Soil Microbial Communities Show Different Patterns under Different Land Use Types in the Coastal Area of Nantong, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Soil Chemical Properties Determination
2.3. DNA Extraction and Amplicon Sequencing
2.4. Sequenced Data Processing
2.5. Statistical Analyses
3. Results
3.1. Characteristics of Soils
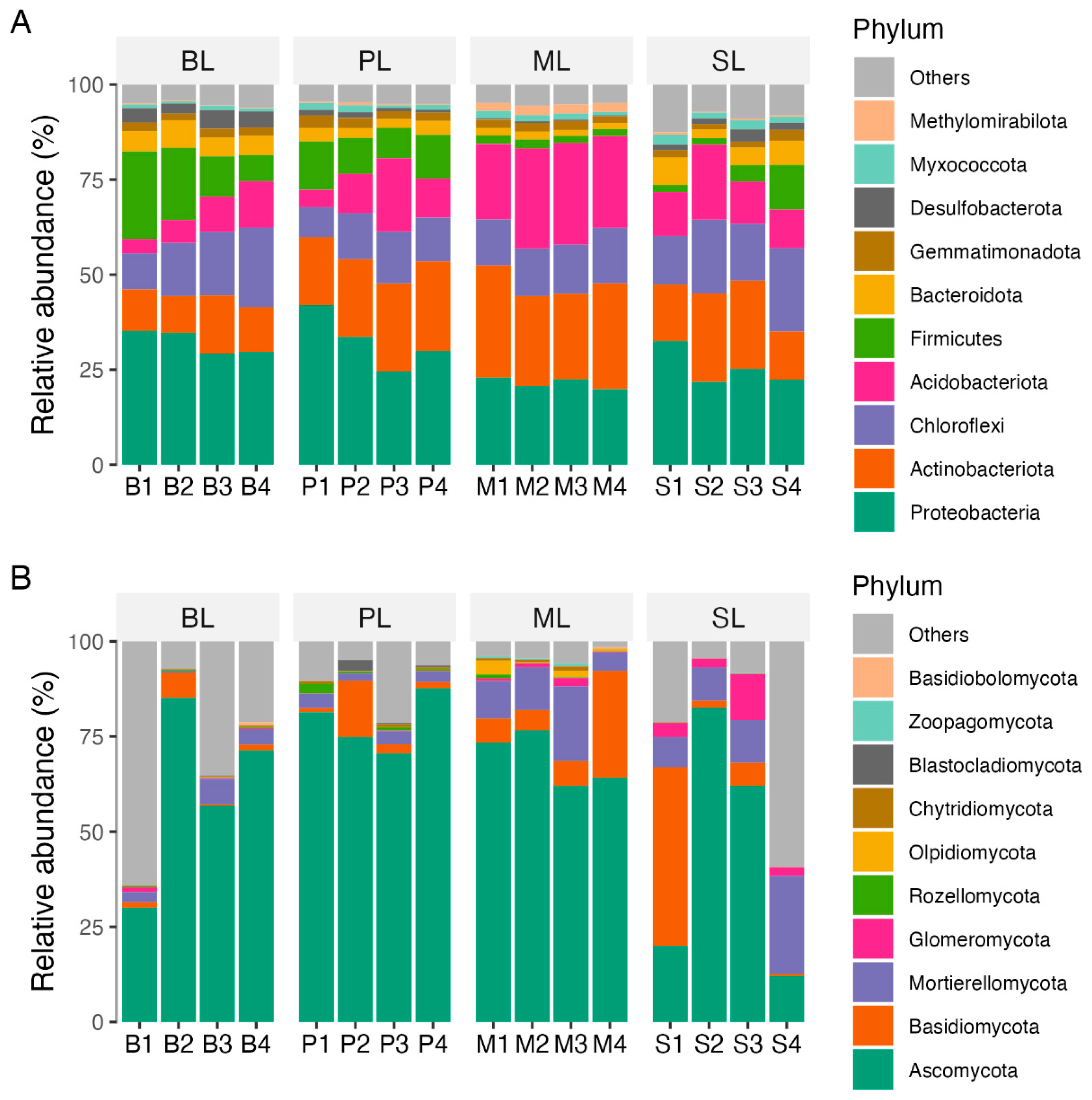
3.2. Bacterial and Fungal Taxa in Soils of Different Land-Use Types
3.3. Alpha and Beta Diversity of the Microbial Communities
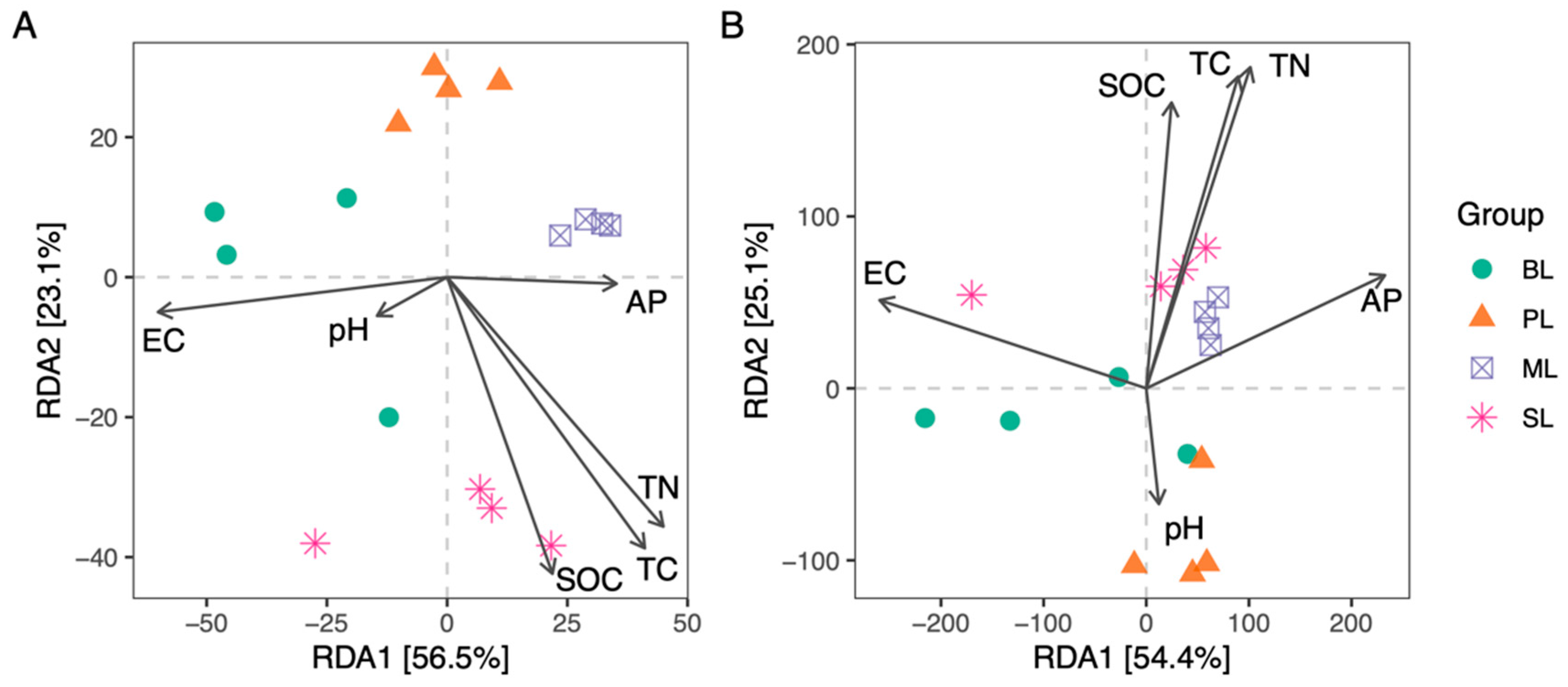
3.4. Relationship between Soil Properties and the Microbial Communities
3.5. Bacterial and Fungal Ecological Functions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil Salinity under Climate Change: Challenges for Sustainable Agriculture and Food Security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, C.; Xue, R.; Wang, L. Effects of Salinity on the Soil Microbial Community and Soil Fertility. J. Integr. Agric. 2019, 18, 1360–1368. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Yao, R.; Xie, W.; Zhang, X. Manure plus Plastic Film Mulch Reduces Soil Salinity and Improves Barley-Maize Growth and Yield in Newly Reclaimed Coastal Land, Eastern China. Water 2022, 14, 2944. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, S.; Liu, L.; Wu, L.; Ding, X. Combined Organic Amendments and Mineral Fertilizer Application Increase Rice Yield by Improving Soil Structure, P Availability and Root Growth in Saline-Alkaline Soil. Soil Tillage Res. 2021, 212, 105060. [Google Scholar] [CrossRef]
- Stagg, C.L.; Baustian, M.M.; Perry, C.L.; Carruthers, T.J.B.; Hall, C.T. Direct and Indirect Controls on Organic Matter Decomposition in Four Coastal Wetland Communities along a Landscape Salinity Gradient. J. Ecol. 2018, 106, 655–670. [Google Scholar] [CrossRef]
- Hadrich, J.C. Managing the Economics of Soil Salinity; Department of Agribusiness and Applied Economics, Agricultural Experiment Station, North Dakota State University: Fargo, ND, USA, 2011. [Google Scholar]
