Abstract
The mineralization of nitrogen in apple orchard soil will increase the soil supply. An incubation study to test the soil potential and the validity of analytical methods was conducted at 3, 8, 15, and 20 °C for up to 128 days on soils from western and south-eastern Norway. Soils with the highest pH showed the highest mineralization. The mineralization increased with increasing temperature and time, but start-up N reduced mineralization. The mineralization cannot be estimated from standard soil chemical parameters because the different C/N ratio indicates organic material of different origin and quality. The increase in NO3-N started very quickly and ranged from 17 to 182% and 12 to 64% after 8 days at 3 °C and 20 °C, respectively. There was no correlation between total N in the soil and the amount of mineralized N. On average, the mineralization increased by 5–7% for a change of 1 °C in the interval from 8 to 15 °C in the soil. The chemical extraction method using heated KCl correlated well with the mineralization data. On average, the chemical method estimated 30 kg N ha−1, which corresponded to 0.48% of total N. Recommendations for N fertilization based on total N in the soil overestimate the contribution of plant-available N in most cases.
1. Introduction
It is well known that nitrogen (N) is often a limiting factor in crop production and that fertilization is necessary to obtain annual vegetative growth and a high yield of good quality. Nitrogen is the main component of proteins, amino acids, nucleic acids, chlorophyll, and phytohormones, and therefore it plays a major role in plant metabolic processes [1]. In fruit production, however, too much N provokes a vigorous vegetative growth; reduced amount of flowers, yield, and fruit coloration; poor fruit quality; and storage problems [2,3]. N deficiencies, on the other hand, induce poor growth and low yield, and leads to small fruits for fruit trees in general [4]. Therefore, in fruit production it is important to balance the N supply, considering plants needs from soil reserves and applied fertilizer.
Thus, nitrogen supply to fruits relies on mineral reserves stored in perennial plant parts, nitrogen in the soil, and nitrogen from fertilizers. An important soil N fraction is nitrogen derived from the mineralization of soil organic matter, which can contribute significantly to fruit nitrogen supply. This mineralized nitrogen is defined as the amount of available soil nitrogen (NH4+ and NO3−) released during a given period, and has been studied by various chemical and biological methods [5].
In Nordic countries, high annual precipitation and a cool climate have led to an accumulation of organic matter in soil. In Norwegian cultivated mineral soils, 5–6% organic matter is normal [6]. In orchard soils, organic matter is often due to added compost or other organic fertilizers [7]. Organic matter contains a significant amount of total nitrogen, and mineralization can result in a significant supply during the growing season. Therefore, the accurate estimation of soil nitrogen supply is required to optimize fertilizer N management and to achieve high fruit quality.
The ability to supply nitrogen through soil mineralization depends on several factors. Soil temperature [8], moisture, clay content and aeration of the soil [9,10], and crop rotation and cropping history [11] strongly influence on the N mineralization. A common method for studying mineralization is the incubation technique, in which moist soil is stored for an extended period of time to test the effect of microorganisms on the release of nutrients. It appears that soil pH most strongly affects microbial population activity [12], with microbial activity usually increasing when acid soils are limed to higher pH levels indicating that an increase in the rate of mineralization of N was the main effect of lime [13]. An older literature review concluded that the mineralization of organic N occurs over the entire pH range, but the rate progressively decreases below about pH 6 [14]. Also, a 6-year incubation study on acidic Norwegian mineral soils (pH 4.7–6.0) demonstrated the positive effect of N mineralization by increasing the pH by liming to pH about 7, probably due to increased microbial activity [15]. A newer study showed that the interaction between different soil factors is important, and that organic matter alone is not a good estimation indicator of mineralization. Both organic matter content and quality, as well as soil texture and mineralogy, influence mineralization and must be included in mineralization models [16].
