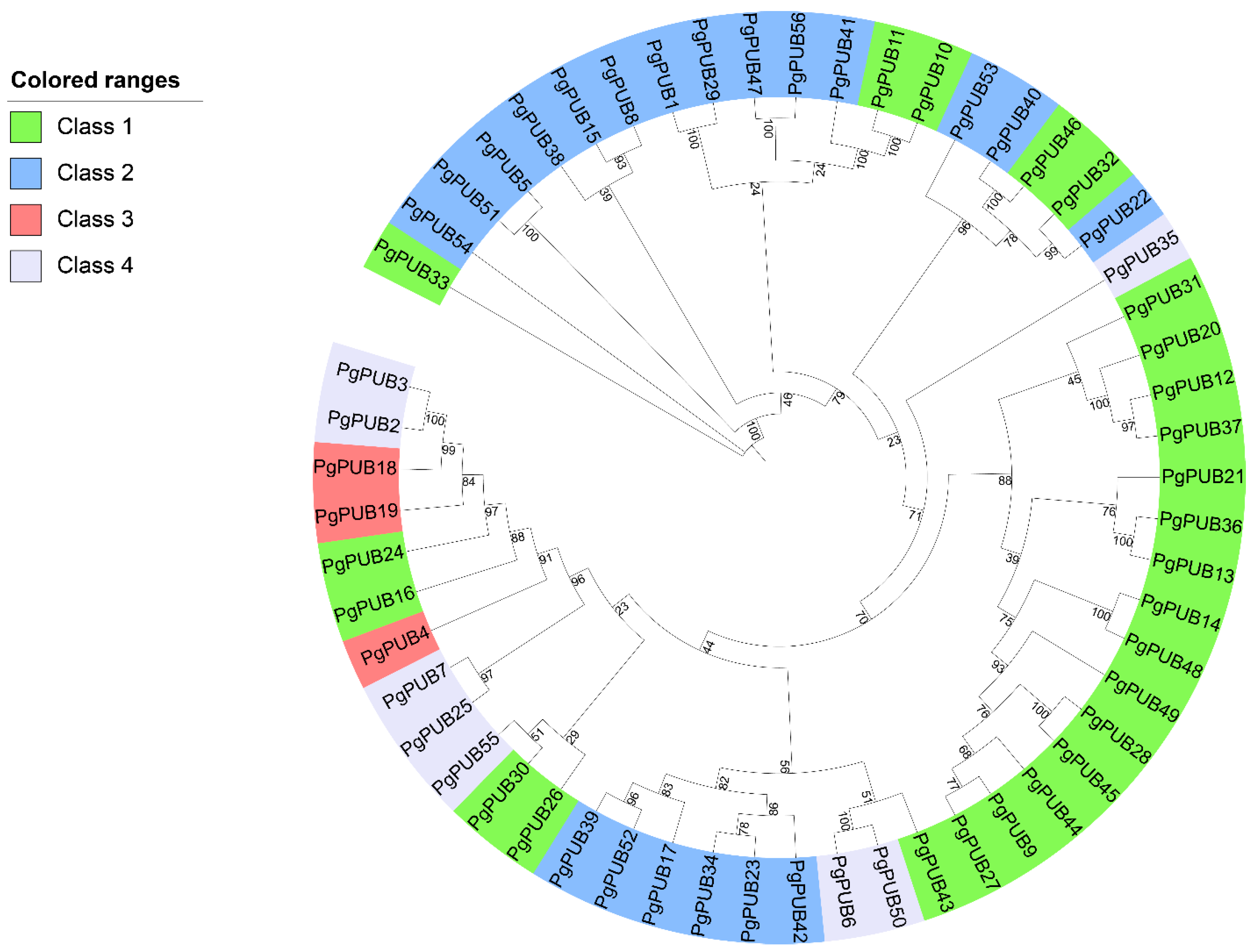
3.2. Phylogenetic Analysis of U-Box Genes in Pomegranate
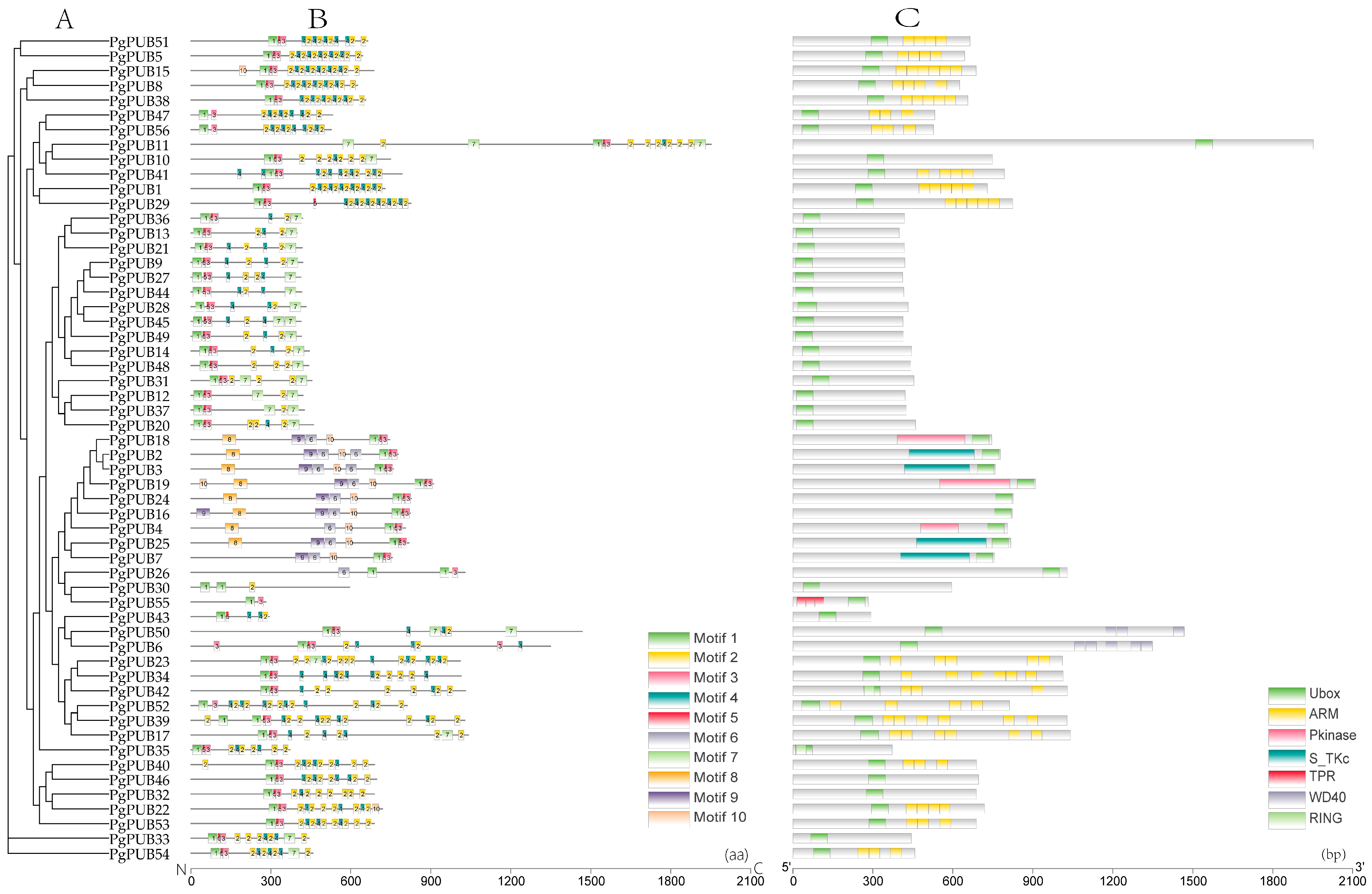
3.3. Conserved Motifs and Domain Organization Analysis
3.4. Cis-Acting Regulatory Element Prediction in Promoter Regions of U-Box Family Members
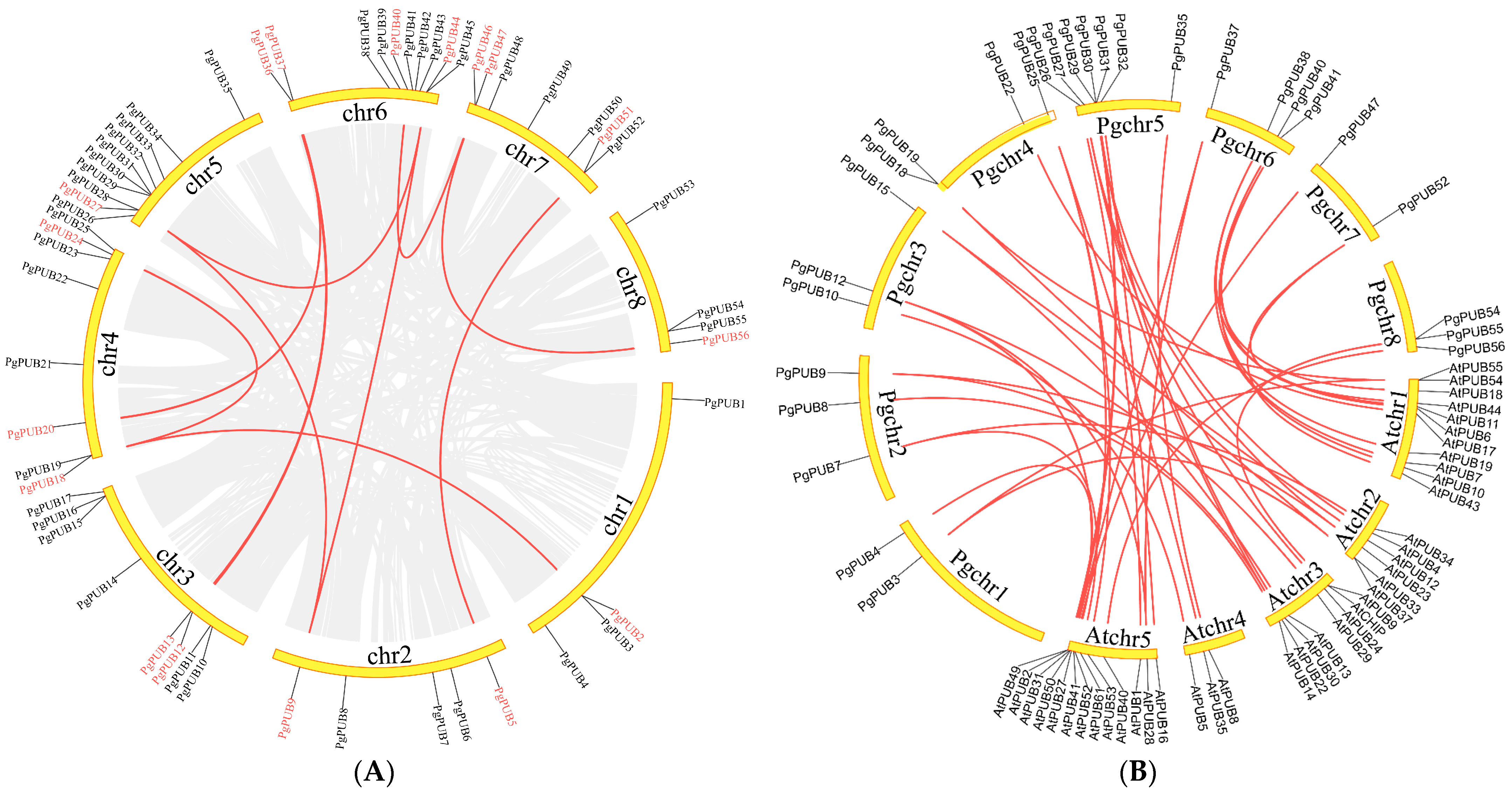
3.5. Chromosomal Distribution and Synteny Analysis of U-Box Family Members
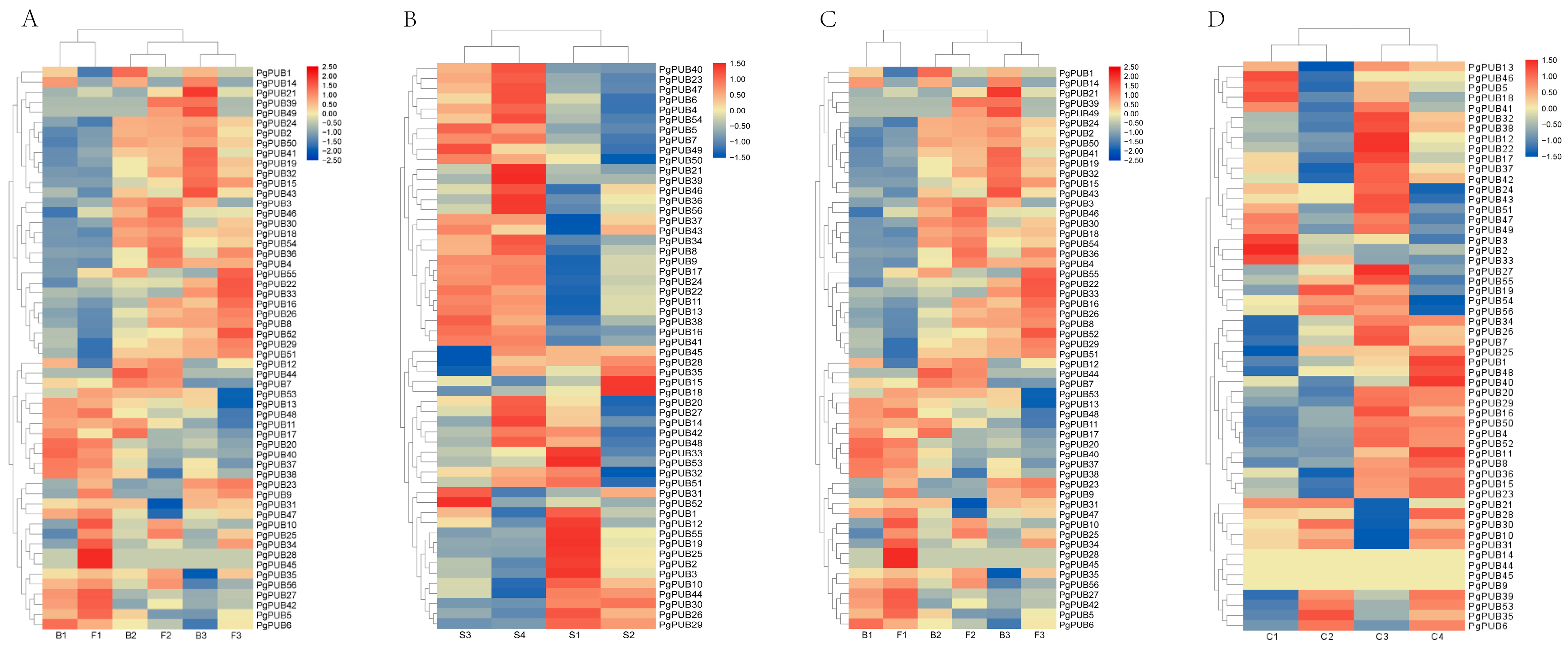
3.6. Expression Patterns of U-Box Family
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Callis, J. The ubiquitination machinery of the ubiquitin system. Arab. Book-Am. Soc. Plant Biol. 2014, 12, e0174. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; Swatek, K.N.; Thelen, J.J. Regulation of the regulators: Post-translational modifications, subcellular, and spatiotemporal distribution of plant 14-3-3 proteins. Front. Plant Sci. 2016, 7, 611. [Google Scholar] [CrossRef]
- Zheng, N.; Shabek, N. Ubiquitin ligases: Structure, function, and regulation. Annu. Rev. Biochem. 2017, 86, 129–157. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.; Shirasu, K. Ubiquitination in plant immunity. Curr. Opin. Plant Biol. 2010, 13, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Duan, W.; Riquicho, A.R.; Jing, Z.; Liu, T.; Hou, X.; Li, Y. Genome-wide survey and expression analysis of the PUB family in Chinese cabbage (Brassica rapa ssp. pekinesis). Mol. Genet. Genom. 2015, 290, 2241–2260. [Google Scholar] [CrossRef]
- Richburg, J.H.; Myers, J.L.; Bratton, S.B. The role of E3 ligases in the ubiquitin-dependent regulation of spermatogenesis. Semin. Cell Dev. Biol. 2014, 30, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Dye, B.T.; Schulman, B.A. Structural Mechanisms Underlying Posttranslational Modification by Ubiquitin-Like Proteins. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M. News from the PUB: Plant U-box type E3 ubiquitin ligases. J. Exp. Bot. 2018, 69, 371–384. [Google Scholar] [CrossRef]
- Vierstra, R.D. The ubiquitin–26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell. Bio. 2009, 10, 385–397. [Google Scholar] [CrossRef]
- Cyr, D.M.; Höhfeld, J.; Patterson, C. Protein quality control: U-box-containing E3 ubiquitin ligases join the fold. Trends Biochem. Sci. 2002, 27, 368–375. [Google Scholar] [CrossRef]