- Tejada, M.; Garcia, C.; Gonzalez, J.L.; Hernandez, M.T. Use of Organic Amendment as a Strategy for Saline Soil Remediation: Influence on the Physical, Chemical and Biological Properties of Soil. Soil Biol. Biochem. 2006, 38, 1413–1421. [Google Scholar] [CrossRef]
- Liu, L.; Wang, B. Protection of Halophytes and Their Uses for Cultivation of Saline-Alkali Soil in China. Biology 2021, 10, 353. [Google Scholar] [CrossRef]
- Xie, X.; Pu, L.; Wang, Q.; Zhu, M.; Xu, Y.; Zhang, M. Response of Soil Physicochemical Properties and Enzyme Activities to Long-Term Reclamation of Coastal Saline Soil, Eastern China. Sci. Total Environ. 2017, 607–608, 1419–1427. [Google Scholar] [CrossRef]
- Rath, K.M.; Rousk, J. Salt Effects on the Soil Microbial Decomposer Community and Their Role in Organic Carbon Cycling: A Review. Soil Biol. Biochem. 2015, 81, 108–123. [Google Scholar] [CrossRef]
- Aislabie, J.; Deslippe, J.; Dymond, J. Soil Microbes and Their Contribution to Soil Services. Ecosystem Services in New Zealand: Conditions and Trends; Manaaki Whenua Press: Lincoln, New Zealand, 2013; pp. 143–161. [Google Scholar]
- Bárcenas-Moreno, G.; Bååth, E.; Rousk, J. Functional Implications of the pH-Trait Distribution of the Microbial Community in a Re-Inoculation Experiment across a pH Gradient. Soil Biol. Biochem. 2016, 93, 69–78. [Google Scholar] [CrossRef]
- He, Z.; Yuan, C.; Chen, P.; Rong, Z.; Peng, T.; Farooq, T.H.; Wang, G.; Yan, W.; Wang, J. Soil Microbial Community Composition and Diversity Analysis under Different Land Use Patterns in Taojia River Basin. Forests 2023, 14, 1004. [Google Scholar] [CrossRef]
