Genome-Wide Analysis of Amino Acid Transporter Gene Family Revealed That the Allele Unique to the Aus Variety Is Associated with Amino Acid Permease 17 (OsAAP17) Amplifies Both the Tiller Count and Yield in Indica Rice (Oryza sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification and Characterization of Members of the OsAAP Gene Family within the Indica Rice Genome
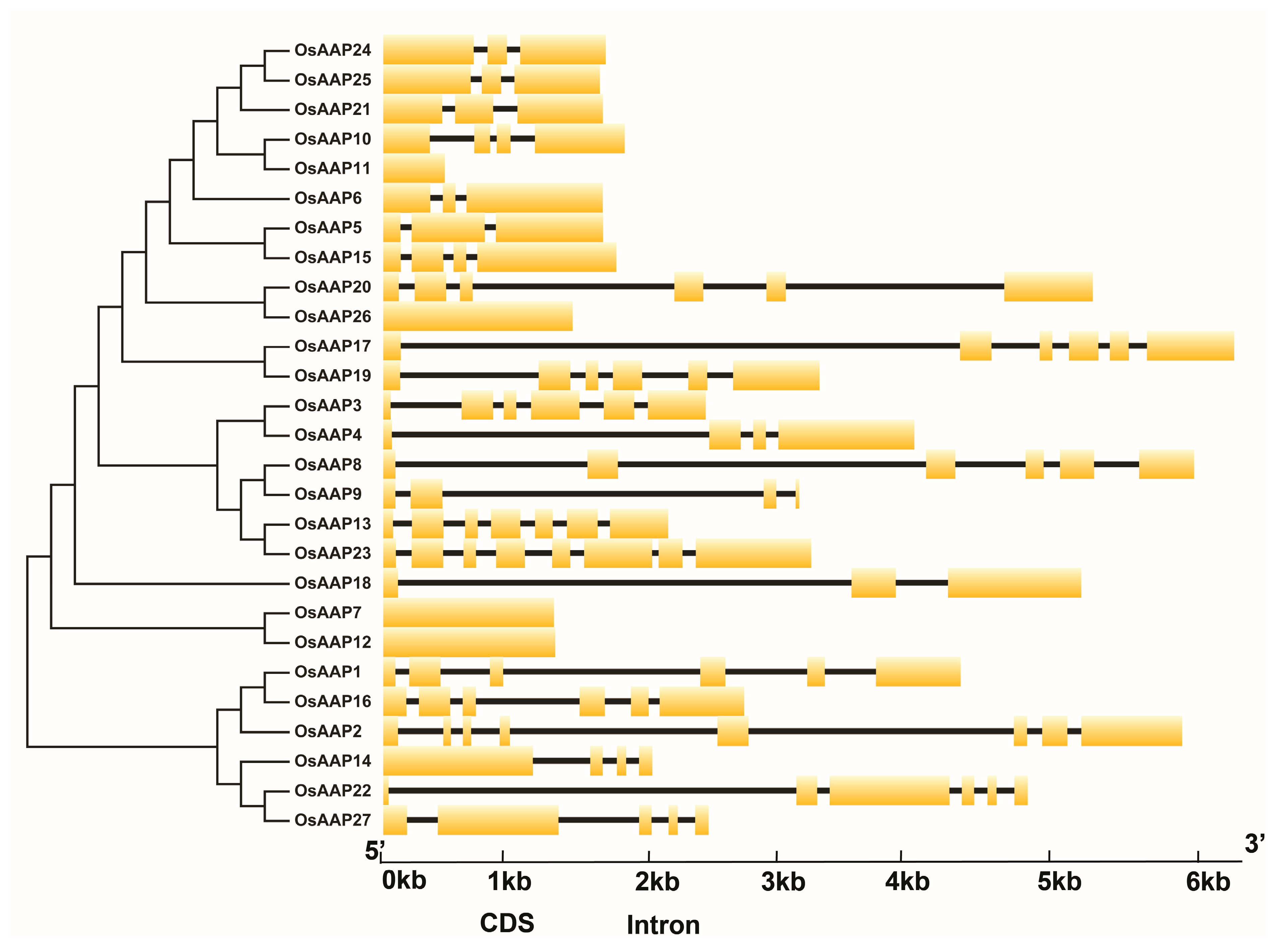
2.2. Phylogenetic Tree, Syntenic Relationships, and Gene Structure Analysis
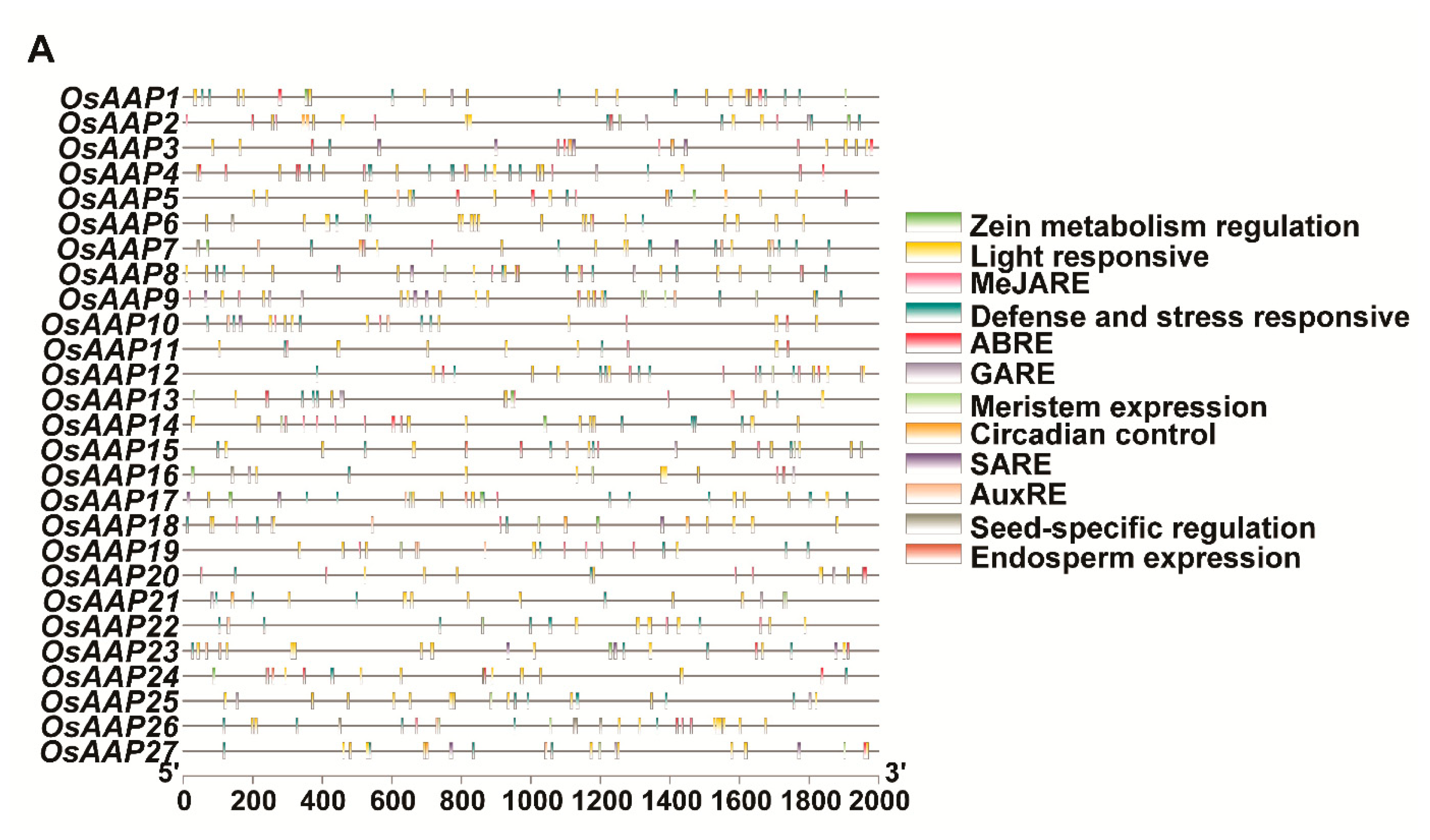
2.3. Localization of Chromosomes, Duplication of Genes, Cis-Regulatory Elements, and Gene Ontology Analysis
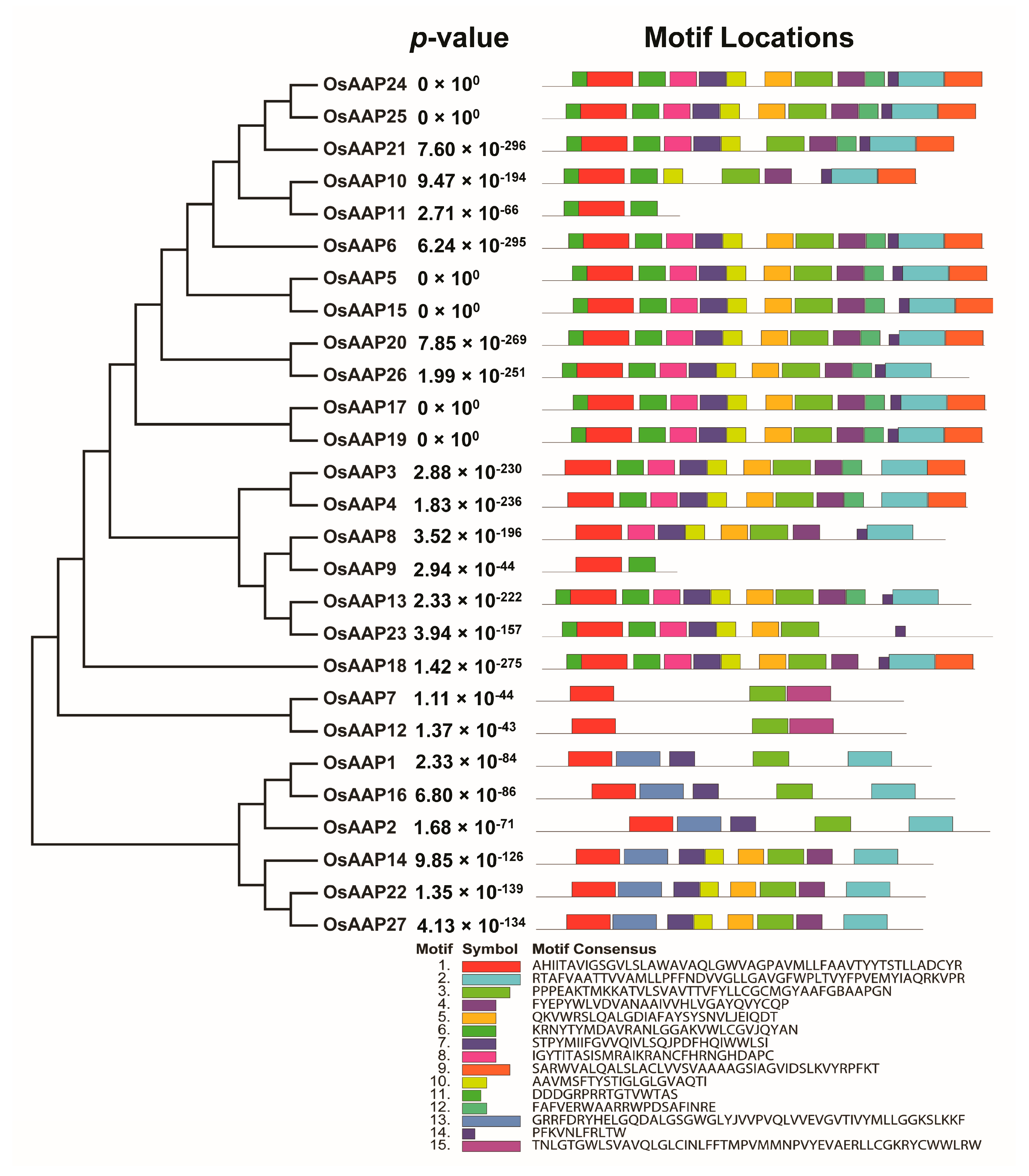
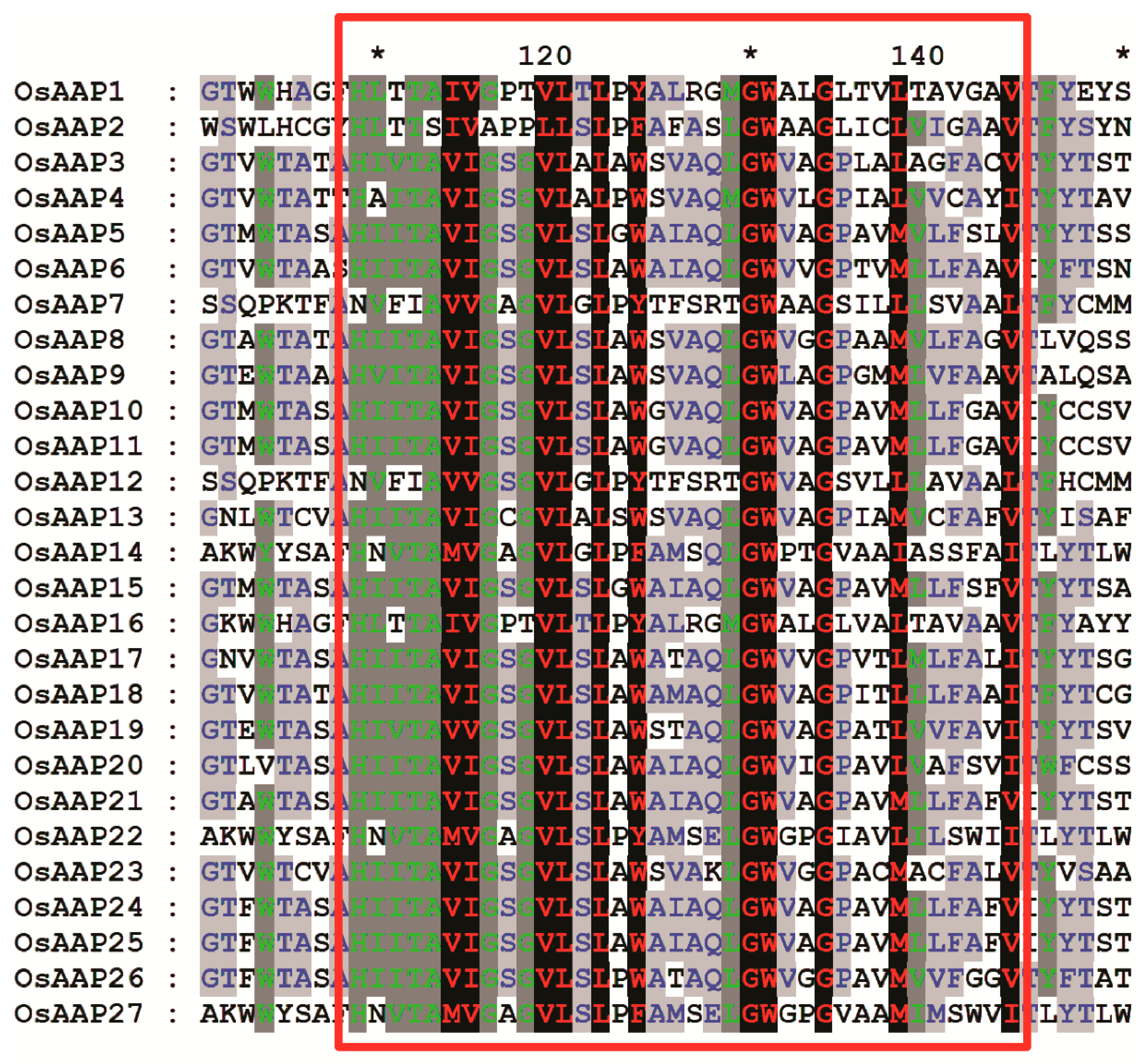
2.4. Conserved Motifs, Transmembrane Domains, and the 3D Structure
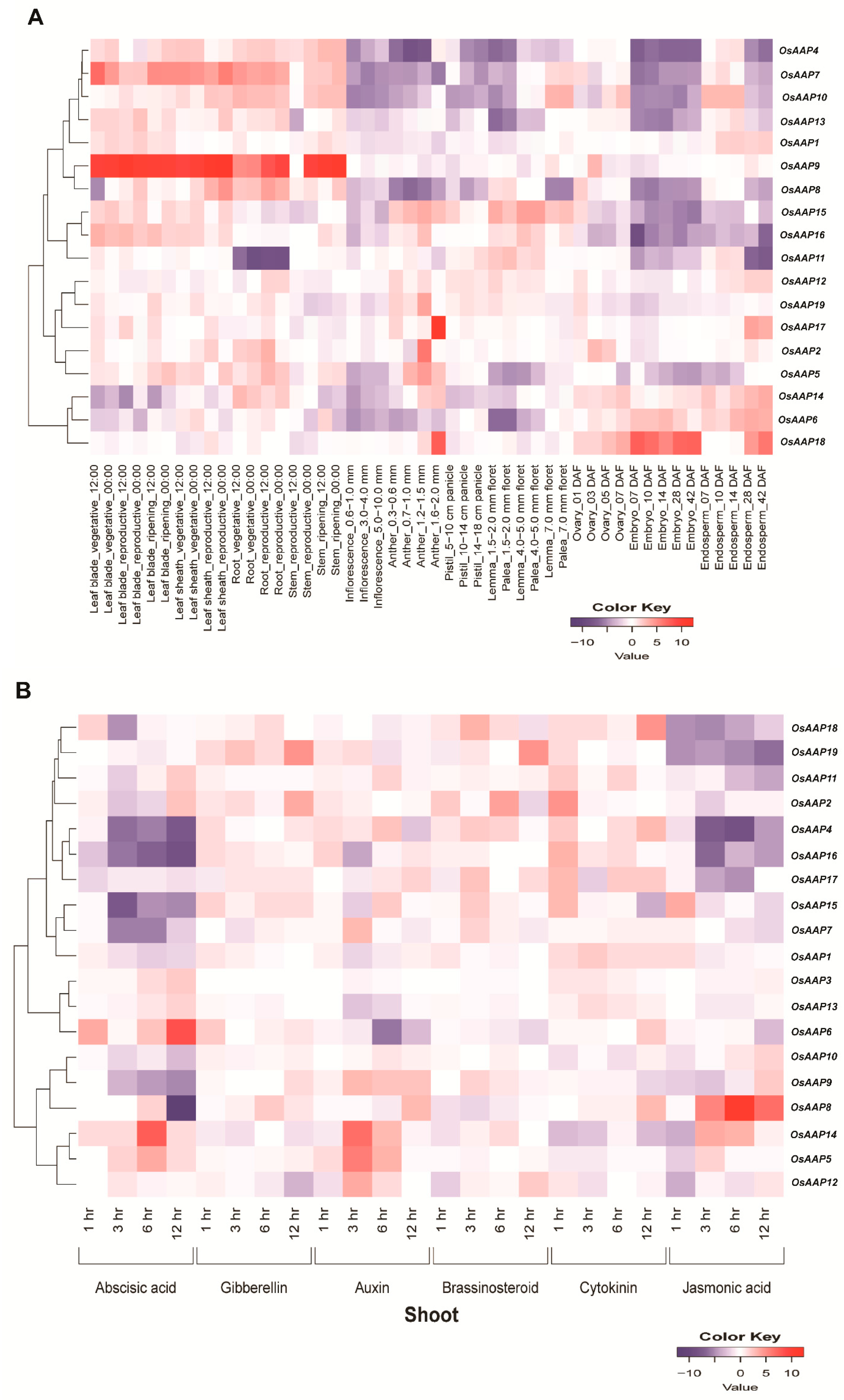
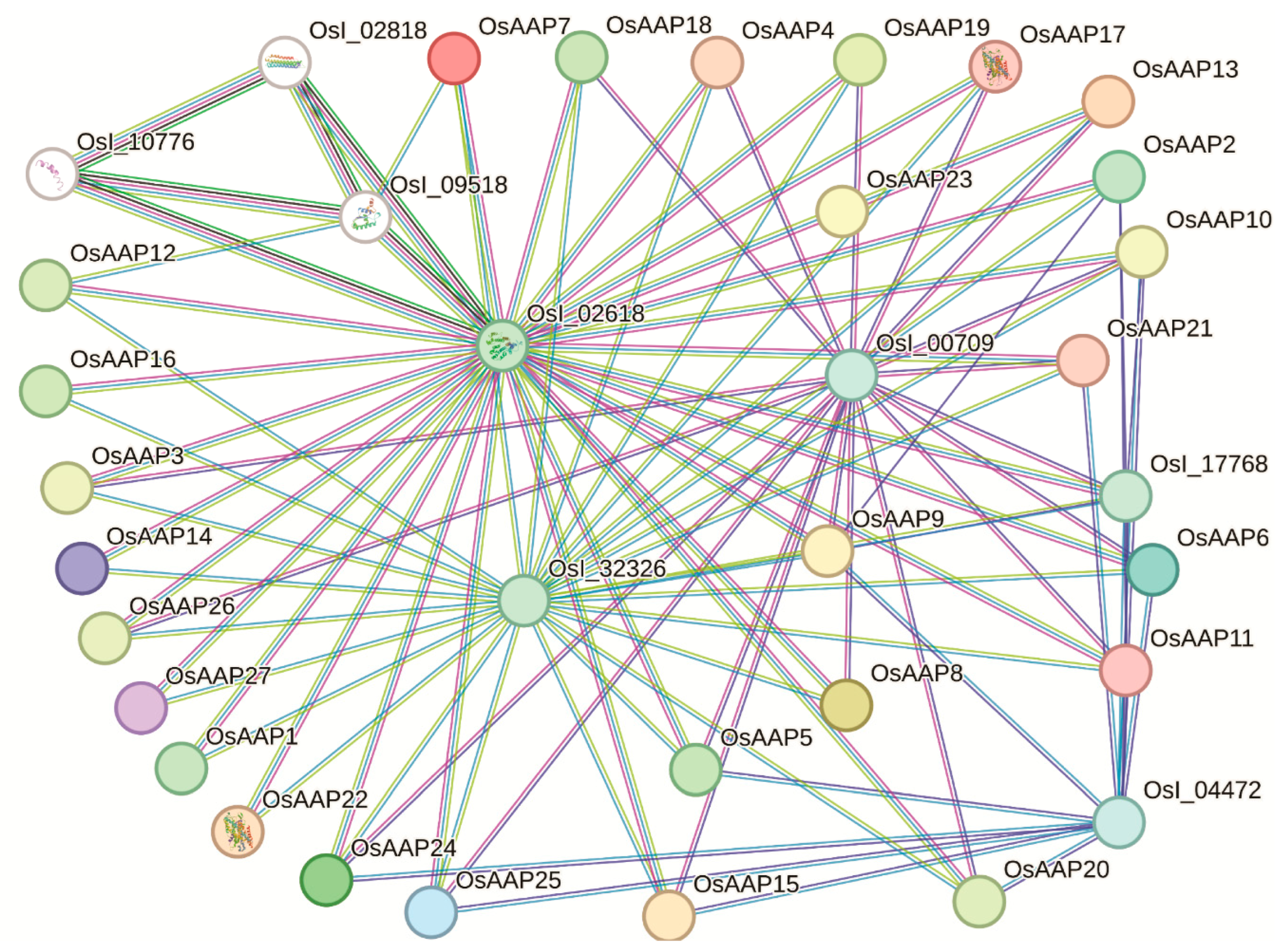
2.5. Gene Expression, miRNA, and the Analysis of Protein–Protein Network
2.6. Primer Development of OsAAP17 Gene (LOC_Os06g36180)
2.7. OsAAP17 Gene Diversity Analysis in Rice Varieties and Aus Genotypes
2.8. Development of Mapping Population between N22 and JR201
2.9. Screening of Mapping Population for Tiller Number and Yield-Related Traits
2.10. Linear Regression and Classification Analysis
2.11. Statistical Analysis
3. Results
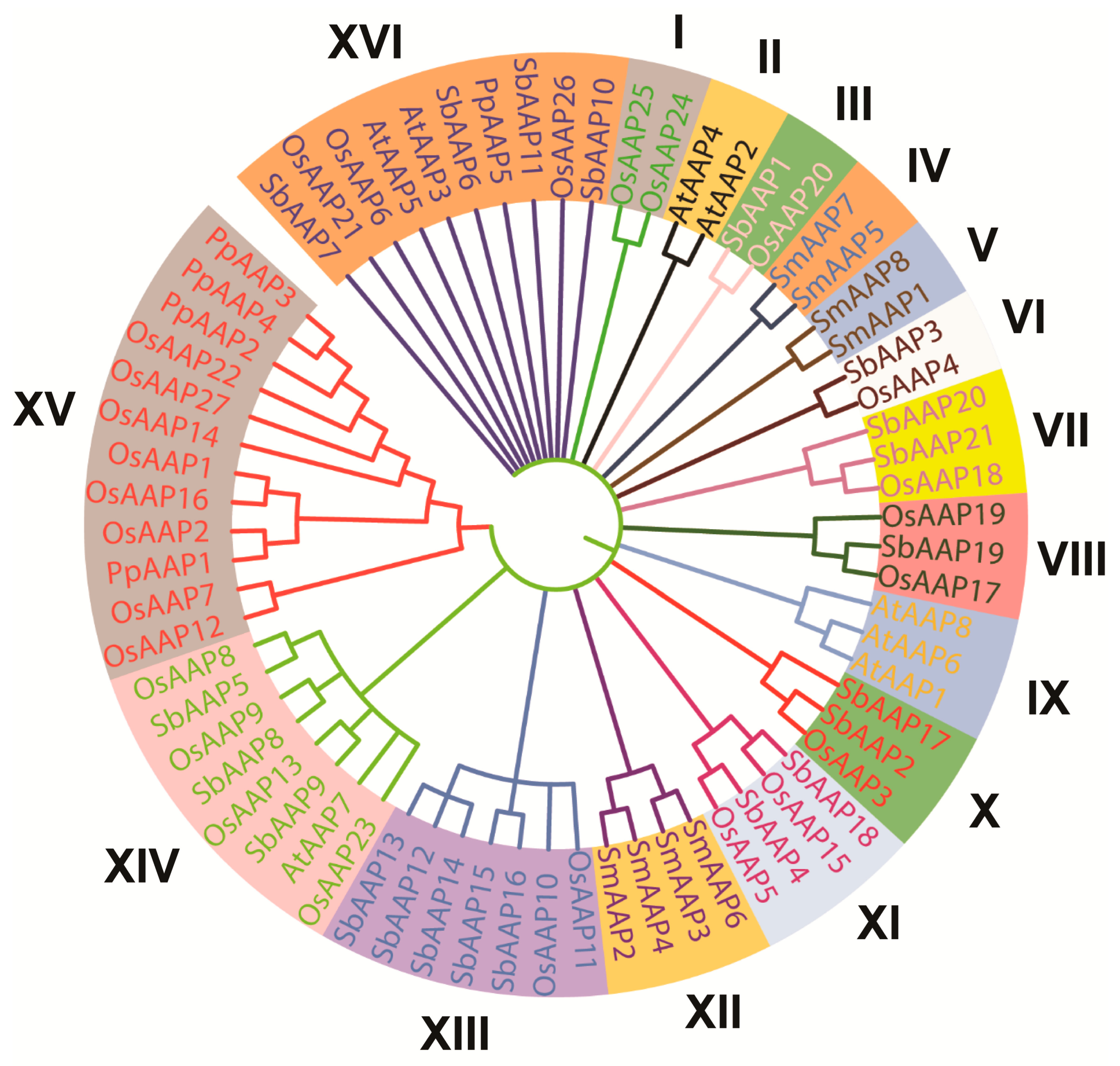
3.1. Identification and Characterization of OsAAP Genes across the Entire Genome of Indica Rice and Evolutionary Analysis
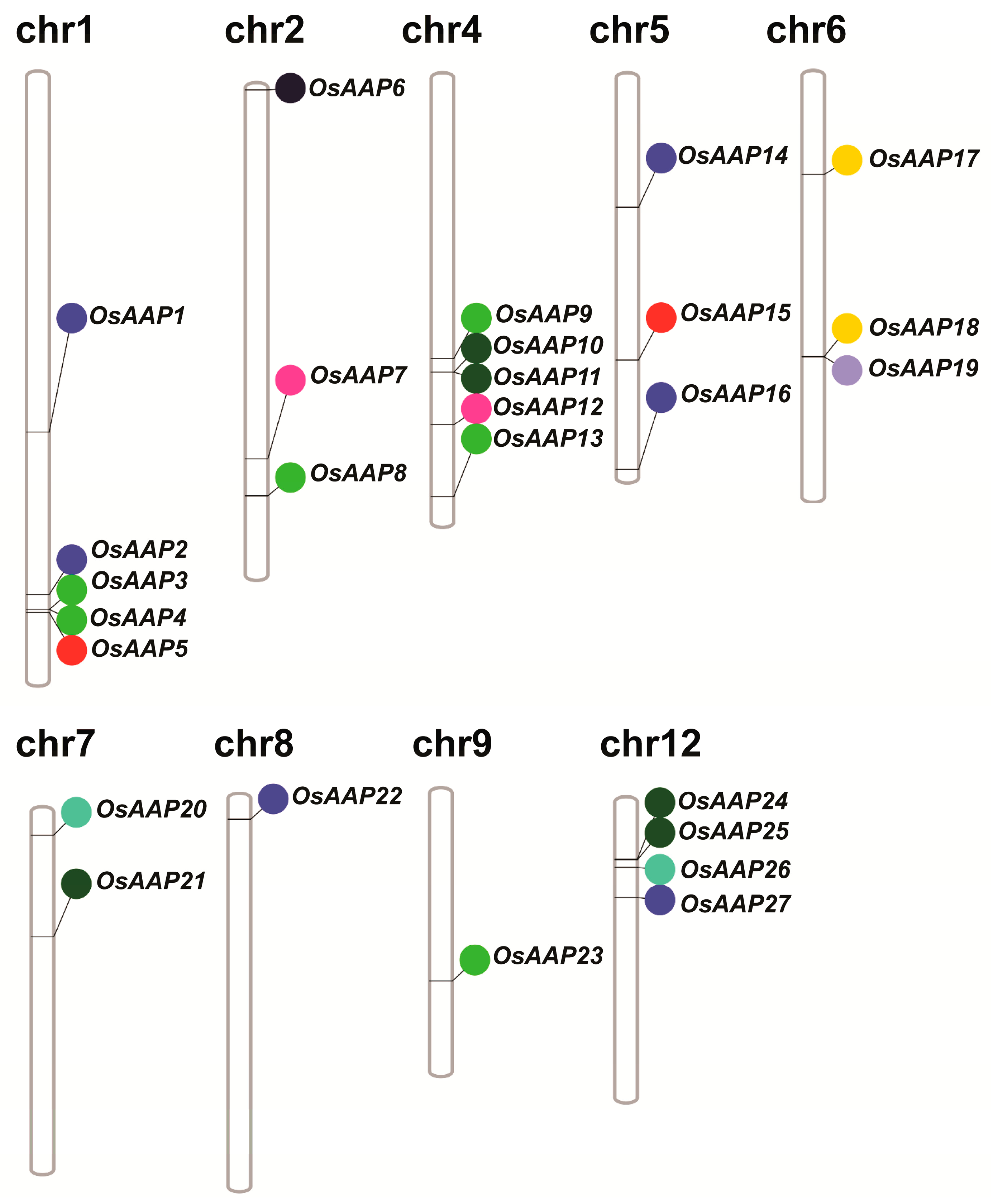
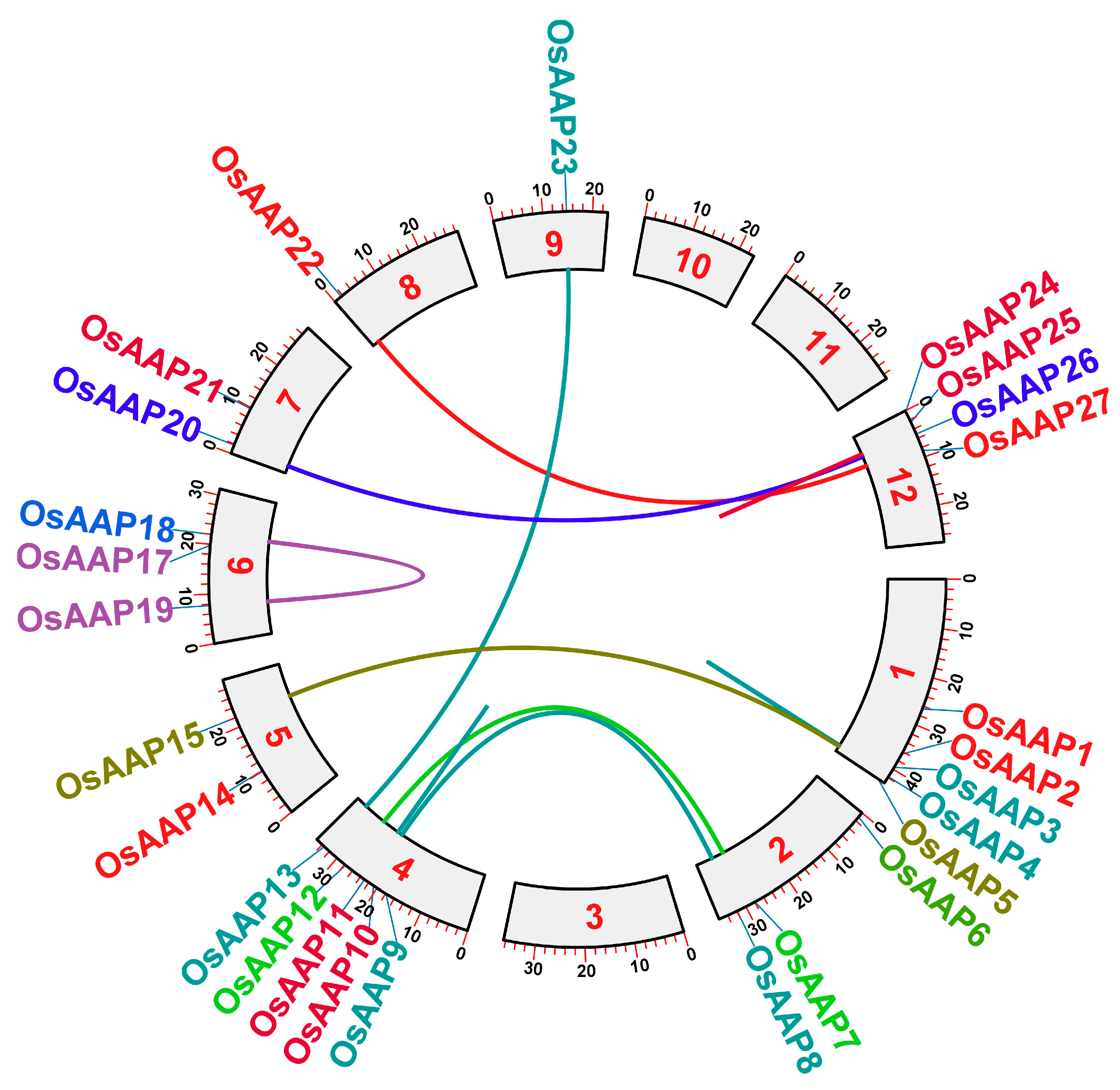
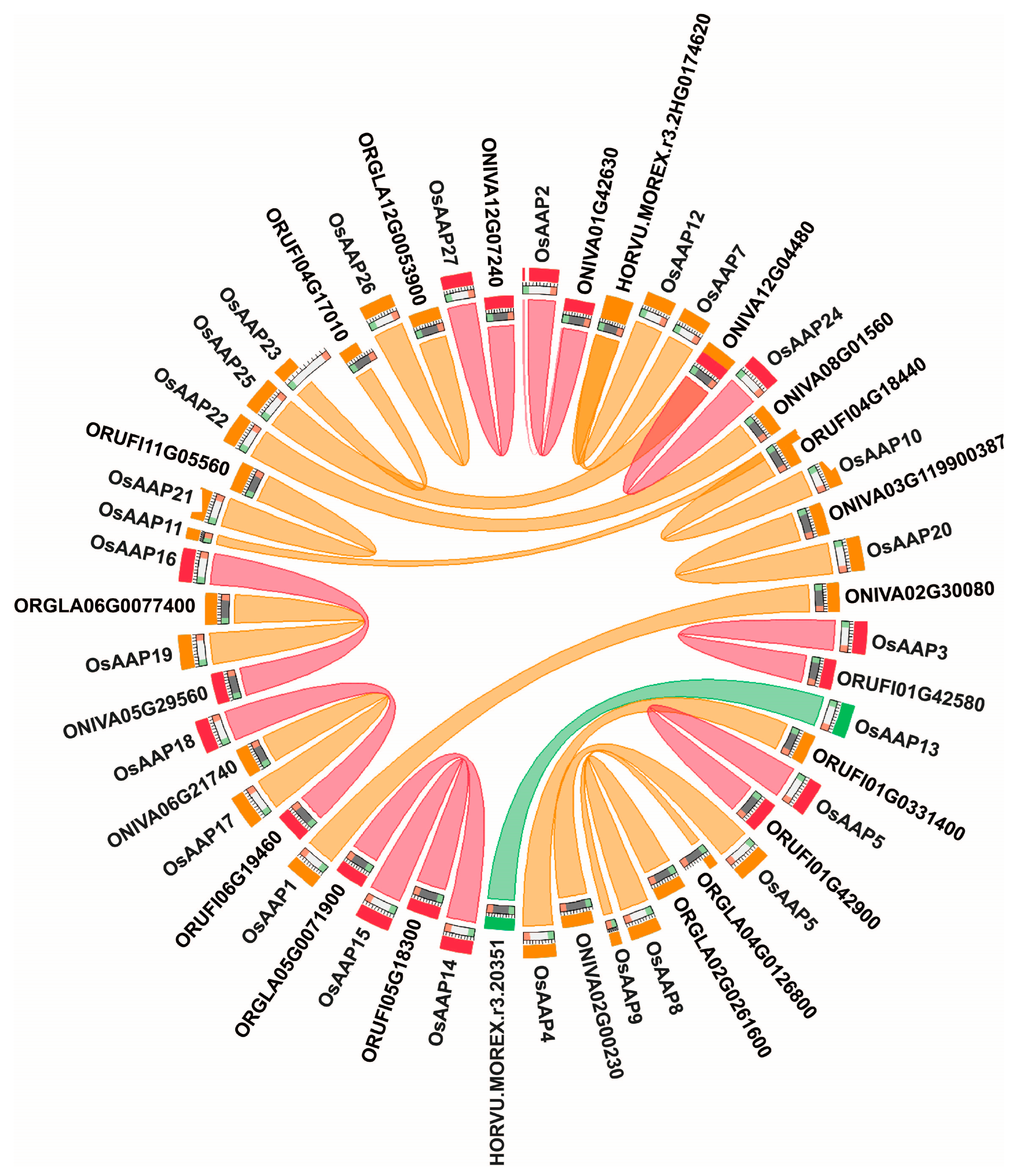
3.2. Analysis of Gene Duplication and Chromosome Distribution of the OsAAP Genes
3.3. Gene Structure and Basic Motif Analysis of OsAAP Gene
3.4. Analysis of Promoter Elements and Gene Ontology (GO) Enrichment
3.5. Transcriptome Profiles of OsAAP Family Genes across Distinct Tissues and under Varying Phytohormone Treatments in Rice
3.6. Networking between miRNAs and the AAP Gene Family
3.7. Analysis of the Protein–Protein Interactions among the OsAAP Family Genes
3.8. Regression Analysis of OsAAP17 Gene with Culm Number in Rice
3.9. Classification Analysis of Important Genetic Variants of OsAAP17 Gene
3.10. Diversity Analysis of OsAAP17 Gene in Rice Varieties and Aus Genotypes
3.11. Descriptive Statistics of Mapping Population between N22 and JR201 in Two Different Seasons
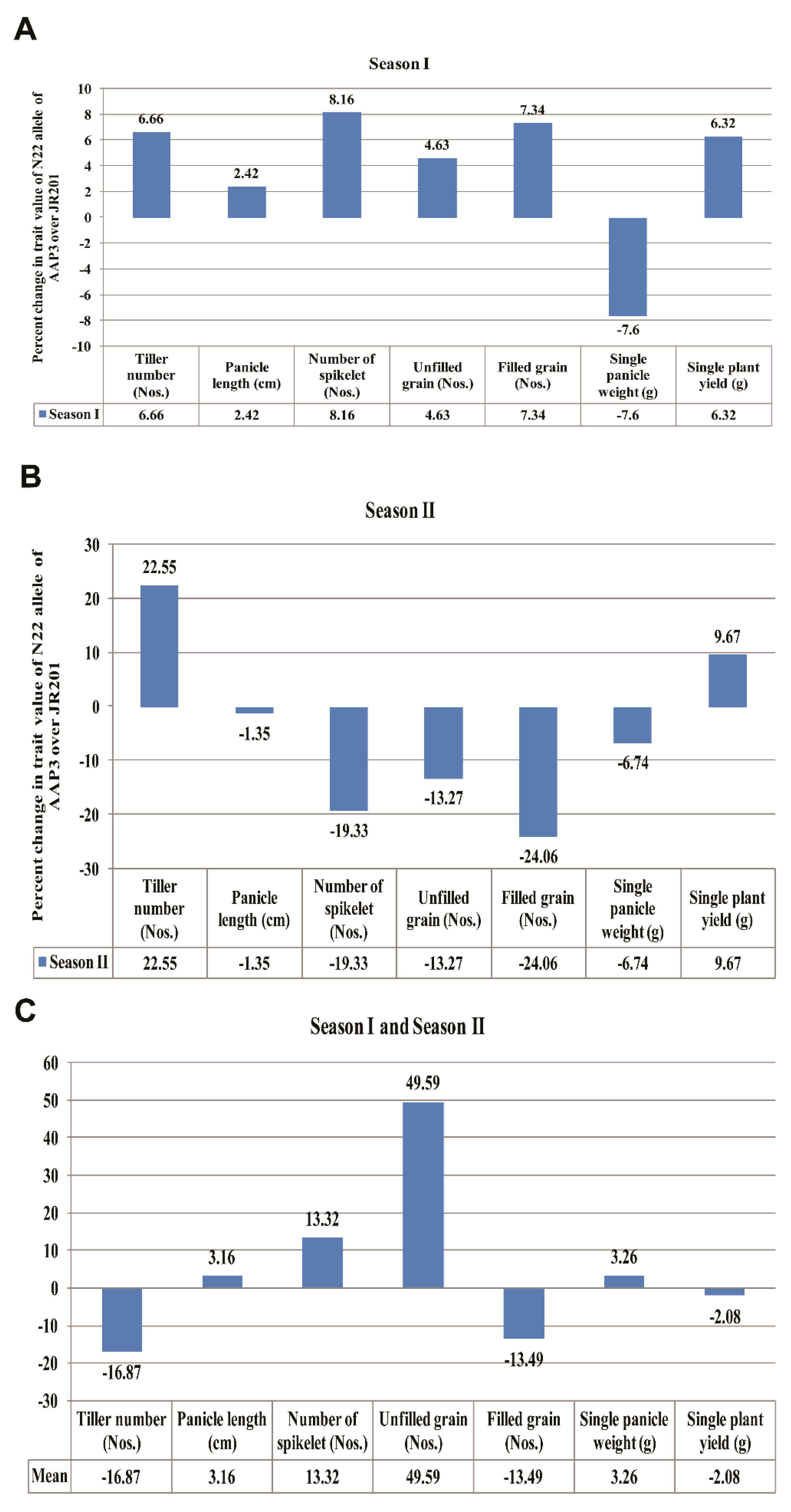
3.12. Allelic Effect of the Alleles of the OsAAP17 Gene for Yield-Related Traits
4. Discussion
4.1. The OsAAP Gene Family in Rice: Identification, Characterization, and Evolutionary Analysis
4.2. AAP Gene Family Encompasses Multiple CAREs and Implicated in Plant Growth and Developmental Processes in Rice
4.3. Aus Rice Has a Unique Genotypic Constitution for the OsAAP17 Gene in Rice
4.4. Aus Allele of OsAAP17 Enhances the Tiller Number and Yield in Rice
4.5. Environmental Effect of the Aus OsAAP17 Allele in Rice
4.6. Scope of Aus OsAAP17 Allele for Enhancing the Genetic Gain in Indica Rice Varieties
4.7. Intron Diversity of OsAAP17 Gene and Functional Relationship
4.8. Global Polymorphic In/Dels for Ecotype-Specific Haplotype-Introgression Breeding in Rice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fischer, W.-N.; André, B.; Rentsch, D.; Krolkiewicz, S.; Tegeder, M.; Breitkreuz, K.; Frommer, W.B. Amino acid transport in plants. Trends Plant Sci. 1998, 3, 188–195. [Google Scholar] [CrossRef]
- Pratelli, R.; Pilot, G. Regulation of amino acid metabolic enzymes and transporters in plants. J. Exp. Bot. 2014, 65, 5535–5556. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M. Transporters for amino acids in plant cells: Some functions and many unknowns. Curr. Opin. Plant Biol. 2012, 15, 315–321. [Google Scholar] [CrossRef]
- Yang, G.; Wei, Q.; Huang, H.; Xia, J. Amino acid transporters in plant cells: A brief review. Plants 2020, 9, 967. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M.; Rentsch, D. Uptake and partitioning of amino acids and peptides. Mol. Plant 2010, 3, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Sun, Y.; Shi, Y.; Zhang, Y.; Chen, K.; Li, Y.; Liu, G.; Yao, F.; Cheng, D.; Li, J. Branched-chain amino acids regulate plant growth by affecting the homeostasis of mineral elements in rice. Sci. China Life Sci. 2019, 62, 1107–1110. [Google Scholar] [CrossRef]
- Lu, Y.; Song, Z.; Lü, K.; Lian, X.; Cai, H. Molecular characterization, expression and functional analysis of the amino acid transporter gene family (OsAATs) in rice. Acta Physiol. Plant 2012, 34, 1943–1962. [Google Scholar] [CrossRef]
- Wu, M.; Wu, S.; Chen, Z.; Dong, Q.; Yan, H.; Xiang, Y. Genome-wide survey and expression analysis of the amino acid transporter gene family in poplar. Tree Genet. Genomes 2015, 11, 83. [Google Scholar] [CrossRef]
