Comparative Genetic Diversity Assessment and Marker–Trait Association Using Two DNA Marker Systems in Rice (Oryza sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Agro-Morphological Characteristics of Plant Materials and Their Evaluations in the Field
2.2. Field Trials
2.3. Statistical Analysis of Agricultural Morphological Features
2.4. Agro-Morphological Trait Principal Component Analysis
2.5. Extraction of Genomic DNA and Genotyping with Genetic Markers
2.6. Molecular Characterization
2.7. Association between Agro-Morphological Characteristics and Molecular Markers
3. Results
3.1. Analysis of Variance of Agro-Morphological Traits
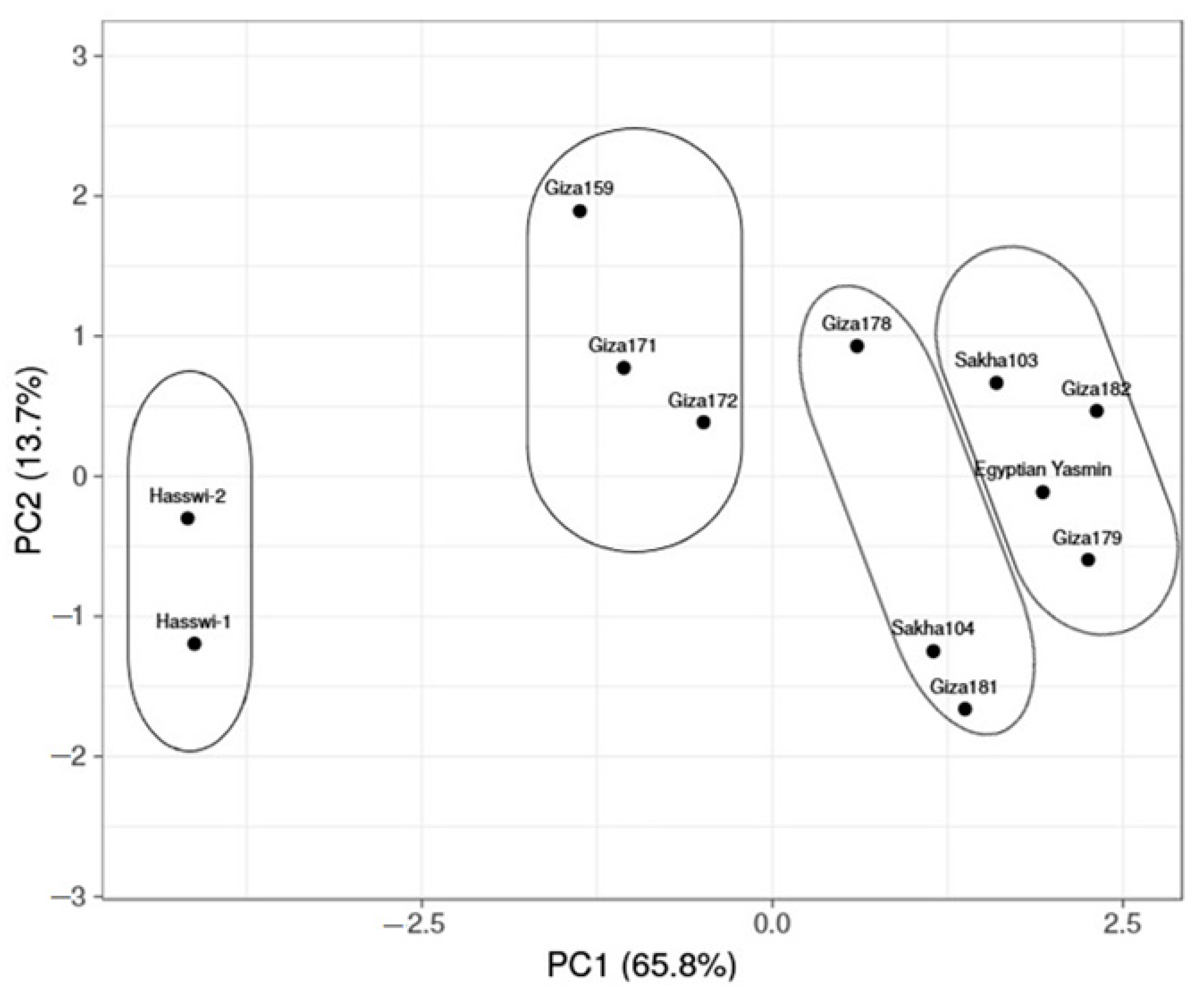
3.2. Principal Component Analysis
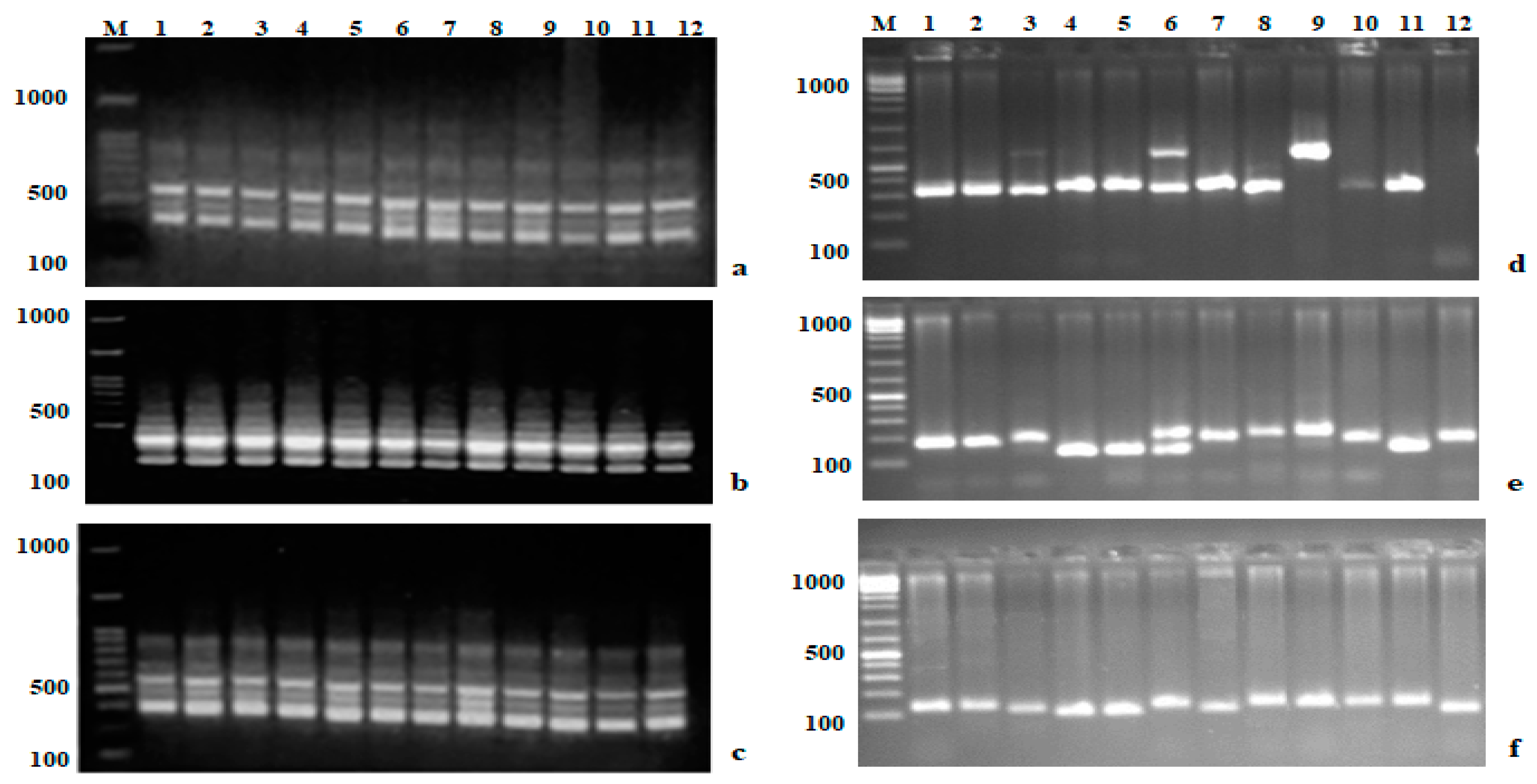
3.3. Analysis of Genetic Variations and the Efficiency of Markers
3.4. Association between Molecular Markers and Agro-Morphological Traits
4. Discussion
4.1. Principal Component Analysis
4.2. Genetic Variation and Marker Efficiency
4.3. Association of Agro-Morphological Features with Molecular Markers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vieira, M.B.; Faustino, M.V.; Lourenço, T.F.; Oliveira, M.M. DNA-Based Tools to Certify Authenticity of Rice Varieties—An Overview. Foods 2022, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Al-Daej, M.I.; El-Malky, M.M.; Sattar, M.N.; Rezk, A.A.; Naqqash, M.N.; Al-Khayri, J.M. Rice (Oryza sativa L.) Breeding among Hassawi Landrace and Egyptian Genotypes for Stem Borer (Chilo agamemnon Bles.) Resistance and Related Quantitative Traits. Phyton-Int. J. Exp. Bot. 2022, 91, 1905–1922. [Google Scholar] [CrossRef]
- Hossain, M.K.; Jena, K.K.; Bhuiyan, M.A.R.; Wickneswari, R. Association between QTLs and morphological traits toward sheath blight resistance in rice (Oryza sativa L.). Breed. Sci. 2016, 15154. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Liu, S.; Zhao, G.; Zhang, C.; Peng, Z.; Wang, Z.; Yang, J.; Li, Y. Genetic Diversity Relationship Between Grain Quality and Appearance in Rice. Front. Plant Sci. 2021, 1490. [Google Scholar] [CrossRef]
- Metwally, T.; Al-Daej, M.; El-Malky, M.; Ragab, A. Genetic analysis for rice seedling vigor and yeild characterestics of Hassawi landrace compared with some rice varaiteis. Fresenius Environ. Bull. 2022, 31, 3793–3804. [Google Scholar]
- Rathnathunga, E.U.U.; Geekiyanage, S. Morphological diversity of Sri Lankan traditional rice varieties “Pachchaperumal” and “Suduru samba”. Open Agric. 2017, 2, 552–560. [Google Scholar] [CrossRef]
- Roy, S.C.; Shil, P. Assessment of genetic heritability in rice breeding lines based on morphological traits and caryopsis ultrastructure. Scientific reports 2020, 10, 7830. [Google Scholar] [CrossRef]
- Shamim, M.; Sharma, V. Study of genetic diversity among different rice varieties using quantitative characters. ORYZA-Int. J. Rice 2014, 51, 86–89. [Google Scholar]
- Singh, B.; Mishra, M.; Naik, R. Genetic diversity among some traditional aromatic rice (Oryza sativa L) varieties of orissa. Indian J. Agric. Res. 2010, 44, 141–145. [Google Scholar]
