Synthesis and Application of Modified Lignin Polyurea Binder for Manufacturing a Controlled-Release Potassium Fertilizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
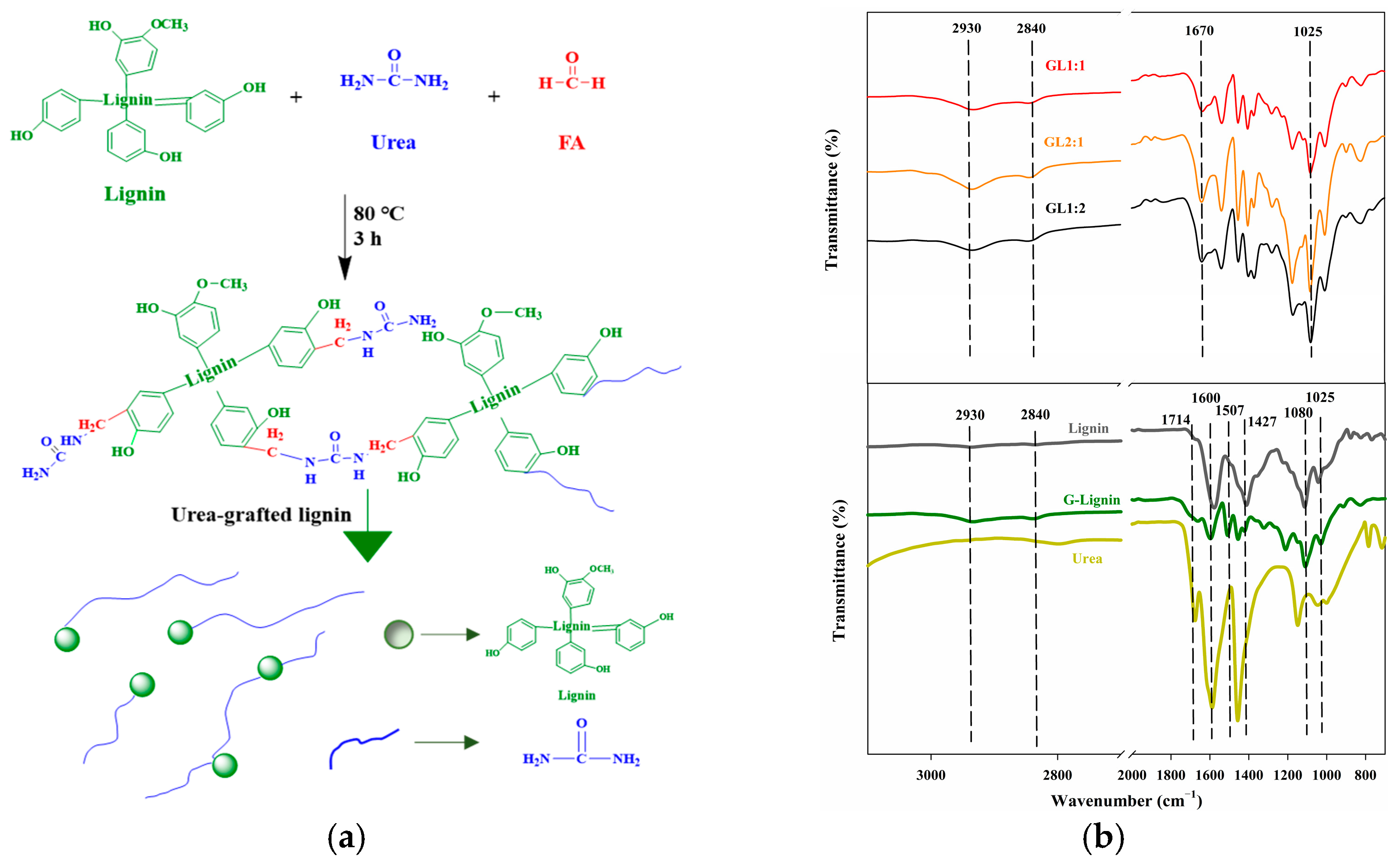
2.2. Preparation of Urea-Grafted Lignin and the Determination of Lignin-Grafted Ratio
2.3. Preparation and Characterization of Lignin Modified Polyurea Binder
2.4. Preparation of Fertilizer Cores
2.5. Evaluation of Fertilizer Coating Effect
2.6. Characterization of Fertilizer Core Granules
2.7. Flowchart of the Modified Polyurea Binder Controlled-Release Potassium Chloride Process
2.8. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Modified Lignin-Based Polyurea Binder
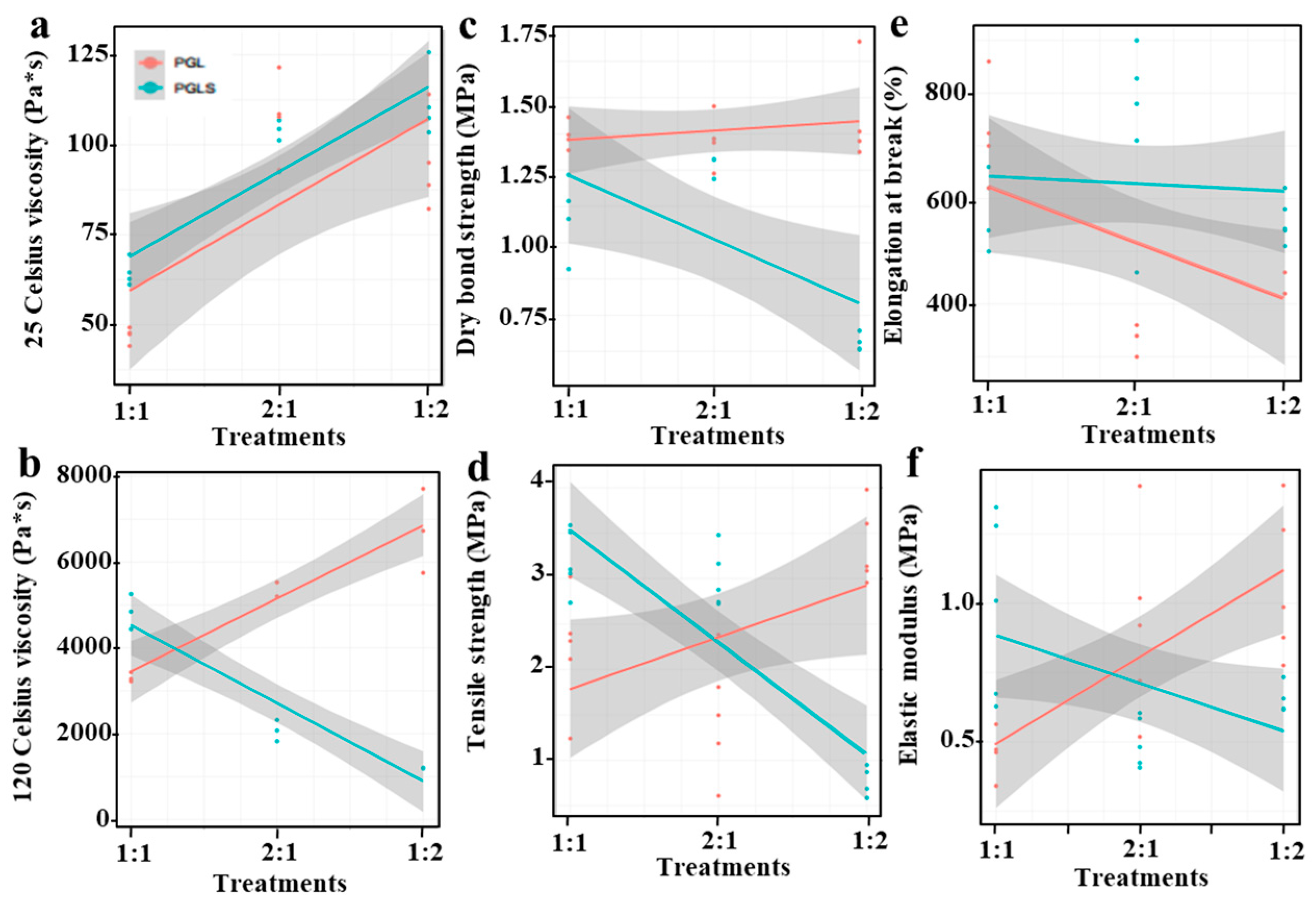
3.2. Performance Characterization of Modified Lignin-Based Polyurea Binder
3.3. TGA and DSC Analysis of Lignin-Based Polyurea Binder
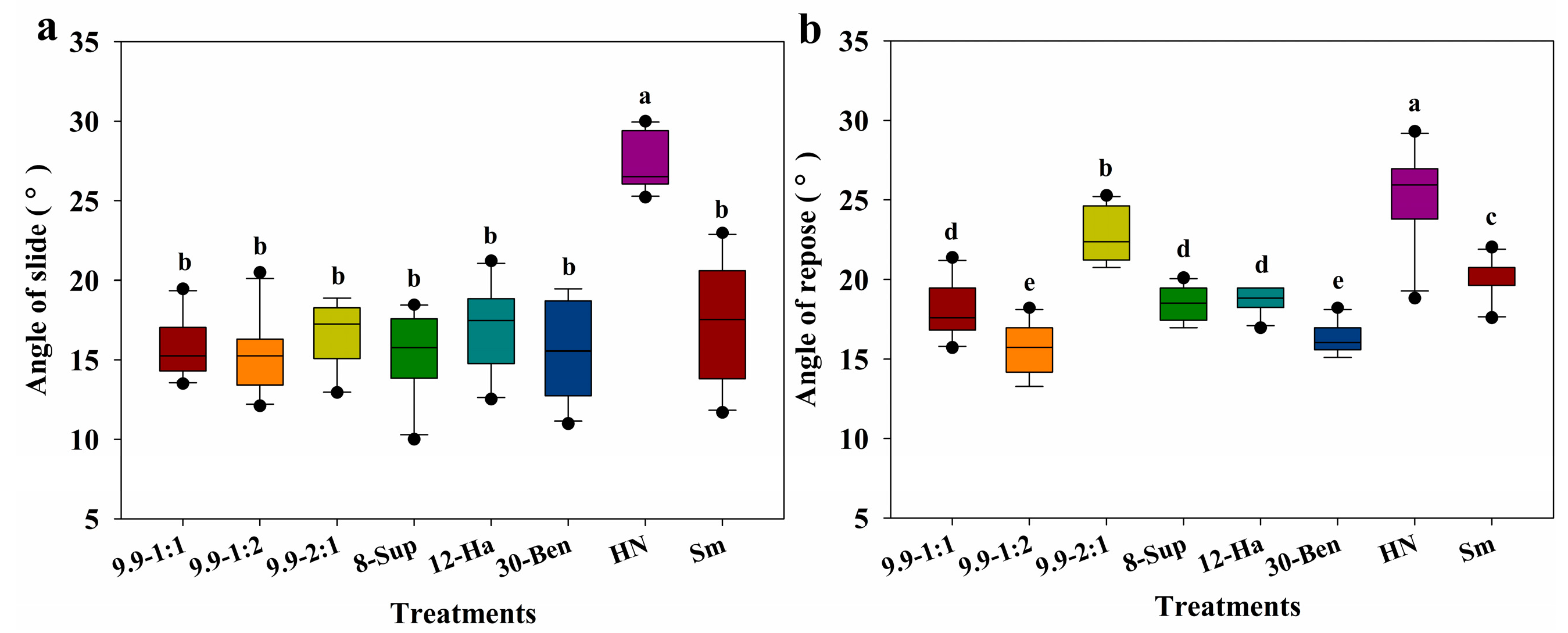
3.4. Granule Strength and Fluidity of Modified Polyurea Potassium Chloride Fertilizer Cores
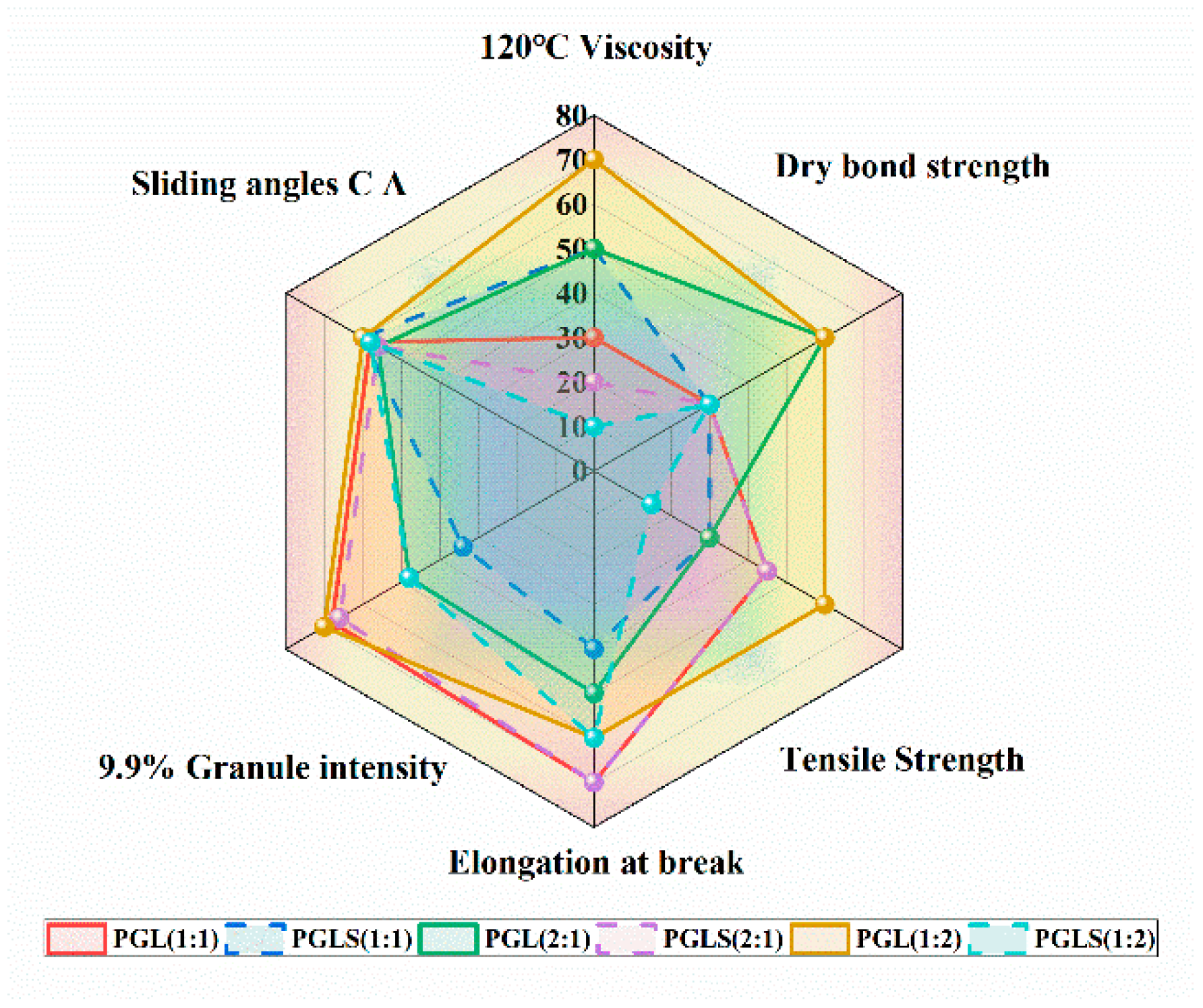
3.5. Radar Image of Lignin-Based Polyurea Binder
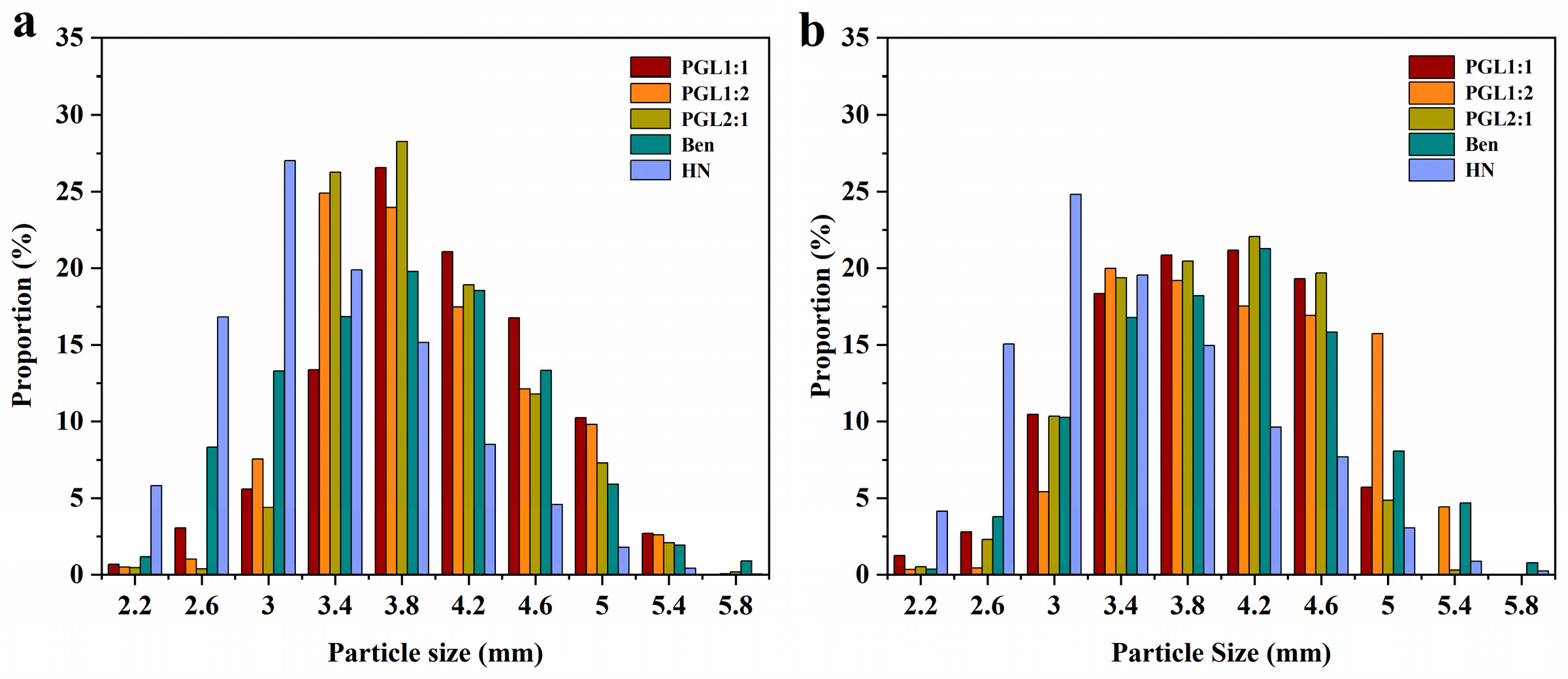
3.6. Analysis of Granule Size and Roundness of Coated Potassium Chloride Fertilizers and Fertilizer Cores
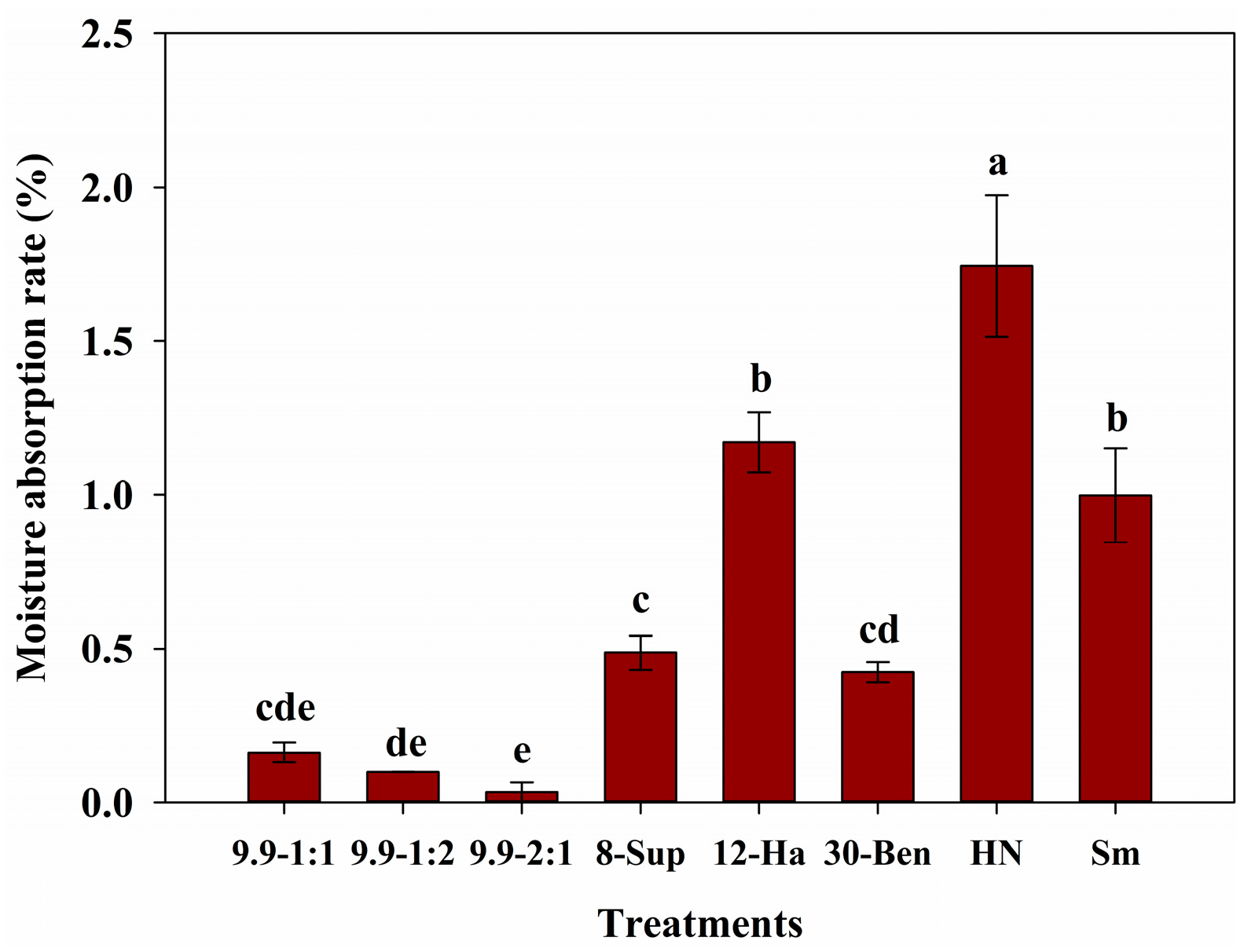
3.7. Hygroscopicity of Potassium Chloride Fertilizer Cores
3.8. Nutrient Release Rate of Potassium Chloride Granules with Different Membrane Thickness
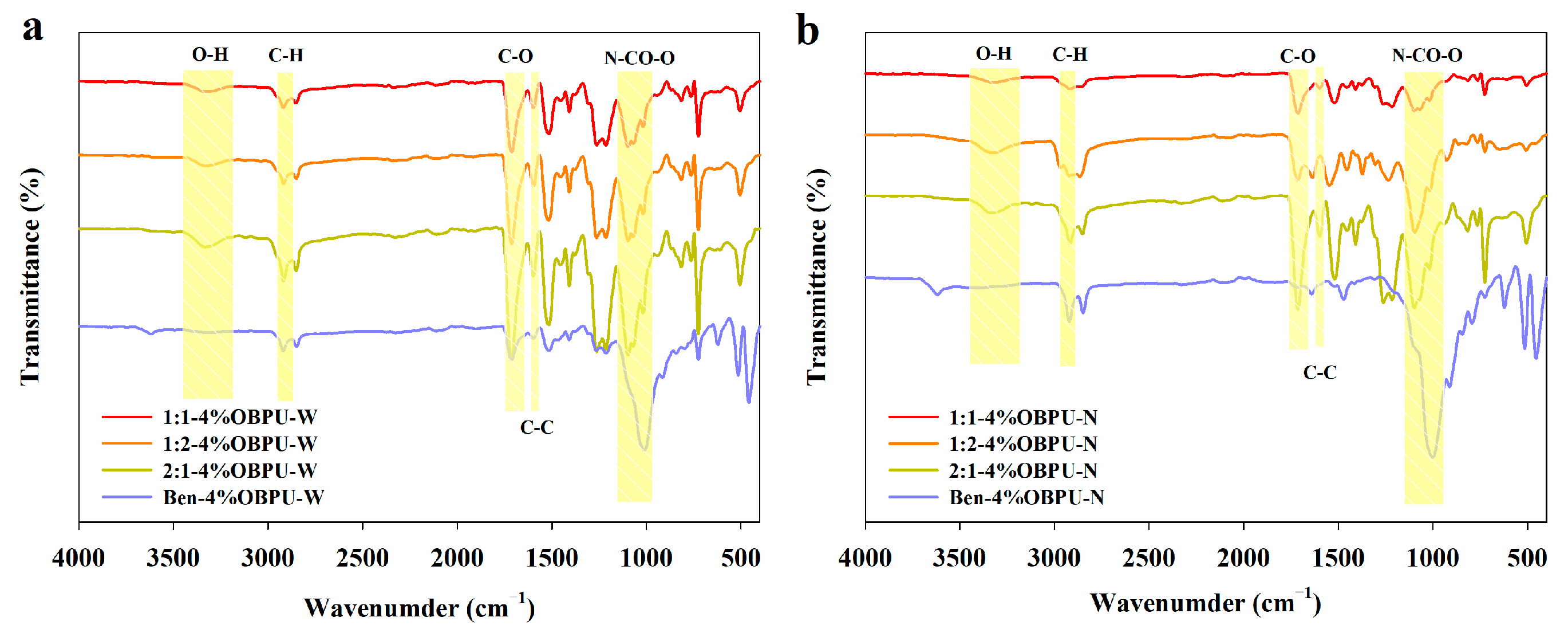
3.9. Fourier-Transform Infrared Spectrum of Membrane Shell of Controlled-Release Potassium Chloride Fertilizers
3.10. Scanning Electron Microscopy of Controlled-Release Potassium Chloride Fertilizers and Membrane Shells
3.11. Laser Scanning of the Morphology of Potassium Chloride Fertilizer Cores
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nadarajan, S.; Sukumaran, S. Chemistry and Toxicology behind Chemical Fertilizers. In Controlled Release Fertilizers for Sustainable Agriculture; Academic Press: Cambridge, MA, USA, 2021; pp. 195–229. [Google Scholar]