- Ma, H.; Cao, X.; Shi, S.; Li, S.; Gao, J.; Ma, Y.; Zhao, Q.; Chen, Q. Genome-wide survey and expression analysis of the amino acid transporter superfamily in potato (Solanum tuberosum L.). Plant Physiol. Biochem. 2016, 107, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Hirner, A.; Ladwig, F.; Stransky, H.; Okumoto, S.; Keinath, M.; Harms, A.; Frommer, W.B.; Koch, W. Arabidopsis LHT1 is a high-affinity transporter for cellular amino acid uptake in both root epidermis and leaf mesophyll. Plant Cell 2006, 18, 1931–1946. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Foster, J.; Chen, J.; Voll, L.M.; Weber, A.P.; Tegeder, M. AAP1 transports uncharged amino acids into roots of Arabidopsis. Plant J. 2007, 50, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.; Collier, R.; Trethewy, A.; Gould, G.; Sieker, R.; Tegeder, M. AAP1 regulates import of amino acids into developing Arabidopsis embryos. Plant J. 2009, 59, 540–552. [Google Scholar] [CrossRef]
- Fischer, W.N.; Loo, D.D.; Koch, W.; Ludewig, U.; Boorer, K.J.; Tegeder, M.; Rentsch, D.; Wright, E.M.; Frommer, W.B. Low and high affinity amino acid H+-cotransporters for cellular import of neutral and charged amino acids. Plant J. 2002, 29, 717–731. [Google Scholar] [CrossRef]
- Okumoto, S.; Koch, W.; Tegeder, M.; Fischer, W.N.; Biehl, A.; Leister, D.; Stierhof, Y.D.; Frommer, W.B. Root phloem-specific expression of the plasma membrane amino acid proton co-transporter AAP3. J. Exp. Bot. 2004, 55, 2155–2168. [Google Scholar] [CrossRef]
- Svennerstam, H.; Ganeteg, U.; Näsholm, T. Root uptake of cationic amino acids by Arabidopsis depends on functional expression of amino acid permease 5. New Phytol. 2008, 180, 620–630. [Google Scholar] [CrossRef]
- Hunt, E.; Gattolin, S.; Newbury, H.J.; Bale, J.S.; Tseng, H.-M.; Barrett, D.A.; Pritchard, J. A mutation in amino acid permease AAP6 reduces the amino acid content of the Arabidopsis sieve elements but leaves aphid herbivores unaffected. J. Exp. Bot. 2010, 61, 55–64. [Google Scholar] [CrossRef]
- Schmidt, R.; Stransky, H.; Koch, W. The amino acid permease AAP8 is important for early seed development in Arabidopsis thaliana. Planta 2007, 226, 805–813. [Google Scholar] [CrossRef]
- Miranda, M.; Borisjuk, L.; Tewes, A.; Heim, U.; Sauer, N.; Wobus, U.; Weber, H. Amino acid permeases in developing seeds of Vicia faba L.: Expression precedes storage protein synthesis and is regulated by amino acid supply. Plant J. 2001, 28, 61–71. [Google Scholar] [CrossRef]
- Koch, W.; Kwart, M.; Laubner, M.; Heineke, D.; Stransky, H.; Frommer, W.B.; Tegeder, M. Reduced amino acid content in transgenic potato tubers due to antisense inhibition of the leaf H+/amino acid symporter StAAP1. Plant J. 2003, 33, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Grennan, A.K.; Pélissier, H.C.; Rentsch, D.; Tegeder, M. Characterization and expression of French bean amino acid transporter PvAAP1. Plant Sci. 2008, 174, 348–356. [Google Scholar] [CrossRef]
- Couturier, J.; De Faÿ, E.; Fitz, M.; Wipf, D.; Blaudez, D.; Chalot, M. PtAAP11, a high affinity amino acid transporter specifically expressed in differentiating xylem cells of poplar. J. Exp. Bot. 2010, 61, 1671–1682. [Google Scholar] [CrossRef]
- Perchlik, M.; Tegeder, M. Improving plant nitrogen use efficiency through alteration of amino acid transport processes. Plant Physiol. 2017, 175, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Garneau, M.G.; Tan, Q.; Tegeder, M. Function of pea amino acid permease AAP6 in nodule nitrogen metabolism and export, and plant nutrition. J. Exp. Bot. 2018, 69, 5205–5219. [Google Scholar] [CrossRef]
- Fang, Z.; Wu, B.; Ji, Y. The amino acid transporter OsAAP4 contributes to rice tillering and grain yield by regulating neutral amino acid allocation through two splicing variants. Rice 2021, 14, 2. [Google Scholar] [CrossRef]
- Lu, K.; Wu, B.; Wang, J.; Zhu, W.; Nie, H.; Qian, J.; Huang, W.; Fang, Z. Blocking amino acid transporter Os AAP 3 improves grain yield by promoting outgrowth buds and increasing tiller number in rice. Plant Biotechnol. J. 2018, 16, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, K.; Nie, H.; Zeng, Q.; Wu, B.; Qian, J.; Fang, Z. Rice nitrate transporter OsNPF7. 2 positively regulates tiller number and grain yield. Rice 2018, 11, 12. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Yu, Y.; Duan, J.; Liao, Z.; Xiong, G.; Meng, X.; Liu, G.; Qian, Q.; Li, J. Degradation of MONOCULM 1 by APC/CTAD1 regulates rice tillering. Nat. Commun 2012, 3, 750. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q. Boosting rice yield by fine-tuning SPL gene expression. Trends Plant Sci. 2017, 22, 643–646. [Google Scholar] [CrossRef]
- Yeh, S.-Y.; Chen, H.-W.; Ng, C.-Y.; Lin, C.-Y.; Tseng, T.-H.; Li, W.-H.; Ku, M.S. Down-regulation of cytokinin oxidase 2 expression increases tiller number and improves rice yield. Rice 2015, 8, 36. [Google Scholar] [CrossRef]
- Tan, L.; Li, X.; Liu, F.; Sun, X.; Li, C.; Zhu, Z.; Fu, Y.; Cai, H.; Wang, X.; Xie, D. Control of a key transition from prostrate to erect growth in rice domestication. Nat. Genet. 2008, 40, 1360–1364. [Google Scholar] [CrossRef]
- Yu, J.; Xuan, W.; Tian, Y.; Fan, L.; Sun, J.; Tang, W.; Chen, G.; Wang, B.; Liu, Y.; Wu, W. Enhanced OsNLP4-OsNiR cascade confers nitrogen use efficiency by promoting tiller number in rice. Plant Biotechnol. J. 2021, 19, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, C.; Fu, Y.; Hu, G.; Si, H.; Zhu, L.; Luan, W.; He, Z.; Sun, Z. Identification and characterization of HTD2: A novel gene negatively regulating tiller bud outgrowth in rice. Planta 2009, 230, 649–658. [Google Scholar] [CrossRef]