- Aravind, L.; Koonin, E.V. The U box is a modified RING finger—A common domain in ubiquitination. Curr. Biol. 2000, 10, R132–R134. [Google Scholar] [CrossRef] [PubMed]
- Wiborg, J.; O’Shea, C.; Skriver, K. Biochemical function of typical and variant Arabidopsis thaliana U-box E3 ubiquitin-protein ligases. Biochem. J. 2008, 413, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Park, C.H.; Venu, R.C.; Gough, J.; Wang, G. Classification, expression pattern, and E3 ligase activity assay of rice U-box-containing proteins. Mol. Plant 2008, 1, 800–815. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Taganna, J. Genome-wide analysis of the U-box E3 ubiquitin ligase enzyme gene family in tomato. Sci. Rep. 2020, 10, 9581. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Mo, X.; Yang, H.; Yue, L.; Song, J.; Mo, B. The U-box family genes in Medicago truncatula: Key elements in response to salt, cold, and drought stresses. PLoS ONE 2017, 12, e182402. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Cong, Y.; Wang, T.; Zhong, X.; Yang, S.; Li, Y.; Gai, J. Genome-wide identification of soybean U-box E3 ubiquitin ligases and roles of GmPUB8 in negative regulation of drought stress response in Arabidopsis. Plant Cell Physiol. 2016, 57, 1189–1209. [Google Scholar] [CrossRef]
- Vega-Sánchez, M.E.; Zeng, L.; Chen, S.; Leung, H.; Wang, G. SPIN1, a K homology domain protein negatively regulated and ubiquitinated by the E3 ubiquitin ligase SPL11, is involved in flowering time control in rice. Plant Cell 2008, 20, 1456–1469. [Google Scholar] [CrossRef]
- Li, W.; Ahn, I.; Ning, Y.; Park, C.; Zeng, L.; Whitehill, J.G.; Lu, H.; Zhao, Q.; Ding, B.; Xie, Q. The U-Box/ARM E3 ligase PUB13 regulates cell death, defense, and flowering time in Arabidopsis. Plant Physiol. 2012, 159, 239–250. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Huang, X.; Sun, J.; Xie, Q. AtPUB19, a U-box E3 ubiquitin ligase, negatively regulates abscisic acid and drought responses in Arabidopsis thaliana. Mol. Plant 2011, 4, 938–946. [Google Scholar] [CrossRef]
- Jung, C.; Zhao, P.; Seo, J.S.; Mitsuda, N.; Deng, S.; Chua, N. Plant U-box protein10 regulates MYC2 stability in Arabidopsis. Plant Cell 2015, 27, 2016–2031. [Google Scholar] [CrossRef]