- Al Rawashdeh, R.; Maxwell, P. Analysing the world potash industry. Resour. Policy 2014, 41, 143–151. [Google Scholar] [CrossRef]
- Fixen, P.E.; Johnston, A.M. World fertilizer nutrient reserves: A view to the future. J. Sci. Food Agric. 2012, 92, 1001–1005. [Google Scholar] [CrossRef]
- Al Rawashdeh, R.; Xavier–Oliveira, E.; Maxwell, P. The potash market and its future prospects. Resour. Policy 2016, 47, 154–163. [Google Scholar] [CrossRef]
- Blanco, M. Supply of and Access to Key Nutrients NPK for Fertilizers for Feeding the World in 2050; UPM: Madrid, Spain, 2011. [Google Scholar]
- Dobermann, A. Nutrient use efficiency—Measurement and management. In Fertilizer Best Management Practices: General Principles, Strategy for Their Adoption and Voluntary Initiatives Versus Regulations; Krauss, A., Isherwood, K., Heffer, P., Eds.; International Fertilizer Industry Association: Paris, France, 2007; pp. 1–28. [Google Scholar]
- Al-Rawajfeh, A.E.; Alrbaihat, M.R.; AlShamaileh, E.M. Characteristics and types of slow-and controlled-release fertilizers. In Controlled Release Fertilizers for Sustainable Agriculture; Academic Press: Cambridge, MA, USA, 2021; pp. 57–78. [Google Scholar]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef]
- Abbas, A.; Wang, Z.; Zhang, Y.; Zhang, Y.R.; Peng, P.; She, D. Lignin-based controlled release fertilizers: A review. Int. J. Biol. Macromol. 2022, 222, 1801–1817. [Google Scholar] [CrossRef]
- Yang, X.Y.; Geng, J.B.; Li, C.L.; Zhang, M.; Chen, B.C.; Tian, X.F.; Zheng, W.K.; Liu, Z.G.; Wang, C. Combined application of polymer coated potassium chloride and urea improved fertilizer use efficiencies, yield and leaf photosynthesis of cotton on saline soil. Field Crops Res. 2016, 197, 63–73. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, X.G.; Font, O.; Moreno, N.; Vallejo, V.R.; Querol, X.; Tobias, A. Synthesis of merlinoite from Chinese coal fly ashes and its potential utilization as slow releases K-fertilizer. J. Hazard. Mater. 2014, 265, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.A. Potassium sources, use, and potential. In Potassium in Agriculture; Wiley: Hoboken, NJ, USA, 1985; pp. 83–98. [Google Scholar]