- Lin, H.; Wang, R.; Qian, Q.; Yan, M.; Meng, X.; Fu, Z.; Yan, C.; Jiang, B.; Su, Z.; Li, J. DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 2009, 21, 1512–1525. [Google Scholar] [CrossRef]
- Li, Z.; Liang, Y.; Yuan, Y.; Wang, L.; Meng, X.; Xiong, G.; Zhou, J.; Cai, Y.; Han, N.; Hua, L. OsBRXL4 regulates shoot gravitropism and rice tiller angle through affecting LAZY1 nuclear localization. Mol. Plant 2019, 12, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, C.; Lin, J.; Liu, J.; Liu, B.; Wang, J.; Huang, A.; Li, H.; Zhao, T. OsMPH1 regulates plant height and improves grain yield in rice. PLoS ONE 2017, 12, e0180825. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, S.; Zhang, W.; Li, G.; Chen, Z.; Zhai, W.; Zhao, X.; Pan, X.; Xie, Q.; Zhu, L. The rice HIGH-TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. Plant J. 2006, 48, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Xu, Y.; Liu, H.; Mao, Z.; Zhang, C.; Ma, Y.; Zhang, Q.; Meng, Z.; Chong, K. The interaction between OsMADS57 and OsTB1 modulates rice tillering via DWARF14. Nat. Commun 2013, 4, 1566. [Google Scholar] [CrossRef]
- Yu, B.; Lin, Z.; Li, H.; Li, X.; Li, J.; Wang, Y.; Zhang, X.; Zhu, Z.; Zhai, W.; Wang, X. TAC1, a major quantitative trait locus controlling tiller angle in rice. Plant J. 2007, 52, 891–898. [Google Scholar] [CrossRef]
- Ishikawa, S.; Maekawa, M.; Arite, T.; Onishi, K.; Takamure, I.; Kyozuka, J. Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol. 2005, 46, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhu, L.; Shou, H.; Wu, P. A PIN1 family gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiol. 2005, 46, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Wang, R.; Ou, X.; Fang, Z.; Tian, C.; Duan, J.; Wang, Y.; Zhang, M. OsTIR1 and OsAFB2 downregulation via OsmiR393 overexpression leads to more tillers, early flowering and less tolerance to salt and drought in rice. PLoS ONE 2012, 7, e30039. [Google Scholar] [CrossRef]
- Gamuyao, R.; Chin, J.H.; Pariasca-Tanaka, J.; Pesaresi, P.; Catausan, S.; Dalid, C.; Slamet-Loedin, I.; Tecson-Mendoza, E.M.; Wissuwa, M.; Heuer, S. The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency. Nature 2012, 488, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, K.H.; Quiatchon, L.A.; Vikram, P.; Swamy, B.M.; Dixit, S.; Ahmed, H.; Hernandez, J.E.; Borromeo, T.H.; Kumar, A. Identification and mapping of a QTL (qDTY1. 1) with a consistent effect on grain yield under drought. Field Crops Res. 2012, 131, 88–96. [Google Scholar] [CrossRef]
- Ye, C.; Tenorio, F.A.; Redoña, E.D.; Morales–Cortezano, P.S.; Cabrega, G.A.; Jagadish, K.S.; Gregorio, G.B. Fine-mapping and validating qHTSF4. 1 to increase spikelet fertility under heat stress at flowering in rice. Theor. Appl. Genet. 2015, 128, 1507–1517. [Google Scholar] [CrossRef]
- Ara, I.; Lewis, M.; Ostendorf, B. Understanding the spatially variable effects of climate change on rice yield for three ecotypes in Bangladesh, 1981–2010. Adv. Agric. 2017, 2017, 6287156. [Google Scholar] [CrossRef]
- Schatz, M.C.; Maron, L.G.; Stein, J.C.; Wences, A.H.; Gurtowski, J.; Biggers, E.; Lee, H.; Kramer, M.; Antoniou, E.; Ghiban, E. Whole genome de novo assemblies of three divergent strains of rice, Oryza sativa, document novel gene space of aus and indica. Genome Biol. 2014, 15, 506. [Google Scholar] [PubMed]
- Kesawat, M.S.; Das, B.K.; Bhaganagare, G.R.; Manorama. Genome-wide identification, evolutionary and expression analyses of putative Fe–S biogenesis genes in rice (Oryza sativa). Genome 2012, 55, 571–583. [Google Scholar] [CrossRef]
- Kesawat, M.S.; Kherawat, B.S.; Katara, J.L.; Parameswaran, C.; Misra, N.; Kumar, M.; Chung, S.-M.; Alamri, S.; Siddiqui, M.H. Genome-wide analysis of proline-rich extensin-like receptor kinases (PERKs) gene family reveals their roles in plant development and stress conditions in Oryza sativa L. Plant Sci. 2023, 334, 111749. [Google Scholar] [CrossRef]
- Sahoo, B.; Nayak, I.; Parameswaran, C.; Kesawat, M.S.; Sahoo, K.K.; Subudhi, H.; Balasubramaniasai, C.; Prabhukarthikeyan, S.; Katara, J.L.; Dash, S.K. A Comprehensive Genome-Wide Investigation of the Cytochrome 71 (OsCYP71) Gene Family: Revealing the Impact of Promoter and Gene Variants (Ser33Leu) of OsCYP71P6 on Yield-Related Traits in Indica Rice (Oryza sativa L.). Plants 2023, 12, 3035. [Google Scholar] [CrossRef] [PubMed]
- Khush, G.S. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol. Biol. 2005, 59, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.; Griffiths-Jones, S.; Bateman, A. Identifying protein domains with the Pfam database. Curr Protoc Bioinformatics 2003, 1, 2.5.1–2.5.19. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef]
- Letunic, I.; Doerks, T.; Bork, P. SMART 6: Recent updates and new developments. Nucleic Acids Res. 2009, 37, D229–D232. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, L.P. IPC–isoelectric point calculator. Biol. Direct 2016, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Horton, P. PSORT: A program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem. Sci. 1999, 24, 34–35. [Google Scholar] [CrossRef]