N fertilization practices and recommended amounts of N for growing trees vary widely. For a site with sandy loam and clayey soil and a yield of 45 t ha−1, the nutrient requirement in apple orchards in the USA is 75–100 kg N ha−1 [17]. A newly published article [18] concluded that the applied annual dose should not exceed 50 kg N ha−1 to obtain the high quality of fruit with minimized leaching of N to groundwater. They also concluded that the mineralization of N in humus-rich soil may completely cover the demand of apple trees for N. Nowadays, nitrogen recommendations in Norwegian orchards vary between 20 and 65 kg N ha−1, based on a yield of 20 t ha−1, depending on local soil variations and tree characteristics [19,20]. However, the soil’s N-supplying capacity, based on mineralization process, is evaluated only upon the soil organic matter content and is therefore an approximate method. When soil organic matter content ranges from 4.6 to 20.5%, as is common in Norwegian orchard soils [7], the N mineralization supply during the growing season is about 10 kg N ha-1 [21], which is rarely considered in fertilizer planning. Better estimates of soil N mineralization in Norwegian orchard soils are not yet available but need to be better utilized in fertilization practice.
The influence of soil properties and climatic factors makes site-specific mineralization rates challenging. These soil factors vary in Norwegian orchards, from sandy soils with high rainfall influenced by the mild climate in the fjord area on the west coast of Norway, to soils with finer texture and lower rainfall in the southeastern part of the country. Studies show that laboratory methods are difficult to extrapolate to field conditions because the amount of mineralized N depends on the methods used and the handling and storage of soil samples, which in most cases must be adjusted in the field to serve as a model [21]. However, many soil tests have been developed over the years to predict N mineralization rates, most of them based on chemical extraction. A general review and meta-analysis based on 218 papers was conducted by Ros, Temminghoff, and Hoffland in 2011 [22]. The best predictions of mineralized N, with an R2 of 57–74%, were made with chemical tests of CaCl2, acidic KMnO4, acidic K2Cr2O7, hot water, or hot KCl extraction. This is in accordance with the study of Øien and Selmer-Olsen [23] on Norwegian soils, where extraction was performed with the 2 M KCl (80 °C) hydrolysis method and a good correlation was obtained between N mineralization and N uptake by plants. However, this method has not been adopted in Norwegian agriculture mainly because of the demanding requirements in sampling procedure and pre-treatments, which did not match the routine work of commercial laboratories. Indeed, the drying and storage of the soil samples will significantly affect the content of mineralized N [24,25,26].
However, commercial European soil laboratories have begun to analyze soil N supply capacity based on dried and stored soil. Results are reported in kg N ha−1 mineralized and available to plants during their growing season. However, there is a lack of description of the analytical method and the basis for calculating plant-available N. A data set of 42 soil samples analyzed by a commercial laboratory in central Europe used by Norwegian fruit farmers showed an average N mineralization capacity of 105 kg N ha−1 during the growing season, with a wide range of variation from 55 to 199 kg N ha−1 [27]. There was a perfect correlation with total N with an average seasonal mineralization of 1.7% of total N in the soil, which is much higher than usual for Norwegian cultivated soils. Other studies are also available, but the plant uptake in relation to Kjeldahl-N in 36 Norwegian cultivated soils was 0.47% and probably less is mineralized from organic matter [28]. Also, a 3-year field study with plots in all the Nordic countries concluded that the N mineralization during the growing season corresponded on average to 0.50% of total soil N [29]. Since many Norwegian fruit farmers use laboratory results from abroad, it is important to conduct a study to test the suitability of recommendations influenced by the different climate and soil conditions in Norway, which consequently emphasize the too high N supply potential of the soil, resulting in low N fertilizer use and thus low yield.
Soil samples from the main apple growing regions in Norway are collected to investigate N mineralization potential by using soil incubation method. The focus of the study is to verify previous results from foreign commercial laboratories.
The objectives of the investigation are:
- To give an overview of the nitrogen mineralization potential in Norwegian orchard soils.
- To examine the possibilities of using existing soil extraction methods to estimate the N supplying capacity of the soil.
