Abstract
With the frequent occurrence of extreme weather such as typhoons and rainstorms, waterlogging has become one of the most important threats to global crop growth and production. Waterlogging limits plants’ access to oxygen and light, leading to disadvantageous changes in metabolism to disturb plant growth and development. To escape the damage of hypoxia or promote the diffusion of oxygen to submerged organs, plants respond to waterlogging stress by regulating their morphological structure, photosynthesis, respiration, energy metabolism, and endogenous plant hormone biosynthesis/signal transduction. The adventitious roots (AR), aerenchyma, and stem internode are the major target structure for waterlogging adaptation. The molecular mechanism of crop survival under waterlogging conditions and the key genes related photosynthesis, reactive oxygen species (ROS) homeostasis, and ethylene signal transduction are reviewed. We also elucidated recent advances in the study of interactions between various regulatory pathways and emphasized the important role of stress memory and cross-stress mechanisms in plant response to abiotic stress, indicating the importance of epigenetic modifications. On the basis of above, the research direction and focus of plants coping with waterlogging stress in the future are proposed. This review provides rich genetic resources and a theoretical basis for improving the genetic breeding of crop resistance to waterlogging.
1. Introduction
Waterlogging stress refers to the “flood” phenomenon that occurs when the soil water content exceeds 20% of the field water capacity, causing damage to plant growth [1]. In recent years, the continuous increase in precipitation caused by the intensification of the global greenhouse effect, coupled with poor soil drainage makes waterlogging stress one of the most important abiotic stresses affecting crop growth, development, and yield [2,3]. Flooding affects about 10% of the world’s arable land, resulting in a 15–80% decline in crop yield. With the continuous change in climate, the global waterlogging area is expected to increase, posing a great threat to global food security [4,5]. Submergence leads to low oxygen content and diffusion rate in plant cells, and the closure of leaf stomata leads to the limitation of gas exchange, which damages the photosynthetic organs of plant leaves, reduces the activity of mesophyll cells and chlorophyll content, leading to the impaired photosynthetic performance, and aggravated membrane lipid peroxidation and plant chlorosis, causing plant leaves to wither and fall off [6,7]. The decrease in photosynthetic rate leads to the decrease in dry matter synthesis, thus affecting the development of reproductive organs including cotton boll formation stagnation and increased boll shedding [8]. The decrease in dry matter synthesis also reduce panicles per plant and grains per panicle in barley [9] and rice [10], and reduce total protein and soluble sugar content of corn grains [11], which is closely related to the growth stage of crops [12]. As the most direct and initial part of sensing waterlogging, the low-oxygen environment, especially in poorly drained soil, leads to the accumulation of a large number of anaerobic respiratory products, which inhibits root development. Consistently, the length, surface area, and vitality of root all decrease significantly and then gradually turn brown and rot [13]. Instead, AR and aerenchyma are formed [14]. Furthermore, waterlogging stress reduces the amount of oxygen and other gases in the soil and leads to the accumulation of harmful salt components and other hazards, leading to soil alkalization and further hindering the growth and development of crops.
Although plants evolved from aquatic plants after artificial domestication and long-term direct selection, the genetic diversity of crop germplasm resources has decreased significantly, and many crops have lost their waterlogging tolerance. The well-known flood-tolerant locus 1 (SUB1) was obtained from the FR13A local variety, and great progress has been made in breeding flood-tolerant rice varieties [15]. In this review, we mainly introduce and summarize the latest research progress in plant flood response, explore potential research methods and integrated breeding strategies, and discuss future research directions for crop improvement in this field.
2. Morphological Adaptation Mechanism of Plants in Response to Waterlogging Stress
Plants undergo changes in external morphological structure and internal biochemical pathways in response to hypoxia caused by flooding (Figure 1). Land and Wetland plants respond to a flooded environment by changing their morphological structure, which is usually manifested as the formation of AR, aerenchyma, and accelerated growth of stem internode under waterlogging [16]. Waterlogging stress can lead to the death of a large number of primary roots, root hairs, and formation of many ARs in the basal stem on the water surface. Plant roots promote water and nutrients absorption, and crops such as tomatoes, cotton, and barley adapt to flood environments by rapidly forming AR in water [17,18]. A deep understanding reveals that the balance between the synergistic effects of ethylene and Gibberellin (GA) and the inhibitory effects of Abscisic acid (ABA) is crucial for promoting plant epidermal cell death and the emergence of AR [19]. In rice, ethylene induces the formation of hydrogen peroxide, which in turn leads to cell death in the epidermal cell layer of the AR primordium, thus promoting the primordial appearance of AR and the formation of solute gas tissue [20,21,22]. Auxin enhanced the expression of ethylene biosynthesis genes ACS1, ACS2, and ACO5 (1-aminocyclopropane-1-carboxylate synthase/oxidase), resulting in the interception of ethylene-by flooded plants, and the formation of auxin-induced AR depend on ethylene [23]. Ethylene and auxin accumulation induce ROS signal by up-regulating of CsRBOHB and CsRBOHF3 genes, ultimately inducing AR formation [24]. In addition, other factors including the group ethylene response factor family VII (ERFVIIs) [25,26], exogenous ABA [27,28], brassinolide (BR) gene [29], and Ca2+ signal transduction also affect the formation of AR [30]. In some aquatic plants, waterlogging and hypoxia can induce the AR system to secrete oxygen into the rhizosphere with the process known as radial oxygen loss (ROL), enabling it to survive in a flooded environment [31]. However, the differences in ROL barriers in AR among plants are significant, suggesting that plants regulate AR formation through various molecular mechanisms. In addition, CsARN6.1 encodes the AAA ATPase domain and has been identified as the main effective quantitative trait loci (QTL) involved in waterlogging resistance by mediating AR formation by forward genetic studies [4]. Therefore, cloning more QTLs related to waterlogging resistance traits will be a future focus and research direction.

Figure 1.
Responses of land and wetland plants to waterlogging at both morphological and physiological levels. Under waterlogging conditions, different tissues of plants show different phenotypes. For land plants such as cotton, waterlogging results in leaf yellowing, stem easily lodging, decreased bolls, and bad fibers. In wetland plants such as rice, some symptoms such as radial oxygen loss, delaying flowering, and reduced panicle number are observed. The above indicates that both vegetative and reproductive growth are affected significantly with different external phenotypes. However, the internal physiological mechanisms are almost similar including photosynthesis damage, reduced transpiration, altered phytohormones, non-structural carbohydrates level, breakdown of ROS homeostasis, and elevated Malondialdehyde (MDA) content, etc., regardless of plant species.
Under hypoxia conditions, cortical cells of plant roots degrade leading to radial cell wall aggregation and parenchyma formation. Parenchyma is a gas channel composed of specific cells that transport oxygen to the root system, effectively alleviating the inhibition of oxygen transport from buds to hydrous roots and improving the plant’s ability to withstand hypoxia stress [32,33]. For example, after the flood, a large amount of parenchyma tissue forms in the roots, leaf sheaths, leaves, and middle parts of stems of corn [34], rice [35], peanut [36], wheat [37] and other plants. Furthermore, compared with other parenchymatous cells, parenchyma cells have less starch, larger vacuoles and thinner cell walls, which are conducive to gas transport [38]. Similar to ARs mediations, the plant hormone ethylene is also directly or indirectly involved in inducing programmed cell death (PCD) processes, thus contributing to inducing aerated tissue formation under hypoxic conditions. For example, the coordination of ethylene and ROS signaling induces PCD to occur and xyloglucan endotransglucosylase (XET) increase, which regulates plant cell wall degradation and leads to adventitious root and parenchyma formation [39].
Under waterlogging stress, plants also show accelerated elongation in stem internodes, so that plant stems and leaves can reach out from the water surface to contact the air, which is conducive to gas exchange [40]. Deepwater rice can rapidly elongate by 20 to 25 cm in a few days [41]. However, lowland dryland rice presents a tendency to grow slowly and remain viable during waterlogging to avoid the energy expenditure related to elongated growth [42]. Rice OsUGT75A regulates coleoptile length by reducing free ABA and jasmonic acid (JA) levels [43] (Figure 2). A survival strategy of plants is that waterlogging has little effect on the elongation of young leaves compared with mature leaves, making mature leaves senescence and maintaining young leaves growth [44]. This process involves gene expression related to photosystem II, thylakoid membrane, and carbohydrate-binding pathway. For instance, the leaf age in barley is specifically regulated by the key genes related to chlorophyll metabolism, such as CURT1B, RNSL4, and PSY1. Under infrequent but prolonged flood events, plants that rapidly extend out of the flood surface display more biomass production than those that remain below the flood [45], as lower petiole porosity of the latter limits internal gas transport and reduces biomass accumulation [46]. When waterlogging is frequent but lasts for a short period, plants with slow elongation ability show obvious advantages over rapid elongation, because the elongated leaves have a weaker biomechanical adaptability and will tip over and partially break after submergence and the leaves will quickly dry [45]. By studying the physiological mechanism of waterlogging avoidance, it shows that GA and ethylene are the main factors contributing to the rapid elongation of deepwater rice after waterlogging [28]. The research on the waterlogging avoidance mechanism mainly focuses on deepwater rice, while research on other crops is less and should be strengthened. The formation of adventitious roots and AR mainly contributes to the improvement of waterlogging resistance.
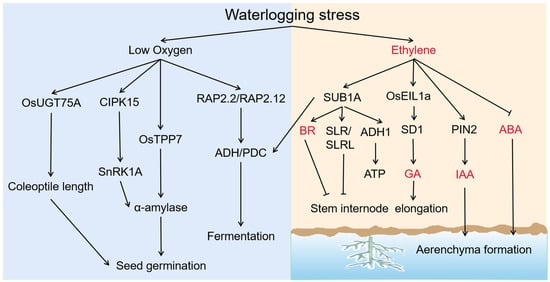
Figure 2.
Molecular and physiological mechanisms in response to hypoxia and ethylene caused by submergence in plants. Suffering from low oxygen, OsUGT75A regulates coleoptile length during rice seed germination. The Amylase gene plays a central role in cereal seeds germination. The rice CIPK15 gene is the upstream regulatory gene of SnRK1A and can induce the expression of α-amylase. RAP2.2/RAP2.12 regulates the alcohol dehydrogenase gene ADH and PDC and involves alcohol fermentation. Phytohormones are involved in plant response to waterlogging stress. Increased ethylene can coordinate the biosynthesis and signaling pathways of GA, ABA, and indole acetic acid (IAA) during submergence. Ethylene promotes the transcription of rice SUB1A, and the overexpression of SUB1A enhances the transcription of ADH1 in transgenic rice and produce more ATP, which up-regulate the expression of Brassinosteroids (BR) biosynthesis genes to inhibits internode elongation. The SUB1A gene also inhibits stem elongation by increasing the accumulation of DELLA protein SLR1 and non-DELLA protein SLRL1. Rice SEMIDWARF1 (SD1) is transcriptionally activated by ethylene transcription factor OsEIL1 and catalyzes the biosynthesis of GA. Ethylene induce the expression of the auxin polar transporter PIN2 and increase the cell activity in AR primordium, by which ethylene interception under waterlogging stimulates the transport of auxin to the submerged part and promotes AR production. ‘→’, positive regulation, ‘⟞’, negative regulation.
3. Physiological Changes and Metabolic Regulation of Plants in Response to Waterlogging Stress
At the cellular level, waterlogging stress mainly affects internal metabolism, internal material flow out, cell membrane destruction, and photosynthetic rate [47]. Hypoxic immobility means that some crops can grow slowly underwater to conserve energy, and cells maintain their intact structure and essential functions through metabolic regulation. In addition, plants can retain a certain number of green leaves and generate new leaves to restore growth after submergence, which has a relatively lower impact on crop agronomic traits [48].
3.1. Hypoxic Response of Plants to Waterlogging Stress
Waterlogging stress causes anoxia in plant tissues, and the diffusion rate of atmospheric gas to roots is cut by 104 times, limiting the tricarboxylic acid (TCA) cycle and the oxygen demand on the mitochondrial electron transport chain. Anaerobic respiration becomes an important source of ATP in plants [49]. Some cells strongly increase metabolic flux through glycolysis, while others consume more conserved carbohydrates. Pyruvic acid accumulation during glycolysis can be used in anaerobic fermentation. Glyceraldehyde phosphate dehydrogenase (GAPDH), ethanol dehydrogenase (ADH), and pyruvate decarboxylase (PDC) in plant roots play a key role in the ethanol fermentation pathway, whose activities are generally considered as one of the important indicators of plant waterlogging resistance [50,51]. The GmADH2 gene is induced during glycolysis and ethanol fermentation and enhanced the germination ability of transgenic GmADH2 soybean seeds under waterlogged conditions [50]. Lactate dehydrogenase (LDH) is also involved in waterlogging resistance together with PDC. The overexpression of LDH significantly enhances the PDC activity and hypoxia-resistance of Arabidopsis [52]. In addition, the overexpression of AdRAP2.3 in tobacco can increase the activity of PDC and ADH enzymes in the root system, as well as the expression levels of waterlogging marker genes [52]. Nevertheless, the intermediate product acetaldehyde and the final product ethanol produced by anaerobic respiration also accumulate correspondingly [53], and the high concentration of toxic anaerobic substances destroy the metabolic balance of plants [54]. Studies on the protein profiles of maize seedlings under anaerobic conditions showed that the synthesis of most proteins decreased sharply [55]. Hypoxia also affects the survival of bacteria in the soil, and the death of bacteria leads to the reduction in nitrate in plants, resulting in a decrease in plant yield [56].