- Savojardo, C.; Martelli, P.L.; Fariselli, P.; Profiti, G.; Casadio, R. BUSCA: An integrative web server to predict subcellular localization of proteins. Nucleic Acids Res. 2018, 46, W459–W466. [Google Scholar] [CrossRef]
- Kumar, S.; Tamura, K.; Nei, M. MEGA: Molecular evolutionary genetics analysis software for microcomputers. Bioinformatics 1994, 10, 189–191. [Google Scholar] [CrossRef]
- Guo, A.-Y.; Zhu, Q.-H.; Chen, X.; Luo, J.-C. GSDS: A gene structure display server. Yi Chuan Hered. 2007, 29, 1023–1026. [Google Scholar] [CrossRef]
- Wolfe, D.; Dudek, S.; Ritchie, M.D.; Pendergrass, S.A. Visualizing genomic information across chromosomes with PhenoGram. BioData Min. 2013, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-h.; Jin, H.; Marler, B.; Guo, H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Du, Z.; Zhou, X.; Ling, Y.; Zhang, Z.; Su, Z. agriGO: A GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010, 38, W64–W70. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nature Proto. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Mansueto, L.; Fuentes, R.R.; Borja, F.N.; Detras, J.; Abriol-Santos, J.M.; Chebotarov, D.; Sanciangco, M.; Palis, K.; Copetti, D.; Poliakov, A. Rice SNP-seek database update: New SNPs, indels, and queries. Nucleic Acids Res. 2017, 45, D1075–D1081. [Google Scholar] [CrossRef]
- Murray, M.; Thompson, W. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef]
- Babu, B.K.; Meena, V.; Agarwal, V.; Agrawal, P. Population structure and genetic diversity analysis of Indian and exotic rice (Oryza sativa L.) accessions using SSR markers. Mol. Biol. Rep. 2014, 41, 4329–4339. [Google Scholar] [CrossRef]
- Chidambaranathan, P.; Balasubramaniasai, C.; Behura, N.; Purty, M.; Samantaray, S.; Subudhi, H.; Ngangkham, U.; Devanna, B.; Katara, J.L.; Kumar, A. Effects of high temperature on spikelet sterility in rice (Oryza sativa L.): Association between molecular markers and allelic phenotypic effect in field condition. Genet. Resour. Crop Evol. 2021, 68, 1923–1935. [Google Scholar] [CrossRef]
- Kanbar, A.; Kondo, K.; Shashidhar, H. Comparative efficiency of pedigree, modified bulk and single seed descent breeding methods of selection for developing high-yielding lines in rice (Oryza sativa L.) under aerobic condition. Electron. J. Plant Breed 2011, 2, 184–193. [Google Scholar]
- Williams, L.M.; Ma, X.; Boyko, A.R.; Bustamante, C.D.; Oleksiak, M.F. SNP identification, verification, and utility for population genetics in a non-model genus. BMC Genet. 2010, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Vejchasarn, P.; Shearman, J.R.; Chaiprom, U.; Phansenee, Y.; Suthanthangjai, A.; Jairin, J.; Chamarerk, V.; Tulyananda, T.; Amornbunchornvej, C. Population structure of nation-wide rice in Thailand. Rice 2021, 14, 88. [Google Scholar] [CrossRef]
- Muthukrishnan, R.; Rohini, R. LASSO: A feature selection technique in predictive modeling for machine learning. In Proceedings of the 2016 IEEE International Conference on Advances in Computer Applications (ICACA), Coimbatore, India, 24 October 2016; pp. 18–20. [Google Scholar]
- Schumacher, K.; Krebs, M. The V-ATPase: Small cargo, large effects. Curr. Opin. Plant Biol. 2010, 13, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Seidel, T. The Plant V-ATPase. Front. Plant Sci. 2022, 13, 931777. [Google Scholar] [CrossRef] [PubMed]
- Gakière, B.; Hao, J.; de Bont, L.; Pétriacq, P.; Nunes-Nesi, A.; Fernie, A.R. NAD+ biosynthesis and signaling in plants. Crit Rev. Plant Sci. 2018, 37, 259–307. [Google Scholar] [CrossRef]
- Hashida, S.-N.; Takahashi, H.; Uchimiya, H. The role of NAD biosynthesis in plant development and stress responses. Ann. Bot. 2009, 103, 819–824. [Google Scholar] [CrossRef]
- Sawada, Y.; Kinoshita, K.; Akashi, T.; Aoki, T.; Ayabe, S.i. Key amino acid residues required for aryl migration catalysed by the cytochrome P450 2-hydroxyisoflavanone synthase. Plant J. 2002, 31, 555–564. [Google Scholar] [CrossRef]
- Lawton-Rauh, A. Evolutionary dynamics of duplicated genes in plants. Mol. Phylogenet. Evol. 2003, 29, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L. The evolution of functionally novel proteins after gene duplication. Proc. R. Soc. B Biol. Sci. 1994, 256, 119–124. [Google Scholar]
- Lin, H.; Zhu, W.; Silva, J.C.; Gu, X.; Buell, C.R. Intron gain and loss in segmentally duplicated genes in rice. Genome Biol. 2006, 7, R41. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.W.; Penny, D. Patterns of intron loss and gain in plants: Intron loss–dominated evolution and genome-wide comparison of O. sativa and A. thaliana. Mol. Biol. Evol. 2007, 24, 171–181. [Google Scholar] [CrossRef]
- William Roy, S.; Gilbert, W. The evolution of spliceosomal introns: Patterns, puzzles and progress. Nat. Rev. Genet. 2006, 7, 211–221. [Google Scholar] [CrossRef]
- Kesawat, M.S.; Kherawat, B.S.; Ram, C.; Singh, A.; Dey, P.; Gora, J.S.; Misra, N.; Chung, S.-M.; Kumar, M. Genome-Wide Identification and Expression Profiling of Aconitase Gene Family Members Reveals Their Roles in Plant Development and Adaptation to Diverse Stress in Triticum aestivum L. Plants 2022, 11, 3475. [Google Scholar] [CrossRef]