- Tian, H.Y.; Zhang, L.N.; Dong, J.J.; Wu, L.; Fang, F.L.; Wang, Y.F.; Li, H.; Xie, C.S.; Li, W.J.; Wei, Z.B.; et al. A one-step surface modification technique improved the nutrient release characteristics of controlled-release fertilizers and reduced the use of coating materials. J. Clean. Prod. 2022, 369, 133331. [Google Scholar] [CrossRef]
- Guo, Y.L.; Liu, Z.G.; Zhang, M.; Tian, X.F.; Chen, J.Q.; Sun, L.L. Synthesis and application of urea-formaldehyde for manufacturing a controlled-release potassium fertilizer. Ind. Eng. Chem. Res. 2018, 57, 1593–1606. [Google Scholar] [CrossRef]
- Loganathan, P.; Hedley, M.J.; Clark, S.A. The manufacture and evaluation of granular potassium chloride fertilizers. Fert. Res. 1992, 31, 291–304. [Google Scholar] [CrossRef]
- Prasai, T.P.; Walsh, K.B.; Midmore, D.J.; Jones, B.E.H.; Bhattarai, S.P. Manure from biochar, bentonite and zeolite feed supplemented poultry: Moisture retention and granulation properties. J. Environ. Manag. 2018, 216, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Washino, K.; Tan, H.S.; Hounslow, M.J.; Salman, A.D. A new capillary force model implemented in micro-scale CFD–DEM coupling for wet granulation. Chem. Eng. Sci. 2013, 93, 197–205. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Lewis, T.D.; Walker, G.M. Granulated polyhalite fertilizer caking propensity. Powder Technol. 2017, 308, 193–199. [Google Scholar] [CrossRef]
- Jacob, M. Spheronization, granulation, pelletization, and agglomeration processes. In Microencapsulation in the Food Industry; Academic Press: Vienna, Austria, 2023; pp. 123–136. [Google Scholar]
- Degrève, J.; Baeyens, J.; Van de Velden, M.; Laet, S.D. Spray-agglomeration of NPK-fertilizer in a rotating drum granulator. Powder Technol. 2006, 163, 188–195. [Google Scholar] [CrossRef]
