Abstract
Glycosylation is a common modification reaction in plants. The products obtained upon glycosylation have different biological functions, making glycosylation an important mechanism affecting and regulating the balance of plant growth and metabolism. In this study, we first speculated that Group I in the apple glycosyltransferase family may have a predicted function like UGT83A1, according to gene chip data published online. Subsequently, by real-time PCR (polymerase chain reaction), we analyzed whether the expression of nine glycosyltransferase genes in Group I was induced by our previously reported ACCase (Acetyl-CoA carboxylase) inhibition-based herbicide QPP ((R)-ethyl·2-(4-((6-fluoro-3-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)oxy) phenoxy) propanoate). It was found that expression of the MdUGT83K2 gene in Group I was significantly increased by QPP. In order to determine whether MdUGT83K2 can glycosylate QPP, we confirmed the enzymatic reaction of MdUGT83K2 in vitro and the presence of QPP glycosides in MdUGT83K2 transgenic apple seedlings by HPLC (High Performance Liquid Chromatography), and found that MdUGT83K2 can transfer glucose to QPP in vivo, which is glycosylated. In this work, we identified a novel apple glycosyltransferase, MdUGT83K2, which functions to glycosylate the ACCase-inhibiting herbicide QPP and may be involved in plant detoxification. Key Contribution: A novel apple glycosyltransferase, MdUGT83K2, was identified, which may be involved in plant detoxification by glycosylation modification of the ACCase-inhibiting herbicide.
1. Introduction
Glycosyltransferase (GT, EC 2.4.x.y) is a class of activated glycosyl donors as substrates, which catalyzes the glycosylation of various substances, such as proteins, lipids, hormones, and phenylpropane compounds. The glycosylation reaction catalyzed by glycosyltransferase is a modification reaction necessary for the growth and development of plant cells and metabolic balance while also playing an important role in other life processes [,]. Glycosyltransferases are divided into 110 different families (CAZy, http://www.cazy.org (accessed on 15 January 2020)) according to amino acid sequence similarity, substrate specificity, and catalytic specificity, among which glycosyltransferase family 1 (GT family 1, GT1) has the largest number of gene members and plays the most important roles. The GT1 family is often referred to as UDP-glycosyltransferase (UGT) because it mainly catalyses the transfer of UDP-sugar to specific receptors (e.g., proteins, nucleic acids, antibiotics, alkaloids and plant hormones), which can regulate plant signaling pathways and cellular homeostasis, with broad roles [].
Bronzel I, the first gene of the plant UGT family, was discovered by Dooner and Nelson when they were studying the genetic traits of maize (Zea mays L) transposons []. The gene encodes a protein with UGT activity, which can synthesize flavonoids and regulate melanin accumulation in maize kernels []. To date, genes encoding the plant UGT family have been found in wheat (Triticum aestivum L.) [], soybean (Glycine max (L.) Merr.) [], and rice (Oryza sativa L.) [].
On the other hand, the application of herbicides has gradually become an indispensable part of agricultural production []. The potential toxic effects of herbicides threaten ecosystem []. It is thus very important to study the reproductive and genotoxicity of herbicides to select safe species for the protection of ecosystems. Among the known herbicides, aryloxyphenoxypropionate (APP) is a class of herbicides that inhibits fatty acid synthesis by inhibiting the activity of acetyl-CoA carboxylase and destroys membrane structures to achieve its herbicidal effect [,]. In our recent study, we discovered the compound QPP could be used as a potential lead structure for the further development of novel ACCase-inhibiting herbicides (Figure 1) []. In this study, we used biological methods to find glycosyltransferases that can glycosylate QPP so as to achieve the purpose of plant detoxification.
Figure 1.
The structure of ACCase-inhibiting herbicide QPP.
2. Results
2.1. MdUGT83K2 Candidate Glycosyltransferase Identification
According to the gene chip database published online and a phylogenetic tree of apple (Malus domestica) glycosyltransferases [], it was found that 7 glycosyltransferase genes MdUGT83L3 (MD09G1064900), MdUGT83L4 (MD09G1064700), MdUGT83L5 (MD17G1058200), MdUGT83L6 (MD17G1058400), MdUGT83L7 (MD17G1058100), MdUGT83L8 (MD09G1065000), and MdUGT83K2 (MD09G1065400) in the apple glycosyltransferase Group I family may predicted function like UGT83A1 according to gene chip data published online []. The ACCase inhibition-based herbicide QPP was used to act on the wild-type “Gala” strain, RNA was extracted and reverse-transcribed into cDNA, confirmed by real-time quantitative PCR, and it was found that MdUGT83K2 is affected by QPP. Among the findings, when QPP treatment was applied for 12 h, the upregulation was up to 40-fold, which is significant difference (p < 0.01) (Figure 2). Therefore, we identified MdUGT83K2 as a candidate glycosyltransferase gene involved in QPP detoxification.