The way plant cells perceive O2 levels is largely dependent on the control of the stability of ERFVIIs transcription factors. Polyunsaturated long-chain acyl-coA is mainly dynamically involved in the activation of hypoxia signals in plants by regulating ACBP-ERF-VII [57]. As a member of the Arabidopsis CDPK family, CPK12 is activated by calcium-dependent phosphorylation, then CPK12 is transported from the cytoplasm to the nucleus, interacts and phosphorylates a regulator of the plant hypoxic sensing core ERF-VII, to improve hypoxia tolerance [58]. The ability of plants to maintain high levels of non-structural carbohydrates is also an important phenotypic characteristic of tolerant varieties. Amylase gene plays a central role in the degradation of endosperm starch during aerobic germination of cereal seeds [59] (Figure 2). Amy3 subfamily gene expression is upregulated under hypoxic conditions. The rice CIPK15 gene is the main positive and upstream regulatory gene of SnRK1A, which can induce the expression of Amy3D encoding α-amylase and actively regulate carbohydrate catabolism under hypoxia conditions [60,61]. Moreover, the trehalose 6-phosphatase gene is the main QTL for the anaerobic germination tolerance of rice [62]. OsTPP7 induces transcription of α-amylase related genes, leading to the decomposability of starch to provide energy for seed germination [62]. In summary, genes related to sugar and energy metabolism are activated by flood stress to regulate the plant’s response to hypoxia stress. The stratum corneum is the first barrier to gas exchange in plants, especially when the stomata are tightly closed under water flooding conditions. The increase in stratum corneum permeability induced by flooding is closely related to the down-regulation of genes involved in epidermal lipid synthesis in Arabidopsis under hypoxia stress [63]. Previous studies show that carbon dioxide (CO2) and oxygen (O2) can enter plant cells directly through the stratum corneum when the stomata are closed, and the thinner stratum corneum helps more CO2 and O2 flow in and stimulate photosynthesis. Long-chain acyl-CoA synthetase (LACS2) regulates the submergence tolerance by regulating the permeability of the stratum corneum in Arabidopsis cells, which is physiologically related to chlorophyll exudation, ion leakage, and gas exchange [64]. In addition, changes in cellular ion homeostasis and membrane transporters induced by hypoxia may be crucial for cell fate determination and formation of the lysigenous aerenchyma in plant roots, as well as the formation of root structure and adventitious root development [65].
3.2. Osmotic Regulation of Plants in Response to Waterlogging Stress
Waterlogging can affect the osmotic regulation process of plants. An important physiological response for plants to adapt to waterlogging stress is that during short-term waterlogging, osmoregulatory factors (such as soluble protein, sugar, and free proline) are mainly involved in regulating osmotic pressure of plant cells and can be stimulated to accumulate rapidly, which can protect plants under waterlogging conditions by optimizing cell environmental protection enzymes and membrane systems, but the contents of soluble protein, sugar, and free proline decreased gradually with prolonged waterlogging [55,66,67,68,69]. The decreased profiles of osmoregulatory factors in waterlogged grains is related to the growth stage. The loss of total protein (protein, albumin, and gluten) of waxy maize at the jointing stage is the most severe, and the starch content of grains increased but the soluble sugar content decreased at the V6 stage and heading stage, the amylopectin and amylose content increased, while the starch content decreased at maturity [55]. Waterlogging at flowering period or after flowering period can increase starch content and decrease total protein content [70], and plants with different genotypes showed different contents of osmoregulatory factors [71]. Therefore, paying attention to the changes and the internal regulation mechanism of total protein, sugar, and free proline contents in plants with different genotypes at different stages under waterlogging conditions is significantly crucial.
The expression of genes involved in osmotic stress can ameliorate plant cell damage caused by osmotic potential imbalance between plants and the environment. Notably, recent studies have shown that osmotic stress gene expression was influenced by inhibiting Arabidopsis PLANT U-BOX44 (PUB44) mediated calc-dependent protein kinase (CPK4) responding to osmotic stress [72]. The overexpression of maize transcription factor ZmNAC2 in Arabidopsis upregulates the expression of many osmotic stress related genes and plant hormone signaling genes [73]. Therefore, these genes can serve as target genes for improving osmotic stress resistance in crop breeding.
3.3. Active Oxygen Scavenging Mechanism of Plants in Response to Waterlogging Stress
During waterlogging stress, ROS such as hydroxyl radical (OH−), superoxide anion radical (O2−), and hydrogen peroxide (H2O2) accumulate excessively, leading to irreversible oxidation of membrane lipids and proteins, and severely damaging protective barriers of cell membranes and plants [24,74,75]. As the product of lipid peroxidation, MDA is an important physiological index to measure plant tolerance and sensitivity to abiotic stress [76]. MDA can bind to proteins and enzymes on the cell membrane and denature or inactivate them, thereby destroying the biofilm structure and function and affecting cell substances metabolism [77]. For example, MDA accumulation leads to an increase in cell membrane selective permeability and electrolyte exosmosis, further affecting other physiological and biochemical metabolic activities [78]. In addition, the activities of protective enzymes such as SOD and POD in plant tissues are increased in response to waterlogging, and plants maintain the normal level of ROS by coordinating the content of SOD, POD, and catalase (CAT), [79,80,81]. Under waterlogging stress, the antioxidant enzyme activity patterns of different crops vary greatly. For instance, POD activity in cotton first increases and then decreases with the prolongation of flooding time, while SOD activity first decreases and then increases [82]. The activities of SOD and CAT in rapeseed leaves decreases first and then increases, while POD activity always increases with the prolongation of waterlogging time [83,84], which suggests that the mechanism of the plant antioxidant enzyme system is not always same, and there may be different regulatory networks responsible for the antioxidant enzyme system in response to different types of waterlogging in different plants. In plant cells, the expression of some antioxidant genes can maintain the redox balance of cells by increasing the enzymatic antioxidants such as SOD and POD, as well as non-enzymatic antioxidants including ascorbic acid and glutathione (Figure 2). For example, the induced expression of ethylene response factor BnERF2.4 in Brassica napus is involved in regulating the antioxidant system against waterlogging stress [85]. The overexpression of the barley Phytoglobin 1 (HvPgb1) gene is involved in central carbon metabolism, carbon assimilation, and ethylene synthesis, thus improving the tolerance of plant roots to waterlogging [86]. The interaction between the CmRCD1 gene and CmSOS1 in chrysanthemums enhances the enzyme activities of SOD, POD, and CAT to regulate the waterlogging tolerance [87]. Furthermore, exogenous growth regulatory substances application also improve the antioxidant capacity of flooded crops [88]. The exogenous administration of glutathione (GSH) [89] and glycine betaine (GB) [90] in the field can significantly improve the transcription level and enzyme activity of antioxidant defense-related genes to reduce oxidative stress. The utilization of gamma-aminobutyric acid (GABA) can activate the activity of maize antioxidant enzymes, improve the ultrastructure of chloroplasts, enhance photosynthetic characteristics, and promote the growth of maize seedlings under flooded conditions [91].
Mitochondria are the main source of ROS in plants under hypoxic conditions. Chang et al. [92] demonstrated that mitochondrial electron transport chain (mETC) inhibitors can stimulate mitochondria to produce ROS in Arabidopsis seedlings. Under hypoxia conditions, damaged mitochondria can significantly destroy the dynamic equilibrium of cell metabolism, then accumulate excessive ROS and activate hypoxia-inducible factor HIF-1α, to initiate signaling transduction and regulate mitochondrial autophagy processes. There are many studies on the process of autophagy induced by hypoxia in animal cells, but few studies on the molecular process of hypoxia-induced autophagy in plant cells. Meanwhile, the concentration of free Ca2+ in the cytoplasm under waterlogging is increased, thus encoding calcium signals to activate NADPH oxidase and produce excessive ROS, which damages plant cell activity, physiological metabolism, and development [93].
3.4. Photosynthetic Signal Transduction in Response to Waterlogging Stress in Plants
The first response of plants to waterlogging stress is the closure of the leaf stomata. The decrease in gas exchange rate leads to a decrease in photosynthetic performance, and the reduction in water absorption leads to a decrease in transpiration [94,95]. Sucrose and starch are the main end products of photosynthesis in most plants, while flooding reduces the photosynthesis rate and affects the transportation of photosynthetic products from source tissues (leaves) to sink tissues (roots). Waterlogging-resistant plants maintain respiration and growth in the absence of photosynthesis, resulting in the accumulation of more carbohydrates in the roots, while waterlogging-sensitive plants reduce their demand for sucrose due to the obstruction of phloem transport and lead to more starch accumulation in the chloroplasts of leaves [95,96]. Therefore, maintaining low leaf starch concentration, high root tissue starch concentration, and high photosynthetic rate are important characteristics for plant survival in submerged environments [95].
Photochrome is a signal molecule acting as a photoreceptor in response to changes in the ambient light quality and level that regulates plant metabolic pathways and growth and development [97]. The degradation of phytochrome in a waterlogged environment is a common feature of plants. Flooding leads to decreased light intensity and the ratio of red to far-red light (R: FR). The specific transcriptional regulatory factor WRKY6 in Arabidopsis is regulated by phytochrome and directly combines with the promoter of the senescence-induced receptor-like protein kinase (SIRK) gene to induce leaf senescence [98]. This result was confirmed in another literature that WRKY6 positively regulates leaf senescence by up-regulating the expression of senescence-related gene SAG [99]. The expression of photopigment-interacting factor-like protein (OsPIL1) in rice under waterlogging was relatively down-regulated compared with normal light exposure, resulting in a decrease in total chlorophyll (Chl) and Chlb contents, an increase in Chla/b ratio, and a decrease in light-trapping capacity, leading to a decrease in leaf biomass and grain yield [100].
Waterlogging causes stomatal closure and intercellular carbon dioxide cannot be fully absorbed and transformed by plants. Mitochondrial respiration decreases and the activities of photosynthetic-related proteins in plants are significantly down-regulated, resulting in the decomposition of photopigments, which directly affects the photosynthetic rate of plants by disrupting light signal recognition [101,102]. Moreover, the activities of the key carbon assimilation enzymes ribulose-1, 5-diphosphate carboxylase (Rubisco), and phosphoenolpyruvate (PEP) carboxylase decrease, which negatively affect the carbon dioxide assimilation and photosynthesis rate [103]. The reduction in carbohydrate allocation caused by low photosynthetic performance under waterlogging conditions leads to poor spike differentiation and development of plants and reduces plant yield and quality [104]. Moreover, such adverse effects involve different growth stages of plants which increase with the prolongation of waterlogging, and seedlings are usually more sensitive than older plants [105,106,107]. Plants maintain internal ventilation and photosynthesis of leaves by forming air film. A rice gene LEAF GAS FILM 1 (LGF1/OsHSD1) controls the retention of air film in leaves to contribute to internal ventilation by regulating the synthesis of primary alcohol under waterlogging [108]. In addition, the exogenous application of glutathione can protect photosynthetic pigments for photosynthesis [109].
3.5. Changes of Plant Hormone and Signal Molecules-Mediated Pathways under Waterlogging Stress
Ethylene has been proven to play an important role in controlling rapid hypoxia stress responses and help plants adapt to hypoxic environments [110] (Figure 2). The ethylene signaling pathway is involved in various cellular reactions during hypoxia, including enhancing the vitality of apical hypoxic cells, maintaining ROS homeostasis and antioxidant activity [111,112], promoting the callus formation of auxin-induced xylem sheath cells [113], as well as controlling hypoxia induction [110]. In plants, cysteine oxidase (PCOs) acts as an oxygen sensor, mediating the abundance of three ERF-VII protein members [114]. Under hypoxic conditions, PCO cannot oxidize ERF-VII proteins and these proteins are stabilized and enter the nucleus, activating hypoxic response genes and triggering plant hypoxia response [114,115]. During hypoxia, ethylene regulates protein abundance through transcription. The overexpression of the Arabidopsis root tip ethylene regulatory proteins PGB1, HUP26, and HUP36 genes can enhance root tip cells’ hypoxia tolerance [112]. Ethylene enhances nitric oxide (NO) scavenger PHYTOGLOBIN1 (PGB1) and inhibits MetCys2 (MC)-activated ERFVII protein NO-dependent proteolysis through PRT6 N-degron pathway to promote ERFVII accumulation before hypoxia, and this process is involved in an increase in RAP2.2 and RAP2.12 genes expression, which allows plants to preadapt to the impending hypoxia [116,117,118]. RAP2.2 is an important ethylene reaction factor, which regulates the alcohol dehydrogenase gene ADH1, pyruvic acid decarboxylase PDC1, and ethylene synthesis-related genes to counteract hypoxia stress in Arabidopsis [119,120]. RAP2.12 affects the direction of root growth by limiting the flux of IAA from the root tip to the elongation zone [121]. Other ERF factors, including PhERF2, HvERF2.11, ZmEREB180, and OsSublA, have also been shown to play important roles in waterlogging tolerance, supporting the important functions of ethylene and related genes in waterlogging tolerance [15,25,122,123,124]. In addition, ethylene promotes the transcription of rice SUB1A and the overexpression of SUB1A enhances the transcription of ADH1 in transgenic rice, leading to more ATP production [125]. The SUB1A gene also inhibits stem elongation by increasing the accumulation of DELLA protein SLENDER rice-1 (SLR1) and non-DELLA protein SLR1-like-1 (SLRL1) and internode elongation by up-regulating the expression of BR biosynthesis genes [48] (Figure 2).
Under submerged conditions, ethylene contributes to promoting branch elongation, aerenchyma formation and adventitial root growth during submergence by coordinating the biosynthesis and signaling pathways of GA, ABA and IAA [126,127]. Rice SD1 protein is a key factor in the gibberellin synthesis pathway and is transcriptionally activated by ethylene transcription factor OsEIL1a, which mainly catalyzes the biosynthesis of GA and significantly stimulates internode elongation in deep-water rice under flooding conditions [128]. Flooding leads to the accumulation of ethylene in the lower part of the stem, resulting in the decrease in the ABA level in the stem and AR primordium tissues and the induced expression of the auxin polar transporter PIN2, thereby increasing the cell activity in AR primordium [127]. Under ABA treatment, the overexpression of AdPDC1 in Arabidopsis inhibited seed germination and root length [129]. Ethylene interception under waterlogging stimulates the transport of auxin to the submerged part, and the accumulation of auxin in the roots induces the division of meristem cells in AR primordium and promotes AR production [130] (Figure 2). Notably, flooded tomato plants do not produce AR after being treated with the auxin transport inhibitor NPA, which confirmed that ethylene-mediated auxin transport is necessary for AR formation [130].