- To evaluate soil nitrogen data given by commercial laboratories as a tool for fertilization planning in Norwegian orchards.
2. Materials and Methods
2.1. Sampling of Soils
Soils for the mineralization study were sampled from apple orchards in 3 main fruit districts in Norway, Hardanger (Ullensvang) and Sogn in the western part of Norway and from Lier district in the southeastern Norway, in spring 2018. Well-managed and mature high-density apple orchards with 5–10 years old trees in full production were chosen. Four sample sites at each location covering the main soil types and organic amendments used in the orchards were used. In each orchard, one representative row of apple trees was soil sampled from the upper soil layer by taking 9 subsamples from 20 m of the row. Samples were mixed in a bucket, stones and pieces of branches or bark were removed, and approximately 5 kg of soil was sent to the laboratory in sealed bags as soon as possible. All soils had low moisture content and were sieved through a 4 mm stainless steel sieve and frozen at −18 °C until mineralization studies were performed.
2.2. Soil Characherization
A subsample of the frozen samples was dried at 40 °C and sieved through a 2 mm sieve before analysis. Soil texture was analyzed using the pipette method of Elonen [30]. pH was measured in a suspension of soil to deionized water ratio of 1:2.5 using an Orion Ross electrode after being stored staying overnight at room temperature. Total C and total N were analyzed using a dry combustion method [31] and analyzed on the instrument LECO CHN-628. Available nutrients in soil, such as Na, K, Mg, Ca, and P, were extracted by the ammonium-acetate-lactate method at pH 3.75 [32] and measured using ICP-AES 4200 Agilent (Agilent Technologies, Melbourne, Australia).
The Øiens method [23] was used for measuring extractable nitrogen, and involves an extraction of soil nitrogen in 2 M KCl after heating the soil suspension at 80 °C for 20 h [23]. Frozen soils of <4 mm are used for the analysis. Nitrate and ammonium were analyzed in the filtered extract by spectrophotometric flow injection method using FIA Star 5000.
2.3. Soil Incubation
The incubation experiment was performed 3 times with 4 soils each time to cover all 12 soils. A total of 15.00 g of frozen soil was weighed into in 50 mL Sarstedt centrifuge tubes with 3 replicates. The dry matter of the soils varied from 72 to 98%. Deionized water was added to 60% of the water holding capacity. The water holding capacity was measured by carefully adding 15 mL deionized water to 15 g of soil in a centrifuge tube with a drainage hole in the bottom and measuring the weight of the soil when no water dripped from the tube. The tubes were placed in racks covered with perforated parafilm to reduce water evaporation but allowed oxygen inflow. Four racks of tubes were made for incubation temperatures of 3, 8, 15, and 20 °C, respectively, and placed in the dark in 4 incubation chambers (Termaks series KB 8000) adjusted to the desired temperatures with an accuracy of ±0.1 °C. One liter of deionized water to be used to compensate for evaporation was placed in the chamber containing the samples. Tree tubes of each soil at each temperature were taken out of the chambers for extraction of nitrate and ammonium after 0, 1, 2, 4, 8, 16, 32, 64, and 128 days. A total of 108 tubes per soil type covered all temperatures and sampling times. All tubes were weighed before incubation, and water evaporation was controlled during the experiment. Every second week, the racks were taken out of the incubation chambers and deionized water with incubation temperature added to the initial weight. There was almost no evaporation in the soils with a temperature of 3 °C, while the soils with a temperature of 20 °C had lost about 5–10% of the initial weight after two weeks of incubation.
Samples taken out for analysis were added to 30 mL 2 M KCl, shaken for 1 h, and filtered through blue ribbon paper filter (Whatman 589/3); then, concentration of nitrate N and ammonium N were measured spectrophotometrically by using FIA Star 5000. The net mineralization was calculated as the difference between the mineral N concentration (NO3-N + NH4-N) in the soil before and after incubation.