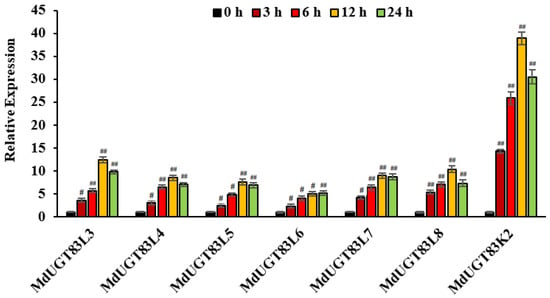
Figure 2.
Expression of apple glycosyltransferase Group I family related genes induced by herbicide QPP. For the treatment of wild-type “Gala” seedlings (normal culture for 3 weeks), QPP synthesized was prepared in a solution with a final concentration of 10 μmol/L. After 12 h, the materials were collected and stored at −80 °C for subsequent RNA extraction. Note: # p < 0.05, ## p < 0.01, n = 3.
2.2. Analysis of the Spatiotemporal Expression Pattern of MdUGT83K2
To further understand the expression pattern of MdUGT83K2, real-time PCR was further performed, the results of which are shown in Figure 3. The expression levels of MdUGT83K2 vary relatively widely across the different parts of a plant, with the lowest expression level in roots and the highest in young seeds.
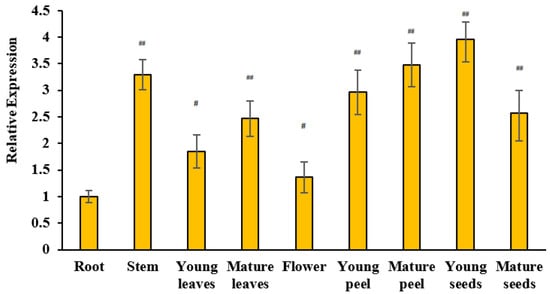
Figure 3.
MdUGT83K2 spatiotemporal expression pattern. Material was collected from the root, stem, leaf, flower, and fruit of a 10-year-old “Gala” apple tree. Note: # p < 0.05, ## p < 0.01, n = 3.
2.3. In Vitro Enzymatic Reaction to Determine MdUGT83K2 Glycosylation Modification of QPP
In the above study, we learned that MdUGT83K2 was induced to be upregulated by QPP. To further verify whether QPP was modified by MdUGT83K2 glycosylation, we constructed a MdUGT83K2 prokaryotic expression vector and expressed it into E. coli protein strain BL-21. After obtaining the active purified enzyme protein of MdUGT83K2, it was subjected to in vitro enzymatic reaction with QPP. HPLC detection showed that MdUGT83K2 could glycosylate QPP (Figure 4; a: HPLC image, b: mass spectrum). The specific enzyme activity of MdUGT74BP1 to QPP was 1.83 ± 0.32 (nkat/mg protein).
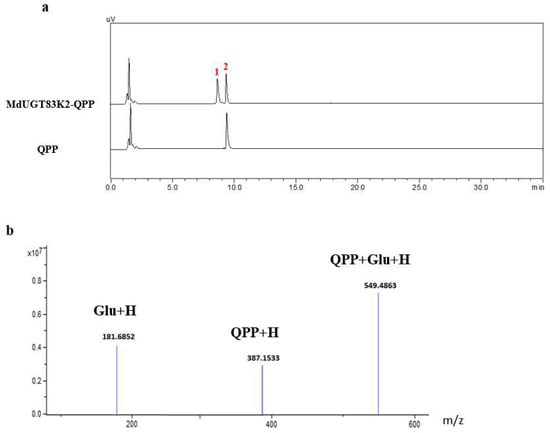
Figure 4.
HPLC analysis of reaction products from induced by herbicide QPP by MdUGT83K2. The MdUGT83K2 prokaryotic expression vector was constructed and expressed into E. coli protein strain BL-21, and the MdUGT83K2 enzymatic protein was purified and in vitro enzymatic reaction demonstrated that MdUGT83K2 glycosylation modifies QPP. (a): HPLC image, (b): mass spectrum. 1, QPP glycosides; 2, QPP.
2.4. Acquisition of Transgenic MdUGT83K2 and Determination of QPP Glycoside Content
In the above results, we demonstrated that MdUGT83K2 can glycosylate and modify QPP in vitro, so in vivo to further determine whether MdUGT83K2 glycosylation modifies QPP in plants, we constructed a plant expression vector of MdUGT83K2 and obtained transgenic “Gala” lines OE6 and OE11 (Figure 5). After extracting QPP glycosides, HPLC analysis was performed. Compared to the level in the wild-type, the level of free QPP in OE6 and OE11 decreased, while the level of QPP glycoside increased, indicating that MdUGT83K2 can still glycosylate QPP in plants (Figure 6).