Scientists from different geographical regions of the world are actively involved in the use of exogenous nutrients and plant hormones to make plants tolerant to flood stress. Exogenous plant growth regulators can alleviate waterlogging stress in many ways, including increasing leaf water content and cell viability, regulating canopy temperature and stomatal opening, and promoting root detoxification and wound healing [82,107,131,132]. An exogenous gas hydrogen sulfide (H2S) application can enhance the function of endogenous H2S in plants, and is involved in plant responses to waterlogging stress by increasing the expression of alternative oxidase genes and inducing Ca2+ influx to activate the expression of intracellular Ca2+-sensing-related genes [133,134]. Moreover, the synergistic application of plant hormones and biochar can enhance the resilience and survival ability of plants under flood conditions [135].
3.6. Energy Metabolism of Plants in Response to Waterlogging Stress
The process of energy metabolism is closely related to seed germination, and plant development. Previous studies have shown that hypoxic concentration caused by flooding changes the energy metabolism pattern, and the aerobic respiration pathways such as tricarboxylic acid cycle (TCA cycle) and oxidative phosphorylation are damaged. Anaerobic respiration dominates but only provides limited energy for seed germination and plant growth under flooding conditions, thus affecting plant growth and the reproduction process. In many submerged plants, genes involved in β-oxidation and tricarboxylic acid cycles, as well as most genes involved in sucrose to acetyl-CoA are down-regulated [136]. On the contrary, hypoxia induces up-regulation of ADH and PDC genes related to ethanol fermentation, resulting in the formation of physiologically toxic substances such as ethanol and acetaldehyde [136]. The plants’ responses to waterlogging are coordinated by regulating interconnected signal pathways in different metabolic networks. The upregulated activities of alanine aminotransferase, ubiquitin-activating enzyme E1, mitogen-activated protein kinase, and pyruvate kinase in waterlogging-resistant maize indicate that genes involved in protein degradation, signal transduction, and carbon metabolism are involved in the adaptive mechanisms under waterlogging conditions [137]. PsERF/PsCIPK genes in cherries regulate glucose metabolism to resist waterlogging stress by enhancing the activities of sucrose synthetase (SUS), α-amylase, ADH, and PDC in leaves [138]. The overexpression of MaRAP2-4 of Mentha showed enhanced flooding tolerance and oxidative stress tolerance and regulated the bidirectional sugar transporter AtSWEET10 to meet the sugar supply of transgenic plants [139].
Lipids are widely involved in regulating plant responses to hypoxia. The Arabidopsis CoA-binding protein ACBP3 is involved in plant response to hypoxia by regulating ultra-long chain fatty acids metabolism [140]. The polyunsaturated long-chain acyl-CoA regulates hypoxic perception in plants by regulating the kinetics of the acyl-CoA-binding protein-Group VII ethylene reaction factor [141]. In addition, unsaturated ultra-long chain ceramide species protect plants from hypoxia-induced cell damage by modulating the kinase activity of the assembled triple RESPONSE1 in the ethylene signaling pathway. Jasmonic acid oxylipin specifically regulates plant response to reoxygenation stress through the transcriptional regulation of the antioxidant biotin [141]. Hypoxia reduces the membrane barrier function and induces changes in lipid composition [140]. Phosphatidyl acid (PA) is an important lipid required for plant growth and development and response to biotic and abiotic stresses. In Arabidopsis, PA regulates plant tolerance to flooding by regulating membrane integrity and MPK3/6-mediated hypoxia signaling pathway [142].
3.7. Nitrogen Uptake and Utilization under Waterlogging Stress
Nitrogen is an essential nutrient for plant growth and development, and nitrogen metabolism has also been proven to help cells adapt to hypoxic stress. Under waterlogging stress, the assimilation of ammonium nitrogen in rice and soybean requires less energy than nitrate nitrogen, and the utilization efficiency of ammonium nitrogen is higher than that of nitrate nitrogen [143]. There is a 30% reduction in ammonium uptake of nitrogen-deficient roots in AtAMT1 (NH4+ translocator) Arabidopsis mutant, and the function of these genes showed additive effects [144]. Evidence show that the accumulation of NO and nitrate/nitrite plays an important and multifaceted role in plant adaptation to a hypoxic environment, such as morphological changes, hypotonia, mitochondrial structure protection, ATP production, and ROS clearance [145,146]. Nitrate fertilization increases NO accumulation and partially restores ATP levels in submerged plants, while reducing the content of reactive oxygen species and toxic products such as lactic acid and ethanol [147]. An important mechanism of plant survival in anoxic environments is the circulation of non-symbiotic hemoglobin and nitric oxide generated by nitrate supply. Nitrate is converted to nitrite by nitrate reductase (NR) to produce NO in the cytoplasm, and nitrite acts as an electron transport chain acceptor in mitochondria to promote proton pumping and ATP production under hypoxic conditions [145,148]. NO signaling by nitrite can inhibit CAT and maintain ROS levels under hypoxia conditions, on the other hand, NO signaling interacts with hypoxia-induced hemoglobin during NADH regeneration cycle to regulate ATP homeostasis [149].
NO is involved in plants response to abiotic stress in many ways, including promoting root aerenchyma formation [150], ethylene biosynthesis, mitochondrial electron transfer, antioxidant enzyme activity, antioxidant production, and regulating ROS content [145,151,152]. In Arabidopsis, changes in oxygen and nitric oxide (NO) control the stability of ERFVII transcription factors. ERFVII protein hydrolysis is regulated by the N-degron pathway and mediates adaptation to flood-induced hypoxia [116]. The application of NO donor sodium nitroprusside on leaves can increase NO concentration in plant tissues, reduce membrane lipid peroxidation and hypoxia damage caused by waterlogging, induce the expression of hormone metabolism-related genes, and reduce plant yield loss under waterlogging [153]. Exogenous spermidine application can maintain the integrity of root cells and the normal morphology and function of roots [154]. Nitrogen application changed the enzyme activity of nitrogen metabolism after alternating drought and flood and increased nitrogen accumulation in organs, as well as improving the microbial environment of rice rhizosphere soil [155]. There is evidence that the increase in rhizosphere anaerobic microorganisms under flooding may have potential negative effects on plants due to their pathogenic behavior or soil denitrification capacity [156]. At present, the positive effects of nitrogen application on flooded plant roots are well known, but the effects of the interaction between hypoxia with carbon and nitrogen metabolism are still not fully illustrated.
4. Transcription Factors in Plant Response to Waterlogging Stress
Transcription factors are crucial for gene network regulation in plants. Transcriptome analysis show that transcription factors AP2-ERF, WRKY, MYB, NAC, and basic helix-loop-helix (bHLH) are identified as key genes involved in response to flood tolerance [157] (Figure 3). So far, studies on the response of AP2/ERF transcription factors in rice and Arabidopsis to waterlogging stress are significantly comprehensive [158]. Transcription factors AvERF73 and AvERF78 in kiwifruit show transcriptional activation activity under waterlogging conditions and regulate the expression of downstream structural genes [159]. LACS2 in Arabidopsis is involved in plant hypoxia tolerance under submerged conditions by regulating the translocation of ERF-VII transcription factor from cell membrane to nucleus and the kinetics of ACBP-ERF-VII ACyl-CoA binding protein [57]. In Arabidopsis, ERF1 directly upregulates the expression of anthranilate synthase α1 (ASA1) by binding to its promoter, leading to auxin accumulation and ethylene-induced root growth inhibition [160]. In addition to promoting auxin synthesis, ERF1 also enhances auxin transport by directly up-regulating the expression of PIN1 and AUX1, leading to excessive accumulation in the endoderm, cortex, and epidermal cells around the lateral root primordium. Moreover, ERF1 inhibits the transcription of ARF7, downregulating the expression of cell wall remodeling genes that promote the emergence of lateral root (LR), inhibiting the appearance of LR [161]. The constitutive expression of the hexaploid wheat ERFVⅡ gene TaerFVI.1 enhances the tolerance to waterlogging [162].
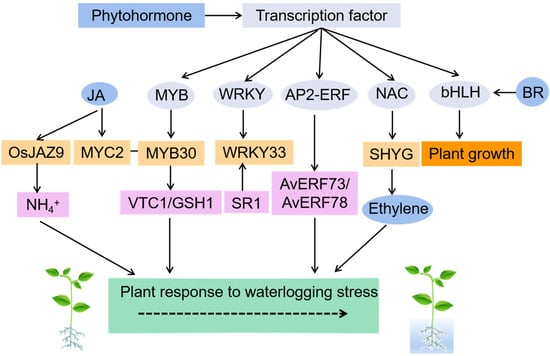
Figure 3.
Transcription factors are involved in waterlogging-resistance and are regulated by plant hormones. MYB30 interacts with MYC2 involved in JA signaling transduction and activates the expression of vitamin C deficiency 1 (VTC1) and GSH1 gene families to enhance antioxidant biosynthesis. Arabidopsis E3 ubiquitin ligase anti-submergence SR1 regulates the stability of transcription factor WRKY33 to regulate the submergence response. Transcription factors AvERF73 and AvERF78 show transcriptional activation activity under waterlogging conditions and regulate the expression of downstream structural genes. The SHYG gene combines with the promoter of the key enzyme gene ACO5 to promote ethylene biosynthesis. OsJAZ9 in the JA signaling pathway is a key transcription factor in response to low NH4+. The interaction between the basic bHLH homolog expression enhanced by BR is one of the first transcription factors identified to regulate plant growth.
Arabidopsis E3 ubiquitin ligase anti-submergence SR1 regulates the stability of transcription factor WRKY33 to regulate the submergence response [163]. Under waterlogging stress, the expression level of MdWRKY gene in apple roots is significantly up-regulated as in apple callus [164]. Previous research has shown that a single transcription factor may play different roles in several seemingly distinct signaling pathways. For example, AtWRKY6 in Arabidopsis plays a role in at least three different processes, including boron deficiency response, pathogen defense, and aging, suggesting that the same factor may mediate different pathways or regulate cross-talk between different pathways in different tissues.
The abundance of MYB domain protein 30 (MYB30) decreased during submergence and accumulated during reoxygenation. Under submergence conditions, the central regulator of light signal constitutive photomorphogenesis 1 (COP1) leads to the ubiquitination and degradation of MYB30 [165]. To response to de-flooding, light-induced MYB30 interacts with the main transcription factor MYC2 involved in jasmonic acid signaling transduction and activates the expression of VTC1 and GSH1 gene families to enhance antioxidant biosynthesis [165]. Similarly, MYB30 knockout mutants showed increased sensitivity to flooding, which is partially alleviated by the overexpression of VTC1 or GSH1 [165].
The overexpression of the NAC transcription factor SPEEDY HYPONASTIC GROWTH (SHYG) in Arabidopsis under waterlogging enhanced cell expansion on the back of petioles. Moreover, the expression of several expansion proteins encoding cell wall loosening proteins and xyloglucan transglycosylase/hydrolase genes increased, which combined with the promoter of the key enzyme gene ACC oxidase5 (ACO5) to promote ethylene biosynthesis [166].
Transcription factors are regulated by plant hormones and affect plant immunity, branch development, root development, and meristem function [167]. In a plant hormone pathway, BR signal transduction is closely related to plant growth and immunity. The interaction between the bHLH homolog expression enhanced by BR interacts with HTH IBH 1 (HBI1) and is one of the first transcription factors identified to regulate plant immunity and growth [168]. JA and flavonoids are important signaling molecules in response to low NH4+ in roots, and the gene expression required for amino acid and carbohydrate metabolism is up-regulated under low NH4+ supplementation. OsJAZ9 in the JA signaling pathway is a key transcription factor in response to low NH4+ and promotes root growth under low NH4+ conditions by regulating NH4+ absorption, sugar, and amino acid biosynthesis [169]. The overexpression of the transcription factor MYC2 of Arabidopsis enhances the tolerance to post-hypoxic stress and serves as a key regulator in JA-mediated reoxygenation response, alleviating oxidative damage caused by reoxygenation [170].
5. Stress Memory and Cross-Stress in Plants
5.1. Waterlogging Stress Memory in Plants
Prime is considered to be a promising strategy for crop production to cope with future climate change. Plants can generate “stress memory” by pre-exposing to predisposing factors, and respond to biotic or abiotic stress events by combining antioxidant protection, osmotic regulation, and plant hormone responses [171,172,173]. Recent advances in elucidating the mechanistic basis of plant stress memory provide new opportunities for crop improvement. In plants, stress memory seems to be involved in epigenetic chromatin modification, such as DNA methylation, histone modification, chromatin remodeling, plant hormones, and microRNA [174,175,176,177]. Moreover, plants have stress memories of transcriptomic, proteomic, and metabolomic changes, enabling enhanced responses to additional stress exposure [176]. Waterlogged-activated plants show higher enzyme activity in the ascorbic acid glutathione cycle, and the expression levels of energy metabolism and stress defense-related proteins are significantly higher than those of non-waterlogged activated plants [178]. Meanwhile, the abundance of biosynthetic protein methionine synthetase and S-adenosyl methionine synthetase are significantly increased in ethylene activated plants, which alleviated the negative effects of waterlogging on leaf photosynthsis by increasing stomatal conductance [131].