- Abdel-Lateif, K.; Hewedy, O. Genetic diversity among Egyptian wheat cultivars using SCoT and ISSR markers. SABRAO J. Breed. Genet. 2018, 50, 36–45. [Google Scholar]
- Al-Turki, T.; Basahi, M.A. Assessment of ISSR based molecular genetic diversity of Hassawi rice in Saudi Arabia. Saudi J. Biol. Sci. 2015, 22, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, Z.J.; Aslam, K.; Septiningsih, E.M.; Collard, B.C. Evaluation of SSR and SNP markers for molecular breeding in rice. Plant Breed. Biotechnol. 2015, 3, 139–152. [Google Scholar] [CrossRef]
- Yogi, R.; Kumar, N.; Kumar, R.; Jain, R. Genetic diversity analysis among important rice (Oryza sativa L.) genotypes using SSR markers. Adv. Biores. 2020, 11, 68–74. [Google Scholar] [CrossRef]
- Alhasnawi, A.N.; Kadhimi, A.A.; Isahak, A.; Ashraf, M.F.; Doni, F.; Mohamad, A.; Mohtar, W.; Yusoff, W.; Radziah, C.; Zain, C. Application of inter simple sequence repeat (ISSR) for detecting genetic analysis in rice (Oryza sativa L.). J. Pure Appl. Microbiol. 2015, 9, 1091–1101. [Google Scholar]
- Moonsap, P.; Laksanavilat, N.; Sinumporn, S.; Tasanasuwan, P.; Kate-Ngam, S.; Jantasuriyarat, C. Genetic diversity of Indo-China rice varieties using ISSR, SRAP and InDel markers. J. Genet. 2019, 98, 80. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, S.; Kanagarajan, S.; Ponnusamy, S. Efficiency of RAPD and ISSR markers system in accessing genetic variation of rice bean (Vigna umbellata) landraces. Electron. J. Biotechnol. 2008, 11, 32–41. [Google Scholar] [CrossRef]
- Ramchander, S.; Leon, M.P.; Souframanien, J.; Pillai, M.A. Genetic diversity allelic variation and marker trait associations in gamma irradiated mutants of rice (Oryza sativa L). Int. J. Radiat. Biol. 2021, 98, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Rezk, A.A.; El-Malky, M.M.; El-Beltagi, H.S.; Al-daej, M.; Attia, K.A. Conventional Breeding and Molecular Markers for Blast Disease Resistance in Rice (Oryza sativa L.). Phyton-Int. J. Exp. Bot. 2022, 92, 725–746. [Google Scholar]
- Chettri, D.; Anandan, A.; Nagireddy, R.K.; Sabarinathan, S.; Sathyanarayana, N. Genetic diversity inrice (Oryza sativa L.) landraces of Sikkim-Himalaya and early insight into their use in genome-wide association analysis. Plant Genet. Resour. 2021, 19, 347–356. [Google Scholar] [CrossRef]
- Silva, J.M.; Lima, P.R.L.; Souza, F.V.D.; Ledo, C.A.S.; Souza, E.H.; Pestana, K.N.; Ferreira, C.F. Genetic diversity and nonparametric statistics to identify possible ISSR marker association with fiber quality of pineapple. Anais da Academia Brasileira de Ciências 2019, 91, e2018074. [Google Scholar] [CrossRef]
- IRRI. Standard Evaluation System for Rice; International Rice Research Institute: Laguna, Philippines, 2014; 52p, ISBN 97897122030469712203042. [Google Scholar]