- Cho, S.K.; Ryu, M.Y.; Song, C.; Kwak, J.M.; Kim, W.T. Arabidopsis PUB22 and PUB23 are homologous U-Box E3 ubiquitin ligases that play combinatory roles in response to drought stress. Plant Cell 2008, 20, 1899–1914. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Yi, J.; Yoon, J.; Cho, L.H.; Ping, J.; Jeong, H.J.; Cho, S.K.; Kim, W.T.; An, G. OsPUB15, an E3 ubiquitin ligase, functions to reduce cellular oxidative stress during seedling establishment. Plant J. 2011, 65, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, G.; Kang, H.; Zhou, S.; Wang, W. TaPUB1, a Putative E3 Ligase Gene from Wheat, Enhances Salt Stress Tolerance in Transgenic Nicotiana benthamiana. Plant Cell Physiol. 2017, 58, 1673–1688. [Google Scholar] [CrossRef]
- Yuan, Z.; Fang, Y.; Zhang, T.; Fei, Z.; Han, F.; Liu, C.; Liu, M.; Xiao, W.; Zhang, W.; Wu, S. The pomegranate (Punica granatum L.) genome provides insights into fruit quality and ovule developmental biology. Plant Biotechnol. J. 2018, 16, 1363–1374. [Google Scholar] [CrossRef]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Chidambara Murthy, K.N.; Jayaprakasha, G.K.; Singh, R.P. Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J. Agr. Food Chem. 2002, 50, 4791–4795. [Google Scholar] [CrossRef] [PubMed]
- Holland, D.; Hatib, K.; Bar-Ya’Akov, I. 2 Pomegranate: Botany, Horticulture, Breeding. Hortic. Rev. 2009, 35, 127–191. [Google Scholar]
- Luo, X.; Li, H.; Wu, Z.; Yao, W.; Zhao, P.; Cao, D.; Yu, H.; Li, K.; Poudel, K.; Zhao, D.; et al. The pomegranate (Punica granatum L.) draft genome dissects genetic divergence between soft- and hard-seeded cultivars. Plant Biotechnol. J. 2020, 18, 955–968. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Li, H.; Niu, J.; Xue, H.; Liu, B.; Wang, Q.; Luo, X.; Zhang, F.; Zhao, D. Transcriptomic analysis reveals candidate genes for female sterility in pomegranate flowers. Front. Plant Sci. 2017, 8, 1430. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Zhao, X.; Wang, J.; Gu, M.; Yuan, Z. Transcriptomic Profiling of Pomegranate Provides Insights into Salt Tolerance. Agronomy 2020, 10, 44. [Google Scholar] [CrossRef]
- Kashash, Y.; Doron Faigenboim, A.; Holland, D.; Porat, R. Effects of low-temperature conditioning and cold storage on development of chilling injuries and the transcriptome of ‘Wonderful’pomegranate fruit. Int. J. Food Sci. Technol. 2018, 53, 2064–2076. [Google Scholar] [CrossRef]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef] [PubMed]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Springer: Cham, Switzerland, 2005; pp. 571–607. [Google Scholar]