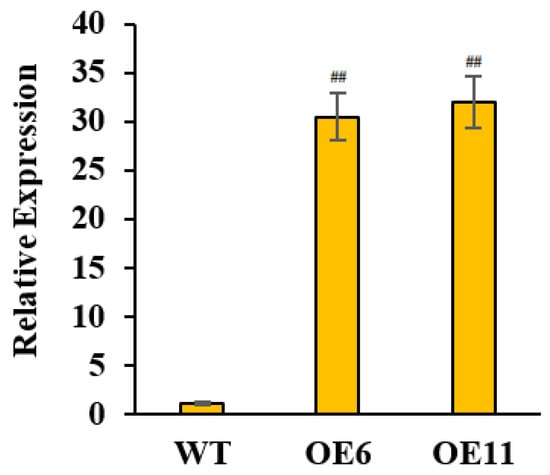
Figure 5.
Expression intensity of MdUGT83K2 OE lines (normal culture for 3 weeks). Note: ## p < 0.01, n = 3.
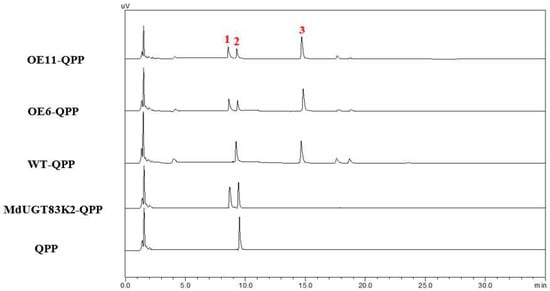
Figure 6.
HPLC detection of QPP glycoside of various strains. For QPP glycoside extraction, tissue culture seedlings (normal culture for 3 weeks) of wild-type “Gala” and transgenic apple lines (OE6 and OE11) were treated with 10 μmol/L QPP for 12 h. 1, QPP glycosides; 2, QPP; 3, Internal reference “IBA”.
3. Discussion
Herbicides play an important role in agricultural production, but they also bring new challenges to the breeding of crops for herbicide resistance and environmental restoration. Research on herbicide resistance of plants has increasingly become a hot filed. Most known plant resistance to herbicides is derived from the mutation or modification of herbicide target proteins [,,,], while less research has been performed on herbicide detoxification enzymes. Known herbicide detoxification enzymes include GST in maize and ketoaldehyde reductase in barnyard grass (Echinochloa crus galli (L.) Beauv.) []. Microbial herbicide detoxification enzymes include PAT, glyphosate oxidoreductase, and the products of genes such as bxn, glpA, glpB, atzA, atzB, atzC, and trzN. Mouse-derived cytochrome oxidase P450 has also been used for the detoxification of plant herbicides [,]. This is the focus of current research on herbicide detoxifying. This study found that the apple glycosyltransferase MdUGT83K2 can glycosylate QPP and participate in the process of plant detoxification. It can be used alone or in combination with other herbicide-resistance genes in crop herbicide-resistance breeding, which can expand the range of QPP applications. This work also provides new ideas and methods for research on herbicide selectivity, and it is expected to lead to the cultivation of new crop varieties that are resistant to herbicides. Therefore, the results of this study have potential value for applications in research on the herbicide resistance of crops.
Numerous studies have shown that plant glycosyltransferases can glycosylate small-molecule toxins to detoxify them [,]. Toxin molecules include not only biologically derived molecules, but also some non-biologically derived small-molecule substances, such as 2,4,5-trichlorophenol and 3,4-dichlorobenzamide. The identification of the plant glycosyltransferase MdUGT83K2 and its herbicide activity not only enriches the functional study of plant glycosyltransferases, but also provides a theoretical basis for an understanding of the functional evolution of glycosyltransferases. Glycosyltransferases first appeared in unicellular algae, and with the evolution of plants, the members of the glycosyltransferase family continued to expand in mosses, ferns, gymnosperms, and angiosperms, with the number increasing exponentially []. This reflects the growing importance of glycosyltransferases in adaptation to terrestrial life and their increasingly diverse functions in response to unfavorable internal and external environments. With the development of human social activities and industrial production, the external environment faced by plant growth is now constantly changing, and unfavorable factors continue to appear. It may be that plants must use existing glycosyltransferase family members to deal with emerging harmful substances and achieve detoxification through the functional evolution of enzymes so that plants can survive and develop better. Studies have shown that glycosyltransferases play an important role in the process of removing exogenous toxins, so the glycosylation and detoxification of herbicides may be a further manifestation of this function. Therefore, it is believed that the modification activity of glycosyltransferases on herbicides may be the result of their functional evolution. However, it cannot be ruled out that the glycosyltransferase MdUGT83K2 focused on in this study has other diverse substrates and exerts diverse functions enabling plants to cope with unfavorable environments. This is a topic that requires further exploration and research.