- Le Clerg, E.; Leonard, W.; Erwin, L.; Warren, H.; Andrew, G. Field Plot Technique; Burgess Publishing Company: Minneapolis, MN, USA, 1962. [Google Scholar]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, A.; Pokhrel, A.; Sharma, S.; Poudel, A. Multivariate analysis of phenotypic diversity of rice (Oryza sativa L.) landraces from Lamjung and Tanahun Districts. Nepal. Int. J. Agron. 2020, 2020, 8867961. [Google Scholar] [CrossRef]
- McCouch, S.R.; Kochert, G.; Yu, Z.; Wang, Z.; Khush, G.; Coffman, W.; Tanksley, S. Molecular mapping of rice chromosomes. Theor. Appl. Genet. 1988, 76, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Jahan, P.; Hasan, Q.; Rao, P. Relationship between PTEN and gestational diabetes in Asian Indians womens. J. Health Spec. 2015, 3, 184. [Google Scholar]
- Sabouri, A.; Alinezhad, F.; Mousanejad, S. Association analysis using SSR markers and identification of resistant aerobic and Iranian rice cultivars to blast disease. Eur. J. Plant Pathol. 2020, 158, 561–570. [Google Scholar] [CrossRef]
- Sanghamitra, P.; Barik, S.R.; Bastia, R.; Mohanty, S.P.; Pandit, E.; Behera, A.; Mishra, J.; Kumar, G.; Pradhan, S.K. Detection of Genomic Regions Controlling the Antioxidant Enzymes, Phenolic Content, and Antioxidant Activities in Rice Grain through Association Mapping. Plants 2022, 11, 1463. [Google Scholar] [CrossRef]
- Sitoe, H.M.; Zhang, Y.; Chen, S.; Li, Y.; Ali, M.; Sowadan, O.; Karikari, B.; Liu, E.; Dang, X.; Qian, H.; et al. Detection of QTLs for Plant Height Architecture Traits in Rice (Oryza sativa L.) by Association Mapping and the RSTEP-LRT Method. Plants 2022, 11, 999. [Google Scholar] [CrossRef]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. iMEC: Online marker efficiency calculator. Appl. Plant Sci. 2018, 6, e01159. [Google Scholar] [CrossRef]
- Nei, M. Genetic distance between populations. Am. Nat. 1972, 106, 283–292. [Google Scholar] [CrossRef]
- Rohlf, F.J. NTSYS-pc: Numerical Taxonomy and Multivariate Analysis System; Version 2.1; Exeter Software; Exeter Publishing Setauket: New York, NY, USA, 2000. [Google Scholar]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Hardy, O.J. Estimation of pairwise relatedness between individuals and characterization of isolation by distance processes using dominant genetic markers. Mol. Ecol. 2003, 12, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Hardy, O.J.; Vekemans, X. SPAGeDi: A versatile computer program to analyze spatial genetic structure at the individual or population levels. Mol. Ecol. Notes 2002, 2, 618–620. [Google Scholar] [CrossRef]
- Gattepaille, L.M.; Jakobsson, M. Combining markers into haplotypes can improve population structure inference. Genetics 2012, 190, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Pressoir, G.; Briggs, W.; Vroh, B.I.; Yamasaki, M.; Doebley, J.F.; McMullen, M.D.; Gaut, B.S.; Nielsen, D.M.; Holland, J.B.; et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 2006, 38, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Cappa, E.P.; Martínez, M.C.; Garcia, M.N.; Villalba, P.V.; Marcucci Poltri, S.N. Effect of population structure and kinship relationships on the results of association mapping tests of growth and wood quality traits in four Eucalyptuspopulations. BMC Proc. 2011, 5, P23. [Google Scholar] [CrossRef]