- Savojardo, C.; Martelli, P.L.; Fariselli, P.; Profiti, G.; Casadio, R. BUSCA: An integrative web server to predict subcellular localization of proteins. Nucleic Acids Res. 2018, 46, W459–W466. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2007, 23, 127–128. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.; Jin, H.; Marler, B.; Guo, H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiao, J.; Wu, J.; Zhang, H.; Liu, G.; Wang, X.; Dai, L. ParaAT: A parallel tool for constructing multiple protein-coding DNA alignments. Biochem. Bioph. Res. Commun. 2012, 419, 779–781. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A toolkit incorporating gamma-series methods and sliding window strategies. Genom. Proteom. Bioinform. 2010, 8, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Lohani, N.; Golicz, A.A.; Singh, M.B.; Bhalla, P.L. Genome-wide analysis of the Hsf gene family in Brassica oleracea and a comparative analysis of the Hsf gene family in B. oleracea, B. rapa and B. napus. Funct. Integr. Genom. 2019, 19, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Yang, W. E3 ubiquitin ligases: Ubiquitous actors in plant development and abiotic stress responses. Plant Cell Physiol. 2017, 58, 1461–1476. [Google Scholar] [CrossRef]
- Pareek, S.; Valero, D.; Serrano, M. Postharvest biology and technology of pomegranate. J. Sci. Food Agr. 2015, 95, 2360–2379. [Google Scholar] [CrossRef]
- Sayyari, M.; Aghdam, M.S.; Salehi, F.; Ghanbari, F. Salicyloyl chitosan alleviates chilling injury and maintains antioxidant capacity of pomegranate fruits during cold storage. Sci. Hortic. 2016, 211, 110–117. [Google Scholar] [CrossRef]
- Duplan, V.; Rivas, S. E3 ubiquitin-ligases and their target proteins during the regulation of plant innate immunity. Front. Plant Sci. 2014, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.; Santos-Rosa, M.J.; Shirasu, K. The U-box protein family in plants. Trends Plant Sci. 2001, 6, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Biłas, R.; Szafran, K.; Hnatuszko-Konka, K.; Kononowicz, A.K. Cis-regulatory elements used to control gene expression in plants. Plant Cell Tissue Organ Cult. 2016, 127, 269–287. [Google Scholar] [CrossRef]
