Management of Water Supply in the Cultivation of Different Agaricus bisporus Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genetic Characterization of the A. bisporus Strains
2.2. Spawn and Compost Production
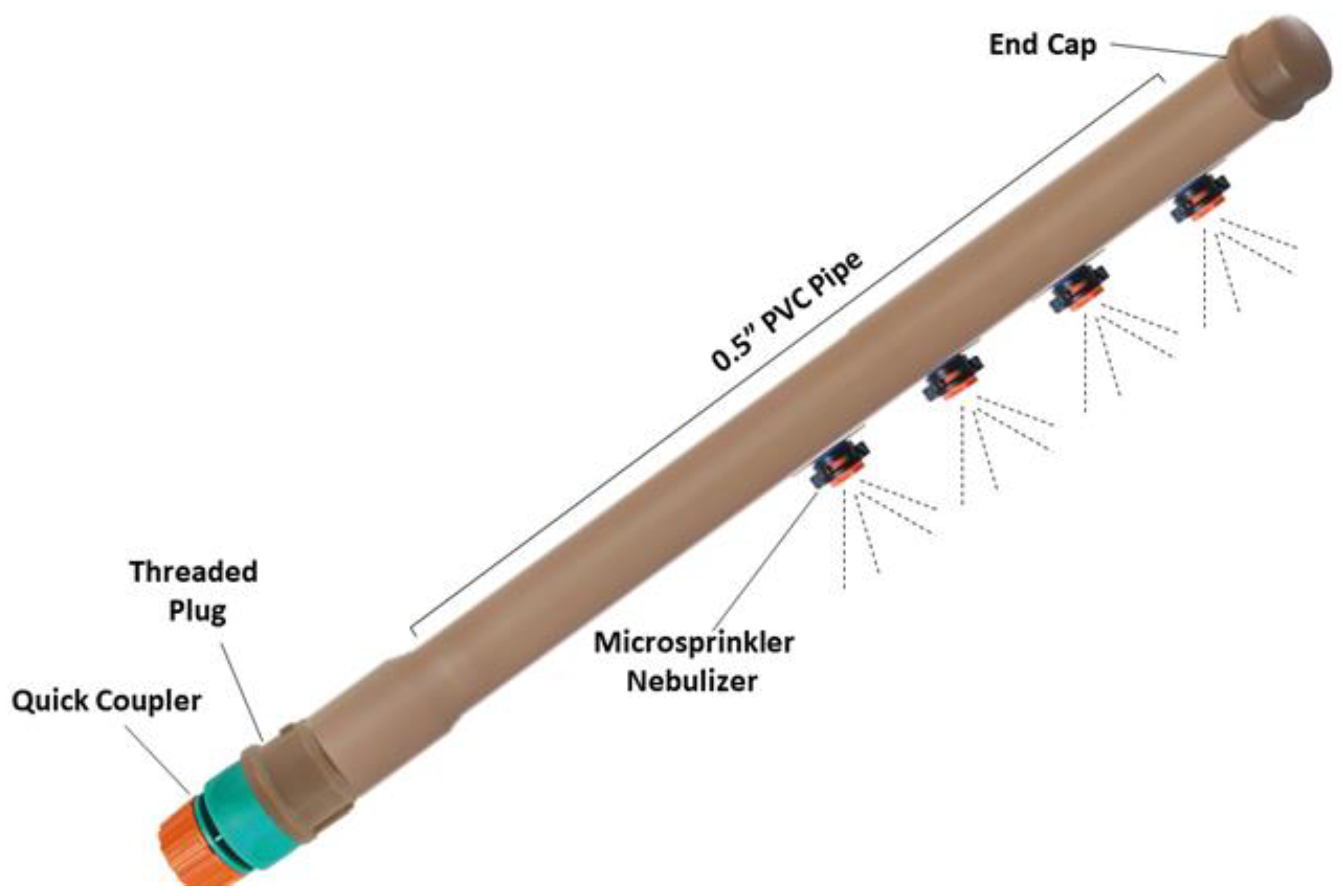
2.3. Experimental Design
2.4. Cultivation Crop
2.5. Agronomic Traits
2.6. Statistical Analysis
3. Results
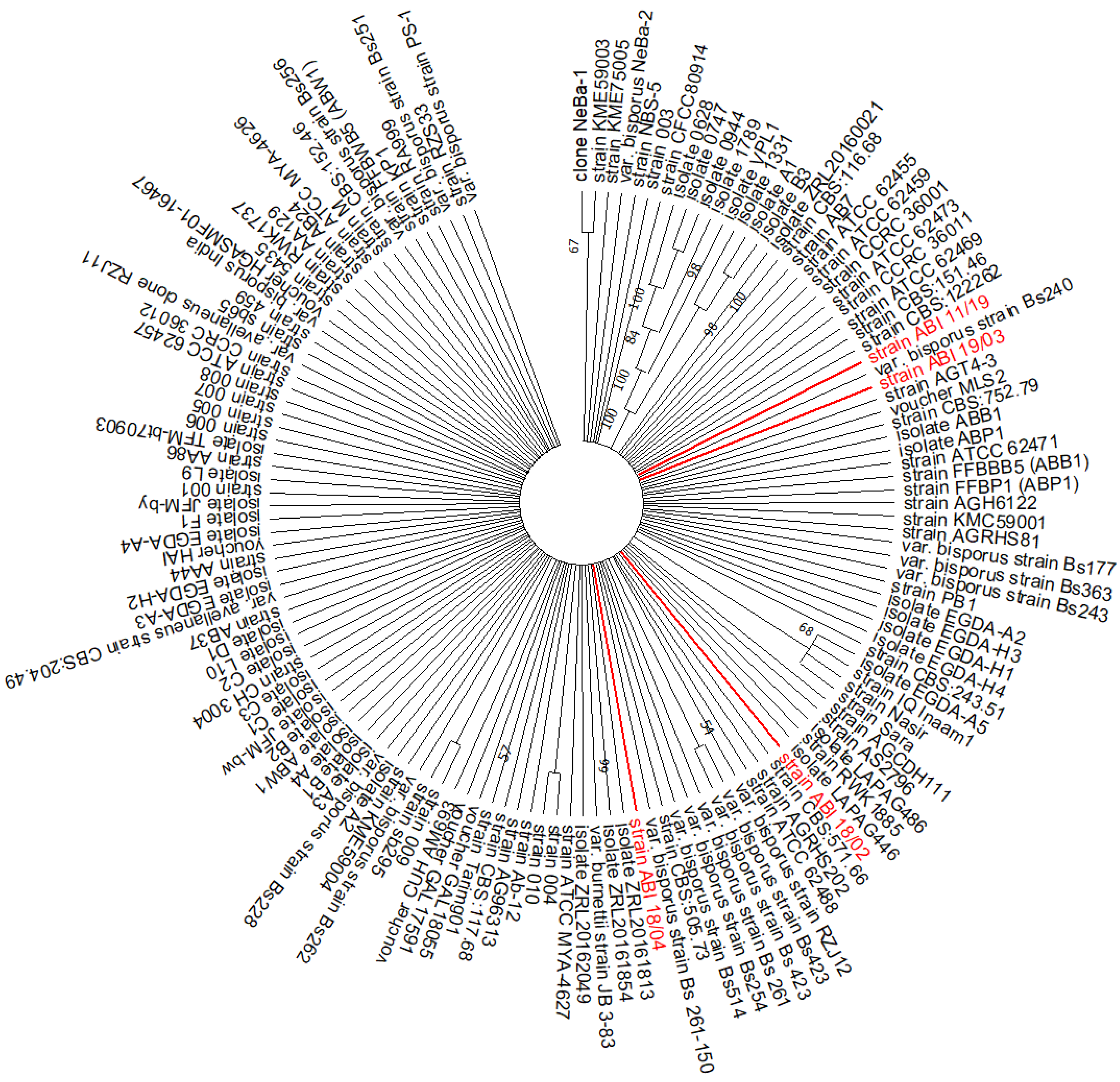
3.1. ITS Sequencing of A. bisporus Strains
3.2. Agronomic Traits
4. Discussion
5. Conclusions
- (a)
- The selection of appropriate strains based on their agronomic characteristics, market demands, and climatic prerequisites;
- (b)
- The optional application of water to the phase III compost before the casing layer, primarily influenced by the initial moisture content of the substrate;
- (c)
- The assessment of economic expenditure on employees and working time combined with the moderate use of water aiming for better sustainability.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Lei, K.; Khu, S.; Meng, W. Assessment of water resources carrying capacity for sustainable development based on a system dynamics model: A case study of Tieling City, China. Water Resour. Manag. 2015, 29, 885–899. [Google Scholar] [CrossRef]
- Scanlon, B.R.; Fakhreddine, S.; Rateb, A.; de Graaf, I.; Famiglietti, J.; Gleeson, T.; Grafton, R.Q.; Jobbagy, E.; Kebede, S.; Kolusu, S.R.; et al. Global water resources and the role of groundwater in a resilient water future. Nat. Rev. Earth Environ. 2023, 4, 87–101. [Google Scholar] [CrossRef]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of stomatal density and morphology on water-use efficiency in a changing world. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water scarcity in agriculture: An overview of causes, impacts and approaches for reducing the risks. Heliyon 2023, 9, e18507. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, H.; Gosling, S.N.; Kummu, M.; Flörke, M.; Pfister, S.; Hanasaki, N.; Wada, Y.; Zhang, X.; Zheng, C.; et al. Water scarcity assessments in the past, present, and future. Earth’s Future 2017, 5, 545–559. [Google Scholar] [CrossRef] [PubMed]
- ONU. Objetivo de Desenvolvimento Sustentável 6. Água Potável e Saneamento: Garantir a Disponibilidade e a Gestão Sustentável da Água Potável e do Saneamento Para Todos. 2023. Available online: https://brasil.un.org/pt-br/sdgs/6 (accessed on 6 April 2023).