- Andersen, R.; Wells, C.; Macrae, M.; Price, J. Nutrient Mineralisation and Microbial Functional Diversity in a Restored Bog Approach Natural Conditions 10 Years Post Restoration. Soil Biol. Biochem. 2013, 64, 37–47. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Kim, M.; Singh, D.; Lee-Cruz, L.; Lai-Hoe, A.; Ainuddin, A.N.; Go, R.; Rahim, R.A.; Husni, M.H.A.; Chun, J.; et al. Tropical Soil Bacterial Communities in Malaysia: pH Dominates in the Equatorial Tropics Too. Microb. Ecol. 2012, 64, 474–484. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.; Yang, R.; Yang, L.; Ge, B. Land-Use Conversion Altered Topsoil Properties and Stoichiometry in a Reclaimed Coastal Agroforestry System. Agronomy 2022, 12, 1143. [Google Scholar] [CrossRef]
- He, H.; Miao, Y.; Gan, Y.; Wei, S.; Tan, S.; Rask, K.A.; Wang, L.; Dai, J.; Chen, W.; Ekelund, F. Soil Bacterial Community Response to Long-Term Land Use Conversion in Yellow River Delta. Appl. Soil Ecol. 2020, 156, 103709. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, X.; Huang, L.; Shan, Q.; Hu, A.; Yan, D.; Zhang, J.; Long, X.-E. Soil Profile Rather than Reclamation Time Drives the Mudflat Soil Microbial Community in the Wheat-Maize Rotation System of Nantong, China. J. Soils Sediments 2021, 21, 1672–1687. [Google Scholar] [CrossRef]
- Lee, A.W.-T.; Chan, C.T.-M.; Wong, L.L.-Y.; Yip, C.-Y.; Lui, W.-T.; Cheng, K.-C.; Leung, J.S.-L.; Lee, L.-K.; Wong, I.T.-F.; Ng, T.T.-L.; et al. Identification of Microbial Community in the Urban Environment: The Concordance between Conventional Culture and Nanopore 16S rRNA Sequencing. Front. Microbiol. 2023, 14, 1164632. [Google Scholar] [CrossRef]
- Wani, G.A.; Khan, M.A.; Dar, M.A.; Shah, M.A.; Reshi, Z.A. Next Generation High Throughput Sequencing to Assess Microbial Communities: An Application Based on Water Quality. Bull. Environ. Contam. Toxicol. 2021, 106, 727–733. [Google Scholar] [CrossRef]
- Li, L.; Li, G.; Du, J.; Wu, J.; Cui, L.; Chen, Y. Effects of Tidal Flat Reclamation on the Stability of Coastal Wetland Ecosystem Services: A Case Study in Jiangsu Coast, China. Ecol. Indic. 2022, 145, 109697. [Google Scholar] [CrossRef]