- Ringnér, M. What is principal component analysis? Nat. Biotechnol. 2008, 26, 303–304. [Google Scholar] [CrossRef]
- Shoba, D.; Vijayan, R.; Robin, S.; Manivannan, N.; Iyanar, K.; Arunachalam, P.; Nadarajan, N. Assessment of genetic diversity in aromatic rice (Oryza sativa L.) germplasm using PCA and cluster Analysis. Electron. J. Plant Breed. 2019, 10, 1095–1104. [Google Scholar] [CrossRef]
- Mia, S.; Ahmed, N.U.; Islam, M.Z.; Rashad, M.; Islam, M.; Islam, M.; Zaman, A. Genetic diversity and yield performance among T. Aman rice (Oryza sativa L.) landraces in Barishal region of Bangladesh. J. Crop Sci. Biotechnol. 2022, 25, 123–132. [Google Scholar] [CrossRef]
- Panda, D.; Behera, P.K.; Mishra, S.; Mishra, B.S. Differential drought tolerance responses in short-grain aromatic rice germplasms from Koraput valley of Eastern Ghats of India. Plant Physiol. Rep. 2022, 27, 119–131. [Google Scholar] [CrossRef]
- Soe, I.; Tamu, A.; Asante, M.D.; Nyadanu, D.; Akromah, R. Genetic diversity analyses of rice germplasm using morphological traits. J. Plant Breed. Crop Sci. 2019, 11, 128–136. [Google Scholar]
- Ponsiva, S.T.; Sabarinathan, S.; Senthilkumar, N.; Thangavel, P.; Manivannan, K.; Thirugnanakumar, S. Principal components analysis in rice genotypes grown over seasons. Plant Arch. 2019, 19, 3118–3120. [Google Scholar]
- Suvi, W.T.; Shimelis, H.; Laing, M.; Mathew, I.; Shayanowako, A.I. Variation among Tanzania Rice Germplasm Collections Based on Agronomic Traits and Resistance to Rice Yellow Mottle Virus. Agronomy 2021, 11, 391. [Google Scholar] [CrossRef]
- Al-Daej, M.; Ismail, M.; Rezk, A.; El-Malky, M. Molecular identification of blast resistance genes in rice genotypes using gene-specific markers. BioTechnologia 2019, 100, 311–322. [Google Scholar] [CrossRef]
- Yang, Q.; Lyu, X.; Zhao, F.; Liu, Y. Effects of codon usage on gene expression are promoter context dependent. Nucleic Acids Res. 2021, 49, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, H.; Talebi, R.; Keshavarzi, F. Comparative efficiency of functional gene-based markers, start codon targeted polymorphism (SCoT) and conserved DNA-derived polymorphism (CDDP) with ISSR markers for diagnostic fingerprinting in wheat (Triticum aestivum L.). Cereal Res. Commun. 2014, 42, 558–567. [Google Scholar] [CrossRef]
- Ashfaq, M.; Khan, A. Genetic diversity in basmati rice (Oryza sativa L.) germplasm as revealed by microsatellite (SSR) markers. Russ. J. Genet. 2012, 48, 53–62. [Google Scholar] [CrossRef]
- Salgotra, R.; Gupta, B.; Bhat, J.A.; Sharma, S. Genetic diversity and population structure of Basmati rice (Oryza sativa L.) germplasm collected from North Western Himalayas using trait linked SSR markers. PLoS ONE 2015, 10, e0131858. [Google Scholar] [CrossRef]