Stress prime in plants also presents the potential of transgenerational adaptation. When plants undergo stress priming, their progeny will perform better under stress [179,180]. This transgenerational stress memory is mediated by the up-regulation of antioxidant systems [181,182], biochemical modifications, and changes in hormonal pathways [176]. The determination of the stage and degree of priming effect is crucial, for example, pre-flowering initiation of waterlogging stress can effectively mitigate the negative effects of post-flowering waterlogging stress on wheat yield by increasing the activities of antioxidant enzymes (SOD, APX, CAT) [178]. The treatment of maize seedlings with ethylene before waterlogging stress can trigger a programmed cell death process and induce stomatal formation, thus improving maize tolerance to subsequent waterlogging stress [183]. Plants can improve their defense systems by retaining and maintaining stress memory, making stronger or faster responses to repeated stress situations. Therefore, elucidating epigenetic marks, clarifying the stress prime method and the application stage and degree, as well as managing the balance of stress optimization between mother and offspring to develop stress memory will be the future research focus of plant breeding.
5.2. Effect of Temperature on Waterlogging
The environment in which plants grow is dynamically changing with the simultaneous occurrence of many stress events during the entire plant life cycle. It has been clear that plants have cross-tolerance to environmental abiotic stresses (drought, flood, high temperature, cold, salinity, etc.) [184,185]. To cope with cross-stress, plants use cross-tolerance genes when stress occurs, which closely relate to hormones, photosynthesis, antioxidant system, and transcription factors [184,186,187]. The global average temperature is rising year by year under the backdrop of rapid global climate change. Heavy rainfall and chronic high temperature occurrences from May to October every year in the Yangtze River Basin in China, and the winter snowmelt in the Northern Hemisphere cause temporary flooding of crops. In the actual production process, the growth of many plants often suffers from chronic waterlogging and simultaneous stress of high/low temperatures, which can significantly affect crop production [11,188].
Studies have shown that transient soil waterlogging promotes photosynthesis under chronic high-temperature stress, changing leaf growth and promoting sucrose accumulation [189]. The enhancement of photosynthesis by moderate waterlogging may be related to changes in the antioxidant defense system of the affiliated leaves, including decreased MDA content, increased glutathione reductase (GR) and ascorbic acid (ASA) concentrations in leaves, thus limiting the accumulation of H2O2, O2− and membrane lipid peroxidation [190]. However, another study shows that the stress of high temperature and waterlogging may have a synergistic effect, leading to the reduction in plant stem development and total biomass which cause more severe yield loss than single stress [105]. The combination of high temperature and waterlogging limits the synthesis of primary cell wall by regulating the GhCESAs gene, and changes fiber sucrose content by regulating the sucrose transporter gene GhSUT-1, thus shortening cotton fiber length [191].
Plants have different modes to cope with high/low temperature flood stress with various regulatory mechanisms in terms of photosynthetic efficiency and carbohydrate allocation. The carbohydrate accumulation in leaves decreases at low temperature and increases at high temperature, while that is opposite in the crown [192]. High temperature results in higher enzyme activity and is more harmful to plants than low temperature [192]. Low temperature reduces the photoinhibition damage of flooded plants, and the activation of non-photochemical mechanism genes of photosynthetic adaptation to cold during low temperature floods may lead to higher frost resistance in plants. In contrast, the decrease in frost resistance in some low-temperature flooding genotypes may be related to decreased photochemical activity and photoinhibition of photosynthesis [193,194]. In addition, over 40 target genes are activated by cold stress, as among which transcription factors in the CBF family have been identified in Arabidopsis [195,196]. Under low temperature and flood conditions, changes in the induction kinetics of transcription factors encoding cold regulatory genes are related to transient freezing tolerance of plants, and the expression of freezing tolerance gene CBF6 may trigger the expression of downstream genes to cause changes in circadian rhythm [194].
6. Challenges and Prospects
Breeding new varieties is the most direct and effective way to improve plant waterlogging tolerance. Plants with water-resistant genotypes can maintain CO2 assimilation rate, stomatal conductance, biomass accumulation, chlorophyll content, and Rubisco activity under inundation stress [197]. Due to the complexity of waterlogging tolerance factors and differences in growth stages, further expanding the research on flood tolerance in different growth periods is necessary. In addition, combining physiology, ecology, and breeding methods to explore the genetic differences under complete and partial waterlogging of filtrate and cultivate flood tolerance crops is important to synergistically improve the flood tolerance, yield, quality, and other valuable traits of target crops [198]. Compared with traditional breeding methods, the use of QTL markers to assist backcross can be incorporated into the breeding of many excellent varieties in a short period. Despite some progress being made in the flood tolerance of crops, practical methods for combining genome-wide association studies (GWA) and QTL with specific waterlogging tolerance traits need to be explored in future studies [16].
Hypoxia is one of the main challenges for flooded plants. A series of processes caused by hypoxia, such as decreased plant metabolism, energy production, photosynthesis, and ROS disorder, are several major factors contributing to plant growth restriction and yield reduction. Wetland plants have stronger tolerance to hypoxia. By studying the morphology characteristics, physiological changes, and related gene expression of wild wetland plants, we can obtain abundant anaerobic biodiversity data and provide a new gene pool for improving the waterlogging tolerance of economic plants [199]. The survival ability of wetland plants in water and their ability to ensure safe seed production is beneficial to agricultural practices and economic value [33]. The isolation and identification of hypoxic responsive genes from wetland wild-type plants will be a research hotspot to explore waterlogging tolerance mechanisms in the future. Transcriptional reprogramming and expression changes of transcription factors and cooperative regulation of plant hormones under flooding conditions are also noteworthy.
Cross stress is common in the actual growing environment of crops, and waterlogging often occurs accompanied by high or low temperature stress at the same time. Therefore, it is necessary to consider the influence of different temperatures on waterlogging, involving the expression of cross-tolerance genes related to hormones, photosynthesis, antioxidant system, and transcription factors [184,186,187]. The adaptation mechanisms of diverse crop species under flood stress are quite different. Therefore, it is necessary to conduct a comprehensive and systematic study on the basic knowledge of morphological, physiology, and biochemistry characteristics in various crops under waterlogging stress and to lay a foundation for elucidating the molecular mechanism of crop waterlogging tolerance.
Soil tillage affects the response of plants to waterlogging stress. Compared with flat tillage, ridge tillage is conducive to the reproduction and growth of surface soil microorganisms, thus improving soil vitality, water retention, and water use efficiency [200,201]. Wet seeding at the beginning of the rainy season allows for more timely and rapid establishment of crop early growth [202]. Similar to the idea of the ridge-ditch system, raised bed planting is often used to reduce waterlogging damage when planting wheat after a rice harvest in the Yangtze River Plain of China [203]. Raised bed planting can accelerate the development of stem tillers and delay the aging of roots and leaves, and significantly increase crop yield [204]. Therefore, improving cultivation or field management measures are promising approaches to combat abiotic stress. Meanwhile, exogenous nutrients and plant hormones application to make plants withstand flood stress is a widely used method by scientists around the world to improve crop flood tolerance [107,131]. Moreover, using a machine learning algorithm to establish a crop waterlogging tolerance model and an LoT monitoring system to obtain timely crop growth information data under flooded conditions are auxiliary methods for the future development of smart agriculture [205,206]. For the waterlogged soil, the government should strengthen the protection and utilization of submerged arable land, implement the policy of replanting, give interest discounts to loans for replanting and give incentives to cultivated land that reach a certain area to promote agricultural development. Guansheng Town took four measures to rectify the submerged land, changing small fields into large fields through the construction of production roads thus improving agricultural production conditions.
Previous studies have shown that the synergistic regulation of waterlogging tolerance traits is important and increasingly promising. Effective integrated strategies for waterlogging tolerance can be established through a detailed understanding of the interactions and regulation of different pathways. For example, ethylene in plants induces a morphological adaptation to flooding and actively regulates ROS levels in plants [111,112]. Accordingly, it is important to enhance plant tolerance under waterlogging stress conditions using transgenic approaches. Relevant molecular biology studies should be strengthened to isolate and identify the key enzyme genes, enlist crops which have a genetic resistance to waterlogging to realize large areas of cultivation (Table 1). In conclusion, the perfect unity of crop quality, yield, and flood tolerance can be achieved by combining traditional and molecular breeding, expanding and strengthening the identification and utilization of germplasm resources, and using more advanced sequencing and plant transformation technologies, thus providing a new research strategy and theoretical basis for ensuring national food security.

Table 1.
Recent advances in transgenic approaches to enhance plant tolerance under waterlogging stress conditions.
Author Contributions
Conceptualization, writing—original draft preparation, project administration L.Y. and N.L. Methodology, formal analysis, Y.L. investigation, visualization, software, P.M. Supervision, resources, funding acquisition, J.L. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Nanfan special project, CAAS (YBXM2310, YBXM07 and YBXM09), the Hainan Yazhou Bay Seed Lab (B23CJ0208), and National Natural Science Foundation of China (32072022).
Data Availability Statement
All data analysed during this study are included in this published article.
Acknowledgments
We appreciate Zhen Jiao for providing administrative and technical support. We also thank the staff of National Nanfan Research Institute, Chinese Academy of Agricultural Sciences for assisting with various aspects of the field work.
Conflicts of Interest
The authors declared no potential conflict of interest concerning the research, authorship, and/or publication of this article.
Abbreviations
| AR | Adventitial roots |
| ROS | Reactive oxygen species |
| ROL | Radial oxygen loss |
| BR | Brassinolide |
| TCA | Tricarboxylic acid |
| ADH | Ethanol dehydrogenase |
| PCD | Programmed cell death |
| PDC | Pyruvate decarboxylase |
| ERF-VII | Ethylene response factor VII |
| QTL | Quantitative trait loci |
| XET | Xyloglucan endotransglucosylase |
| LDH | Lactate dehydrogenase |
| ADH | Ethanol dehydrogenase |
| QTL | Quantitative trait loci |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| CAT | Catalase |
| GSH | Glutathione |
| GB | Glycine betaine |
| GABA | Gamma-aminobutyric acid |
| LACS2 | Long-chain acyl-CoA synthetase |
| PUB44 | PLANT U-BOX44 |
| CPK4 | Calc-dependent protein kinase |
| HvPgb1 | Phytoglobin 1 in Hordeum vulgare |
| mETC | Mitochondrial electron transport chain |