Statistical analysis was conducted using SAS v.9.4 [33]. To test on changes in mineral N during incubation, the GLM procedure with Tukey grouping of the methods was used. The level of significance is tested on 5% level of probability. The PROC REG procedure was used to make a linear regression analysis between the methods. To calculate basic parameters like mean and standard deviation, the PROC MEANS procedures were used.
3. Results
The characterization of the soils in the orchards is shown in Table 1. All soils are characterized as loamy soils with some variation in sand and silt content, with only L1 characterized as loamy sand. Soil organic matter is important for nitrogen mineralization, with the average total C content being highest in the Ullensvang soils (U), while the lowest values were found in the Lier soils (L). Total nitrogen content varied from 0.05% to 0.41%, with little variation in the U soils and the greatest variation in the L soils. However, little variation was observed in the average values for the C/N ratio between regions, with a range of 11.4 (S3) to 18 (L2) for all soils. The sampled soils had different pH values, with the lowest pH in the U soils ranging from 4.68 to 5.34, while the highest value in the Sogn soils (S) ranged from 5.76 to 6.82. The L soils had the greatest variation in pH, which ranged from 4.96 to 7.04.

Table 1.
Characterization of the soils in the different apple orchards from three Norwegian regions Ullensvang (U), Lier (L) and Sogn (S).
Plant-available Mg and Ca content, measured as Mg-AL and Ca-AL, varies among regions, with average content lowest in U soils and highest in S soils. Acid-soluble K content, reflecting soil mineralogy, is highest in U soils and lowest in S soils. For the nutrients Na, K, and P, measured as AL-extractable elements, differences between regions were small. However, there were large differences in some elements within regions. In L soils, P- AL varied in the range of 68 to 840 mg kg−1 soil, and in U soils, K- AL varied in the range of 74 to 380 mg kg−1 soil, indicating the intensity of fertilization during the apple growing season.
Table 2 shows the increase in mineral nitrogen (percent increase over initial concentration) for incubation at 2, 4, and 8 days. The mineralization of nitrogen started very quickly in all soils and at all temperatures, except for the soils U3 and U4. Soil U3 had a very high concentration of mineral N, measured as nitrate and ammonium in the KCl extract at the beginning of the experiment, while U4 is a very acidic soil. Soils L, S, and U1 showed a significant increase at 5% level in mineral N after only 2 days of incubation. Similar results were observed for soils U2 at 20 °C and for U4 at 15 °C and 20 °C. For U2, it took more than 2 days to show a significant increase in mineral N, while for U3 it took more than 4 days at all temperatures. In the first week of the experiment, the increase in mineral N generally meant an increase in both nitrate and ammonium ions. Nitrate concentration was elevated at all temperatures from the first day throughout the experiment. However, for most soils, the ammonium concentration was highest after 2 to 4 days of incubation, and then decreased to zero during the first 32 days. Exceptions were soils U2 and U4, with soil U2 still containing a small amount of ammonium after 64 days of the experiment at all temperatures. U4 still had a small amount of ammonium even after 128 days at 3 °C, while the other temperature regimes still had some ammonium in the system after 64 days. Compared to nitrate concentrations, these amounts of ammonium are very small, so their contribution to total mineral N is marginal.

Table 2.
The increase in mineral N after 2, 4, and 8 days expressed as a percentage from the start incubation day in mg N l−1 in the extraction solution from three Norwegian regions (Ullensvang (U), Lier (L), and Sogn (S). No significant difference at 5% level from the initial concentration is marked as ns.
Figure 1 shows that nitrogen mineralization increased with time (32 to 128 days) and temperature. Except for soil L2, the mineralization increased relatively more as temperature increased. Soil U3 started with a high content of mineral N, and the influence of temperature and time seemed to be less than for the other soils. Since the contribution of ammonium is negligible at incubation times of 32 days or more, the curves in Figure 1 are based on the nitrate content of the samples. The standard deviation showed very good replicates for all soils except S2 at 20 °C and 128 days of incubation.