- Xu, N.; Wang, Y.; Huang, C.; Jiang, S.; Jia, M.; Ma, Y. Monitoring Coastal Reclamation Changes across Jiangsu Province during 1984–2019 Using Landsat Data. Mar. Policy 2022, 136, 104887. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.; Du, W.; Liu, Q.; Zhao, Y.; Xu, M. Research on the Limit Values of Reclamation Based on Ecological Security: A Case Study of Tongzhou Bay in Rudong, Jiangsu Province. Int. J. Environ. Res. Public Health 2022, 19, 8301. [Google Scholar] [CrossRef]
- China Soil Science Data Center. Available online: http://vdb3.soil.csdb.cn/extend/jsp/eng (accessed on 1 June 2023).
- Cui, L.; Li, G.; Liao, H.; Ouyang, N.; Li, X.; Liu, D. Remote Sensing of Coastal Wetland Degradation Using the Landscape Directional Succession Model. Remote Sens. 2022, 14, 5273. [Google Scholar] [CrossRef]
- Lu, R. Analytical Methods of Soil Agrochemistry; Chinese Agriculture Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Rodriguez, J.B.; Self, J.R.; Soltanpour, P.N. Optimal Conditions for Phosphorus Analysis by the Ascorbic Acid-Molybdenum Blue Method. Soil Sci. Soc. Am. J. 1994, 58, 866–870. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global Patterns of 16S rRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Op De Beeck, M.; Lievens, B.; Busschaert, P.; Declerck, S.; Vangronsveld, J.; Colpaert, J.V. Comparison and Validation of Some ITS Primer Pairs Useful for Fungal Metabarcoding Studies. PLoS ONE 2014, 9, e97629. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Posit Team. RStudio: Integrated Development Environment for R; Posit Software, PBC: Boston, MA, USA, 2023; Available online: http://www.posit.co/ (accessed on 1 July 2023).
- Callahan, B.J.; Sankaran, K.; Fukuyama, J.A.; McMurdie, P.J.; Holmes, S.P. Bioconductor Workflow for Microbiome Data Analysis: From Raw Reads to Community Analyses. F1000Research 2016, 5, 1492. [Google Scholar] [CrossRef]
- McLaren, M.R. Silva SSU Taxonomic Training Data Formatted for DADA2 (Silva Version 138). Available online: https://zenodo.org/record/3986799 (accessed on 1 April 2023).