- Mathur, M.A.; Dias, M.F.; Mathur, M.P. Importance of green technology in fertilizer quality improvement. Procedia Eng. 2016, 138, 308–313. [Google Scholar] [CrossRef]
- Subero-Couroyer, C.; Ghadiri, M.; Brunard, N.; Kolenda, F. Analysis of catalyst particle strength by impact testing: The effect of manufacturing process parameters on the particle strength. Powder Technol. 2005, 160, 67–80. [Google Scholar] [CrossRef]
- Kabiri, S.; Baird, R.; Tran, D.N.H.; Andelkovic, I.; McLaughlin, M.J.; Losic, D. Cogranulation of low rates of graphene and graphene oxide with macronutrient fertilizers remarkably improves their physical properties. ACS Sustain. Chem. Eng. 2018, 6, 1299–1309. [Google Scholar] [CrossRef]
- Secco, M.; Valentini, L.; Addis, A. Ancient and modern binders: Naturally nanostructured materials. In Nanotechnologies and Nanomaterials for Diagnostic. Conservation and Restoration of Cultural Heritage; Elsevier: Amsterdam, The Netherlands, 2019; pp. 205–237. [Google Scholar]
- Zhang, G.J.; Sun, Y.H.; Xu, Y. Review of briquette binders and briquetting mechanism. Renew. Sustain. Energy Rev. 2018, 82, 477–487. [Google Scholar] [CrossRef]
- Xue, B.C.; Liu, T.; Huang, H.; Liu, E.B. The effect of the intimate structure of the solid binder on material viscosity during drum granulation. Powder Technol. 2014, 253, 584–589. [Google Scholar] [CrossRef]
- De La Grée, G.C.H.D.; Florea, M.V.A.; Keulen, A.; Brouwers, H.J.H. Contaminated biomass fly ashes–Characterization and treatment optimization for reuse as building materials. Waste Manag. 2016, 49, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Skeist, I.; Miron, J. History of adhesives. J. Macromol. Sci. Chem. 1981, 15, 1151–1163. [Google Scholar] [CrossRef]
- Liu, K.K.; Su, C.Q.; Ma, W.W.; Li, H.L.; Zeng, Z.; Li, L.Q. Free formaldehyde reduction in urea-formaldehyde resin adhesive: Modifier addition effect and physicochemical property characterization. BioResources 2020, 15, 2339–2355. [Google Scholar] [CrossRef]