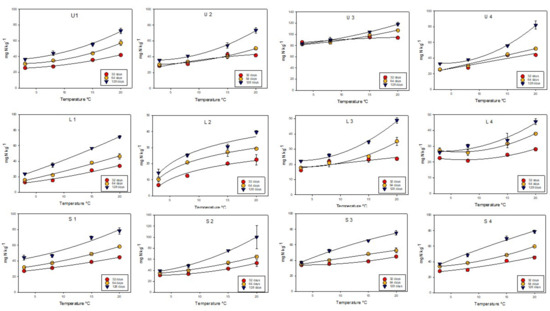
Figure 1.
Mineralization of nitrogen (based on the NO3-N results) as function of incubation time and temperature from 12 apple orchard soils located in three Norwegian regions (Ullensvang (U), Lier (L), and Sogn (S). All results are given as mg N kg−1 soil. Standard deviation is given for all plots.
Table 3 shows the potential for N mineralization at a temperature of 8 °C and 15 °C during incubation periods of 64 and 128 days (2 and 4 months). Although the growing season can last several months, studies have shown that the most active nitrogen uptake by apple trees occurs in the first part of the season, i.e., after dormancy, during flowering and fruit set and their growth development [34]. Therefore, the two-month incubation period (64 days) may be the most important period to focus on. Temperatures of 8 °C and 15 °C were chosen for the treatments because they reflect the natural variation of soil temperature in the root zone during the growing season of fruit trees, i.e., from early spring to mid-summer in the studied areas [35]. Even though the incubation was carried out under ideal conditions in terms of humidity and oxygenation, it may differ from real field conditions, reflecting the relative differences between soils. The numbers in Table 3 are based on the calculation of concentration values obtained from the fitting curve (Figure 1) (equations not shown). Soil density in the field at a soil depth of 25 cm is the depth used by routine laboratories when reporting N supply capacity per ha to farmers.

Table 3.
N mineralization based on incubation at different temperatures and times on soils from three Norwegian regions (Ullensvang (U), Lier (L), and Sogn (S)). Results at different temperatures are the change in mineral N from start of incubation. All results are given as kg N ha−1 to a soil depth of 25 cm.
For all soils, an average of 56 kg N ha−1 was mineralized in 64 days at 8 °C, which increased by 39% to 78 kg N ha−1 in the following two months. At 15 °C, 83 and 121 kg N ha−1 were mineralized, respectively, during the same period, an increase of 46%. This shows that an increase in temperature also increases the rate of N mineralization.
A correlation analysis showed no significant relationship between mineralization of N and total N content or C/N ratio at all selected temperatures and incubation times. However, it was found that there was a trend with increasing temperature. For example, at 128 days of incubation, the correlation at 8, 15, and 20 °C was 0.12, 0.31, and 0.51, respectively. The soil parameters that had significant effects on N mineralization were pH and Ca-AL. The effect of pH increased over the incubation period from 32 to 128 days, regardless of temperature, but was significant only at 128 days (r = 0.51–0.59). The positive correlation with Ca- AL exists only at the longest incubation time under the temperatures of 15 °C and 20 °C (r = 0.56–0.57).
Routine soil testing for available nitrogen has not been used in Norwegian agriculture, mainly because of the mineralization of organic nitrogen, which is difficult to measure, and because the results are difficult to relate the field conditions. However, a method called the Øien method, based on anaerobic incubation in 2 M KCl under heating, was developed about 50 years ago by Selmer-Olsen et al. [36]. The estimated mineralization of organic matter gave the amount of N released for plant nutrition during the growing season. The method has not been used in practice, but showed good correlation with plant uptake by grass and cereals in both laboratory experiments [28] and field trials [37]. Soil organic matter may be different in grassland soils than in orchard soils due to differences in fertilization and management practices. It is still of interest to see whether incubation results for orchard soils correlate with the anaerobic KCl method to provide a tool for better N fertilizer planning.