| SIRK | Senescence-induced receptor-like protein kinase |
| OsPIL1 | Photopigment-interacting factor-like protein |
| Chl | Chlorophyll |
| PEP | Phosphoenolpyruvate |
| LGF1 | Leaf gas film 1 |
| PCO | Cysteine oxidase |
| PGB1 | PHYTOGLOBIN1 |
| IAA | Indole acetic acid |
| ABA | Abscisic acid |
| GA | Gibberellin |
| SLR1 | SLENDER rice-1 |
| SLRL1 | SLR1-like-1 |
| SD1 | SEMIDWARF1 |
| H2S | Hydrogen sulfide |
| SUS | Sucrose synthetase |
| PA | Phosphatidyl acid |
| NR | Nitrate reductase |
| NO | Nitric oxide |
| ASA | Ascorbic acid |
| COP1 | Constitutive photomorphogenesis 1 |
| VTC1 | vitamin C deficiency 1 |
| GSH1 | Glutathione synthase 1 |
| SHYG | SPEEDY HYPONASTIC GROWTH |
| ACO5 | ACC oxidase5 |
| bHLH | Basic helix-loop-helix |
| HBI1 | HTH IBH 1 |
| GR | Glutathione reductase |
| GWA | Genome-wide association |
References
- Sharkey, T.D. The Physiology of Plants under Stress: Abiotic Factors; Nilsen, E.T., Orcutt, D.M., Eds.; The Quarterly Review of Biology; The University of Chicago Press: Chicago, IL, USA, 1996; Volume 72, pp. 476–477. ISBN 0-471-03512-6. [Google Scholar]
- Choudhary, P.; Muthamilarasan, M. Modulating physiological and transcriptional regulatory mechanisms for enhanced climate resilience in cereal crops. J. Plant Physiol. 2022, 278, 153815. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wang, S.; Yang, P.; Tao, Y. Experimental and modeling evaluation of siphon-type subsurface drainage performance in flooding and waterlogging removal. Agric. Water Manag. 2023, 275, 108031. [Google Scholar] [CrossRef]
- Xu, X.; Ji, J.; Xu, Q.; Qi, X.; Weng, Y.; Chen, X. The major-effect quantitative trait locus CsARN6.1 encodes an AAA ATPase domain-containing protein that is associated with waterlogging stress tolerance by promoting adventitious root formation. Plant J. 2018, 93, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Pais, I.P.; Moreira, R.; Semedo, J.N.; Ramalho, J.C.; Lidon, F.C.; Coutinho, J.; Macas, B.; Scotti-Campos, P. Wheat crop under waterlogging: Potential soil and plant effects. Plants 2022, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, M.; Sivaraju, K.; Krishnamurthy, V. Effect of waterlogging on physiological characteristics, yield and quality of flue-cured tobacco. Indian J. Plant Physiol. 2013, 18, 67–70. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Fukao, T.; Gibbs, D.J.; Holdsworth, M.J.; Lee, S.C.; Licausi, F.; Perata, P.; Voesenek, L.A.; Van Dongen, J.T. Making sense of low oxygen sensing. Trends Plant Sci. 2012, 17, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, U.; Bange, M.P.; Tan, D.K.Y.; Atwell, B.J. Consequences of waterlogging in cotton and opportunities for mitigation of yield losses. AoB Plants 2015, 7, plv080. [Google Scholar] [CrossRef] [PubMed]
- De San Celedonio, R.P.; Abeledo, L.G.; Miralles, D.J. Physiological traits associated with reductions in grain number in wheat and barley under waterlogging. Plant Soil 2018, 429, 469–481. [Google Scholar] [CrossRef]
- Zhen, B.; Li, H.; Niu, Q.; Qiu, H.; Tian, G.; Lu, H.; Zhou, X. Effects of combined high temperature and waterlogging stress at booting stage on root anatomy of rice (Oryza sativa L.). Water 2020, 12, 2524. [Google Scholar] [CrossRef]
- Shao, J.; Liu, P.; Zhao, B.; Zhang, J.; Zhao, X.; Ren, B. Combined effects of high temperature and waterlogging on yield and stem development of summer maize. Crop J. 2022, 11, 651–660. [Google Scholar] [CrossRef]
- Ren, B.; Yu, W.; Liu, P.; Zhao, B.; Zhang, J. Responses of photosynthetic characteristics and leaf senescence in summer maize to simultaneous stresses of waterlogging and shading. Crop J. 2023, 11, 269–277. [Google Scholar] [CrossRef]
- Song, M.; Li, X.; Saikkonen, K.; Li, C.; Nan, Z. An asexual epichloë endophyte enhances waterlogging tolerance of Hordeum brevisubulatum. Fungal Ecol. 2015, 13, 44–52. [Google Scholar] [CrossRef]
- Ashraf, M.A. Waterlogging stress in plants: A review. Afr. J. Agric. Res. 2012, 7, 1976–1981. [Google Scholar] [CrossRef]
- Xu, K.; Xu, X.; Fukao, T.; Canlas, P.; Maghirang-Rodriguez, R.; Heuer, S.; Ismail, A.M.; Bailey-Serres, J.; Ronald, P.C.; Mackill, D.J. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 2006, 442, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Oladosu, Y.; Rafii, M.Y.; Arolu, F.; Chukwu, S.C.; Muhammad, I.; Kareem, I.; Salisu, M.A.; Arolu, I.W. Submergence tolerance in rice: Review of mechanism, breeding, and future prospects. Sustainability 2020, 12, 1632. [Google Scholar] [CrossRef]
- Vidoz, M.L.; Mignolli, F.; Aispuru, H.T.; Mroginski, L.A. Rapid formation of adventitious roots and partial ethylene sensitivity result in faster adaptation to flooding in the aerial roots (aer) mutant of tomato. Sci. Hortic. 2016, 201, 130–139. [Google Scholar] [CrossRef]
- Qian, L.; Chen, X.; Wang, X.; Huang, S.; Luo, Y. The effects of flood, drought, and flood followed by drought on yield in cotton. Agronomy 2020, 10, 555. [Google Scholar] [CrossRef]
- Negi, S.; Ivanchenko, M.G.; Muday, G.K. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J. 2008, 55, 175–187. [Google Scholar] [CrossRef]
- Sasidharan, R.; Voesenek, L.A. Ethylene-mediated acclimations to flooding stress. Plant Physiol. 2015, 169, 3–12. [Google Scholar] [CrossRef]
- Steffens, B.; Rasmussen, A. The physiology of adventitious roots. Plant Physiol. 2016, 170, 603–617. [Google Scholar] [CrossRef]
- Mergemann, H.; Sauter, M. Ethylene induces epidermal cell death at the site of adventitious root emergence in rice. Plant Physiol. 2000, 124, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, Q.; Ma, X.; Qian, C.; Wang, H.; Ren, N.; Shen, C.; Huang, S.; Xu, X.; Xu, Q.; et al. Waterlogging-induced adventitious root formation in cucumber is regulated by ethylene and auxin through reactive oxygen species signalling. Plant Cell Environ. 2019, 42, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Che, Q.; Su, S.; Liu, Y.; Wang, Y.; Xu, X. Genome-wide identification and characterization of Respiratory Burst Oxidase Homolog genes in six Rosaceae species and an analysis of their effects on adventitious rooting in apple. PLoS ONE 2020, 15, e0239705. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Liang, K.; Fang, T.; Zhao, H.; Han, X.; Cai, M.; Qiu, F. A group VII ethylene response factor gene, ZmEREB180, coordinates waterlogging tolerance in maize seedlings. Plant Biotechnol. J. 2019, 17, 2286–2298. [Google Scholar] [CrossRef] [PubMed]
- Eysholdt-Derzso, E.; Sauter, M. Hypoxia and the group VII ethylene response transcription factor HRE2 promote adventitious root elongation in Arabidopsis. Plant Biol. 2019, 21 (Suppl. S1), 103–108. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, S.; Yoshioka, T.; Yamamoto, R.; Hiraga, S.; Nakamura, T.; Shimada, S.; Komatsu, S. Role of abscisic acid in flood-induced secondary aerenchyma formation in soybean (Glycine max) hypocotyls. Plant Prod. Sci. 2014, 17, 131–137. [Google Scholar] [CrossRef]
- Kim, Y.H.; Hwang, S.J.; Waqas, M.; Khan, A.L.; Lee, J.H.; Lee, J.D.; Nguyen, H.T.; Lee, I.J. Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Front. Plant Sci. 2015, 6, 714. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.H.; Guo, S.R. 24-epibrassinolide improves cucumber photosynthesis under hypoxia by increasing CO2 assimilation and photosystem II efficiency. Photosynthetica 2014, 52, 96–104. [Google Scholar] [CrossRef]
- Trewavas, A.J.; Malho, R. Ca2+ signalling in plant cells: The big network. Curr. Opin. Plant Biol. 1998, 1, 428–433. [Google Scholar] [CrossRef]
- Lai, W.-L.; Zhang, Y.; Chen, Z.-H. Radial oxygen loss, photosynthesis, and nutrient removal of 35 wetland plants. Ecol. Eng. 2012, 39, 24–30. [Google Scholar] [CrossRef]
- Colmer, T.D. Long-distance transport of gases in plants: A perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ. 2003, 26, 17–36. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Shen, Q.F.; Zhang, G.P. The mechanisms for the difference in waterlogging tolerance among sea barley, wheat and barley. Plant Growth Regul. 2022, 96, 431–441. [Google Scholar] [CrossRef]
- Liang, K.; Tang, K.; Fang, T.; Qiu, F. Waterlogging tolerance in maize: Genetic and molecular basis. Mol. Breed. 2020, 40, 111. [Google Scholar] [CrossRef]
- Matsukura, C.; Kawai, M.; Toyofuku, K.; Barrero, R.A.; Yamaguchi, J. Transverse vein differentiation associated with gas space formation—Fate of the middle cell layer in leaf sheath development of rice. Ann. Bot. 2000, 85, 19–27. [Google Scholar] [CrossRef]
- Shimamura, S.; Mochizuki, T.; Nada, Y.; Fukuyama, M. Formation and function of secondary aerenchyma in hypocotyl, roots and nodules of soybean (Glycine max) under flooded conditions. Plant Soil 2003, 251, 351–359. [Google Scholar] [CrossRef]
- Sundgren, T.; Uhlen, A.; Waalen, W.; Lillemo, M. Field Screening of Waterlogging Tolerance in Spring Wheat and Spring Barley. Agronomy 2018, 8, 38. [Google Scholar] [CrossRef]
- Seago, J.L., Jr.; Marsh, L.C.; Stevens, K.J.; Soukup, A.; Votrubova, O.; Enstone, D.E. A re-examination of the root cortex in wetland flowering plants with respect to aerenchyma. Ann. Bot. 2005, 96, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, D.; Lin, Z.; Guan, B.; Liu, D.; Yang, L.; Deng, X.; Mei, F.; Zhou, Z. Histone acetylation modification affects cell wall degradation and aerenchyma formation in wheat seminal roots under waterlogging. Plant Growth Regul. 2019, 87, 149–163. [Google Scholar] [CrossRef]
- Sakagami, J.; Joho, Y.; Ito, O. Contrasting physiological responses by cultivars of Oryza sativa and O. glaberrima to prolonged submergence. Ann. Bot. 2009, 103, 171–180. [Google Scholar] [CrossRef]
- Matthews, G. Rice in Deep Water. Crop Prot. 1994, 13, 316–317. [Google Scholar] [CrossRef]
- Colmer, T.D. Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Ann. Bot. 2003, 91, 301–309. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sun, S.; Zhao, J.; Huang, Z.; Peng, L.; Huang, C.; Tang, Z.; Huang, Q.; Wang, Z. UDP-glucosyltransferase OsUGT75A promotes submergence tolerance during rice seed germination. Nat. Commun. 2023, 14, 2296. [Google Scholar] [CrossRef] [PubMed]
- Pantin, F.; Simonneau, T.; Muller, B. Coming of leaf age: Control of growth by hydraulics and metabolics during leaf ontogeny. New Phytol. 2012, 196, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Visser, E.J.; De Kroon, H.; Pierik, R.; Voesenek, L.A.; Huber, H. Fitness consequences of natural variation in flooding-induced shoot elongation in Rumex palustris. New Phytol. 2011, 190, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Pierik, R.; Van Aken, J.M.; Voesenek, L.A.C.J. Is elongation-induced leaf emergence beneficial for submerged Rumex species? Ann. Bot. 2009, 103, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Colmer, T.D. Plant tolerance of flooding stress—Recent advances. Plant Cell Environ. 2014, 37, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Nagai, K.; Furukawa, S.; Song, X.J.; Kawano, R.; Sakakibara, H.; Wu, J.; Matsumoto, T.; Yoshimura, A.; Kitano, H.; et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 2009, 460, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, H.; Akman, M.; Jamar, D.C.; Vreugdenhil, D.; Kooiker, M.; Van Tienderen, P.; Voesenek, L.A.; Schranz, M.E.; Sasidharan, R. Group VII ethylene response factor diversification and regulation in four species from flood-prone environments. Plant Cell Environ. 2014, 37, 2421–2432. [Google Scholar] [CrossRef]
- Tougou, M.; Hashiguchi, A.; Yukawa, K.; Nanjo, Y.; Hiraga, S.; Nakamura, T.; Nishizawa, K.; Komatsu, S. Responses to flooding stress in soybean seedlings with the alcohol dehydrogenase transgene. Plant Biotechnol. 2012, 29, 301–305. [Google Scholar] [CrossRef]
- Xuewen, X.; Huihui, W.; Xiaohua, Q.; Qiang, X.; Xuehao, C. Waterlogging-induced increase in fermentation and related gene expression in the root of cucumber (Cucumis sativus L.). Sci. Hortic. 2014, 179, 388–395. [Google Scholar] [CrossRef]
- Pan, D.L.; Wang, G.; Wang, T.; Jia, Z.H.; Guo, Z.R.; Zhang, J.Y. AdRAP2.3, a novel ethylene response factor VII from Actinidia deliciosa, enhances waterlogging resistance in transgenic tobacco through improving expression levels of PDC and ADH genes. Int. J. Mol. Sci. 2019, 20, 1189. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.J.; Harrison-Murray, R.S.; Taylor, J.M. Rapid flood-induced stomatal closure accompanies xylem sap transportation of root-derived acetaldehyde and ethanol in Forsythia. Environ. Exp. Bot. 2008, 64, 196–205. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, F.; Meng, Y.; Chandrasekaran, U.; Luo, X.; Yang, W.; Shu, K. Plant waterlogging/flooding stress responses: From seed germination to maturation. Plant Physiol. Biochem. 2020, 148, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Ning, D.F.; Zheng, H.J.; Liu, Z.D. Effects of waterlogging at different stages on growth and ear quality of waxy maize. Agric. Water Manag. 2022, 266, 107603. [Google Scholar] [CrossRef]