- Qin, Q.; Wang, Y.; Huang, L.; Du, F.; Zhao, X.; Li, Z.; Wang, W.; Fu, B. A U-box E3 ubiquitin ligase OsPUB67 is positively involved in drought tolerance in rice. Plant Mol. Biol. 2020, 102, 89–107. [Google Scholar] [CrossRef]
- Antignani, V.; Klocko, A.L.; Bak, G.; Chandrasekaran, S.D.; Dunivin, T.; Nielsen, E. Recruitment of PLANT U-BOX13 and the PI4Kβ1/β2 Phosphatidylinositol-4 Kinases by the Small GTPase RabA4B Plays Important Roles during Salicylic Acid-Mediated Plant Defense Signaling in Arabidopsis. Plant Cell 2015, 27, 243–261. [Google Scholar] [CrossRef]

| Accession No. | Sample Name | Cultivars | Sample Type | Note | Library Layout | Instrument | Reference |
|---|---|---|---|---|---|---|---|
| SRR5446598 | B1 | ‘Tunisia’ | Floral pistil | 3.0–5.0 mm bisexual buds | PAIRED | Illumina HiSeq 2500 | [29] |
| SRR5446595 | B2 | ‘Tunisia’ | Floral pistil | 5.1–13.0 mm bisexual buds | PAIRED | Illumina HiSeq 2500 | [29] |
| SRR5446592 | B3 | ‘Tunisia’ | Floral pistil | 13.1–25.0 mm bisexual buds | PAIRED | Illumina HiSeq 2500 | [29] |
| SRR5446607 | F1 | ‘Tunisia’ | Floral pistil | 3.0–5.0 mm functional buds | PAIRED | Illumina HiSeq 2500 | [29] |
| SRR5446604 | F2 | ‘Tunisia’ | Floral pistil | 5.1–13.0 mm functional buds | PAIRED | Illumina HiSeq 2500 | [29] |
| SRR5446601 | F3 | ‘Tunisia’ | Floral pistil | 13.1–25.0 mm functional buds | PAIRED | Illumina HiSeq 2500 | [29] |
| SRR11268902 | S1 | ‘Tunisia’ | Seed | Germination stage1 | PAIRED | HiSeq X Ten | |
| SRR11268897 | S2 | ‘Tunisia’ | Seed | Germination stage 2 | PAIRED | HiSeq X Ten | |
| SRR11268894 | S3 | ‘Tunisia’ | Seed | Germination stage 3 | PAIRED | HiSeq X Ten | |
| SRR11268891 | S4 | ‘Tunisia’ | Seed | Germination stage 4 | PAIRED | HiSeq X Ten | |
| SRR7187397 | R0 | ‘Taishanhong’ | Root | 0d NaCl stress | PAIRED | Illumina HiSeq 4000 | [30] |
| SRR7187384 | R1 | ‘Taishanhong’ | Root | 3d NaCl stress | PAIRED | Illumina HiSeq 4000 | [30] |
| SRR7187385 | R2 | ‘Taishanhong’ | Root | 6d NaCl stress | PAIRED | Illumina HiSeq 4000 | [30] |
| SRR7187388 | L0 | ‘Taishanhong’ | Leaf | 0d NaCl stress | PAIRED | Illumina HiSeq 4000 | [30] |
| SRR7187390 | L1 | ‘Taishanhong’ | Leaf | 3d NaCl stress | PAIRED | Illumina HiSeq 4000 | [30] |
| SRR7187394 | L2 | ‘Taishanhong’ | Leaf | 6d NaCl stress | PAIRED | Illumina HiSeq 4000 | [30] |
| SRR6024695 | C1 | ‘Wonderful’ | Fruits | Immediately after harvest | PAIRED | Illumina HiSeq 2000 | [31] |