- Kesawat, M.S.; Kherawat, B.S.; Singh, A.; Dey, P.; Kabi, M.; Debnath, D.; Saha, D.; Khandual, A.; Rout, S.; Manorama. Genome-wide identification and characterization of the brassinazole-resistant (BZR) gene family and its expression in the various developmental stage and stress conditions in wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2021, 22, 8743. [Google Scholar] [CrossRef] [PubMed]
- Kesawat, M.S.; Kherawat, B.S.; Singh, A.; Dey, P.; Routray, S.; Mohapatra, C.; Saha, D.; Ram, C.; Siddique, K.H.; Kumar, A. Genome-wide analysis and characterization of the proline-rich extensin-like receptor kinases (PERKs) gene family reveals their role in different developmental stages and stress conditions in wheat (Triticum aestivum L.). Plants 2022, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Riewe, D.; Jeon, H.J.; Lisec, J.; Heuermann, M.C.; Schmeichel, J.; Seyfarth, M.; Meyer, R.C.; Willmitzer, L.; Altmann, T. A naturally occurring promoter polymorphism of the Arabidopsis FUM2 gene causes expression variation, and is associated with metabolic and growth traits. Plant J. 2016, 88, 826–838. [Google Scholar] [CrossRef]
- Roy, A.L.; Sen, R.; Roeder, R.G. Enhancer–promoter communication and transcriptional regulation of Igh. Trends Immunol. 2011, 32, 532–539. [Google Scholar] [CrossRef][Green Version]
- Kumar, M.; Kherawat, B.S.; Dey, P.; Saha, D.; Singh, A.; Bhatia, S.K.; Ghodake, G.S.; Kadam, A.A.; Kim, H.-U.; Manorama. Genome-wide identification and characterization of PIN-FORMED (PIN) gene family reveals role in developmental and various stress conditions in Triticum aestivum L. Int. J. Mol. Sci. 2021, 22, 7396. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bai, G.; Huang, W.; Wang, Z.; Wang, X.; Zhang, M. The rice peptide transporter OsNPF7. 3 is induced by organic nitrogen, and contributes to nitrogen allocation and grain yield. Front. Plant Sci. 2017, 8, 1338. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Huang, W.; Wu, B.; Fang, Z.; Wang, X. The amino acid transporter AAP1 mediates growth and grain yield by regulating neutral amino acid uptake and reallocation in Oryza sativa. J. Exp. Bot. 2020, 71, 4763–4777. [Google Scholar] [CrossRef]
- Liu, X.; Bush, D. Expression and transcriptional regulation of amino acid transporters in plants. Amino Acids 2006, 30, 113–120. [Google Scholar] [CrossRef]
- Rentsch, D.; Hirner, B.; Schmelzer, E.; Frommer, W.B. Salt stress-induced proline transporters and salt stress-repressed broad specificity amino acid permeases identified by suppression of a yeast amino acid permease-targeting mutant. Plant Cell 1996, 8, 1437–1446. [Google Scholar] [PubMed]
- Popova, O.V.; Dietz, K.-J.; Golldack, D. Salt-dependent expression of a nitrate transporter and two amino acid transporter genes in Mesembryanthemum crystallinum. Plant Mol. Biol. 2003, 52, 569–578. [Google Scholar] [CrossRef]
- Jain, M.; Nijhawan, A.; Arora, R.; Agarwal, P.; Ray, S.; Sharma, P.; Kapoor, S.; Tyagi, A.K.; Khurana, J.P. F-box proteins in rice. Genome-wide analysis, classification, temporal and spatial gene expression during panicle and seed development, and regulation by light and abiotic stress. Plant Physiol. 2007, 143, 1467–1483. [Google Scholar] [CrossRef]
- Cooper, B.; Clarke, J.D.; Budworth, P.; Kreps, J.; Hutchison, D.; Park, S.; Guimil, S.; Dunn, M.; Luginbühl, P.; Ellero, C. A network of rice genes associated with stress response and seed development. Proc. Natl. Acad. Sci. USA 2003, 100, 4945–4950. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, B.; Lu, K.; Wei, Q.; Qian, J.; Chen, Y.; Fang, Z. The amino acid permease 5 (OsAAP5) regulates tiller number and grain yield in rice. Plant Physiol. 2019, 180, 1031–1045. [Google Scholar] [CrossRef]
- McNally, K.L.; Childs, K.L.; Bohnert, R.; Davidson, R.M.; Zhao, K.; Ulat, V.J.; Zeller, G.; Clark, R.M.; Hoen, D.R.; Bureau, T.E. Genomewide SNP variation reveals relationships among landraces and modern varieties of rice. Proc. Natl. Acad. Sci. USA 2009, 106, 12273–12278. [Google Scholar] [CrossRef]
- Londo, J.P.; Chiang, Y.-C.; Hung, K.-H.; Chiang, T.-Y.; Schaal, B.A. Phylogeography of Asian wild rice, Oryza rufipogon, reveals multiple independent domestications of cultivated rice, Oryza sativa. Proc. Natl. Acad. Sci. USA 2006, 103, 9578–9583. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lawas, L.M.; Malo, R.; Glaubitz, U.; Erban, A.; Mauleon, R.; Heuer, S.; Zuther, E.; Kopka, J.; Hincha, D.K. Metabolic and transcriptomic signatures of rice floral organs reveal sugar starvation as a factor in reproductive failure under heat and drought stress. Plant Cell Environ. 2015, 38, 2171–2192. [Google Scholar] [CrossRef]
- González-Schain, N.; Dreni, L.; Lawas, L.M.; Galbiati, M.; Colombo, L.; Heuer, S.; Jagadish, K.S.; Kater, M.M. Genome-wide transcriptome analysis during anthesis reveals new insights into the molecular basis of heat stress responses in tolerant and sensitive rice varieties. Plant Cell Physiol. 2016, 57, 57–68. [Google Scholar] [CrossRef]