- Tessier, C.; David, J.; This, P.; Boursiquot, J.-M.; Charrier, A. Optimization of the choice of molecular markers for varietal identification in Vitis vinifera L. Theor. Appl. Genet. 1999, 98, 171–177. [Google Scholar] [CrossRef]
- Prevost, A.; Wilkinson, M. A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor. Appl. Genet. 1999, 98, 107–112. [Google Scholar] [CrossRef]
- Serra, I.A.; Procaccini, G.; Intrieri, M.C.; Migliaccio, M.; Mazzuca, S.; Innocenti, A.M. Comparison of ISSR and SSR mark-ers for analysis of genetic diversity in the seagrass Posidonia oceanica. Mar. Ecol. Prog. Ser. 2007, 338, 71–79. [Google Scholar] [CrossRef]
- Ghomi, K.; Rabiei, B.; Sabouri, H.; Gholamalipour Alamdari, E. Association analysis, genetic diversity and population structure of barley (Hordeum vulgare L.) under heat stress conditions using SSR and ISSR markers linked to primary and secondary metabolites. Mol. Biol. Rep. 2021, 48, 6673–6694. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Cardon, L.R. The complex interplay among factors that influence allelic association. Nat. Rev. Genet. 2004, 5, 89–100. [Google Scholar] [CrossRef]
- Chen, X.; Dang, X.; Wang, Y.; Yang, Y.; Yang, G.; Sun, J.; Yin, H.; Liu, E.; Hong, D. Association Mapping of Thousand Grain Weight using SSR and SNP Markers in Rice (Oryza sativa L.) Across Six Environments. Trop. Plant Biol. 2021, 14, 143–155. [Google Scholar] [CrossRef]
- Shirmohammadli, S.; Sabouri, H.; Ahangar, L.; Ebadi, A.A.; Sajjadi, S.J. Genetic diversity and association analysis of rice genotypes for grain physical quality using iPBS, IRAP, and ISSR markers. J. Genet. Resour. 2018, 4, 122–129. [Google Scholar]
- Buu, B.C.; Ha, P.T.T.; Tam, B.P.; Nhien, T.T.; Van Hieu, N.; Phuoc, N.T.; Minh, L.T.; Giang, L.H.; Lang, N.T. Quantitative Trait Loci Associated with Heat Tolerance in Rice (Oryza sativa L.). Plant Breed. Biotechnol. 2014, 2, 14–24. [Google Scholar] [CrossRef]
- Wattoo, J.I.; Liaqat, S.; Mubeen, H.; Ashfaq, M.; Shahid, M.N.; Farooq, A.; Sajjad, M.; Arif, M. Genetic mapping of grain nutritional profile in rice using basmati derived segregating population revealed by SSRs. Int. J. Agric. Biol. 2019, 21, 929–935. [Google Scholar]
- Oladosu, Y.; Rafii, M.; Magaji, U.; Abdullah, N.; Miah, G.; Chukwu, S.C.; Hussin, G.; Ramli, A.; Kareem, I. Genotypic and phenotypic relationship among yield components in rice under tropical conditions. BioMed Res. Int. 2018, 2018, 8936767. [Google Scholar] [CrossRef]
- Hall, D.; Tegstrom, C.; Ingvarsson, P.K. Using association mapping to dissect the genetic basis of complex traits in Plants. Brief. Funct. Genom. 2010, 9, 157–165. [Google Scholar] [CrossRef]
- Zulfiqar, A.; Naseer, S.; Saleem, A.; Sabar, M.; Ahmed, S.; Sardar, R.; Shahzadi, F.; Raza, Q. Genetic diversity studies for grain iron and zinc content analysis for Elite rice (Oryza sativa L.) genotype by using SSR markers. J. Food Compos. Anal. 2023, 115, 104816. [Google Scholar] [CrossRef]