4. Conclusions
In this study, we first speculated that Group I in the apple glycosyltransferase family may predicted function like UGT83A1 to have possible detoxification effects according to gene chip data published online []. By qPCR technology, it was found that MdUGT83K2 in Group I was upregulated by the novel herbicide QPP. In vitro experiments, it was found that MdUGT83K2 can glycosylate the compound QPP, which indicated it may reduce the damage caused by herbicides to plants through the glycosylation of QPP. Therefore, in studies on transgenic plants, we further proved that MdUGT83K2 overexpression in “Gala” can indeed reduce free QPP and increase QPP glycosides.
This study found that the apple glycosyltransferase MdUGT83K2 can glycosylate QPP and participate in the process of plant detoxification. It can be used alone or in combination with other herbicide-resistance genes in crop herbicide-resistance breeding, which can expand the range of QPP applications. This work also provides new ideas and methods for research on herbicide selectivity, and it is expected to lead to the cultivation of new crop varieties that are resistant to herbicides. Therefore, the results of this study have potential value for applications in research on the herbicide resistance of crops.
5. Materials and Methods
5.1. Plant Material
Material for clarifying the spatiotemporal expression pattern of MdUGT83K2 was collected from the root, stem, leaf, flower, and fruit of a 10-year-old “Gala” apple (Malus × domestica Borkh.) tree planted at the School of Life Sciences, Shandong Agricultural University. After sampling, it was quick-frozen in liquid nitrogen and stored at −80 °C. Transgenic apple lines (OE6 and OE11) were obtained by transformation of the leaves of “Gala”, using tissue culture plantlets of the clone “GL-3” apple. The growth conditions of tissue culture seedlings were 22 °C, 60 μmol m−2 s−1, and a 14/10 h photoperiod. After subculture for 30 d and culture with rooting medium for 45 d, the seedlings were cultivated for 1 week, after which the tissue-cultured seedlings were transferred to plastic pots (8.5 × 8.5 × 7.5 cm) and then placed in a light incubator for cultivation.
5.2. Treatment of “Gala” Seedlings with QPP
For the treatment of wild-type “Gala” seedlings (normal culture for 3 weeks), QPP synthesized was prepared in a solution with a final concentration of 10 μmol/L. After 12 h, the materials were collected and stored at −80 °C for subsequent RNA extraction.
For the treatment of transgenic “Gala” seedlings, QPP synthesized in the early stage was prepared in a solution with a final concentration of 10 μmol/L. Then, the wild-type “Gala” and transgenic OE6 and OE11 lines were placed in the solution for 12 h of treatment. After that, the materials were collected and stored at −80 °C for subsequent extraction of QPP glycosides.
5.3. Real-Time PCR
For RNA extraction, 2 g of frozen material was taken and quickly ground into a fine powder in liquid nitrogen. The ground material was quickly transferred to a centrifuge containing 15 mL of CTAB extraction buffer (Weber Technology Co., Ltd., Guangzhou, China) in the tube, immediately vortexed vigorously for 30 s, and then warmed in a 65 °C water bath for 3–5 min. Subsequently, an equal volume of chloroform/isoamyl alcohol (24:1) was added, the sample was shaken evenly, centrifuged at 10,000 rpm and room temperature for 20 min, and then the supernatant was transferred to another centrifuge tube. The chloroform/isoamyl alcohol extraction was repeated one more time, and then the aqueous phase was transferred to another centrifuge tube. According to the solution volume, 1/3 volume of 8 mol/L LiCl was added to make the final concentration of LiCl 2 mol/L, followed by precipitation overnight at 4 °C, centrifugation at 4 °C (12,000 rpm) for 20 min, and then pouring off the supernatant. The precipitate was rinsed with 500 μL of 75% ethanol to remove LiCl, the precipitate was dissolved with 500 μL of SSTE buffer (Weber Technology Co., Ltd., Guangzhou, China), the solution was transferred to a 1.5 mL centrifuge tube, and then the extract was supplemented one more time with an equal volume of chloroform/isoamyl alcohol. Two volumes of 100% ethanol were added to the supernatant, followed by precipitation at −70 °C for 30 min or −20 °C for 2 h, and then centrifugation at full speed (12,000 rpm/min) and 4 °C for 20 min to precipitate RNA. The pellet was first rinsed with 400 μL of 70% ethanol, after which 400 μL of 100% ethanol was added to rinse the pellet. After drying the pellet, it was dissolved in 50 μL of DEPC-treated water.