- Anandan, A.; Panda, S.; Sabarinathan, S.; Travis, A.J.; Norton, G.J.; Price, A.H. Superior haplotypes for early root vigor traits in rice under dry direct seeded low nitrogen condition through genome wide association mapping. Front. Plant Sci. 2022, 13, 911775. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, P.; Travis, A.J.; Hossain, M.; Islam, M.R.; Norton, G.J.; Price, A.H. Identification of genomic loci regulating grain iron content in aus rice under two irrigation management systems. Food Energy Secur. 2022, 11, e329. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.; Lai, K. The effects of temperature and light intensity on the tiller development of rice. Agric. Natl. Taiwan Univ. 1990, 30, 22–30. [Google Scholar]
- Jumaa, S.; Redona, E.; Walker, T.; Gao, W.; Reddy, K. Developing screening tools for early-season high-and low-temperature stress tolerance in rice. Sabrao J. Breed. Genet. 2019, 51, 12–36. [Google Scholar]
- Lyu, J.; Zhang, S.; Dong, Y.; He, W.; Zhang, J.; Deng, X.; Zhang, Y.; Li, X.; Li, B.; Huang, W. Analysis of elite variety tag SNPs reveals an important allele in upland rice. Nat. Commun. 2013, 4, 2138. [Google Scholar] [CrossRef]
- Huang, L.; Bao, Y.; Qin, S.; Ning, M.; Lyu, J.; Zhang, S.; Huang, G.; Zhang, J.; Wang, W.; Fu, B. An ABA Synthesis Enzyme Allele OsNCED2 Promotes the Aerobic Adaption in Upland Rice. bioRxiv 2020. [Google Scholar] [CrossRef]
- Xie, W.; Wang, G.; Yuan, M.; Yao, W.; Lyu, K.; Zhao, H.; Yang, M.; Li, P.; Zhang, X.; Yuan, J. Breeding signatures of rice improvement revealed by a genomic variation map from a large germplasm collection. Proc. Natl. Acad. Sci. USA 2015, 112, E5411–E5419. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, C.; Li, M.; Cui, Y.; Shi, Y.; Wu, Z.; Hu, Z.; Wang, W.; Xu, J.; Li, Z. The landscape of gene–CDS–haplotype diversity in rice: Properties, population organization, footprints of domestication and breeding, and implications for genetic improvement. Mol. Plant 2021, 14, 787–804. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, R.; Singh, A.K.; Singh, V.K.; Abbai, R.; Habde, S.V.; Singh, U.M.; Kumar, A. Superior haplotypes towards development of low glycemic index rice with preferred grain and cooking quality. Sci. Rep. 2021, 11, 10082. [Google Scholar] [CrossRef] [PubMed]


| Proposed Gene Name | Gene ID | Genomic Location | Orientation | CDS Length (bp) | Protein Length (aa) | Molecular Weight (KDa) | Isoelectric Point (pI) | GRAVY | Predicted Subcellular Localization |
|---|---|---|---|---|---|---|---|---|---|
| OsAAP1 | BGIOSGA001188 | 1:27819467–27819727 | Reverse | 1365 | 454 | 48.57 | 8.26 | 0.72 | plasma membrane |
| OsAAP2 | BGIOSGA000460 | 1:40537731–40537886 | Reverse | 1566 | 521 | 56.07 | 8.15 | 0.63 | plasma membrane |
| OsAAP3 | BGIOSGA000395 | 1:41725345–41726478 | Reverse | 1398 | 465 | 49.94 | 7.72 | 0.54 | plasma membrane |
| OsAAP4 | BGIOSGA000394 | 1:41739541–41742697 | Reverse | 1401 | 466 | 50.02 | 7.42 | 0.62 | plasma membrane |
| OsAAP5 | BGIOSGA000378 | 1:41942366–41943340 | Reverse | 1467 | 488 | 52.87 | 7.65 | 0.5 | plasma membrane |
| OsAAP6 | BGIOSGA007327 | 2:125686–126493 | Forward | 1455 | 484 | 53.22 | 6.64 | 0.37 | plasma membrane |
| OsAAP7 | BGIOSGA008784 | 2:29016053–29016202 | Forward | 1269 | 422 | 44.84 | 7.2 | 0.8 | plasma membrane |
| OsAAP8 | BGIOSGA008950 | 2:31930792–31931241 | Forward | 1329 | 442 | 47.07 | 5.72 | 0.42 | plasma membrane |
| OsAAP9 | BGIOSGA014910 | 4:21924811–21927625 | Reverse | 447 | 148 | 15.31 | 7.76 | 0.44 | organelle membrane |
| OsAAP10 | BGIOSGA016659 | 4:22997818–22998441 | Forward | 1236 | 411 | 43.11 | 7.67 | 0.56 | plasma membrane |
| OsAAP11 | BGIOSGA016661 | 4:23016996–23017175 | Forward | 456 | 151 | 15.89 | 6.97 | 0.45 | endomembrane system |
| OsAAP12 | BGIOSGA014538 | 4:27155165–27155314 | Reverse | 1278 | 425 | 44.76 | 7.78 | 0.8 | plasma membrane |
| OsAAP13 | BGIOSGA017288 | 4:32739933–32740566 | Forward | 1413 | 470 | 51.4 | 8.31 | 0.44 | plasma membrane |
| OsAAP14 | BGIOSGA018471 | 5:10135284–10135993 | Reverse | 1371 | 456 | 49.94 | 7.13 | 0.54 | plasma membrane |
| OsAAP15 | BGIOSGA019901 | 5:22018146–22018484 | Forward | 1491 | 496 | 53.51 | 8.73 | 0.54 | plasma membrane |
| OsAAP16 | BGIOSGA020459 | 5:30590867–30591034 | Forward | 1446 | 481 | 51.04 | 7.06 | 0.72 | chloroplast outer membrane |
| OsAAP17 | BGIOSGA023082 | 6:21947609–21953508 | Forward | 1464 | 487 | 52.78 | 7.42 | 0.4 | plasma membrane |
| OsAAP18 | BGIOSGA023083 | 6:21972799–21972954 | Forward | 1425 | 474 | 50.72 | 7.44 | 0.52 | plasma membrane |
| OsAAP19 | BGIOSGA021614 | 6:7712971–7714521 | Reverse | 1455 | 484 | 51.9 | 7.74 | 0.46 | endomembrane system |
| OsAAP20 | BGIOSGA024866 | 7:1787401–1788046 | Reverse | 1458 | 485 | 52.59 | 8.09 | 0.43 | plasma membrane |
| OsAAP21 | BGIOSGA025496 | 7:9759616–9760347 | Forward | 1356 | 451 | 48.41 | 8.52 | 0.54 | plasma membrane |
| OsAAP22 | BGIOSGA027915 | 8:1609038–1609402 | Forward | 1344 | 447 | 49.83 | 7.61 | 0.52 | plasma membrane |
| OsAAP23 | BGIOSGA030830 | 9:14727692–14728921 | Forward | 2310 | 769 | 82.7 | 7.89 | −0.23 | organelle membrane |