- ONU. Recursos de Água Doce Disponíveis por Pessoa Baixam mais de 20% em duas Décadas. 2020. Available online: https://news.un.org/pt/story/2020/11/1734182 (accessed on 6 April 2023).
- Robinson, B.; Winans, K.; Kendall, A.; Dlott, J.; Dlot, F. A life cycle assessment of Agaricus bisporus production in the USA. Int. J. Life Cycle Assess. 2019, 24, 456–467. [Google Scholar] [CrossRef]
- Zhang, K.; Pu, Y.Y.; Sun, D.W. Recent advances in quality preservation of postharvest mushrooms (Agaricus bisporus): A review. Trends Food Sci. Technol. 2018, 78, 72–82. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Zhao, Y.; Kakumyan, P. Impact of cultivation substrate and microbial community on improving mushroom productivity: A review. Biology 2022, 11, 569. [Google Scholar] [CrossRef]
- Navarro, M.J.; Gea, F.J.; Pardo-Giménez, A.; Martínez, A.; Raz, D.; Levanon, D.; DanaY, O. Agronomical valuation of a drip irrigation system in a commercial mushroom farm. Sci. Hortic. 2020, 265, 109234. [Google Scholar] [CrossRef]
- Gómez, A. New technology in Agaricus bisporus cultivation. In Edible and Medicinal Mushrooms: Technology and Applications, 1st ed.; Zied, D.C., Pardo-Giménez, A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 211–220. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, V.; Adelodun, B.; Bedeković, D.; Kos, I.; Širić, I.; Alamri, S.A.; Alrumman, S.A.; Eid, E.M.; Abou Fayssal, S.; et al. Sustainable use of sewage sludge as a casing material for button mushroom (Agaricus bisporus) cultivation: Experimental and prediction modeling studies for uptake of metal elements. J. Fungi 2022, 8, 112. [Google Scholar] [CrossRef]
- Baars, J.J.P.; Scholtmeijer, K.; Sonnenberg, A.S.M.; van Peer, A. Critical factors involved in primordia building in Agaricus bisporus: A review. Molecules 2020, 25, 2984. [Google Scholar] [CrossRef] [PubMed]
- McGee, C.F. Microbial ecology of the Agaricus bisporus mushroom cropping process. Appl. Microbiol. Biotechnol. 2018, 102, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhao, X.; Wang, R.; Zhao, K.; Ma, H.; Jin, X. Effects of water stress on dynamic development quality of Agaricus bisporus and water efficiency in greenhouse. Trans. Chin. Soc. Agric. Eng. 2021, 37, 205–213. [Google Scholar] [CrossRef]
- Danay, O.; Raz, D.; Ezoz, N.; Barski, I.; Levanon, D. Drip irrigation, a new way for watering, during Agaricus bisporus cultivation: Increased production and lower carbon footprint. In Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products, WSMBMP-ICAR-Mushroom Society of India, New Delhi, India, 19–22 November 2014; pp. 325–329. [Google Scholar]
- Idrees, M.; Yaqoob, S.; Leo, S.F.; Khan, A.A.; Sun, L.; Yang, Y.; Li, D.; Fu, Y.; Li, Y. A comparison of the physical, chemical, and structural properties of wild and commercial strains of button mushroom, Agaricus bisporus (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 611–625. [Google Scholar] [CrossRef]
- Singh, B.; Kapoor, S.; Sharma, S.; Sodhi, H.S. Screening of Agaricus bisporus strains and casing variables for improving the yield potential of mushrooms. Indian J. Hortic. 2020, 77, 301–306. [Google Scholar] [CrossRef]