Figure 2 shows the relationship between the available N after mineralization at 8 and 15 °C and 64 days of incubation and the N extracted using the Øiens method. The correlation between the methods is very good, with an R2 of 0.85 and 0.90 for the two temperature regimes, respectively.
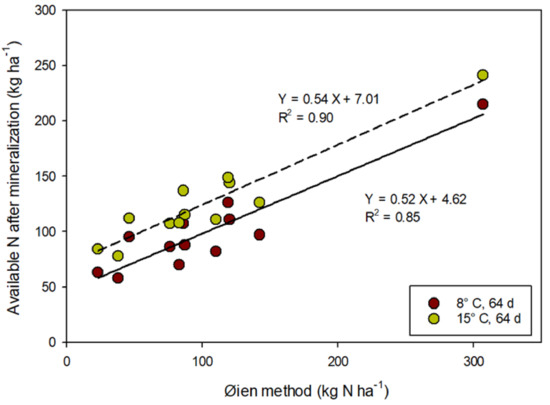
Figure 2.
The correlation between extractable N after Øien method and available N after mineralization of 64 days.
The relationship between the Øiens method and mineral N after incubation is very good, as shown in Table 4. The mineral N extracted after 32 days and at 15 °C and after 64 days and at 8 °C was 103% and 97%, respectively, almost equal to the amount of N extracted using the Øiens method.

Table 4.
The relationship between available N after mineralization and extracted using Øiens method expressed as slope of the regression line, R2, and % of Øien N as average for all 12 soils.
4. Discussion
4.1. Nutrient Status of the Soils
In general, the soils used for the studies have a good nutrient status when macronutrients P, K, Mg, and Ca are compared with the reported optimal values for orchard soils [19]. The soils belong to loamy soils with a low clay content (2–14%), which underlines the importance of organic matter as an important factor for nutrient availability. However, the pH is rather low, as 7 out of 12 soils have a pH lower than the recommended values for these orchard soils, which require a value higher than 5.5 [19]. All soils in the Ullensvang area had a pH below 5.5, with the lowest value being 4.68, while two other soils in the Lier area also had a pH below 5.5. Aside from the fact that low pH can affect nutrient availability, this pH also has a significant effect on nitrogen mineralization. As Lyngstad has shown, soils limed from pH of 5 to about 7 result in increased mineralization due to increased microbial activity [15]. Raising the pH in regions of low pH by liming, therefore, also increases N supply capacity through increased mineralization.
Low pH can cause a problem with aluminum toxicity, which affects root cells and causes stress growth of apple trees. A pH lower than 5 results in the most toxic positively charged Al species in the soil solution, especially when clay minerals are present [38].
In this study, the top 25 cm of soil was examined. However, apple tree roots also take up nutrients from deeper layers, where pH is usually higher and toxicity problems are less important. Most common apple tree roots penetrate the upper 80 cm of the soil, and the 20–40 cm layer is the central zone for nutrient uptake if dwarf roots are involved [39]. Nitrate is readily leached from the uppermost layer by irrigation or precipitation, and mineralized N can contribute significantly to N supply even in deeper soil layers than the layer sampled in this study. On average, readily available mineral N for all original soils was 44 kg N ha−1, with a wide range of variation from 11 (L1 and L3) to 185 kg N ha−1 (U3) (Table 3). Incubation increased these amounts. Nitrate was the major source of N resulting from incubation, and the leaching of N in the loamy soil is likely.
Soil mineral N content varied by region, with the lowest average values in Lier (Table 2). Compared to total nitrogen content, these values are very low, and except for soil U3, which contains 97.6% organic nitrogen, all other soils have an average organic nitrogen content of 99.5%, indicating a high potential for nitrogen mineralization in all soils.
4.2. Mineralization of Nitrogen
The acidity of the soil affects mineralization, as discussed in Section 4.1. In our material, there is a positive effect of increased pH on mineralization, as a significant increase in mineralization was found when comparing soils with a pH < 5.6 and soils with a pH > 5.6. This was confirmed by other studies on cultivated mineral soils with a similar pH as an effect of increased microbial activity [15,40]. Raising the pH of acidic soils is therefore a good strategy to increase the soil’s contribution to plant-available nitrogen.