| OsAAP24 | BGIOSGA037101 | 12:4448712–4448945 | Forward | 1449 | 482 | 51.9 | 7.9 | 0.51 | plasma membrane |
| OsAAP25 | BGIOSGA036516 | 12:4465477–4465710 | Reverse | 1428 | 475 | 51.11 | 7.18 | 0.55 | plasma membrane |
| OsAAP26 | BGIOSGA036484 | 12:5104622–5104741 | Reverse | 1407 | 468 | 50.01 | 8.29 | 0.52 | plasma membrane |
| OsAAP27 | BGIOSGA037215 | 12:7432040–7433174 | Forward | 1335 | 444 | 48.83 | 9.08 | 0.59 | plasma membrane |
| Gene Name | Number of Targeting miRNA | miRNA | miRNA Sequence | Length (nt) | Inhibition |
|---|---|---|---|---|---|
| OsAAP1 | 1 | OIn-miRN6163 | UUUUGAACGACUUGCACGAGA | 21 | Cleavage |
| OsAAP2 | 2 | OIn-miR159a | CUUGGACUGAAGGGUGCUCCCU | 22 | Cleavage |
| OIn-miR159b | UUGGACUGAAGGGUGCUCCCU | 21 | Cleavage | ||
| OsAAP4 | 5 | OIn-miR397a | UUGAGUGCAGCGUUGAUGAACC | 22 | Cleavage |
| OIn-miR397b | UUGAGUGCAGCGUUGAUGAAC | 21 | Cleavage | ||
| OIn-miRN1147a | CGUUCCCCAGCGGAGUCGCCA | 21 | Cleavage | ||
| OIn-miRN1147b | CGUUCCCCAGCGGAGUCGCCA | 21 | Cleavage | ||
| OIn-miRN1147c | CGUUCCCCAGCGGAGUCGCCA | 21 | Cleavage | ||
| OsAAP5 | 2 | OIn-miR1850a | UGGAAAGUUGGGAGAUUGGGG | 21 | Cleavage |
| OIn-miR166a | UCUCGGAUCAGGCUUCAUUCC | 21 | Cleavage | ||
| OsAAP8 | 4 | OIn-miRN6152 | CCAGUGAAGAGUACUUUGGCU | 21 | Cleavage |
| OIn-miR482c | UUCCCGAUGCCUCCCAUGCCUA | 22 | Cleavage | ||
| OIn-miRN2235b | UUUUUUAAUAGAACCGACACCU | 22 | Cleavage | ||
| OIn-miRN2235c | UUUUUUAAUAGAACCGACACCU | 22 | Cleavage | ||
| OsAAP11 | 5 | OIn-miRN6120 | UUGUUGUACUGUAUCAGCACCU | 22 | Cleavage |
| OIn-miRN6121 | UUGUUGUACUGUAUCAGCACCU | 22 | Cleavage | ||
| OIn-miRN6122 | UUGUUGUACUGUAUCAGCACCU | 22 | Cleavage | ||
| OIn-miRN6127 | UUGUUGUACUGUAUCAGCACCU | 22 | Cleavage | ||
| OIn-miRN6128 | UUGUUGUACUGUAUCAGCACCU | 22 | Cleavage | ||
| OsAAP13 | 1 | OIn-miR1846b | UCCCACCGAGCAGCCGGAUCUC | 22 | Cleavage |
| OsAAP14 | 6 | OIn-miR164a | UGGAGAAGCAGGGC-ACGUGCA | 21 | Cleavage |
| OIn-miR164b | UGGAGAAGCAGGGC-ACGUGCA | 21 | Cleavage | ||
| OIn-miR164c | UGGAGAAGCAGGGC-ACGUGCA | 21 | Cleavage | ||
| OIn-miR164d | UGGAGAAGCAGGGC-ACGUGCU | 21 | Cleavage | ||
| OIn-miR169l | AGCCAAGGAUGACUUGCCGGC | 21 | Cleavage | ||
| OIn-miRN2219a | AUCGGAGGCCAUGGUGCAGCC | 21 | Cleavage | ||
| OsAAP15 | 1 | OIn-miRN6160 | UACCUCGGGCAACUGAAGACU | 21 | Cleavage |
| OsAAP16 | 1 | OIn-miRN2274a | CACCAGGGAUUUCAUCGACUC | 21 | Cleavage |
| OsAAP17 | 1 | OIn-miRN6160 | UACCUCGGGCAACUGAAGACU | 21 | Cleavage |
| OsAAP20 | 1 | OIn-miRN6168 | UGCGAGGUUCACCAUGUUCUG | 21 | Translation |
| OsAAP22 | 4 | OIn-miR164a | UGGAGAAGCAGGGCACGUGCA | 21 | Translation |
| OIn-miR164b | UGGAGAAGCAGGGCACGUGCA | 21 | Translation | ||
| OIn-miR164c | UGGAGAAGCAGGGCACGUGCA | 21 | Translation | ||
| OIn-miR164d | UGGAGAAGCAGGGCACGUGCU | 21 | Translation | ||
| OsAAP23 | 3 | OIn-miRN2248a | CUUUUUCCUUGGGAAGGUGGU | 21 | Translation |
| OIn-miRN6133 | CGGUUCCUGUCCCAAGAUCGAG | 22 | Cleavage | ||
| OIn-miRN6138 | CGGUUCCUGUCCCAAGAUCGAG | 22 | Cleavage | ||
| OsAAP24 | 1 | OIn-miR2118a | CUCCUGAUGCCUCCCAAGCCUA | 22 | Translation |
| OsAAP25 | 1 | OIn-miR2118a | CUCCUGAUGCCUCCCAAGCCUA | 22 | Translation |
| OsAAP27 | 4 | OIn-miR172a | AGAAUCUUGAUGAUGCUGCAU | 21 | Cleavage |
| OIn-miR172b | AGAAUCUUGAUGAUGCUGCAU | 21 | Cleavage | ||
| OIn-miR172c | AGAAUCUUGAUGAUGCUGCAU | 21 | Cleavage | ||
| OIn-miR172d | GGAAUCUUGAUGAUGCUGCAU | 21 | Cleavage |
| Sl.No | OsAAP17 Gene SNP Ids | Mean Value of Culm Number of SNP1 (Nos.) | Mean Value of Culm Number of SNP2 (Nos.) | p-Value * |
|---|---|---|---|---|
| Linear Regression model | ||||
| 1 | 21186420 | 17.62 | 15.56 | 2.22 × 10−16 |
| 2 | 21187038 | 15.31 | 17.29 | 9.99 × 10−16 |
| 3 | 21187201 | 17.31 | 16.40 | 0.004662 |
| 4 | 21188911 | 15.17 | 17.29 | 0 |
| LASSO | ||||
| 1 | 21186274 | 16.46 | 19.91 | 0.003351 |
| 2 | 21186676 | 14.46 | 16.49 | 0.010262 |
| 3 | 21187004 | 17.32 | 14.69 | 0 |
| 4 | 21188056 | 17.32 | 14.98 | 0 |
| 5 | 21188057 | 16.43 | 18.5 | 0.001057 |
| 6 | 21188177 | 19.16 | 16.48 | 0.041175 |
| 7 | 21188591 | 16.49 | 21.5 | 0.030368 |
| 8 | 21189128 | 16.37 | 16.52 | 0.357885 ns |
| Random Forest | ||||
| 1 | 21187038 | 17.98 | 15.55 | 0 |
| 2 | 21185768 | 16.49 | 18.68 | 0.035122 |
| 3 | 21188179 | 17.22 | 16.02 | 1.29 × 10−6 |
| 4 | 21188056 | 17.32 | 14.98 | 0 |
| 5 | 21186226 | 15.29 | 17.25 | 0 |
| Ecotypes * | Admix | Aro | Aus | Indica | Japonica | Error Rate |
|---|---|---|---|---|---|---|
| admix | - | - | 11 | 15 | 9 | 1.0 |
| aro | - | - | 13 | 1 | 21 | 1.0 |
| aus | - | - | 119 | 1 | - | 0.008 |
| indica | 1 | - | 23 | 719 | 16 | 0.055 |
| japonica | - | - | 7 | 31 | 351 | 0.108 |
| Marker | Major Allele Frequency | Genotype No | Sample Size | No. of Obs. | Allele No | Availability | Gene Diversity | Hetero Zygosity | PIC |
|---|---|---|---|---|---|---|---|---|---|
| OsAAP17_1 | 1.00 | 1.0 | 155 | 155 | 1.0 | 1.0 | 0.00 | 0 | 0.00 |
| OsAAP17_2 | 1.00 | 1.0 | 155 | 155 | 1.0 | 1.0 | 0.00 | 0 | 0.00 |
| OsAAP17_3 | 0.94 | 2.0 | 155 | 155 | 2.0 | 1.0 | 0.11 | 0 | 0.10 |
| OsAAP17_4 | 0.87 | 2.0 | 155 | 155 | 2.0 | 1.0 | 0.21 | 0 | 0.19 |
| OsAAP17_5 | 0.94 | 2.0 | 155 | 155 | 2.0 | 1.0 | 0.09 | 0 | 0.09 |