- Conner, A.H. Urea-formaldehyde adhesive resins. In Polymeric Materials Encyclopedia; Salamone, J.C., Ed.; CRC Press: New York, NY, USA, 1996; Volume 11. [Google Scholar]
- Balčiūnas, G.; Vėjelis, S.; Vaitkus, S.; Kairytė, A. Physical properties and structure of composite made by using hemp hurds and different binding materials. Procedia Eng. 2013, 57, 159–166. [Google Scholar] [CrossRef]
- Gironès, J.; López, J.P.; Mutjé, P.; Curvelo, A.A.S.; Vilaseca, F. Natural fiber-reinforced thermoplastic starch composites obtained by melt processing. Compos. Sci. Technol. 2012, 72, 858–863. [Google Scholar] [CrossRef]
- Kremensas, A.; Kairytė, A.; Vaitkus, S.; Vėjelis, S. Physical-mechanical properties of Composites from Hemp Shives and Starch. Int. J. Eng. Sci. Invent. 2018, 7, 43–49. [Google Scholar]
- Shim, J.; Mohr, D. Using split Hopkinson pressure bars to perform large strain compression tests on polyurea at low, intermediate and high strain rates. Int. J. Impact Eng. 2009, 36, 1116–1127. [Google Scholar] [CrossRef]
- Liu, W.; Fang, C.; Chen, F.T.; Qiu, X.Q. Strong, reusable, and self-healing lignin-containing polyurea adhesives. ChemSusChem 2020, 13, 4691–4701. [Google Scholar] [CrossRef]
- Li, T.; Xie, Z.N.; Xu, J.; Weng, Y.X.; Guo, B.H. Design of a self-healing cross-linked polyurea with dynamic cross-links based on disulfide bonds and hydrogen bonding. Eur. Polym. J. 2018, 107, 249–257. [Google Scholar] [CrossRef]
- Li, T.H.; Zhang, B.G.; Jiang, S.Y.; Zhou, X.J.; Du, G.B.; Wu, Z.G.; Cao, M.; Yang, L. Novel highly branched polymer wood adhesive resin. ACS Sustain. Chem. Eng. 2020, 8, 5209–5216. [Google Scholar] [CrossRef]
- Shojaei, B.; Najafi, M.; Yazdanbakhsh, A.; Abtahi, M.; Zhang, C.W. A review on the applications of polyurea in the construction industry. Polym. Adv. Technol. 2021, 32, 2797–2812. [Google Scholar] [CrossRef]