Orchards are often not established on soil with the highest organic matter content, and the variation in total C content among our soils is quite wide, ranging from 0.81 to 5.13%. Soil organic matter has many important properties for plant nutrition, such as improving the soil structure, water-holding capacity, nutrient adsorption, and potential for nitrogen supply through the mineralization of organic matter [41]. The mineralization potential is strongly influenced by temperature and time, as our results have shown. However, mineralization studies are often conducted at quite high temperatures, such as 30–40 °C [15,25], to measure N mineralization potential. If we try to estimate soil mineralization potential under Nordic temperature conditions during the growing season, measurements should be made in the root zone. For this reason, our study was carried out in the range of 3 to 20 °C to cover the most common temperatures in Norwegian orchard soils. It is evident that mineralization starts rapidly, even at 3 °C, and has already significantly increased the mineral N content in all soils after 2 days, with one exception in sample 3 of Hardanger soils (Table 2). This soil U3 has a high initial concentration of mineral N, and it appears that this suppresses mineralization throughout the experiment. This means that U3 has an initial concentration 3 to 25 times higher than the other soil samples. Even though the difference between the other samples is relatively large, there was no significant correlation between the initial concentration and the amount of mineralized, N regardless of temperature and time. The low pH may also affect the start of mineralization of these soils, as microbiological activity is likely to be lower than at higher pH values. At the beginning of the incubation period, NH4-N accounted for an average of 35% of the mineral N. Both NO3-N and NH4-N increased during the first few days of incubation. However, NH4-N decreased rapidly and nitrification increased, which is a normal process in aerobic incubation [42].
The longest incubation period of 128 days should reflect a full growing season. N uptake is most active in the first part of the growing season, so an incubation period of 64 days may be more realistic [34]. Temperature has a strong influence on N mineralization, as shown in Figure 1 and Table 3. At 64 days of incubation, N release at 8 °C and 15 °C varied in the range of 30 (U3)-78 (S4) kg N ha−1 and 56 (U3)-108 (S4) kg N ha−1, respectively, with average values of 56 and 83 kg N ha−1 for each temperature. The same soils used for the mineralization tests were analyzed in a routine laboratory where N delivery capacity was reported based on total N measurements. The mineralization results represent only 57 and 83%, respectively, of what was measured by the routine commercial laboratory as plant-available N for a growing season, based on the data shown in Figure 3. In laboratory experiments, soils are brought to optimal conditions that nature does not provide. Even with realistic extraction times, incubation experiments overestimate actual mineralization because soil moisture, soil temperature, and field oxygen conditions often vary.
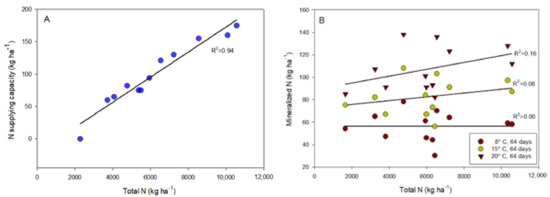
Figure 3.
N supplying capacity given by a routine soil laboratory (A) and N mineralization in the incubation experiments (B) as a function of total N in soil.
A change in soil temperature due to climate change will significantly affect soil N mineralization. Model calculations indicate that the average annual temperature in Vestland and Buskerud counties, which include our study areas, could increase by 4 °C by 2100, and precipitation could increase, with winters milder than in 2000 [43]. Based on the formulas underlying the curves in Figure 1 (formulas not shown), a calculation of the temperature effect on N mineralization is performed. Increasing soil temperature by one degree in the temperature range of 8–10 °C and 13–15 °C increases the amount of mineralized N by 5–7%. A change in climate in these areas can increase the decomposition of organic matter, and thus significantly increase the supply of plant-available N in the soil. Cold climates, as in the Nordic countries, will on the one hand limit the mineralization of nitrogen in the soil, but on the other hand will also limit the cultivation of fruit. One positive effect of climate change due to temperature increase is that apple areas will increase, and overall production will increase. Research has shown that an increase in average air temperature in the areas where soils are sampled for our study allows for the cultivation of more heat-demanding varieties [44].