| SRR6024697 | C2 | ‘Wonderful’ | Fruits | LTC treatment | PAIRED | Illumina HiSeq 2000 | [31] |
| SRR6024699 | C3 | ‘Wonderful’ | Fruits | 2 weeks of cold storage at 1 °C | PAIRED | Illumina HiSeq 2000 | [31] |
| SRR6024701 | C4 | ‘Wonderful’ | Fruits | LTC + 2 weeks at 1 °C | PAIRED | Illumina HiSeq 2000 | [31] |
| Gene Name | Protein ID | Chromosomal Position | Size (aa) | MW (Da) | PI | GRAVY | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| PgPUB1 | XP_031377184.1 | chr1:2947864-–2952320 | 729 | 79,787.02 | 6.65 | −0.25 | Nucleus |
| PgPUB2 | XP_031377001.1 | chr1:44172533–44185322 | 778 | 87,360.6 | 4.91 | −0.532 | Nucleus |
| PgPUB3 | XP_031402583.1 | chr1:44186135–44192284 | 760 | 85,622.54 | 4.94 | −0.57 | Nucleus |
| PgPUB4 | XP_031377406.1 | chr1:52940497–52944484 | 805 | 91,804.83 | 6.31 | −0.452 | Nucleus |
| PgPUB5 | XP_031384103.1 | chr2:3755632–3758688 | 644 | 70,869.91 | 7.53 | −0.156 | Nucleus |
| PgPUB6 | XP_031384353.1 | chr2:10835456–10842973 | 1349 | 149,031.6 | 5.82 | −0.174 | Chloroplast |
| PgPUB7 | XP_031382118.1 | chr2:14312179–14317263 | 756 | 84,350.43 | 6.74 | −0.317 | Nucleus |
| PgPUB8 | XP_031384418.1 | chr2:30479382–30482731 | 626 | 67,839.01 | 5.77 | −0.218 | Nucleus |
| PgPUB9 | XP_031379370.1 | chr2:39234572–39236409 | 420 | 46,459.35 | 9 | 0.005 | Nucleus |
| PgPUB10 | XP_031386023.1 | chr3:6739180–6743403 | 749 | 83,936.92 | 6.29 | −0.255 | Nucleus |
| PgPUB11 | XP_031386836.1 | chr3:7122525–7132337 | 1952 | 219,216.6 | 5.85 | −0.309 | Extracellular space |
| PgPUB12 | XP_031389245.1 | chr3:11307289–11308979 | 421 | 45,666.86 | 8.85 | 0.081 | Nucleus |
| PgPUB13 | XP_031385069.1 | chr3:11584904–11586651 | 398 | 44,396.32 | 5.73 | −0.048 | Nucleus |
| PgPUB14 | XP_031385043.1 | chr3:25161277–25162870 | 445 | 49,557.2 | 6.26 | −0.143 | Chloroplast |
| PgPUB15 | XP_031386047.1 | chr3:38561364–38564797 | 687 | 75,352.04 | 5.46 | −0.3 | Nucleus |
| PgPUB16 | XP_031384546.1 | chr3:38592909–38597310 | 823 | 93,294.76 | 6.77 | −0.384 | Nucleus |
| PgPUB17 | XP_031389227.1 | chr3:39375519–39379722 | 1041 | 114,621.7 | 6.11 | −0.196 | Nucleus |
| PgPUB18 | XP_031391630.1 | chr4:666345–671083 | 746 | 84,562.07 | 5.07 | −0.481 | Nucleus |
| PgPUB19 | XP_031391943.1 | chr4:674315–680627 | 911 | 101,269.1 | 5.79 | −0.35 | Chloroplast |