For cDNA reverse transcription, PrimeScript RNA Reverse Transcriptase Reagent Kit with gDNA Eraser Kit (Code No. RR047A; Takara Biotechnology Co., Ltd., Dalian, China) was used in accordance with the kit instructions. The primer design was completed by Beijing Aoke Company (see Supplementary Materials Table S1).
For the real-time PCR, ChemoHS qPCR Mix (MonAmp™) and a real-time PCR instrument (ABI 7500) were used. The 10 µL reaction system included 0.4 µL each of upstream and downstream primers, 5 µL of MonAmp™ ChemoHS qPCR Mix, 0.1 µL of Low ROX Dye, 1–2 µL of cDNA template, and nuclease-free water to bring the volume to 10 µL. The PCR amplification program involved 95 °C for 5 min, followed by 40 cycles of 95 °C for 5 s of denaturation and 60 °C for 34 s of annealing and extension. Three replicates were set for each sample, melting curves were analyzed, and the relative expression levels of each gene were calculated.
5.4. Vector Construction
PCR amplification was performed using Ex Taq enzyme (RR030A; TaKaRa Biotechnology Co., Ltd., Dalian, China). Product recovery was performed using an agarose gel DNA recovery kit (ZY511-100T; Runye Biotechnology Co., Ltd., Shanghai, China). The gel-recovered product was connected to the pMD18-T vector and transformed into E. coli DH5α, and the recombinants were screened by the blue-white spot method. The recombinant plasmids (prokaryotic expression vector PGEX, plant expression vector PBI121) were double digested to identify positive clones and sequenced by UW Genetics (Shenzhen, China).
The PCR amplification system included 2 μL of Ex Taq (5 U/μL), 2.5 μL of 10 × Ex Taq Buffer (Mg2+ plus), 2 μL of dNTP mixture (2.5 mmol/L each), 0.5 μL each of upstream and downstream primers (20 μmol/L), 2 μL of template DNA (50 ng·L−1), and ddH2O to make up the volume to 25 μL. PCR amplification conditions were pre-denaturation at 94 °C for 4 min; followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 40 s and extension at 72 °C for 1.5 min; and then final extension at 72 °C for 10 min. The reaction system included 0.5 μL of MdUGT83K2 restriction endonuclease I (BamHI), 0.5 μL of restriction endonuclease II (HindIII), 2 μL of 10 × buffer, 5 μL of plasmid, and sterile water to make the volume up to 20 μL. Incubation was performed at 37 °C for 1 h, followed by verification by electrophoresis.
5.5. In Vitro Enzymatic Reaction
The correctly validated recombinant plasmid MdUGT83K2-PGEX was transformed into E. coli BL-21 receptor state, selected 3–5 picked single bacteria were inoculated into LB liquid medium (Amp+) and incubated overnight at 37 °C in a 225 rpm/min shaker. The strains were inoculated in 30 mL LB medium containing antibiotics at a ratio of 1: 50 and incubated at 37 °C 225 rpm/min until the OD reached about 0.6. The OD value was then recorded, 1 mL was taken as the sample at 0 h, to which 0.5 mol·L−1 IPTG was added to make the final concentration 0.5 mmol. Samples were taken every 1 h until 5 h, so that the amount of bacteria in each sample was the same. Then, 2 mL of pre-chilled 1 spheroplast buffer (0.5 mM EDTA, 750 mM sucrose, 200 mM Tris-HCl; pH 8.0) was added to the collected cells, pipetting was performed to suspend them, followed by the addition of 14 mL of pre-chilled 1/2 concentration spheroplast buffer solution (containing 2 mg of bacterial lyase). Next, the samples were placed on ice for 30 min with shaking several times during this period. Subsequently, lysed cells were collected by centrifugation at 4500 rpm for 10 min at 4 °C, after which 5 mL of 1 PBS buffer solution was added to the pellet (2.7 mM KCl, 140 mM NaCl, 10 mM Na2HPO4, 1.8 mM KH2PO4) along with 10 μL of 100 mM PMSF(phenylmethyl sulfonylfluoride), followed by mixing by gentle pipetting and then placed in an ice bath for 30 min with shaking several times during this period. Samples were then centrifuged at 7000 rpm and 4 °C for 20 min; the supernatant was transferred to a new centrifuge tube, 100 μL of 50% glutathione Sepharose 4B (BH-S0637; Bohu, Shanghai, China) beads were added, shaking upside down was performed, followed by slowly shaking on a horizontal shaker for 1 h. Subsequently, centrifugation was performed at 500× g for 5 min, the supernatant was discarded, and the pipette tip was used to cut the pellets. The beads were aspirated and transferred to a 1.5 mL centrifuge tube, followed by washing three to four times with PBS solution. Centrifugation was again performed at 500× g for 5 min; the supernatant was discarded, and 50 μL of protein eluent was added, followed by shaking for 15 min. Another round of centrifugation was then performed at 500× g for 5 min, with the supernatant subsequently obtained after centrifugation being the target protein solution. The above steps were repeated to collect the protein several times. Ten microliters of protein were obtained, which was homogenized using 25 μL of 2 × protein loading buffer, followed by electrophoresis through an SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electropheresis) gel. The gel block was gently removed from the gel rack after electrophoresis and put in Coomassie staining solution, shaken for 30 min and then supplemented with decolorization solution or water for decolorizing. The obtained results were then observed in a gel imager after decolorization (Figure S1). Another part of the protein was concentrated and then subjected to an in vitro enzymatic reaction.