- Põlme, S.; Abarenkov, K.; Henrik Nilsson, R.; Lindahl, B.D.; Clemmensen, K.E.; Kauserud, H.; Nguyen, N.; Kjøller, R.; Bates, S.T.; Baldrian, P.; et al. FungalTraits: A User-Friendly Traits Database of Fungi and Fungus-like Stramenopiles. Fungal Divers. 2020, 105, 1–16. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling Function and Taxonomy in the Global Ocean Microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An Open Annotation Tool for Parsing Fungal Community Datasets by Ecological Guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. Microeco: An R Package for Data Mining in Microbial Community Ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Pu, L.; Zhu, M.; Wu, T.; Xu, Y.; Wang, X. Effect of Long-Term Reclamation on Soil Quality in Agricultural Reclaimed Coastal Saline Soil, Eastern China. J. Soils Sediments 2020, 20, 3909–3920. [Google Scholar] [CrossRef]
- Xie, X.; Pu, L.; Zhu, M.; Xu, Y.; Wang, X. Linkage between Soil Salinization Indicators and Physicochemical Properties in a Long-Term Intensive Agricultural Coastal Reclamation Area, Eastern China. J. Soils Sediments 2019, 19, 3699–3707. [Google Scholar] [CrossRef]
- Li, J.; Pokharel, P.; Liu, G.; Chen, J. Reclamation of Desert Land to Different Land-Use Types Changes Soil Bacterial Community Composition in a Desert-Oasis Ecotone. Land Degrad. Dev. 2021, 32, 1389–1399. [Google Scholar] [CrossRef]
- Wei, X.; Shao, M.; Gale, W.; Li, L. Global Pattern of Soil Carbon Losses Due to the Conversion of Forests to Agricultural Land. Sci. Rep. 2014, 4, 4062. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Steenwerth, K.L.; Jackson, L.E.; Scow, K.M. Land Use and Climatic Factors Structure Regional Patterns in Soil Microbial Communities. Glob. Ecol. Biogeogr. 2010, 19, 27–39. [Google Scholar] [CrossRef]
- Somenahally, A.C.; McLawrence, J.; Chaganti, V.N.; Ganjegunte, G.K.; Obayomi, O.; Brady, J.A. Response of Soil Microbial Communities, Inorganic and Organic Soil Carbon Pools in Arid Saline Soils to Alternative Land Use Practices. Ecol. Indic. 2023, 150, 110227. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X.; Zhang, Z.; Zhao, Y.; Yang, J.; Zhu, Y. Species Diversity and Drivers of Arbuscular Mycorrhizal Fungal Communities in a Semi-Arid Mountain in China. PeerJ 2017, 5, e4155. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global Effects of Land Use on Local Terrestrial Biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Zak, D.R.; Blackwood, C.B.; Curtis, C.D.; Tilman, D. Resource Availability Controls Fungal Diversity across a Plant Diversity Gradient: Resource Availability Controls Fungal Diversity. Ecol. Lett. 2006, 9, 1127–1135. [Google Scholar] [CrossRef]
- Xie, Y.; Fan, J.; Zhu, W.; Amombo, E.; Lou, Y.; Chen, L.; Fu, J. Effect of Heavy Metals Pollution on Soil Microbial Diversity and Bermudagrass Genetic Variation. Front. Plant Sci. 2016, 7, 755. [Google Scholar] [CrossRef]
- Ambrosini, A.; de Souza, R.; Passaglia, L.M.P. Ecological Role of Bacterial Inoculants and Their Potential Impact on Soil Microbial Diversity. Plant Soil 2016, 400, 193–207. [Google Scholar] [CrossRef]
- De Silva, S.; Ball, A.S.; Shahsavari, E.; Indrapala, D.V.; Reichman, S.M. The Effects of Vehicular Emissions on the Activity and Diversity of the Roadside Soil Microbial Community. Environ. Pollut. 2021, 277, 116744. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Xie, X.; Chen, X.; Pu, L.; Zhang, X. Soil Microbial Succession with Soil Development since Costal Reclamation. CATENA 2020, 187, 104393. [Google Scholar] [CrossRef]
- Chen, T.; Hu, R.; Zheng, Z.; Yang, J.; Fan, H.; Deng, X.; Yao, W.; Wang, Q.; Peng, S.; Li, J. Soil Bacterial Community in the Multiple Cropping System Increased Grain Yield within 40 Cultivation Years. Front. Plant Sci. 2021, 12, 804527. [Google Scholar] [CrossRef]