- Fazal, U.; Din, I.U.; Khan, A.M.; Khan, F.U.; Khan, M.N.; Iqbal, N.; Ibrahim, M.; Bangash, S.A.K. Evaluation of agro-morphological traits, seed characterization and genetic diversity of local rice (Oryza sativa L.) varieties of Pakistan. Genet. Resour. Crop Evol. 2022, 1–15. [Google Scholar] [CrossRef]
| No. | Genotypes | Parentage | Origin | Type | Grain Shape |
|---|---|---|---|---|---|
| 1 | Giza171 | Nahda (Pure line selection)/Calady40 | Egyptian | Japonica | Short |
| 2 | Egyptian Yasmin | (Jasmin85) IR841-67 | Egyptian | Indica | Long |
| 3 | Giza172 | Nahda/Kinmaza | Egyptian | Japonica | Short |
| 4 | Giza179 | Gz1368/IRAT112 | Egyptian | I.-J1. | Short |
| 5 | Giza181 | IR28/IR22 | Egyptian | Indica | Long |
| 6 | Giza182 | Giza181/IR39422-163-247-2-2-3 | Egyptian | Indica | Long |
| 7 | Sakha104 | Gz4096-8-1/Gz4100-9-1 | Egyptian | Japonica | Short |
| 8 | Sakha103 | Giza177/Suwwon349 | Egyptian | Japonica | Short |
| 9 | Giza178 | Giza175/Milyange49 | Egyptian | I.-J. | Short |
| 10 | Giza159 | Giza14/AgamiM1 | Egyptian | Japonica | Short |
| 11 | Hasswi-1 | Exotic | Kingdom of Saudi Arabia | I.-J. | Short |
| 12 | Hasswi-2 | IRI112/Hasswi-1 | Kingdom of Saudi Arabia | I.-J. | Short |
| No. | Name | Sequence (5′-3′) |
|---|---|---|
| 1 | A44 | CTC TCT CTC TCT CTC TAG |
| 2 | B44 | CTC TCT CTC TCT CTC TGC |
| 3 | A49 | CAC ACA CAC ACA AG |
| 4 | B49 | CAC ACA CAC ACA GG |
| 5 | B89 | CACACACACACAGT |
| 6 | A89 | CACACACACACACA |
| 7 | HB8 | GAGAGAGAGAGAGG |
| 8 | HB15 | GTGGTGGTGGC |
| 9 | HB9 | GTGTGTGTGTGTGG |
| 10 | HB12 | CAC CAC CAC GC |
| 11 | HB11 | GTG TGT GTG TGT TGT CC |
| 12 | HB13 | GAG GAG GAG GC |
| No. | Primer | Primer Sequence (5′-3′) | Repeat Motif | Annealing Temperature (°C) | Ch. No. | Expected |
|---|---|---|---|---|---|---|
| Product Size (bp) | ||||||
| 1F | RM209 | ATATGAGTTGCTGTCGTGCG | (CT)18 | 55 | 11 | 134–150 |
| 1R | RM209 | CAACTTGCATCCTCCCCTCC | ||||
| 2F | RM217 | ATCGCAGCAATGCCTCGT | (CT)20 | 55 | 6 | 133 |
| 2R | RM217 | GGGTGTGAACAAAGACAC | ||||
| 3F | RM224 | ATCGATCGATCTTCACGAGG | (AAG)8(AG)12 | 55 | 11 | 130–157 |
| 3R | RM224 | TGCTATAAAAGGCATTCGGG | ||||
| 4F | RM211 | CCGATCTCATCAACCAACTG | (TC)3A(TC)18 | 55 | 2 | 159–189 |
| 4R | RM211 | CTTCACGAGGATCTCAAAGG | ||||
| 5F | RM3873 | GCTAGCTAGGACCGACATG | (GA)50 | 58 | 1 | 215 |
| 5R | RM3873 | CCTCCTCCTTATCCTCC | ||||
| 6F | RM29 | CAGGGACCCACCTGTCATAC | (GA)7-18(GA)5(AG)4 | 55 | 2 | 250 |
| 6R | RM29 | AACGTTGGTCATATCGGTGG | ||||
| 7F | RM1216 | TTCCCCAATGGAACAGTGAC | (AG)14 | 50 | 1 | 73–80 |
| 7R | RM1216 | GGGTCTACCACCCGATCTC | ||||
| 8F | RM225 | TGCCCATATGGTCTGGATG | (CT)18 | 55 | 6 | 126–164 |
| 8R | RM225 | GAAAGTGGATCAGGAAGGC | ||||
| 9F | RM247 | TAGTGCCGATCGATGTAACG | (CT)16 | 55 | 12 | 180 |
| 9R | RM247 | CATATGGTTTTGACAAAGCG |
| Genotype | Duration (Day) | Plant Height (cm) | No. of Tillers Plant−1 | Panicle Length (cm) | No. of Panicles Plant−1 | Panicle Weight (g) |
|---|---|---|---|---|---|---|
| Giza171 | 152.2 | 135.67 | 27.8 | 24.4 | 25.17 | 2.61 |
| Egyptian Yasmin | 140.70 | 132.32 | 27.73 | 23.36 | 24.53 | 3.62 |