- Chang, W.W.; Huang, L.; Shen, M.; Webster, C.; Burlingame, A.L.; Roberts, J.K. Patterns of protein synthesis and tolerance of anoxia in root tips of maize seedlings acclimated to a low-oxygen environment, and identification of proteins by mass spectrometry. Plant Physiol. 2000, 122, 295–317. [Google Scholar] [CrossRef]
- Zhou, Y.; Tan, W.J.; Xie, L.J.; Qi, H.; Yang, Y.C.; Huang, L.P.; Lai, Y.X.; Tan, Y.F.; Zhou, D.M.; Yu, L.J.; et al. Polyunsaturated linolenoyl-CoA modulates ERF-VII-mediated hypoxia signaling in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 330–348. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Liao, K.; Wang, L.N.; Shi, L.L.; Zhang, Y.; Xu, L.J.; Zhou, Y.; Li, J.F.; Chen, Y.Q.; Chen, Q.F.; et al. Calcium-dependent activation of CPK12 facilitates its cytoplasm-to-nucleus translocation to potentiate plant hypoxia sensing by phosphorylating ERF-VII transcription factors. Mol. Plant 2023, 16, 979–998. [Google Scholar] [CrossRef] [PubMed]
- Whan, A.; Dielen, A.-S.; Mieog, J.; Bowerman, A.F.; Robinson, H.M.; Byrne, K.; Colgrave, M.; Larkin, P.J.; Howitt, C.A.; Morell, M.K.; et al. Engineering alpha-amylase levels in wheat grain suggests a highly sophisticated level of carbohydrate regulation during development. J. Exp. Bot. 2014, 65, 5443–5457. [Google Scholar] [CrossRef]
- Park, M.; Yim, H.K.; Park, H.G.; Lim, J.; Kim, S.H.; Hwang, Y.S. Interference with oxidative phosphorylation enhances anoxic expression of rice alpha-amylase genes through abolishing sugar regulation. J. Exp. Bot. 2010, 61, 3235–3244. [Google Scholar] [CrossRef]
- Lee, K.W.; Chen, P.W.; Lu, C.A.; Chen, S.; Ho, T.H.D.; Yu, S.M. Coordinated responses to oxygen and sugar deficiency allow rice seedlings to tolerate flooding. Plant Biol. 2009, 2, 61. [Google Scholar] [CrossRef]
- Kretzschmar, T.; Pelayo, M.A.; Trijatmiko, K.R.; Gabunada, L.F.; Alam, R.; Jimenez, R.; Mendioro, M.S.; Slamet-Loedin, I.H.; Sreenivasulu, N.; Bailey-Serres, J.; et al. A trehalose-6-phosphate phosphatase enhances anaerobic germination tolerance in rice. Nat. Plants 2015, 1, 15124. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, D.; Suh, M.C. Cuticle ultrastructure, cuticular lipid composition, and gene expression in hypoxia-stressed Arabidopsis stems and leaves. Plant Cell Rep. 2017, 36, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.J.; Tan, W.J.; Yang, Y.C.; Tan, Y.F.; Zhou, Y.; Zhou, D.M.; Xiao, S.; Chen, Q.F. Long-Chain acyl-CoA Synthetase LACS2 Contributes to Submergence Tolerance by Modulating Cuticle Permeability in Arabidopsis. Plants 2020, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shabala, L.; Zhang, X.; Zhou, M.; Voesenek, L.A.C.J.; Hartman, S.; Yu, M.; Shabala, S. Cation transporters in cell fate determination and plant adaptive responses to a low-oxygen environment. J. Exp. Bot. 2022, 73, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Phukan, U.J.; Mishra, S.; Shukla, R.K. Waterlogging and submergence stress: Affects and acclimation. Crit. Rev. Biotechnol. 2016, 36, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qiao, X.; Tian, Z.; Zhang, X.; Zou, X.; Cheng, Y.; Lu, G.; Zeng, L.; Fu, G.; Ding, X.; et al. Proteomic analysis of rapeseed root response to waterlogging stress. Plants 2018, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Barickman, T.C.; Simpson, C.R.; Sams, C.E. Waterlogging causes early modification in the physiological performance, carotenoids, chlorophylls, proline, and soluble sugars of cucumber plants. Plants 2019, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2021, 24, 227–239. [Google Scholar] [CrossRef]
- Yang, H.; Huang, T.; Ding, M.; Lu, D.; Lu, W. Effects of waterlogging around flowering stage on the grain yield and eating properties of fresh waxy maize. Cereal Chem. 2016, 93, 605–611. [Google Scholar] [CrossRef]
- Habibullah, M.; Sarkar, S.; Islam, M.M.; Ahmed, K.U.; Rahman, M.Z.; Awad, M.F.; Elsayed, A.I.; Mansour, E.; Hossain, M.S. Assessing the response of diverse sesame genotypes to waterlogging durations at different plant growth stages. Plants 2021, 10, 2294. [Google Scholar] [CrossRef]
- Fan, W.; Liao, X.L.; Tan, Y.Q.; Wang, X.R.; Schroeder, J.; Li, Z.X. Arabidopsis PLANT U-BOX44 down-regulates osmotic stress signaling by mediating Ca2+-DEPENDENT PROTEIN KINASE4 degradation. Plant Cell 2023, 35, 3870–3888. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Li, X.L.; Xie, X.; Liu, L.J.; Fu, J.Y.; Wang, Q. Maize transcription factor ZmNAC2 enhances osmotic stress tolerance in transgenic Arabidopsis. J. Plant Physiol. 2023, 282, 153948. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.A.D.; Oliveira, L.E.M.D.; Domiciano, D.; Carvalho, J.N.D.; Prudente, D.D.O.; Guimarães, R.J. Effect of nitrogen source and oxygen deficiency on carbon metabolism and antioxidant system of rubber tree plants (Hevea spp.). Aust. J. Crop Sci. 2018, 12, 116–125. [Google Scholar] [CrossRef]
- Basu, S.; Kumar, G.; Kumari, N.; Kumari, S.; Shekhar, S.; Kumar, S.; Rajwanshi, R. Reactive oxygen species and reactive nitrogen species induce lysigenous aerenchyma formation through programmed cell death in rice roots under submergence. Environ. Exp. Bot. 2020, 177, 104118. [Google Scholar] [CrossRef]
- Dantas, B.; Silva, R.; Ribeiro, R.; Aragão, C. Respiration and antioxidant enzymes activity in watermelon seeds and seedlings subjected to salt and temperature stresses. Am. J. Exp. Agric. 2015, 7, 70–77. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Ahmed, A.R.; Farris, F.F.; Ray, S.D. Lipid peroxidation. Biomed. Sci. 2023. [Google Scholar]
- Hodges, D.M.; Andrews, C.J.; Johnson, D.A.; Hamilton, R.I. Antioxidant enzyme responses to chilling stress in differentially sensitive inbred maize lines. J. Exp. Bot. 1997, 48, 1105–1113. [Google Scholar] [CrossRef]
- Ekmekçi, Y.; Tanyolaç, D.; Ayhan, B. Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. J. Plant Physiol. 2008, 165, 600–611. [Google Scholar] [CrossRef]
- Abedi, T.; Pakniyat, H. Antioxidant enzymes changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus L.). Czech J. Genet. Plant Breed. 2010, 46, 27–34. [Google Scholar] [CrossRef]
- Li, M.-F.; Zhu, J.-Q.; Jiang, Z.-H. Plant growth regulators and nutrition applied to cotton after waterlogging. In Proceedings of the Third International Conference on Intelligent System Design and Engineering Applications, Hong Kong, China, 16–18 January 2013; pp. 1045–1048. [Google Scholar] [CrossRef]
- Das, K.K.; Panda, D.; Sarkar, R.K.; Reddy, J.N.; Ismail, A.M. Submergence tolerance in relation to variable floodwater conditions in rice. Environ. Exp. Bot. 2009, 66, 425–434. [Google Scholar] [CrossRef]
- Rich, S.M.; Ludwig, M.; Colmer, T.D. Photosynthesis in aquatic adventitious roots of the halophytic stem-succulent Tecticornia pergranulata (formerly Halosarcia pergranulata). Plant Cell Environ. 2008, 31, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Fu, S.; Chen, S.; Zhang, W.; Qi, C. Ethylene response factor BnERF2-like (ERF2.4) from Brassica napus L. enhances submergence tolerance and alleviates oxidative damage caused by submergence in Arabidopsis thaliana. Crop J. 2016, 4, 199–211. [Google Scholar] [CrossRef]
- Hill, R.D.; De Castro, J.; Mira, M.M.; Igamberdiev, A.U.; Hebelstrup, K.H.; Renault, S.; Xu, W.; Badea, A.; Stasolla, C. Over-expression of the barley Phytoglobin 1 (HvPgb1) evokes leaf-specific transcriptional responses during root waterlogging. J. Plant Physiol. 2023, 283, 153944. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, J.; Zhang, Z.; Liu, W.; Cheng, P.; Mu, W.; Su, T.; Chen, S.; Chen, F.; Jiang, J. Overexpression of CmSOS1 confers waterlogging tolerance in Chrysanthemum. J. Integr. Plant Biol. 2020, 62, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- An, Y.Y.; Lin, Q.; Wang, L.J. ALA pretreatment improves waterlogging tolerance of fig plants. PLoS ONE 2016, 11, e0147202. [Google Scholar] [CrossRef] [PubMed]
- Keya, S.S.; Mostofa, M.G.; Mezanur Rahman, M.; Das, A.K.; Abiar Rahman, M.; Anik, T.R.; Sultana, S.; Arifur Rahman Khan, M.; Robyul Islam, M.; Watanabe, Y.; et al. Effects of glutathione on waterlogging-induced damage in sesame crop. Ind. Crops Prod. 2022, 185, 115092. [Google Scholar] [CrossRef]
- Rasheed, R.; Iqbal, M.; Ashraf, M.A.; Hussain, I.; Shafiq, F.; Yousaf, A.; Zaheer, A. Glycine betaine counteracts the inhibitory effects of waterlogging on growth, photosynthetic pigments, oxidative defence system, nutrient composition, and fruit quality in tomato. J. Hortic. Sci. Biotechnol. 2017, 93, 385–391. [Google Scholar] [CrossRef]
- Salah, A.; Zhan, M.; Cao, C.; Han, Y.; Ling, L.; Liu, Z.; Li, P.; Ye, M.; Jiang, Y. γ-Aminobutyric acid promotes chloroplast ultrastructure, antioxidant capacity, and growth of waterlogged maize seedlings. Sci. Rep. 2019, 9, 484. [Google Scholar] [CrossRef]
- Chang, R.; Jang, C.J.; Branco-Price, C.; Nghiem, P.; Bailey-Serres, J. Transient MPK6 activation in response to oxygen deprivation and reoxygenation is mediated by mitochondria and aids seedling survival in Arabidopsis. Plant Mol. Biol. 2012, 78, 109–122. [Google Scholar] [CrossRef]
- Wan, D. The Study of Calcium Signal Transduction Induced by Oxidative Stress (H2O2). Master’s Thesis, Hangzhou Normal University, Hangzhou, China, 2018. [Google Scholar]
- Bansal, R.; Srivastava, J.P. Effect of waterlogging on photosynthetic and biochemical parameters in pigeonpea. Russ. J. Plant Physiol. 2015, 62, 322–327. [Google Scholar] [CrossRef]
- Gravarr, D.A.; Kirby, C.J. Patterns of photosynthesis and starch allocation in seedlings of four bottomland hardwood tree species subjected to flooding. Tree Physiol. 1998, 18, 411–417. [Google Scholar] [CrossRef]
- Sairam, R.K.; Dharmar, K.; Chinnusamy, V.; Meena, R.C. Waterlogging-induced increase in sugar mobilization, fermentation, and related gene expression in the roots of mung bean (Vigna radiata). J. Plant Physiol. 2009, 166, 602–616. [Google Scholar] [CrossRef] [PubMed]
- Distefano, G.; Cirilli, M.; Las Casas, G.; La Malfa, S.; Continella, A.; Rugini, E.; Thomas, B.; Long, G.; Gentile, A.; Muleo, R. Ectopic expression of Arabidopsis phytochrome B in Troyer citrange affects photosynthesis and plant morphology. Sci. Hortic. 2013, 159, 1–7. [Google Scholar] [CrossRef]
- Lim, J.; Park, J.H.; Jung, S.; Hwang, D.; Nam, H.G.; Hong, S. Antagonistic roles of PhyA and PhyB in far-red light-dependent Leaf senescence in Arabidopsis thaliana. Plant Cell Physiol. 2018, 59, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z.; Wang, X.; Wang, J.; Fan, K.; Li, Z.; Lin, W. DELLA proteins negatively regulate dark-induced senescence and chlorophyll degradation in Arabidopsis through interaction with the transcription factor WRKY6. Plant Cell Rep. 2018, 37, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.; Dash, G.K.; Mohanty, S.; Sekhar, S.; Roy, A.; Tudu, C.; Behera, L.; Tripathy, B.C.; Baig, M.J. Phytochrome A mediated modulation of photosynthesis, development and yield in rice (Oryza sativa L.) in fluctuating light environment. Environ. Exp. Bot. 2023, 206, 105183. [Google Scholar] [CrossRef]
- Jackson, M.B. Long-distance signalling from roots to shoots assessed the flooding story. J. Exp. Bot. 2002, 53, 175–181. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, J.; Dong, S.; Liu, P.; Zhao, B. Effects of waterlogging on leaf mesophyll cell ultrastructure and photosynthetic characteristics of summer maize. PLoS ONE 2016, 11, e0161424. [Google Scholar] [CrossRef]
- Liao, C.T.; Ho, L.C. Effect of flooding stress on photosynthetic activities of momordica charantia. Plant Physiol. Biochem. 1994, 32, 479–485. [Google Scholar]
- Hu, J.; Ren, B.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J. Poor development of spike differentiation triggered by lower photosynthesis and carbon partitioning reduces summer maize yield after waterlogging. Crop J. 2022, 10, 478–489. [Google Scholar] [CrossRef]
- Huang, C.; Gao, Y.; Qin, A.; Liu, Z.; Zhao, B.; Ning, D.; Ma, S.; Duan, A.; Liu, Z. Effects of waterlogging at different stages and durations on maize growth and grain yields. Agric. Water Manag. 2022, 261, 107334. [Google Scholar] [CrossRef]
- Tian, L.; Li, J.; Bi, W.; Zuo, S.; Li, L.; Li, W.; Sun, L. Effects of waterlogging stress at different growth stages on the photosynthetic characteristics and grain yield of spring maize (Zea mays L.) Under field conditions. Agric. Water Manag. 2019, 218, 250–258. [Google Scholar] [CrossRef]