| Mean | 0.95 | 1.6 | 155 | 155 | 1.6 | 1.0 | 0.08 | 0 | 0.07 |
| Traits | Mean | Standard Error | Median | Mode | Kurtosis | Skewness | Range | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Season | SI | SII | SI | SII | SI | SII | SI | SII | SI | SII | SI | SII | SI | SII |
| No. of Tillers (nos) | 10.2 | 8.71 | 0.48 | 0.44 | 10 | 8 | 8 | 6.33 | −0.04 | 1.22 | 0.27 | 1.22 | 9.8–10.5 | 7.59–9.83 |
| Panicle length (cm) | 20.18 | 21.49 | 0.63 | 0.45 | 21.35 | 21.4 | 21.7 | 20.96 | 0.56 | 2.09 | 0.08 | 0.52 | 20.55–21.06 | 21.35–21.64 |
| No. of Spikelet (nos) | 95.16 | 109.8 | 9.71 | 11.68 | 64 | 85.16 | 51 | 61.66 | 1.87 | 4.71 | 1.72 | 2.26 | 91.12–99.2 | 100.11–119.47 |
| Unfilled Grain (nos) | 23.52 | 27.45 | 3.56 | 4.42 | 15.5 | 18.16 | 9 | 13 | 5.04 | 12.05 | 2.26 | 3.32 | 22.2–24.83 | 43.76–49.57 |
| Filled Grain (nos) | 71.64 | 82.33 | 6.85 | 8.09 | 51.5 | 68.16 | 46 | 36 | 1.5 | 2.99 | 1.61 | 1.91 | 68.91–74.37 | 56.34–69.9 |
| Panicle weight (gm) | 1.78 | 1.84 | 0.18 | 0.16 | 1.33 | 1.54 | 0.96 | 1.003 | 6.93 | 3.95 | 2.27 | 2.03 | 1.71–1.84 | 1.78–1.90 |
| Single plant yield (gm) | 17.14 | 15.97 | 1.01 | 1.10 | 16.52 | 13.29 | 9.14 | 13.46 | 6.00 | 0.08 | 1.83 | 0.97 | 7.09–47.04 | 7.69–58.23 |
| Traits | Mean Values of OsAAP17 Alleles of Season I | p-Value | |
|---|---|---|---|
| JR201 Allele (480 bp) | N22 Allele (500 bp) | ||
| Tiller number (Nos.) | 9.8 ± 3.08 | 10.5 ± 3.71 | 0.262629 |
| Panicle length (cm) | 20.55 ± 4.43 | 21.06 ± 4.46 | 0.346168 |
| Number of spikelet (Nos.) | 91.12 ± 68.31 | 99.2 ± 67.54 | 0.340095 |
| Unfilled grain (Nos.) | 22.2 ± 23.28 | 24.83 ± 26.52 | 0.357805 |
| Filled grain (Nos.) | 68.91 ± 50.79 | 74.37 ± 44.89 | 0.346624 |
| Single panicle weight (g) | 1.84 ± 1.48 | 1.71 ± 1.008 | 0.358779 |
| Single plant yield (g) | 16.58 ± 5.11 | 17.7 ± 8.58 | 0.291189 |
| Traits | Mean Values of OsAAP17 Alleles of Season II | p-Value | |
|---|---|---|---|
| JR201 Allele (480 bp) | N22 Allele (500 bp) | ||
| Tiller number (TN) | 7.59 ± 2.4 | 9.8 ± 3.2 | 0.005095 ** |
| Panicle length (PL in cm) | 21.64 ± 3.31 | 21.35 ± 2.9 | 0.464042 |
| Number of spikelet (NOS) | 119.47 ± 94.19 | 100.11 ± 65.69 | 0.254926 |
| Number of unfilled grain (NUG) | 49.57 ± 32.01 | 43.76 ± 22.7 | 0.288966 |
| Number of Filled grain (NFG) | 69.9 ± 62.20 | 56.34 ± 42.96 | 0.238248 |
| Single panicle weight (SPW in g) | 1.9 ± 1.18 | 1.78 ± 1.1 | 0.404013 |
| Single plant yield (SPY in g) | 15.97 ± 7.73 | 17.68 ± 10.39 | 0.279943 |
| Traits | Mean Values in Season I and Season II | p-Value | |
|---|---|---|---|
| Season I | Season II | ||
| Tiller number (TN) | 10.18 ± 3.39 | 8.71 ± 3.07 | 0.012941 * |
| Panicle length (PL in cm) | 20.81 ± 4.41 | 21.49 ± 3.12 | 0.189431 |
| Number of spikelet (NOS) | 95.16 ± 67.32 | 109.79 ± 80.93 | 0.16791 |
| Number of unfilled grain (NUG) | 23.52 ± 24.72 | 46.66 ± 30.63 | 7.67 × 10−6 ** |
| Number of Filled grain (NFG) | 71.64 ± 47.50 | 63.12 ± 56.08 | 0.204173 |
| Single panicle weight (SPW in g) | 1.78 ± 1.25 | 1.84 ± 1.13 | 0.403848 |
| Single plant yield (SPY in g) | 17.14 ± 7.01 | 16.82 ± 7.64 | 0.062107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayak, I.; Sahoo, B.; Pradhan, C.; Balasubramaniasai, C.; Prabhukarthikeyan, S.R.; Katara, J.L.; Meher, J.; Chung, S.-M.; Gaafar, A.-R.Z.; Hodhod, M.S.; et al. Genome-Wide Analysis of Amino Acid Transporter Gene Family Revealed That the Allele Unique to the Aus Variety Is Associated with Amino Acid Permease 17 (OsAAP17) Amplifies Both the Tiller Count and Yield in Indica Rice (Oryza sativa L.). Agronomy 2023, 13, 2629. https://doi.org/10.3390/agronomy13102629
Nayak I, Sahoo B, Pradhan C, Balasubramaniasai C, Prabhukarthikeyan SR, Katara JL, Meher J, Chung S-M, Gaafar A-RZ, Hodhod MS, et al. Genome-Wide Analysis of Amino Acid Transporter Gene Family Revealed That the Allele Unique to the Aus Variety Is Associated with Amino Acid Permease 17 (OsAAP17) Amplifies Both the Tiller Count and Yield in Indica Rice (Oryza sativa L.). Agronomy. 2023; 13(10):2629. https://doi.org/10.3390/agronomy13102629
Chicago/Turabian StyleNayak, Itishree, Bijayalaxmi Sahoo, Chinmay Pradhan, Cayalvizhi Balasubramaniasai, Seenichamy Rathinam Prabhukarthikeyan, Jawahar Lal Katara, Jitendriya Meher, Sang-Min Chung, Abdel-Rhman Z. Gaafar, Mohamed S. Hodhod, and et al. 2023. "Genome-Wide Analysis of Amino Acid Transporter Gene Family Revealed That the Allele Unique to the Aus Variety Is Associated with Amino Acid Permease 17 (OsAAP17) Amplifies Both the Tiller Count and Yield in Indica Rice (Oryza sativa L.)" Agronomy 13, no. 10: 2629. https://doi.org/10.3390/agronomy13102629
APA StyleNayak, I., Sahoo, B., Pradhan, C., Balasubramaniasai, C., Prabhukarthikeyan, S. R., Katara, J. L., Meher, J., Chung, S.-M., Gaafar, A.-R. Z., Hodhod, M. S., Kherawat, B. S., Parameswaran, C., Kesawat, M. S., & Samantaray, S. (2023). Genome-Wide Analysis of Amino Acid Transporter Gene Family Revealed That the Allele Unique to the Aus Variety Is Associated with Amino Acid Permease 17 (OsAAP17) Amplifies Both the Tiller Count and Yield in Indica Rice (Oryza sativa L.). Agronomy, 13(10), 2629. https://doi.org/10.3390/agronomy13102629