| Giza172 | 152.78 | 132.3 | 24.63 | 23.21 | 23.1 | 3.62 |
| Giza179 | 124.12 | 98.15 | 25.67 | 24.36 | 25.05 | 3.45 |
| Giza181 | 126.17 | 102.07 | 21.88 | 26.48 | 21.17 | 3.39 |
| Giza182 | 126.08 | 96.30 | 24.95 | 22.16 | 22.48 | 3.62 |
| Sakha104 | 134.87 | 105.95 | 23.27 | 24.58 | 21.00 | 3.35 |
| Sakha103 | 127.23 | 102.57 | 22.50 | 22.41 | 20.52 | 3.21 |
| Giza178 | 133.90 | 98.58 | 26.60 | 23.41 | 23.63 | 3.49 |
| Giza159 | 148.30 | 142.15 | 25.03 | 25.46 | 18.43 | 2.80 |
| Hasswi-1 | 154.12 | 143.47 | 20.87 | 20.50 | 16.17 | 1.92 |
| Hasswi-2 | 154.52 | 144.13 | 20.57 | 21.4 | 15.80 | 2.02 |
| LSD0.05 * | 3.82 | 3.31 | 4.28 | 0.71 | 1.47 | 0.34 |
| Genotype | 1000-grain weight (g) | No. of filled grains panicle−1 | No. of unfiled Grains panicle−1 | Grain yield (t ha−1) | Blast reaction | |
| Giza171 | 25.49 | 137.67 | 19.33 | 6.38 | 7.16 | |
| Egyptian Yasmin | 27.37 | 145.02 | 11.7 | 7.66 | 1.4 | |
| Giza172 | 26.66 | 138.4 | 20.23 | 7.55 | 6.00 | |
| Giza179 | 25.05 | 168.43 | 9.38 | 10.41 | 2.1 | |
| Giza181 | 26.45 | 156.38 | 7.38 | 10.11 | 2.03 | |
| Giza182 | 27.90 | 170.6 | 7.38 | 10.48 | 2.03 | |
| Sakha104 | 27.71 | 150.12 | 13.88 | 10.45 | 5.96 | |
| Sakha103 | 25.70 | 146.23 | 10.77 | 10.02 | 2.03 | |
| Giza178 | 22.93 | 146.6 | 12.52 | 10.26 | 2.06 | |
| Giza159 | 24.43 | 114.52 | 40.45 | 5.60 | 6.00 | |
| Hasswi-1 | 23.58 | 112.08 | 43.82 | 3.50 | 7.1 | |
| Hasswi-2 | 22.70 | 106.30 | 37.73 | 3.76 | 7.00 | |
| LSD0.05 * | 1.32 | 5.67 | 9.68 | 0.41 | 0.25 | |
| Mean Sum of Squares (MSS) | |||||||
|---|---|---|---|---|---|---|---|
| S.O.V. | df | Duration (Day) | Plant Height (cm) | No. of Tillers Plant−1 | Panicle Length (cm) | No. of Panicles Plant−1 | Panicle Weight (g) |
| Replication | 2 | 2.17 | 0.75 | 43.74 | 1.054 | 0.98 | 0.026 |
| Year | 1 | 4.61 ns | 1.93 ns | 1.46 ns | 0.34 ns | 0.48 ns | 0.044 ns |
| Genotype | 11 | 886.47 ** | 2448.82 ** | 37.16 ** | 17.43 ** | 62.68 ** | 2.26 |
| Error | 44 | 5.467 | 4.253 | 7.287 | 0.1707 | 0.832 | 0.04388 |
| Mean sum of squares (MSS) | |||||||
| S.O.V. | df | 1000-grain weight (g) | No. of filled Grains panicle−1 | No. of unfiled Grainspanicle−1 | Grain yield (t ha−1) | Blast reaction | |
| Replication | 2 | 3.0871 | 18.14 | 48.66 | 0.01556 | 0.005 | |
| Year | 1 | 0.0025 ns | 1.89 ns | 41.05 ns | 0.0155 ns | 0.001 ns | |
| Genotype | 11 | 12.14 ** | 2608.84 ** | 1078.14 ** | 49.02 ** | 35.73 ** | |
| Error | 44 | 0.712 | 12.83 | 33.99 | 0.068 | 0.023 | |
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| Duration (day) | 0.320* | 0.430 | 0.590 |
| Plant height (cm) | 0.520 | 0.230 | 0.310 |
| No. of tillers plant−1 | −0.110 | 0.120 | 0.000 |
| Panicle length (cm) | 0.000 | −0.080 | 0.010 |
| No. of panicles plant−1 | −0.100 | 0.050 | 0.050 |
| Panicle weight (g) | −0.010 | 0.020 | −0.010 |
| 1000-grain weight (g) | −0.020 | 0.020 | 0.110 |
| No. of filled grains panicle−1 | −0.600 | −0.280 | 0.710 |
| No. of unfiled grains | 0.490 | −0.810 | 0.160 |