| PgPUB20 | XP_031392831.1 | chr4:6839162–6840933 | 460 | 50,247.24 | 8.92 | 0.045 | Nucleus |
| PgPUB21 | XP_031394767.1 | chr4:17640750–17642718 | 418 | 44,633.42 | 6.46 | 0.162 | Nucleus |
| PgPUB22 | XP_031393056.1 | chr4:32045714–32048712 | 719 | 78,882.8 | 8.25 | −0.037 | Chloroplast |
| PgPUB23 | XP_031391114.1 | chr4:38020685–38026355 | 1011 | 113,047.9 | 5.77 | −0.093 | Nucleus |
| PgPUB24 | XP_031392769.1 | chr4:38931551–38936679 | 827 | 93,788.41 | 6.1 | −0.45 | Plasma membrane |
| PgPUB25 | XP_031393410.1 | chr4:39793459–39798046 | 818 | 91,700.16 | 6.67 | −0.459 | Nucleus |
| PgPUB26 | XP_031398020.1 | chr5:1314957–1320703 | 1029 | 116,656 | 5.44 | −0.207 | Nucleus |
| PgPUB27 | XP_031395601.1 | chr5:2529317–2530803 | 412 | 45,466.06 | 8.53 | 0.104 | Nucleus |
| PgPUB28 | XP_031398133.1 | chr5:2533727–2535429 | 432 | 48,666.62 | 7.17 | −0.045 | Nucleus |
| PgPUB29 | XP_031395775.1 | chr5:5898712–5903869 | 825 | 89,125.02 | 5.85 | −0.271 | Nucleus |
| PgPUB30 | XP_031396537.1 | chr5:6124869–6132321 | 596 | 65,099.27 | 7.63 | −0.541 | Cytoplasm |
| PgPUB31 | XP_031397642.1 | chr5:6258825–6261510 | 454 | 49,543.71 | 5.97 | 0.128 | Nucleus |
| PgPUB32 | XP_031397910.1 | chr5:7601217–7603669 | 688 | 75,002.67 | 8.88 | −0.023 | Chloroplast |
| PgPUB33 | XP_031398091.1 | chr5:9932189–9935006 | 444 | 48,413.65 | 5.49 | −0.106 | Nucleus |
| PgPUB34 | XP_031397089.1 | chr5:14587663–14592547 | 1014 | 111,988.4 | 5.59 | −0.054 | Endomembrane system |
| PgPUB35 | XP_031395361.1 | chr5:28638947–28640863 | 372 | 40,265.73 | 8.95 | 0.067 | Nucleus |
| PgPUB36 | XP_031399446.1 | chr6:614826–616520 | 419 | 46,306.38 | 5.76 | −0.018 | Nucleus |
| PgPUB37 | XP_031402878.1 | chr6:885626–887478 | 426 | 46,705.74 | 6.07 | −0.019 | Nucleus |
| PgPUB38 | XP_031402110.1 | chr6:19025201–19028432 | 656 | 70,796.77 | 6.14 | −0.141 | Nucleus |
| PgPUB39 | XP_031402746.1 | chr6:21000873–21005128 | 1028 | 114,180.2 | 6.27 | −0.179 | Chloroplast |
| PgPUB40 | XP_031401235.1 | chr6:22423862–22426275 | 689 | 76,335.55 | 8.46 | −0.008 | Chloroplast |
| PgPUB41 | XP_031400912.1 | chr6:23386124–23390792 | 793 | 88,102.57 | 5.67 | −0.349 | Nucleus |
| PgPUB42 | XP_031402432.1 | chr6:23903047–23906932 | 1030 | 114,136.6 | 5.95 | −0.121 | Nucleus |
| PgPUB43 | XP_031401153.1 | chr6:24733246–24735959 | 294 | 32,684.9 | 8.92 | −0.074 | Chloroplast |