- Luo, J.Z.; Wang, T.; Sim, C.; Li, Y.Z. Mini-Review of Self-Healing Mechanism and Formulation Optimization of Polyurea Coating. Polymers 2022, 14, 2808. [Google Scholar] [CrossRef]
- Wu, H.Z.; Liao, D.S.; Chen, X.Y.; Du, G.B.; Li, T.H.; Essawy, H.; Pizzi, A.; Zhou, X.J. Functionalized Natural Tannins for Preparation of a novel non-isocyanate polyurea-based adhesive. Polym. Test. 2023, 117, 107853. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Szalaty, T.; Jesionowski, T. Lignin as Material of the Future: Key Information and Development Prospects. Encycl. Polym. Sci. Technol. 2002, 1–28. [Google Scholar] [CrossRef]
- Zhao, X.H.; Zhang, Y.J.; Hu, H.Y.; Huang, Z.Q.; Qin, Y.B.; Shen, F.; Huang, A.M.; Feng, Z.F. Effect of lignin esters on improving the thermal properties of poly (vinyl chloride). J. Appl. Polym. Sci. 2019, 136, 47176. [Google Scholar] [CrossRef]
- Yang, G.T.; Zhao, H.M.; Liu, Y.L.; Li, Z.L.; Gao, F.; Zhang, Q.; Zou, P.; Liu, Z.G.; Zhang, M. Slow release fertilizers based on polyphosphate/montmorillonite nanocomposites for improving crop yield. Arab. J. Chem. 2023, 16, 104871. [Google Scholar] [CrossRef]
- Tian, H.Y.; Liu, Z.G.; Zhang, M.; Guo, Y.L.; Zheng, L.; Li, Y.C.C. Biobased polyurethane, epoxy resin, and polyolefin wax composite coating for controlled-release fertilizer. ACS Appl. Mater. Interfaces 2019, 11, 5380–5392. [Google Scholar] [CrossRef]
- Dubey, A.; Mailapalli, D.R. Zeolite coated urea fertilizer using different binders: Fabrication, material properties and nitrogen release studies. Environ. Technol. Innov. 2019, 16, 100452. [Google Scholar] [CrossRef]
- Ferreira, I.S.B.; Peruchi, R.S.; Fernandes, N.J.; Junior, P.R. Measurement system analysis in angle of repose of fertilizers with distinct granulometries. Measurement 2021, 170, 108681. [Google Scholar] [CrossRef]
- Fang, C.; Liu, W.F.; Qiu, X.Q. Preparation of polyetheramine-grafted lignin and its application in UV-resistant polyurea coatings. Macromol. Mater. Eng. 2019, 304, 1900257. [Google Scholar] [CrossRef]