| Grain yield t ha−1 | −0.070 | 0.020 | 0.010 |
| Blast reaction | 0.060 | 0.010 | 0.090 |
| Marker | Locus | No. of Alleles | Hexp | PIC | D | R |
|---|---|---|---|---|---|---|
| ISSR | 44A | 4 | 0.117 | 0.110 | 0.122 | 0.500 |
| 49A | 6 | 0.129 | 0.121 | 0.135 | 0.833 | |
| 98A | 7 | 0.228 | 0.202 | 0.246 | 1.167 | |
| 49B | 7 | 0.482 | 0.366 | 0.649 | 4.000 | |
| 44B | 6 | 0.105 | 0.099 | 0.109 | 0.667 | |
| HB8 | 8 | 0.291 | 0.249 | 0.324 | 2.833 | |
| HB9 | 9 | 0.466 | 0.358 | 0.606 | 3.000 | |
| HB11 | 6 | 0.389 | 0.313 | 0.461 | 1.833 | |
| HB12 | 5 | 0.180 | 0.164 | 0.192 | 1.000 | |
| HB15 | 5 | 0.255 | 0.222 | 0.280 | 1.500 | |
| Total | 63 | |||||
| Mean | 6.3 | 0.264 | 0.220 | 0.312 | 1.733 | |
| SSR | RM209 | 2 | 0.500 | 0.375 | 0.761 | 0.667 |
| RM217 | 2 | 0.500 | 0.375 | 0.761 | 1.333 | |
| RM224 | 3 | 0.444 | 0.346 | 0.895 | 1.333 | |
| RM211 | 4 | 0.395 | 0.317 | 0.931 | 1.833 | |
| RM3873 | 3 | 0.444 | 0.346 | 0.895 | 2.000 | |
| Total | 14 | |||||
| Mean | 2.8 | 0.457 | 0.352 | 0.849 | 1.433 |
| Marker System | Trait | p-Value | R2 | |
|---|---|---|---|---|
| ISSR | 44A | No. of unfiled grainspanicle−1 | 0.029 | 0.452 |
| 49A | Panicle length (cm) | 0.048 ns | 0.393 | |
| 98A | Panicle length (cm) | 0.043 | 0.406 | |
| HB8 | No. of tillers plant−1 | 0.020 | 0.493 | |
| No. of panicles plant−1 | 0.027 | 0.433 | ||
| Panicle weight(gm) | 0.036 | 0.433 | ||
| No. of unfiled grains panicle−1 | 0.029 | 0.452 | ||
| HB11 | No. of panicles plant−1 | 0.036 | 0.403 | |
| 1000-grain weight (gm) | 0.028 | 0.292 | ||
| SSR | RM209 | Panicle length (cm) | 0.035 | 0.434 |
| RM211 | No. of tillers plant−1 | 0.038 | 0.419 | |
| No. of panicles plant−1 | 0.046 ns | 0.375 | ||
| Panicle weight (gm) | 0.035 | 0.436 | ||
| RM217 | Panicle length (cm) | 0.035 | 0.434 | |
| 1000-grain weight (gm) | 0.047 ns | 0.252 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-daej, M.I.; Rezk, A.A.; El-Malky, M.M.; Shalaby, T.A.; Ismail, M. Comparative Genetic Diversity Assessment and Marker–Trait Association Using Two DNA Marker Systems in Rice (Oryza sativa L.). Agronomy 2023, 13, 329. https://doi.org/10.3390/agronomy13020329
Al-daej MI, Rezk AA, El-Malky MM, Shalaby TA, Ismail M. Comparative Genetic Diversity Assessment and Marker–Trait Association Using Two DNA Marker Systems in Rice (Oryza sativa L.). Agronomy. 2023; 13(2):329. https://doi.org/10.3390/agronomy13020329
Chicago/Turabian StyleAl-daej, Mohammed I., Adel A. Rezk, Mohamed M. El-Malky, Tarek A. Shalaby, and Mohamed Ismail. 2023. "Comparative Genetic Diversity Assessment and Marker–Trait Association Using Two DNA Marker Systems in Rice (Oryza sativa L.)" Agronomy 13, no. 2: 329. https://doi.org/10.3390/agronomy13020329
APA StyleAl-daej, M. I., Rezk, A. A., El-Malky, M. M., Shalaby, T. A., & Ismail, M. (2023). Comparative Genetic Diversity Assessment and Marker–Trait Association Using Two DNA Marker Systems in Rice (Oryza sativa L.). Agronomy, 13(2), 329. https://doi.org/10.3390/agronomy13020329