- Singh, M.; Kamal, S.; Sharma, V.P. Species and region-wise mushroom production in leading mushroom producing countries-China, Japan, USA, Canada and India. Mushroom Res. 2021, 30, 99–108. [Google Scholar] [CrossRef]
- Wieme, J.; Mollazade, K.; Malounas, I.; Zude-Sasse, M.; Zhao, M.; Gowen, A.; Argyropoulos, D.; Fountas, S.; Van Beek, J. Application of hyperspectral imaging systems and artificial intelligence for quality assessment of fruit, vegetables and mushrooms: A review. Biosyst. Eng. 2022, 222, 156–176. [Google Scholar] [CrossRef]
- Banasik, A.; Kanellopoulos, A.; Bloemhof-Ruwaard, J.M.; Claassen, G.D.H. Accounting for uncertainty in eco-efficient agri-food supply chains: A case study for mushroom production planning. J. Clean. Prod. 2019, 216, 249–256. [Google Scholar] [CrossRef]
- Muhammad, I.; Sossah, F.L.; Yang, Y.; Li, D.; Li, S.; Fu, Y.; Li, Y. Identification of resistance to cobweb disease caused by Cladobotryum mycophilum in wild and cultivated strains of Agaricus bisporus and screening for bioactive botanicals. RSC Adv. 2019, 9, 14758–14765. [Google Scholar] [CrossRef]
- Harfi, T.; Alireza, M.A.; Farzad, R.; Fariborz, Z.N. Induced mutation in Agaricus bisporus by gamma ray to improve genetic variability, degradation enzyme activity, and yield. Int. J. Radiat. Biol. 2021, 97, 1020–1031. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; White, M.M. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Xu, J. Fungal DNA barcoding. Genome 2016, 59, 913–932. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols, a Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press Ltd.: London, UK, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G + C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [PubMed]
- Zied, D.C.; Minhoni, M.T.A.; Kopytowski-Filho, J.; Andrade, M.C.N. Production of Agaricus blazei ss. Heinemann (A. brasiliensis) on different casing layers and environments. World J. Microbiol. Biotechnol. 2010, 26, 1857–1863. [Google Scholar] [CrossRef]
- Aguiar, L.V.B.D.; Sales-Campos, C.; Gouvêa, P.R.D.S.; Vianez, B.F.; Dias, E.S.; Chevreuil, L.R. Substrate disinfection methods on the production and nutritional composition of a wild oyster mushroom from the Amazon. Cienc. E Agrotecnologia 2021, 45, 1–8. [Google Scholar] [CrossRef]
- Pardo-Giménez, A.; Pardo, J.E.; Zied, D.C. Evaluation of harvested mushrooms and viability of Agaricus bisporus growth using casing materials made from spent mushroom substrate. Int. J. Food Sci. Technol. 2011, 46, 787–792. [Google Scholar] [CrossRef]
- Mitchell, A.D.; Bresinsky, A. Phylogenetic relationships of Agaricus species based on ITS-2 and 28S ribosomal DNA sequences. Mycologia 1999, 91, 811–819. [Google Scholar] [CrossRef]
- Gao, W.; Liang, J.; Pizzul, L.; Feng, X.M.; Zhang, K.; del Pilar Castillo, M. Evaluation of spent mushroom substrate as substitute of peat in Chinese biobeds. Int. Biodeterior. Biodegrad. 2015, 98, 107–112. [Google Scholar] [CrossRef]
- Gao, W.; Baars, J.J.; Maliepaard, C.; Visser, R.G.; Zhang, J.; Sonnenberg, A.S. Multi-trait QTL analysis for agronomic and quality characters of Agaricus bisporus (button mushrooms). AMB Express 2016, 6, 67. [Google Scholar] [CrossRef]