The enzymatic reaction system contained 10 μL of 0.5 M Tris-HCl (pH 8.0), 5 μL of 50 mM MgSO4, 5 μL of 200 mM KCl, 2.5 μL of 0.1 M UDP-glucose, 1 μL of 10% β-mercaptoethanol, 1 μL of 100 mM QPP, 2 μL of MdUGT83K2 protein, and ddH2O added to make up the volume to 100 μL.
5.6. Obtaining and Testing Genetically Modified Apples
The test material used for apple genetic transformation was the tissue culture seedlings of clone “GL-3”, with strong regenerative ability and high transformation efficiency, obtained from the “Gala” apple. The leaves of “GL-3” were transformed with Agrobacterium carrying the MdUGT83K2 sequence, as described by the reported method []. MS medium was supplemented with 50 mg·L−1 kanamycin and 250 mg·L−1 cefotaxime sodium for the selection of regenerated shoots. During the screening process, the slowly growing and gradually yellowing regenerated buds were discarded, and the normally growing regenerated buds continued to be subcultured on the screening medium until the regenerated buds were cultivated into robust individuals with stem and leaf tissue.
Identification of transgenic lines at the DNA level was performed as follows. DNA from “GL-3” and transgenic apple plants was extracted using the Wolact Plant Genomic DNA Purification Kit (Wolact, Vicband Life Sciences Company Limited). The partial sequence of the CaMV 35S promoter on the vector was used as the upstream primer, and the downstream primer was designed with the sequence of the MdUGT83K2 gene for PCR amplification. The primers are shown in Table S1.
mRNA level identification of transgenic lines was performed as follows. In accordance with the above RNA extraction method, RNA was extracted from MdUGT83K2 transgenic plants and non-transgenic plants and then reverse-transcribed into cDNA. The expression status of MdUGT83K2 in positive plants was detected by qRT-PCR, and the primers are shown in Table S1. The identified MdUGT83K2-positive transgenic plants and untransformed wild-type plants (“GL-3”) were propagated and rooted for subsequent experimental treatments.
5.7. Extraction and HPLC Analysis of QPP Glycosides in Transgenic Apples
For QPP glycoside extraction, tissue culture seedlings of wild-type “Gala” and transgenic apple lines (OE6 and OE11) were treated with 10 μmol/L QPP for 12 h. Here, 10 g fresh samples were taken and ground into powder with liquid nitrogen; 10 mL of 80% methanol solution was added for extraction, and 2 μL of internal reference (Piclorom) was added at the same time. The samples were shaken well and placed at room temperature for 6 h. During this period, samples were continuously inverted and mixed. After vacuum evaporation in a rotary evaporator, the residual powder on the inner wall of the centrifuge tube was dissolved with 1 mL of methanol solution, centrifuged at 15,000 rpm for 20 min and the glycosides for subsequent HPLC detection.
HPLC analysis conditions involved the analytical instrument Shimadzu LC-20AT (Shimadzu, Japan). The main instruments included a workstation LC solution (Verl.21), diode array detector SPD-M20A, autosampler SIL-20A, system controller CBM-20A, and degasser DGM-20A3. The column was a reverse column. The detection wavelength of each species’ eluted peak was between 190 and 430 nm.
Regarding the elution procedure, the mobile phase was acetonitrile, water (both containing 0.1% trifluoroacetic acid) and 10–100% acetonitrile, the flow rate was 1 mL/min, the elution time was 25 min, and the detection wavelength was 320 nm.