- Wang, S.Y.; Liu, W.F.; Yang, D.J.; Qiu, X.Q. Highly resilient lignin-containing polyurethane foam. Ind. Eng. Chem. Res. 2018, 58, 496–504. [Google Scholar] [CrossRef]
- Dhenge, R.M.; Washino, K.; Cartwright, J.J.; Hounslow, M.J.; Salman, A.D. Twin screw granulation using conveying screws: Effects of viscosity of granulation liquids and flow of powders. Powder Technol. 2013, 238, 77–90. [Google Scholar] [CrossRef]
- Lapprand, A.; Boisson, F.; Delolme, F.; Méchin, F.; Pascault, J.P. Reactivity of isocyanates with urethanes: Conditions for allophanate formation. Polym. Degrad. Stab. 2005, 90, 363–373. [Google Scholar] [CrossRef]
- Dušek, K. Theory of network formation by additional crosslinking of polyurethanes due to biuret and allophanate formation. Polym. Bull. 1987, 17, 481–488. [Google Scholar] [CrossRef]
- Xu, Q.W.; Lu, Q.L.; Zhu, S.; Pang, R.; Shan, W.W. Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating. e-Polymers 2019, 19, 444–452. [Google Scholar] [CrossRef]
- Joulazadeh, M.; Navarchian, A.H. Effect of process variables on mechanical properties of polyurethane/clay nanocomposites. Polym. Adv. Technol. 2010, 21, 263–271. [Google Scholar] [CrossRef]
- Chen, B.; Peng, F.; Zhang, L.; Sun, D.A. Investigation on swelling characteristics of GMZ bentonite with different initial water contents. Ann. Nucl. Energy 2023, 181, 109565. [Google Scholar] [CrossRef]
- Chio, C.L.; Sain, M.; Qin, W.S. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Sensui, K.; Tarui, T.; Miyamae, T.; Sato, C. Evidence of chemical-bond formation at the interface between an epoxy polymer and an isocyanate primer. Chem. Commun. 2019, 55, 14833–14836. [Google Scholar] [CrossRef]
- Lu, P.F.; Zhang, Y.F.; Jia, C.; Li, Y.F.; Zhang, M.; Mao, Z.Q. Degradation of polyurethane coating materials from liquefied wheat straw for controlled release fertilizers. J. Appl. Polym. Sci. 2016, 133, 25. [Google Scholar] [CrossRef]