- Wakchaure, G.C.; Minhas, P.S.; Kumar, S.; Khapte, P.S.; Rane, J.; Reddy, K.S. Bulb productivity and quality of monsoon onion (Allium cepa L.) as affected by transient waterlogging at different growth stages and its alleviation with plant growth regulators. Agric. Water Manag. 2023, 278, 108136. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Nagai, K.; Huan, P.D.; Shimazaki, K.; Qu, H.; Mori, Y.; Toda, Y.; Kuroha, T.; Hayashi, N.; Aiga, S.; et al. Rice leaf hydrophobicity and gas films are conferred by a wax synthesis gene (LGF1) and contribute to flood tolerance. New Phytol. 2018, 218, 1558–1569. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.N.; Mostofa, M.G.; Rahman, M.M.; Tahjib-Ul-Arif, M.; Das, A.K.; Mohi-Ud-Din, M.; Rohman, M.M.; Hafiz, H.R.; Ansary, M.M.U.; Tran, L.P. Glutathione improves rice tolerance to submergence: Insights into its physiological and biochemical mechanisms. J. Biotechnol. 2021, 325, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Perata, P. Ethylene signaling controls fast oxygen sensing in plants. Trends Plant Sci. 2020, 25, 3–6. [Google Scholar] [CrossRef]
- Negi, S.; Sukumar, P.; Liu, X.; Cohen, J.D.; Muday, G.K. Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant J. 2010, 61, 3–15. [Google Scholar] [CrossRef]
- Liu, Z.; Hartman, S.; Van Veen, H.; Zhang, H.; Leeggangers, H.; Martopawiro, S.; Bosman, F.; De Deugd, F.; Su, P.; Hummel, M.; et al. Ethylene augments root hypoxia tolerance via growth cessation and reactive oxygen species amelioration. Plant Physiol. 2022, 190, 1365–1383. [Google Scholar] [CrossRef]
- Shin, S.Y.; Choi, Y.; Kim, S.G.; Park, S.J.; Park, J.S.; Moon, K.B.; Kim, H.S.; Jeon, J.H.; Cho, H.S.; Lee, H.J. Submergence promotes auxin-induced callus formation through ethylene-mediated post-transcriptional control of auxin receptors. Mol. Plant 2022, 15, 1947–1961. [Google Scholar] [CrossRef]
- White, M.D.; Kamps, J.; East, S.; Taylor Kearney, L.J.; Flashman, E. The plant cysteine oxidases from Arabidopsis thaliana are kinetically tailored to act as oxygen sensors. J. Biol. Chem. 2018, 293, 11786–11795. [Google Scholar] [CrossRef] [PubMed]
- Weits, D.A.; Giuntoli, B.; Kosmacz, M.; Parlanti, S.; Hubberten, H.M.; Riegler, H.; Hoefgen, R.; Perata, P.; Van Dongen, J.T.; Licausi, F. Plant cysteine oxidases control the oxygen-dependent branch of the N-end-rule pathway. Nat. Commun. 2014, 5, 3425. [Google Scholar] [CrossRef] [PubMed]
- Hartman, S.; Liu, Z.; Van Veen, H.; Vicente, J.; Reinen, E.; Martopawiro, S.; Zhang, H.; Van Dongen, N.; Bosman, F.; Bassel, G.W.; et al. Ethylene-mediated nitric oxide depletion pre-adapts plants to hypoxia stress. Nat. Commun. 2019, 10, 4020. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.J.; Tedds, H.M.; Labandera, A.M.; Bailey, M.; White, M.D.; Hartman, S.; Sprigg, C.; Mogg, S.L.; Osborne, R.; Dambire, C.; et al. Oxygen-dependent proteolysis regulates the stability of angiosperm polycomb repressive complex 2 subunit VERNALIZATION 2. Nat. Commun. 2018, 9, 5438. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.J.; Md Isa, N.; Movahedi, M.; Lozano-Juste, J.; Mendiondo, G.M.; Berckhan, S.; Marin-De La Rosa, N.; Vicente Conde, J.; Sousa Correia, C.; Pearce, S.P.; et al. Nitric oxide sensing in plants is mediated by proteolytic control of group VII ERF transcription factors. Mol. Cell 2014, 53, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Wilson, I.W.; Yang, J.; Buerstenbinder, K.; Llewellyn, D.; Dennis, E.S.; Sauter, M.; Dolferus, R. Arabidopsis RAP2.2: An ethylene response transcription factor that is important for hypoxia survival. Plant Physiol. 2010, 153, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.P.; Lin, T.Y.; Wang, N.N.; Shih, M.C. Differential expression of genes encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis during hypoxia. Plant Mol. Biol. 2005, 58, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006, 140, 411–432. [Google Scholar] [CrossRef]
- Yin, D.; Sun, D.; Han, Z.; Ni, D.; Jiang, C.Z. PhERF2, an ethylene-responsive element binding factor, plays an essential role in waterlogging tolerance of petunia. Hortic. Res. 2019, 6, 83. [Google Scholar] [CrossRef]
- Luan, H.; Guo, B.; Shen, H.; Pan, Y.; Hong, Y.; Lv, C.; Xu, R. Overexpression of barley transcription factor HvERF2.11 in Arabidopsis enhances plant waterlogging tolerance. Int. J. Mol. Sci. 2020, 21, 1982. [Google Scholar] [CrossRef]
- Sun, D.; Nandety, R.S.; Zhang, Y.; Reid, M.S.; Niu, L.; Jiang, C.Z. A petunia ethylene-responsive element binding factor, PhERF2, plays an important role in antiviral RNA silencing. J. Exp. Bot. 2016, 67, 3353–3365. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Xu, K.; Ronald, P.C.; Bailey-Serres, J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell. 2006, 18, 2021–2034. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Bailey-Serres, J. Ethylene—A key regulator of submergence responses in rice. Plant Sci. 2008, 175, 43–51. [Google Scholar] [CrossRef]
- Dawood, T.; Yang, X.; Visser, E.J.; Te Beek, T.A.; Kensche, P.R.; Cristescu, S.M.; Lee, S.; Flokova, K.; Nguyen, D.; Mariani, C.; et al. A Co-Opted hormonal cascade activates dormant adventitious root primordia upon flooding in solanum dulcamara. Plant Physiol. 2016, 170, 2351–2364. [Google Scholar] [CrossRef] [PubMed]
- Kuroha, T.; Nagai, K.; Gamuyao, R.; Wang, D.R.; Furuta, T.; Nakamori, M.; Kitaoka, T.; Adachi, K.; Minami, A.; Mori, Y.; et al. Ethylene-gibberellin signaling underlies adaptation of rice to periodic flooding. Science 2018, 361, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Huang, S.N.; Wang, G.; Xuan, J.P.; Guo, Z.R. Overexpression of Actinidia deliciosa pyruvate decarboxylase 1 gene enhances waterlogging stress in transgenic Arabidopsis thaliana. Plant Physiol. Biochem. 2016, 106, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Vidoz, M.L.; Loreti, E.; Mensuali, A.; Alpi, A.; Perata, P. Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J. 2010, 63, 551–562. [Google Scholar] [CrossRef]
- Wang, X.; Huang, M.; Zhou, Q.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Physiological and proteomic mechanisms of waterlogging priming improves tolerance to waterlogging stress in wheat (Triticum aestivum L.). Environ. Exp. Bot. 2016, 132, 175–182. [Google Scholar] [CrossRef]
- Jiang, Z.-H.; Zhua, J.-Q.; Yang, W.; Lia, M.-F.; Yua, Y. Effects of remedial measures implemented after waterlogging on cotton. In Proceedings of the Third International Conference on Intelligent System Design and Engineering Applications, Hong Kong, China, 16–18 January 2013; pp. 692–695. [Google Scholar] [CrossRef]
- Li, Y.; Sun, D.; Xu, K.; Jin, L.; Peng, R. Hydrogen sulfide enhances plant tolerance to waterlogging stress. Plants 2021, 10, 1928. [Google Scholar] [CrossRef]
- Zhong, Y.-H.; Guo, Z.-J.; Wei, M.-Y.; Wang, J.-C.; Song, S.-W.; Chi, B.-J.; Zhang, Y.-C.; Liu, J.-W.; Li, J.; Zhu, X.-Y.; et al. Hydrogen sulfide upregulates the alternative respiratory pathway in mangrove plant Avicennia marina to attenuate waterlogging-induced oxidative stress and mitochondrial damage in a calcium-dependent manner. Plant Cell Environ. 2023, 46, 1521–1539. [Google Scholar] [CrossRef]
- Hasnain, M.; Munir, N.; Abideen, Z.; Zulfiqar, F.; Koyro, H.W.; El-Naggar, A.; Caçador, I.; Duarte, B.; Rinklebe, J.; Yong, J.W.H. Biochar-plant interaction and detoxification strategies under abiotic stresses for achieving agricultural resilience: A critical review. Ecotoxicol. Environ. Saf. 2023, 249, 114408. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yang, Y.; Zheng, C.; Luo, X.; Chandrasekaran, U.; Yin, H.; Chen, F.; Meng, Y.; Chen, L.; Shu, K. Flooding represses soybean seed germination by mediating anaerobic respiration, glycometabolism and phytohormones biosynthesis. Environ. Exp. Bot. 2021, 188, 104491. [Google Scholar] [CrossRef]
- Kaur, G.; Vikal, Y.; Kaur, L.; Kalia, A.; Mittal, A.; Kaur, D.; Yadav, I. Elucidating the morpho-physiological adaptations and molecular responses under long-term waterlogging stress in maize through gene expression analysis. Plant Sci. 2021, 304, 110823. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Meng, X.; Wang, M.; Zang, S.; Feng, L. Improvement in submergence tolerance of cherry through regulation of carbohydrate metabolism and pant growth by PsERF and PsCIPK. Appl. Biochem. Biotechnol. 2018, 184, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Phukan, U.J.; Jeena, G.S.; Tripathi, V.; Shukla, R.K. MaRAP2-4, a waterlogging-responsive ERF from Mentha, regulates bidirectional sugar transporter AtSWEET10 to modulate stress response in Arabidopsis. Plant Biotechnol. J. 2018, 16, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.J.; Yu, L.J.; Chen, Q.F.; Wang, F.Z.; Huang, L.; Xia, F.N.; Zhu, T.R.; Wu, J.X.; Yin, J.; Liao, B.; et al. Arabidopsis acyl-CoA-binding protein ACBP3 participates in plant response to hypoxia by modulating very-long-chain fatty acid metabolism. Plant J. 2015, 81, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.J.; Zhou, Y.; Chen, Q.F.; Xiao, S. New insights into the role of lipids in plant hypoxia responses. Prog. Lipid Res. 2021, 81, 101072. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, D.M.; Yu, W.W.; Shi, L.L.; Zhang, Y.; Lai, Y.X.; Huang, L.P.; Qi, H.; Chen, Q.F.; Yao, N.; et al. Phosphatidic acid modulates MPK3- and MPK6-mediated hypoxia signaling in Arabidopsis. Plant Cell 2022, 34, 889–909. [Google Scholar] [CrossRef]
- Bloom, A.J.; Sukrapanna, S.S.; Warner, R.L. Root respiration associated with ammonium and nitrate absorption and assimilation by barley. Plant Physiol. 1992, 99, 1294–1301. [Google Scholar] [CrossRef]
- Loque, D.; Ludewig, U.; Yuan, L.; Von Wiren, N. Tonoplast intrinsic proteins AtTIP2;1 and AtTIP2;3 facilitate NH3 transport into the vacuole. Plant Physiol. 2005, 137, 671–680. [Google Scholar] [CrossRef]
- Gupta, K.J.; Mur, L.A.J.; Wany, A.; Kumari, A.; Fernie, A.R.; Ratcliffe, R.G. The role of nitrite and nitric oxide under low oxygen conditions in plants. New Phytol. 2020, 225, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Borella, J.; Becker, R.; Lima, M.C.; Oliveira, D.D.S.C.D.; Braga, E.J.B.; Oliveira, A.C.B.D.; Amarante, L.D. Nitrogen source influences the antioxidative system of soybean plants under hypoxia and re-oxygenation. Sci. Agric. 2019, 76, 51–62. [Google Scholar] [CrossRef]
- Da-Silva, C.J.; Do Amarante, L. Short-term nitrate supply decreases fermentation and oxidative stress caused by waterlogging in soybean plants. Environ. Exp. Bot. 2020, 176, 104078. [Google Scholar] [CrossRef]
- Pucciariello, C.; Perata, P. New insights into reactive oxygen species and nitric oxide signalling under low oxygen in plants. Plant Cell Environ. 2017, 40, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Prakash, V.; Vishwakarma, K.; Singh, V.P.; Rai, P.; Ramawat, N.; Tripathi, D.K.; Sharma, S. NO and ROS implications in the organization of root system architecture. Physiol. Plant 2020, 168, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Wany, A.; Kumari, A.; Gupta, K.J. Nitric oxide is essential for the development of aerenchyma in wheat roots under hypoxic stress. Plant Cell Environ. 2017, 40, 3002–3017. [Google Scholar] [CrossRef] [PubMed]
- Zottini, M.; Formentin, E.; Scattolin, M.; Carimi, F.; Lo Schiavo, F.; Terzi, M. Nitric oxide affects plant mitochondrial functionality in vivo. FEBS Lett. 2002, 515, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.-G.; Yun, B.-W. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Liu, G.; Xu, S.; Dai, J.; Li, W.; Li, Z.; Zhang, D.; Li, C.; Dong, H. Nitric oxide increases the biomass and lint yield of field-grown cotton under temporary waterlogging through physiological and molecular regulation. Field Crops Res. 2021, 261, 107989. [Google Scholar] [CrossRef]
- Liu, C.; Lan, C.; Li, C.; Li, C.; Huang, J. Exogenous spermidine and calcium alleviate waterlogging stress in cherry tomato at the seedling stage. Sci. Hortic. 2023, 307, 111504. [Google Scholar] [CrossRef]
- Zhu, J.; Li, A.; Zhang, J.; Sun, C.; Tang, G.; Chen, L.; Hu, J.; Zhou, N.; Wang, S.; Zhou, Y.; et al. Effects of nitrogen application after abrupt drought-flood alternation on rice root nitrogen uptake and rhizosphere soil microbial diversity. Environ. Exp. Bot. 2022, 201, 105007. [Google Scholar] [CrossRef]
- Martínez-Arias, C.; Witzell, J.; Solla, A.; Martin, J.A.; Rodríguez-Calcerrada, J. Beneficial and pathogenic plant-microbe interactions during flooding stress. Plant Cell Environ. 2022, 45, 2875–2897. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jiang, Z.; Shi, M.; Zhou, Y.; Huo, L.; Li, X.; Xu, K. Comparative transcriptome provides insight into responding mechanism of waterlogging stress in Actinidia valvata Dunn. Gene 2022, 845, 146843. [Google Scholar] [CrossRef] [PubMed]