- Stone, B.W.; Li, J.; Koch, B.J.; Blazewicz, S.J.; Dijkstra, P.; Hayer, M.; Hofmockel, K.S.; Liu, X.A.; Mau, R.L.; Morrissey, E.M. Nutrients Cause Consolidation of Soil Carbo Flux to Small Proportion of Bacterial Community. Nat. Commun. 2021, 12, 3381. [Google Scholar] [CrossRef]
- Zhong, F.; Fan, X.; Ji, W.; Hai, Z.; Hu, N.; Li, X.; Liu, G.; Yu, C.; Chen, Y.; Lian, B.; et al. Soil Fungal Community Composition and Diversity of Culturable Endophytic Fungi from Plant Roots in the Reclaimed Area of the Eastern Coast of China. J. Fungi 2022, 8, 124. [Google Scholar] [CrossRef]
- Kamble, P.N.; Gaikwad, V.B.; Kuchekar, S.R.; Bååth, E. Microbial Growth, Biomass, Community Structure and Nutrient Limitation in High pH and Salinity Soils from Pravaranagar (India). Eur. J. Soil Biol. 2014, 65, 87–95. [Google Scholar] [CrossRef]
- Zheng, B.; Chen, P.; Du, Q.; Yang, H.; Luo, K.; Wang, X.; Yang, F.; Yong, T.; Yang, W. Soil Organic Matter, Aggregates, and Microbial Characteristics of Intercropping Soybean under Straw Incorporation and N Input. Agriculture 2022, 12, 1409. [Google Scholar] [CrossRef]
- Li, Y.; Heal, K.; Wang, S.; Cao, S.; Zhou, C. Chemodiversity of Soil Dissolved Organic Matter and Its Association with Soil Microbial Communities Along a Chronosequence of Chinese Fir Monoculture Plantations. Front. Microbiol. 2021, 12, 729344. [Google Scholar] [CrossRef]
- Lundell, T.K.; Mäkelä, M.R.; de Vries, R.P.; Hildén, K.S. Chapter Eleven—Genomics, Lifestyles and Future Prospects of Wood-Decay and Litter-Decomposing Basidiomycota. In Advances in Botanical Research; Martin, F.M., Ed.; Fungi; Academic Press: Cambridge, MA, USA, 2014; Volume 70, pp. 329–370. [Google Scholar]
- St. Leger, R.J.; Wang, J.B. Metarhizium: Jack of All Trades, Master of Many. Open Biol. 2020, 10, 200307. [Google Scholar]
- Carvalho, J.C.B.; Sousa, K.C.I.; Brito, D.C.; Chaibub, A.A.; Luzini, A.P.; Côrtes, M.V.C.B.; Filippi, M.C.C.; Kato, L.; Vaz, B.G.; Costa, H.B.; et al. Biocontrol Potential of Waitea Circinata, an Orchid Mycorrhizal Fungus, against the Rice Blast Fungus. Trop. Plant Pathol. 2015, 40, 151–159. [Google Scholar] [CrossRef]
- Khmelevtsova, L.E.; Sazykin, I.S.; Azhogina, T.N.; Sazykina, M.A. Influence of Agricultural Practices on Bacterial Community of Cultivated Soils. Agriculture 2022, 12, 371. [Google Scholar] [CrossRef]



| Land Use 1 | BL | PL | ML | SL |
|---|---|---|---|---|
| EC (mS cm−1) | 14.52 ± 0.81 a | 1.80 ± 1.15 b | 0.73 ± 0.17 bc | 0.17 ± 0.08 c |
| pH | 8.48 ± 0.30 ab | 8.93 ± 0.36 a | 7.99 ± 0.20 b | 8.45 ± 0.09 ab |
| TC (g kg−1) | 12.18 ± 0.57 c | 11.66 ± 0.35 c | 14.71 ± 0.24 b | 16.69 ± 0.91 a |
| TN (g kg−1) | 0.34 ± 0.02 c | 0.32 ± 0.02 c | 0.78 ± 0.08 b | 0.98 ± 0.08 a |
| SOC (g kg−1) | 4.35 ± 0.74 c | 2.94 ± 0.56 d | 6.26 ± 0.33 b | 10.09 ± 0.84 a |
| AP (mg kg−1) | 40.07 ± 3.97 b | 41.79 ± 5.20 ab | 49.70 ± 4.40 a | 43.16 ± 0.95 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Hu, A.; Wang, X.; Zhao, C.; Jin, J.; Liu, G.; Han, Y.; Liu, B. Soil Microbial Communities Show Different Patterns under Different Land Use Types in the Coastal Area of Nantong, China. Agronomy 2023, 13, 2613. https://doi.org/10.3390/agronomy13102613
Li J, Hu A, Wang X, Zhao C, Jin J, Liu G, Han Y, Liu B. Soil Microbial Communities Show Different Patterns under Different Land Use Types in the Coastal Area of Nantong, China. Agronomy. 2023; 13(10):2613. https://doi.org/10.3390/agronomy13102613
Chicago/Turabian StyleLi, Jinbiao, Anyong Hu, Xiuping Wang, Chuang Zhao, Jiarui Jin, Guangming Liu, Yujie Han, and Bo Liu. 2023. "Soil Microbial Communities Show Different Patterns under Different Land Use Types in the Coastal Area of Nantong, China" Agronomy 13, no. 10: 2613. https://doi.org/10.3390/agronomy13102613
APA StyleLi, J., Hu, A., Wang, X., Zhao, C., Jin, J., Liu, G., Han, Y., & Liu, B. (2023). Soil Microbial Communities Show Different Patterns under Different Land Use Types in the Coastal Area of Nantong, China. Agronomy, 13(10), 2613. https://doi.org/10.3390/agronomy13102613