LC-MS (Liquid Chromatograph Mass Spectrometer) analysis conditions involved a Surveyor MSQ (USA) single quadrupole liquid chromatography mass spectrometer as the instrument, and the ESI (electrospray ionization) interface was used in this experiment. Here, 0.1% phosphoric acid or triethylamine acetic acid was replaced with 0.1% formic acid in the mobile phase to reduce ion suppression. The chromatographic conditions were the same as in the HPLC elution procedure, the detection was carried out in positive ion mode, and the surface-induced decomposition intensity was 30 eV.
5.8. Data Analysis
Statistical analysis was performed using SPSS Statistics for Windows version 17.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was assessed by one-way ANOVA and Tukey’s multiple-range test. Different letters indicate significant differences (p < 0.05) and extremely significant differences (p < 0.01).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13020306/s1. Figure S1: MdUGT83K2 purified protein SDS-PAGE gel. 1, GST, 2, MdUGT83K2-GST. Table S1: Primer sequence.
Author Contributions
Conceptualization, L.J. and K.L.; methodology, P.L.; validation, A.Z. and R.L.; formal analysis, P.L. and S.H.; investigation, P.L. and N.L.; data curation, L.J.; writing—original draft preparation, L.J.; writing—review and editing, P.L.; project administration, L.J.; funding acquisition, P.L. and L.J. All authors have read and agreed to the published version of the manuscript.
Funding
This project was financially supported by National Natural Science Foundation of China (No. 32001982, 31701827), Shandong Provincial Natural Science Foundation of China (No. ZR202102180037), National Key Research and Development Program of China (No. SQ2020YFF0422322) and the China Postdoctoral Science Foundation (No. 2020M671984).
Data Availability Statement
Not applicable.
Acknowledgments
This project was supported by grants from the National Natural Science Foundation of China (Grant No. 32001982), Shandong Provincial Natural Science Foundation of China (Grant No. ZR202102180037), National Key Research and Development Program of China (SQ2020YFF0422322). Thank you very much for the plant materials provided by the College of Life Sciences of Shandong Agricultural University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, B.; Liu, X.; Xu, K.; Zhang, B. Genome-wide characterization, evolution and expression profiling of UDP-glycosyltransferase family in pomelo (Citrus grandis) fruit. BMC Plant Biol. 2020, 20, 459. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, F.; Li, P.; Wang, T.; Zheng, C.; Hou, B.K. An Arabidopsis cytokinin-modifying glycosyltransferase UGT76C2 improves drought and salt tolerance in rice. Front Plant Sci. 2020, 11, 560696. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, N.; Gao, T.; Jin, J.; Jing, T.; Wang, J.; Wu, Y.; Wan, X.; Schwab, W.; Song, C. Sesquiterpene glucosylation mediated by glucosyltransferase UGT91Q2 is involved in the modulation of cold stress tolerance in tea plants. New Phytol. 2020, 226, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Dooner, H.K.; Nelson, O.E. Controlling element-induced alterations in UDPglucose: Flavonoid glucosyltransferase, the enzyme specified by the bronze locus in maize. Proc. Natl. Acad. Sci. USA 1977, 74, 5623–5627. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ahmad, D.; Zhang, X.; Zhang, Y.; Wu, L.; Jiang, P.; Ma, H. Genome-wide analysis of family-1 UDP glycosyltransferases (UGT) and identification of UGT genes for FHB resistance in wheat (Triticum aestivum L.). BMC Plant Biol. 2018, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Yano, R.; Takagi, K.; Tochigi, S.; Fujisawa, Y.; Nomura, Y.; Tsuchinaga, H.; Takahashi, Y.; Takada, Y.; Kaga, A.; Anai, T.; et al. Isolation and characterization of the soybean Sg-3 gene that is involved in genetic variation in sugar chain composition at the C-3 position in soyasaponins. Plant Cell Physiol. 2018, 59, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.Q.; Sun, Y.; Guo, T.; Guo, T.; Shi, C.L.; Zhang, Y.M.; Kan, Y.; Xiang, Y.H.; Zhang, H.; Yang, Y.B.; et al. UDP-glucosyltransferase regulates grain size and abiotic stress tolerance associated with metabolic flux redirection in rice. Nat. Commun. 2020, 11, 2629. [Google Scholar] [CrossRef] [PubMed]