- Sonnenberg, A.S.; Baars, J.J.; Gao, W.; Visser, R.G. Developments in breeding of Agaricus bisporus var. bisporus: Progress made and technical and legal hurdles to take. Appl. Microbiol. Biotechnol. 2017, 101, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.L.; Choi, I.G.; Kong, W.S.; Jang, K.Y.; Oh, M.J.; Im, J.H. Evaluating genetic diversity of Agaricus bisporus accessions through phylogenetic analysis using single-nucleotide polymorphism (SNP) markers. Mycobiology 2021, 49, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Dong, O.; Powers, M.; Liu, Z.; Yoshinaga, M. Arsenic metabolism, toxicity and accumulation in the white button mushroom Agaricus bisporus. Toxics 2022, 10, 554. [Google Scholar] [CrossRef] [PubMed]
- Sattar, M.N.; Iftikhar, S.; El-Masri, I.; Aldine, N.J.; El-Sebaaly, Z.; Sassine, Y.N. Breeding of Agaricus bisporus: Strains, spawns, and impact on yield. In Mushrooms: Agaricus bisporus; Sassine, Y.N., Ed.; CAB International: Osfordshire, UK, 2021; pp. 190–239. [Google Scholar] [CrossRef]
- Foulongne-Oriol, M.; Rodier, A.; Rousseau, T.; Savoie, J.-M. Quantitative trait locus mapping of yield-related components and oligogenic control of the cap color of the button mushroom Agaricus bisporus. Appl. Environ. Microbiol. 2012, 78, 2422–2434. [Google Scholar] [CrossRef]
- Pardo-Giménez, A.; Pardo, J.E.; Zied, D.C. Casing materials and techniques in Agaricus bisporus cultivation. In Edible and Medicinal Mushrooms: Technology and Applications, 1st ed.; Zied, D.C., Pardo-Giménez, A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 149–174. [Google Scholar] [CrossRef]
- Dhar, B.L. Mushroom farm design and technology of cultivation. In Edible and Medicinal Mushrooms: Technology and Applications, 1st ed.; Zied, D.C., Pardo-Giménez, A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 271–308. [Google Scholar] [CrossRef]


| Strain | 93 | 252 | 314 | 563 | 604 | 735 |
|---|---|---|---|---|---|---|
| ABI 18/04 | C | R | A | C | C | Y |
| ABI 19/03 | Y | A | A | T | T | T |
| ABI 18/02 | C | A | A | C | C | T |
| ABI 11/19 | Y | A | R | T | T | T |
| 1st Flush | ||||
|---|---|---|---|---|
| Irrigation/Strain | ABI 18/02 | ABI 18/04 | ABI 19/03 | ABI 11/19 |
| Yield (%) | ||||
| Control | 17.15 a | 12.15 a | 7.50 Bb | 14.62 a |
| Added water | 19.9 a | 11.55 b | 15.09 Ab | 20.00 a |
| CV | 25.14 | Average | 14.74 | |
| Biological Efficiency (%) | ||||
| Control | 49.00 a | 34.71 a | 21.43 Bb | 41.77 a |
| Added water | 56.85 a | 33.00 b | 43.11 Ab | 57.14 a |
| CV | 25.14 | Average | 42.12 | |
| Number of Mushrooms (un) | ||||
| Control | 35.5 a | 14.2 b | 6.5 Bb | 23.7 Ba |
| Added water | 29.7 a | 13.5 b | 25.0 Ab | 41.2 Aa |
| CV | 37.52 | Average | 23.6 | |
| Weight of Mushrooms (g) | ||||
| Control | 13.07 c | 21.38 b | 29.45 Aa | 16.11 c |
| Added water | 16.77 b | 23.29 a | 14.68 Bb | 12.73 b |
| CV | 22.01 | Average | 18.44 | |
| 2nd Flush | ||||
|---|---|---|---|---|
| Irrigation/Strain | ABI 18/02 | ABI 18/04 | ABI 19/03 | ABI 11/19 |
| Yield (%) | ||||
| Control | 11.91 A | 14.45 A | 14.89 A | 12.81 A |
| Added water | 6.23 B | 7.85 B | 5.34 B | 6.21 B |
| CV | 32.72 | Average | 9.96 | |
| Biological Efficiency (%) | ||||
| Control | 34.03 A | 41.28 A | 42.54 A | 36.60 A |