| PgPUB44 | XP_031400164.1 | chr6:25957839–25959409 | 416 | 46,219.5 | 9.08 | −0.077 | Nucleus |
| PgPUB45 | XP_031400178.1 | chr6:25963675–25965100 | 413 | 46,889.98 | 8.76 | −0.077 | Nucleus |
| PgPUB46 | XP_031407430.1 | chr7:1054126–1056539 | 697 | 76,458.11 | 8.4 | −0.018 | Chloroplast |
| PgPUB47 | XP_031405927.1 | chr7:1171657–1174042 | 532 | 58,012.45 | 8.2 | −0.217 | Chloroplast |
| PgPUB48 | XP_031403345.1 | chr7:3813240–3814881 | 442 | 48,985.14 | 5.65 | −0.164 | Nucleus |
| PgPUB49 | XP_031404116.1 | chr7:11882839–11884646 | 415 | 46,318.05 | 8.95 | −0.042 | Nucleus |
| PgPUB50 | XP_031407385.1 | chr7:20996670–21003036 | 1468 | 163,030.8 | 5.76 | −0.206 | Endomembrane system |
| PgPUB51 | XP_031407253.1 | chr7:25091236–25093989 | 664 | 74,079.52 | 5.64 | −0.258 | Nucleus |
| PgPUB52 | XP_031406559.1 | chr7:25178052–25182444 | 812 | 89,340.78 | 5.31 | −0.021 | Nucleus |
| PgPUB53 | XP_031373608.1 | chr8:2760493–2762693 | 688 | 75,204.03 | 8.46 | −0.023 | Nucleus |
| PgPUB54 | XP_031373317.1 | chr8:24301646–24304174 | 458 | 50,457.08 | 8.02 | −0.206 | Nucleus |
| PgPUB55 | XP_031373647.1 | chr8:24309251–24313251 | 282 | 32,078.43 | 5.71 | −0.503 | Nucleus |
| PgPUB56 | XP_031407822.1 | chr8:26638009–26640696 | 527 | 57,628.74 | 7.08 | −0.239 | Nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Ge, D.; Ren, Y.; Wang, Y.; Yan, M.; Zhao, X.; Yuan, Z. Genome-Wide Identification, Characterization, and Expression Analysis of the U-Box Gene Family in Punica granatum L. Agronomy 2023, 13, 332. https://doi.org/10.3390/agronomy13020332
Chen L, Ge D, Ren Y, Wang Y, Yan M, Zhao X, Yuan Z. Genome-Wide Identification, Characterization, and Expression Analysis of the U-Box Gene Family in Punica granatum L. Agronomy. 2023; 13(2):332. https://doi.org/10.3390/agronomy13020332
Chicago/Turabian StyleChen, Lide, Dapeng Ge, Yuan Ren, Yuying Wang, Ming Yan, Xueqing Zhao, and Zhaohe Yuan. 2023. "Genome-Wide Identification, Characterization, and Expression Analysis of the U-Box Gene Family in Punica granatum L." Agronomy 13, no. 2: 332. https://doi.org/10.3390/agronomy13020332
APA StyleChen, L., Ge, D., Ren, Y., Wang, Y., Yan, M., Zhao, X., & Yuan, Z. (2023). Genome-Wide Identification, Characterization, and Expression Analysis of the U-Box Gene Family in Punica granatum L. Agronomy, 13(2), 332. https://doi.org/10.3390/agronomy13020332