- Zhang, S.G.; Yang, Y.C.; Gao, B.; Wan, Y.S.; Li, Y.C.C.; Zhao, C.H. Bio-based interpenetrating network polymer composites from locust sawdust as coating material for environmentally friendly controlled-release urea fertilizers. J. Agric. Food Chem. 2016, 64, 5692–5700. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shen, T.L.; Yang, Y.C.; Gao, B.; Li, Y.C.C.; Xie, J.Z.; Tang, Y.F.; Zhang, S.G.; Wang, Z.H.; Chen, J.Q. Bio-based large tablet controlled-release urea: Synthesis, characterization, and controlled-released mechanisms. J. Agric. Food Chem. 2018, 66, 11265–11272. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tian, H.Y.; Liu, Z.G.; Zhang, M.; Zhao, C.H.; Guo, Y.L.; Guan, R.; Chen, Q.; Yu, X.J.; Wang, H.L.; et al. Polyolefin wax modification improved characteristics of nutrient release from biopolymer-coated phosphorus fertilizers. ACS Omega 2019, 4, 20402–20409. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; E, G.; Wang, C.; Shi, R.; Wang, J.; Wang, S.; Wang, Y.; Chen, Q.; Li, Z.; Liu, Z. Synthesis and Application of Modified Lignin Polyurea Binder for Manufacturing a Controlled-Release Potassium Fertilizer. Agronomy 2023, 13, 2641. https://doi.org/10.3390/agronomy13102641
Li M, E G, Wang C, Shi R, Wang J, Wang S, Wang Y, Chen Q, Li Z, Liu Z. Synthesis and Application of Modified Lignin Polyurea Binder for Manufacturing a Controlled-Release Potassium Fertilizer. Agronomy. 2023; 13(10):2641. https://doi.org/10.3390/agronomy13102641
Chicago/Turabian StyleLi, Mingyang, Gaoyang E, Conghui Wang, Ruolin Shi, Junxi Wang, Shuo Wang, Yu Wang, Qi Chen, Zeli Li, and Zhiguang Liu. 2023. "Synthesis and Application of Modified Lignin Polyurea Binder for Manufacturing a Controlled-Release Potassium Fertilizer" Agronomy 13, no. 10: 2641. https://doi.org/10.3390/agronomy13102641
APA StyleLi, M., E, G., Wang, C., Shi, R., Wang, J., Wang, S., Wang, Y., Chen, Q., Li, Z., & Liu, Z. (2023). Synthesis and Application of Modified Lignin Polyurea Binder for Manufacturing a Controlled-Release Potassium Fertilizer. Agronomy, 13(10), 2641. https://doi.org/10.3390/agronomy13102641