| Added water | 17.79 B | 22.43 B | 15.25 B | 17.74 B |
| CV | 32.72 | Average | 28.46 | |
| Number of Mushroom (un) | ||||
| Control | 41.7 A | 32.2 A | 24.2 | 34.7 A |
| Added water | 15.5 B | 19.0 B | 12.5 | 12.2 B |
| CV | 36.09 | Average | 24.0 | |
| Weight of Mushrooms (g) | ||||
| Control | 7.29 b | 11.42 b | 15.18 Aa | 9.49 b |
| Added water | 10.64 | 10.22 | 7.95 B | 12.64 |
| CV | 22.21 | Average | 10.60 | |
| 3rd Flush | ||||
|---|---|---|---|---|
| Irrigation/Strain | ABI 18/02 | ABI 18/04 | ABI 19/03 | ABI 11/19 |
| Yield (%) | ||||
| Control | 3.18 b | 5.65 b | 7.93 Aa | 4.61 b |
| Added water | 4.40 | 5.24 | 4.87 B | 5.27 |
| CV | 39.94 | Average | 5.14 | |
| Biological Efficiency (%) | ||||
| Control | 9.07 b | 16.14 b | 22.65 Aa | 13.17 b |
| Added water | 12.57 | 14.97 | 13.91 B | 15.05 |
| CV | 39.94 | Average | 14.69 | |
| Number of Mushrooms (un) | ||||
| Control | 6.0 | 13.2 | 16.2 | 10.7 |
| Added water | 6.7 | 11.2 | 11.5 | 7.7 |
| CV | 44.83 | Average | 10.4 | |
| Weight of Mushrooms (g) | ||||
| Control | 15.24 | 10.55 | 13.66 | 11.55 B |
| Added water | 16.52 a | 11.48 b | 9.95 b | 19.48 Aa |
| CV | 39.07 | Average | 13.55 | |
| Total | ||||
|---|---|---|---|---|
| Irrigation/Strain | ABI 18/02 | ABI 18/04 | ABI 19/03 | ABI 11/19 |
| Yield (%) | ||||
| Control | 32.24 | 32.25 A | 30.32 | 32.04 |
| Added water | 30.53 a | 24.64 Bb | 25.30 b | 31.48 a |
| CV | 13.67 | Average | 29.85 | |
| Biological Efficiency (%) | ||||
| Control | 92.11 | 92.14 A | 86.62 | 91.54 |
| Added water | 87.22 a | 70.40 Bb | 72.28 b | 89.94 a |
| CV | 13.67 | Average | 85.28 | |
| Number of Mushrooms (un) | ||||
| Control | 83.2 Aa | 59.7 Ac | 47.0 c | 69.2 b |
| Added water | 52.0 B | 43.7 B | 49.0 | 61.2 |
| CV | 15.33 | Average | 58.1 | |
| Weight of Mushrooms (g) | ||||
| Control | 9.72 Bc | 13.52 b | 16.04 Aa | 11.52 c |
| Added water | 14.84 A | 14.16 | 12.88 B | 13.24 |
| CV | 12.07 | Average | 13.25 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caitano, C.E.C.; Vieira Júnior, W.G.; Soares, D.M.M.; Alves, L.d.S.; Nóbrega, B.d.B.; Pardo-Giménez, A.; Stevani, C.V.; Zied, D.C. Management of Water Supply in the Cultivation of Different Agaricus bisporus Strains. Agronomy 2023, 13, 2626. https://doi.org/10.3390/agronomy13102626
Caitano CEC, Vieira Júnior WG, Soares DMM, Alves LdS, Nóbrega BdB, Pardo-Giménez A, Stevani CV, Zied DC. Management of Water Supply in the Cultivation of Different Agaricus bisporus Strains. Agronomy. 2023; 13(10):2626. https://doi.org/10.3390/agronomy13102626
Chicago/Turabian StyleCaitano, Cinthia Elen Cardoso, Wagner Gonçalves Vieira Júnior, Douglas M. M. Soares, Lucas da Silva Alves, Bianca de Barros Nóbrega, Arturo Pardo-Giménez, Cassius V. Stevani, and Diego Cunha Zied. 2023. "Management of Water Supply in the Cultivation of Different Agaricus bisporus Strains" Agronomy 13, no. 10: 2626. https://doi.org/10.3390/agronomy13102626
APA StyleCaitano, C. E. C., Vieira Júnior, W. G., Soares, D. M. M., Alves, L. d. S., Nóbrega, B. d. B., Pardo-Giménez, A., Stevani, C. V., & Zied, D. C. (2023). Management of Water Supply in the Cultivation of Different Agaricus bisporus Strains. Agronomy, 13(10), 2626. https://doi.org/10.3390/agronomy13102626