- Tong, L. Acetyl-coenzyme A carboxylase: Crucial metabolic enzyme and attractive target for drug discovery. Cell Mol. Life Sci. 2005, 62, 1784–1803. [Google Scholar] [CrossRef]
- Délye, C. Weed resistance to acetyl coenzyme A carboxylase inhibitors: An update. Weed Sci. 2005, 53, 728–746. [Google Scholar] [CrossRef]
- Liu, W.; Harrison, D.K.; Chalupska, D.; Gornicki, P.; O’donnell, C.C.; Adkins, S.W.; Haselkorn, R.; Williams, R.R. Single-site mutations in the carboxyltransferase domain of plastid acetyl-CoA carboxylase confer resistance to grass-specific herbicides. Proc. Natl. Acad. Sci. USA 2007, 104, 3627–3632. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Powles, S.B. Six amino acid substitutions in the carboxyltransferase domain of the plastidic acetyl-CoA carboxylase gene are linked with resistance to herbicides in a Lolium rigidum population. New Phytol. 2006, 172, 636–645. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, K.; Li, N.; Fu, S.Y.; Li, P.; Ji, L.S.; Liu, G.Y.; Wang, X.K.; Lei, K. Design, synthesis, mode of action and herbicidal evaluation of quinazolin-4(3H)-one derivatives based on aryloxyphenoxypropionate motif. Agronomy 2022, 12, 1840. [Google Scholar] [CrossRef]
- Li, Y.J.; Li., P.; Zhang, L.; Shu, J.; Court, M.H.; Sun, Z.; Jiang, L.; Zheng, C.; Shu, H.; Ji., L.S.; et al. Genome-wide analysis of the apple family 1 glycosyltransferases identified a flavonoid-modifying UGT, MdUGT83L3, which is targeted by MdMYB88 and contributes to stress adaptation. Plant Sci. 2022, 321, 111314. [Google Scholar] [CrossRef]
- Bai, S.; Zhao, Y.F.; Zhou, Y.M.; Wang, M.L.; Li, Y.H.; Luo, X.Y.; Li, L.X. Identification and expression of main genes involved in non-target site resistance mechanisms to fenoxaprop-p-ethyl in Beckmannia syzigachne. Pest Manag. Sci. 2020, 76, 2619–2626. [Google Scholar] [CrossRef]
- Uzogara, S.G. The impact of genetic modification of human foods in the 21st century: A review. Biotechnol Adv. 2000, 18, 179–206. [Google Scholar] [CrossRef]
- Dong, H.; Huang, Y.; Wang, K. The Development of herbicide resistance crop plants using CRISPR/Cas9-mediated gene dditing. Genes 2021, 12, 912. [Google Scholar] [CrossRef]
- Hou, C.X.; Dirk, L.M.; Pattanaik, S.; Das, N.C.; Maiti, I.B.; Houtz, R.L.; Williams, M.A. Plant peptide deformylase: A novel selectable marker and herbicide target based on essential cotranslational chloroplast protein processing. Plant Biotechnol. J. 2007, 5, 275–281. [Google Scholar] [CrossRef]
- Burns, E.E.; Keith, B.K.; Refai, M.Y.; Bothner, B.; Dyer, W.E. Constitutive redox and phosphoproteome changes in multiple herbicide resistant Avena fatua L. are similar to those of systemic acquired resistance and systemic acquired acclimation. J. Plant Physiol. 2018, 220, 105–114. [Google Scholar] [CrossRef]
- Li, P.; Yu, Q.; Han, H.P.; Mao, L.; Nyporko, A.; Fan, L.; Bai, L.; Powles, S. Aldo-keto Reductase metabolizes glyphosate and confers glyphosate resistance in Echinochloa colona. Plant Physiol. 2019, 181, 1519–1534. [Google Scholar]
- Schuler, M.A. Plant cytochrome P450 monooxygenases. Crit. Rev. Plant Sci. 1996, 15, 235–284. [Google Scholar] [CrossRef]
- Inui, H.; Shiota, N.; Ishige, T.; Yasunobu, O.; Hideo, O. Herbicide metabolism and resistance of transgenic potato plantexpressing rat cytochrome P4501A1. Breed. Sci. 1998, 48, 135–143. [Google Scholar]
- Pflugmacher, D.; Sandermann, H., Jr. The lipid/protein interface as a target site for general anesthetics: A multiple-site kinetic analysis of synaptosomal Ca2+-ATPase. Biochim. Biophys. Acta 1998, 1415, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Vogt, T. Glycosyltransferases in secondary plant metabolism: Tranquilizers andstimulant controllers. Planta 2001, 213, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Caputi, L.; Malnoy, M.; Goremykin, V.; Nikiforova, S.; Martens, S. A genome-wide phylogenetic reconstruction of family 1 UDP-glycosyltransferase revealed the expansion of the family during the adaptation of plants to life on land. Plant J. 2012, 69, 1030–1042. [Google Scholar] [CrossRef]
- Dai, H.; Li, W.; Han, G.; Yang, Y.; Yue, M.; He, L.; Zhang, Z.H. Development of a seedling clone with high regeneration capacity and susceptibility to Agrobacterium in apple. Sci. Hortic. 2013, 164, 202–208. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).