- Hess, N.; Klode, M.; Anders, M.; Sauter, M. The hypoxia responsive transcription factor genes ERF71/HRE2 and ERF73/HRE1 of Arabidopsis are differentially regulated by ethylene. Physiol. Plant 2011, 143, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.-F.; Li, Z.; Hu, C.-G.; Zhang, Y.-J.; Muhammad, A.; Zhong, Y.-P.; Fang, J.-B. Transcriptome-wide identification and expression analysis of ERF family genes in Actinidia valvata during waterlogging stress. Sci. Hortic. 2021, 281, 109994. [Google Scholar] [CrossRef]
- Mao, J.L.; Miao, Z.Q.; Wang, Z.; Yu, L.H.; Cai, X.T.; Xiang, C.B. Arabidopsis ERF1 mediates cross-talk between ethylene and auxin biosynthesis during primary root elongation by regulating ASA1 expression. PLoS Genet. 2016, 12, e1005760. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhang, J.; Chen, S.; Zhang, Z.; Wan, G.; Mao, J.; Wang, Z.; Tan, S.; Xiang, C. ERF1 inhibits lateral root emergence by promoting local auxin accumulation and repressing ARF7 expression. Cell Rep. 2023, 42, 112565. [Google Scholar] [CrossRef]
- Wei, X.; Xu, H.; Rong, W.; Ye, X.; Zhang, Z. Constitutive expression of a stabilized transcription factor group VII ethylene response factor enhances waterlogging tolerance in wheat without penalizing grain yield. Plant Cell Environ. 2019, 42, 1471–1485. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, Y.; Tang, H.; Tong, S.; Lou, S.; Shao, C.; Zhang, J.; Song, Y.; Chen, N.; Bi, H.; et al. The ubiquitin E3 ligase SR1 modulates the submergence response by degrading phosphorylated WRKY33 in Arabidopsis. Plant Cell 2021, 33, 1771–1789. [Google Scholar] [CrossRef]
- Meng, D.; Li, Y.; Bai, Y.; Li, M.; Cheng, L. Genome-wide identification and characterization of WRKY transcriptional factor family in apple and analysis of their responses to waterlogging and drought stress. Plant Physiol. Biochem. 2016, 103, 71–83. [Google Scholar] [CrossRef]
- Xie, L.-J.; Wang, J.-H.; Liu, H.-S.; Yuan, L.-B.; Tan, Y.-F.; Tan, W.-J.; Zhou, Y.; Chen, Q.-F.; Qi, H.; Li, J.-F.; et al. MYB30 integrates light signals with antioxidant biosynthesis to regulate plant responses during postsubmergence recovery. New Phytol. 2023, 237, 2238–2254. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.; Arif, M.; Fisahn, J.; Xue, G.P.; Balazadeh, S.; Mueller-Roeber, B. NAC transcription factor speedy hyponastic growth regulates flooding-induced leaf movement in Arabidopsis. Plant Cell 2013, 25, 4941–4955. [Google Scholar] [CrossRef]
- Berry, H.M.; Argueso, C.T. More than growth: Phytohormone-regulated transcription factors controlling plant immunity, plant development and plant architecture. Curr. Opin. Plant Biol. 2022, 70, 102309. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Dong, X. In vivo interaction between NPR1 and transcription factor TGA2 leads to salicylic acid-mediated gene activation in Arabidopsis. Plant Cell 2002, 14, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Di, D.W.; Li, G.; Li, Y.; Kronzucker, H.J.; Shi, W. Transcriptome analysis of rice (Oryza sativa L.) in response to ammonium resupply reveals the involvement of phytohormone signaling and the transcription factor OsJAZ9 in reprogramming of nitrogen uptake and metabolism. J. Plant Physiol. 2020, 246–247, 153137. [Google Scholar] [CrossRef]
- Yuan, L.B.; Dai, Y.S.; Xie, L.J.; Yu, L.J.; Zhou, Y.; Lai, Y.X.; Yang, Y.C.; Xu, L.; Chen, Q.F.; Xiao, S. Jasmonate regulates plant responses to postsubmergence reoxygenation through transcriptional activation of antioxidant synthesis. Plant Physiol. 2017, 173, 1864–1880. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, F.-L.; Jiang, D. Priming: A promising strategy for crop production in response to future climate. J. Integr. Agric. 2017, 16, 2709–2716. [Google Scholar] [CrossRef]
- Kartashov, A.V.; Zlobin, I.E.; Pashkovskiy, P.P.; Pojidaeva, E.S.; Ivanov, Y.V.; Ivanova, A.I.; Ivanov, V.P.; Marchenko, S.I.; Nartov, D.I.; Kuznetsov, V.V. Effects of drought stress memory on the accumulation of stress-protective compounds in naturally grown pine and spruce. Plant Physiol. Biochem. 2023, 200, 107761. [Google Scholar] [CrossRef]
- Luis De La Fuente, J.; Zunzunegui, M.; Barradas, M.C.D. Physiological responses to water stress and stress memory in Argania spinosa. Plant Stress 2023, 7, 100133. [Google Scholar] [CrossRef]
- Sharma, M.; Kumar, P.; Verma, V.; Sharma, R.; Bhargava, B.; Irfan, M. Understanding plant stress memory response for abiotic stress resilience: Molecular insights and prospects. Plant Physiol. Biochem. 2022, 179, 10–24. [Google Scholar] [CrossRef]
- Kinoshita, T.; Seki, M. Epigenetic memory for stress response and adaptation in plant. Plant Cell Physiol. 2014, 55, 1859–1863. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Able, A.J.; Able, J.A. Priming crops for the future: Rewiring stress memory. Trends Plant Sci. 2022, 27, 699–716. [Google Scholar] [CrossRef] [PubMed]
- Gallusci, P.; Agius, D.R.; Moschou, P.N.; Dobranszki, J.; Kaiserli, E.; Martinelli, F. Deep inside the epigenetic memories of stressed plants. Trends Plant Sci. 2023, 28, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, D.; Wollenweber, B.; Li, Y.; Dai, T.; Cao, W. Waterlogging pretreatment during vegetative growth improves tolerance to waterlogging after anthesis in wheat. Plant Sci. 2011, 180, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Sharma, K.D.; Siddique, K.H.M.; Nayyar, H. Cold priming the chickpea seeds imparts reproductive cold tolerance by reprogramming the turnover of carbohydrates, osmo-protectants and redox components in leaves. Sci. Hortic. 2020, 261, 108929. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; An, J.; Yin, M.; Jia, X.; Guan, Y.; He, F.; Hu, J. Cold plasma treatment and exogenous salicylic acid priming enhances salinity tolerance of Oryza sativa seedlings. Protoplasma 2019, 256, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Lukić, N.; Schurr, F.M.; Trifković, T.; Kukavica, B.; Walter, J. Transgenerational stress memory in plants is mediated by upregulation of the antioxidative system. Environ. Exp. Bot. 2023, 205, 105129. [Google Scholar] [CrossRef]
- Mohammadi, M.; Afshari, R.T.; Nabati, J.; Oskoueian, E. Stress memory in seedlings of primed seed chickpea (Cicer arietinum L.) using sodium nitroprusside under cold stress. Plant Stress 2023, 8, 100163. [Google Scholar] [CrossRef]
- Geng, S.; Lin, Z.; Xie, S.; Xiao, J.; Wang, H.; Zhao, X.; Zhou, Y.; Duan, L. Ethylene enhanced waterlogging tolerance by changing root architecture and inducing aerenchyma formation in maize seedlings. J. Plant Physiol. 2023, 287, 154042. [Google Scholar] [CrossRef]
- Rossatto, T.; Souza, G.M.; Do Amaral, M.N.; Auler, P.A.; Pérez-Alonso, M.-M.; Pollmann, S.; Braga, E.J.B. Cross-stress memory: Salt priming at vegetative growth stages improves tolerance to drought stress during grain-filling in rice plants. Environ. Exp. Bot. 2023, 206, 105187. [Google Scholar] [CrossRef]
- Harshavardhan, V.T.; Govind, G.; Kalladan, R.; Sreenivasulu, N.; Hong, C.-Y. Cross-Protection by Oxidative Stress: Improving Tolerance to Abiotic Stresses Including Salinity. In Salinity Responses and Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1, pp. 283–305. [Google Scholar] [CrossRef]
- Johnson, R.; Puthur, J.T. Seed priming as a cost effective technique for developing plants with cross tolerance to salinity stress. Plant Physiol. Biochem. 2021, 162, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gai, Z.; Qiu, X.; Liu, T.; Li, S.; Ye, F.; Jian, S.; Shen, Y.; Li, X. Salt stress improves the low-temperature tolerance in sugar beet in which carbohydrate metabolism and signal transduction are involved. Environ. Exp. Bot. 2023, 208, 105239. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Hu, W.; Wang, S.; Snider, J.L.; Zhou, Z. Co-occurring elevated temperature and waterlogging stresses disrupt cellulose synthesis by altering the expression and activity of carbohydrate balance-associated enzymes during fiber development in cotton. Environ. Exp. Bot. 2017, 135, 106–117. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Hu, W.; Wang, S.; Snider, J.L.; Zhou, Z. Carbohydrate metabolism in the subtending leaf cross-acclimates to waterlogging and elevated temperature stress and influences boll biomass in cotton (Gossypium hirsutum). Physiol. Plant 2017, 161, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Y.; Hu, W.; Snider, J.L.; Zhou, Z. Short-term soil-waterlogging contributes to cotton cross tolerance to chronic elevated temperature by regulating ROS metabolism in the subtending leaf. Plant Physiol. Biochem. 2019, 139, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, H.; Hu, W.; Wang, S.; Wang, Y.; Snider, J.L.; Zhou, Z. Combined elevated temperature and soil waterlogging stresses inhibit cell elongation by altering osmolyte composition of the developing cotton (Gossypium hirsutum L.) fiber. Plant Sci. 2017, 256, 196–207. [Google Scholar] [CrossRef]
- Pociecha, E.; Rapacz, M.; Dziurka, M.; Kolasinska, I. Mechanisms involved in the regulation of photosynthetic efficiency and carbohydrate partitioning in response to low- and high-temperature flooding triggered in winter rye (Secale cereale) lines with distinct pink snow mold resistances. Plant Physiol. Biochem. 2016, 104, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Jurczyk, B.; Rapacz, M.; Krępski, T. Photosynthetic apparatus responses to short-term low-temperature flooding may contribute to freezing tolerance changes in forage grasses. J. Agron. Crop Sci. 2015, 201, 49–56. [Google Scholar] [CrossRef]
- Jurczyk, B.; Krępski, T.; Kosmala, A.; Rapacz, M. Different mechanisms trigger an increase in freezing tolerance in Festuca pratensis exposed to flooding stress. Environ. Exp. Bot. 2013, 93, 45–54. [Google Scholar] [CrossRef]
- Maruyama, K.; Sakuma, Y.; Kasuga, M.; Ito, Y.; Seki, M.; Goda, H.; Shimada, Y.; Yoshida, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Identification of cold-inducible downstream genes of the Arabidopsis DREB1A/CBF3 transcriptional factor using two microarray systems. Plant J. 2004, 38, 982–993. [Google Scholar] [CrossRef]
- Fowler, S.; Thomashow, M.F. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 2002, 14, 1675–1690. [Google Scholar] [CrossRef] [PubMed]
- Olorunwa, O.J.; Adhikari, B.; Brazel, S.; Shi, A.; Popescu, S.C.; Popescu, G.V.; Barickman, T.C. Growth and photosynthetic responses of cowpea genotypes under waterlogging at the reproductive stage. Plants 2022, 11, 2315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-M.; Duan, S.-G.; Xia, Y.; Li, J.-T.; Liu, L.-X.; Tang, M.; Tang, J.; Sun, W.; Yi, Y. Transcriptomic, physiological, and metabolomic response of an alpine Plant, Rhododendron delavayi, to waterlogging stress and post-waterlogging recovery. Int. J. Mol. Sci. 2023, 24, 10509. [Google Scholar] [CrossRef] [PubMed]
- Loreti, E.; Veen, H.V.; Perata, P. Plant responses to flooding stress. Curr. Opin. Plant Biol. 2016, 33, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Guo, J.; Zhang, Z.; Kou, T.; Deng, A.; Zheng, C.; Ren, J.; Zhang, W. Impacts of planting systems on soil moisture, soil temperature and corn yield in rainfed area of Northeast China. Eur. J. Agron. 2013, 50, 66–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Liu, G.; Lian, T.; Li, Z.; Liang, T.; Zhang, D.; Cui, Z.; Zhan, L.; Sun, L.; et al. Ridge intertillage alters rhizosphere bacterial communities and plant physiology to reduce yield loss of waterlogged cotton. Field Crops Res. 2023, 293, 108849. [Google Scholar] [CrossRef]
- Devkota, K.P.; Futakuchi, K.; Mel, V.C.; Humphreys, E. Does wet seeding combined with Sub1 varieties increase yield in submergence prone lowlands of West Africa? Field Crops Res. 2022, 276, 108375. [Google Scholar] [CrossRef]
- Fischer, R.A.; Moreno Ramos, O.H.; Ortiz Monasterio, I.; Sayre, K.D. Yield response to plant density, row spacing and raised beds in low latitude spring wheat with ample soil resources: An update. Field Crops Res. 2019, 232, 95–105. [Google Scholar] [CrossRef]
- Du, X.; He, W.; Wang, Z.; Xi, M.; Xu, Y.; Wu, W.; Gao, S.; Liu, D.; Lei, W.; Kong, L. Raised bed planting reduces waterlogging and increases yield in wheat following rice. Field Crops Res. 2021, 265, 108119. [Google Scholar] [CrossRef]
- Den Besten, N.; Steele Dunne, S.; Mahmud, A.; Jackson, D.; Aouizerats, B.; De Jeu, R.; Burger, R.; Houborg, R.; Mcglinchey, M.; Van Der Zaag, P. Understanding Sentinel-1 backscatter response to sugarcane yield variability and waterlogging. Remote Sens. Environ. 2023, 290, 113555. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, X.; Shen, J.; Du, K. Poplar’s waterlogging resistance modeling and evaluating: Exploring and perfecting the feasibility of machine learning methods in plant science. Front. Plant Sci. 2022, 13, 821365. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).