As previously reported, the N recommendation in Norwegian orchards is 55–75 kg N ha−1 per year for normal yields [19], which may indicate that mineralization normally provides some amount of N from the soil, but it is not the main contribution to plant N requirements. A Nordic project concluded that the average N mineralization in cultivated soils from early spring to the end of the growing season is 5 kg ha−1, and that a 1% increase in soil organic matter in the top soil layer increases net mineralization by about 5 kg N ha−1 during the growing season [29]. However, there was wide variation within sites from year to year, reflecting climatic differences between years. Variations were greater within sites from year to year than between sites in different countries.
Some investigations have found a significant positive correlation between total soil and N mineralization [8,45], but opposite results have also been reported [29]. A compilation of data from various studies in the USA and Europe showed a very weak correlation between total N and mineralization [46]. Some incubation studies from California gave a similar result, where R2 did not exceed 0.57, as in our experiment [47]. The results of the large field experiments in the Nordic countries showed no significant correlation between total soil nitrogen content and N mineralization rate [29], and these results include Norwegian soils with this very low positive correlation [23,28]. This is consistent with the results of our study, which are shown in Figure 3. The lack of this correlation in our experiment may be caused by the low temperature in the incubation tests in addition to variations in pH, both of which affect the biological activity in the soil. Another influencing factor could be the character of the organic matter, which usually varies in composition and quality due to the use of compost and different types of manure over the years. These results show that total nitrogen is not a reliable parameter that can be used alone as an indicator of nitrogen mineralization in the soil.
In the soils, the C/N ratio is in the range of 11 to 18, which is considered to be an optimal ratio for N mineralization [48], and it is assumed that this was not a limiting factor in this experiment. It has been documented that different types of organic material such as sewage sludge, animal manure, and plant residues have different effects on N mineralization related to the different biologically active components in the organic material. This was confirmed in both laboratory incubation [49,50] and comparative tests between laboratory and field incubation [51]. A recent literature review and model fit showed that after 100 days of incubation, 16% of the total N is in the form of mineralized N in poultry manure, and up to 39% is in the form of mineralized N in poultry compost, compared to less than 10% in other composts made from more stable material [52]. This also shows how difficult it is to use organic amendments to meet nutrient requirements in orchards.
4.3. Soil Analysis for Available N
Soil analysis of plant-available N has not been offered to Norwegian farmers, mainly because of the difficulty of estimating mineralization in cold climates, the leaching of nitrogen during autumn precipitation and spring snowmelt, and the lack of available laboratory methods. In the widely used fertilizer-planning computer program in Norway [53], the correction for N fertilization based on organic matter content is shown in Table 5. The correction is used for all crops and is constructed as a staircase function, which gives more of an indication of what the organic matter may mean for the mineralization of N rather than a professionally good estimation. Organic matter content in the soils studied varied from 1.5 to 10% (Table 1). No mineralization is counted in Table 5, which is obviously incorrect given the mineralization data reported. The current practice of mineralization is only considered when the soil has an organic content of 12.6% or more. This is also not consistent with the previously cited results of a Nordic field study [29].

Table 5.
Correction of N fertilization (kg ha−1) based on content of organic matter in soil.
For the last 5–10 years, fruit farmers in Norway have been sending soil samples abroad for analysis, and commercial laboratories perform the analysis according to local agriculture practices, which are not always adapted to Norwegian climate and soil conditions. One example is analysis of nitrogen supply capacity, expressed in kg N ha−1 mineralized from organic matter during the growing season. This is a figure that does not take into account local conditions on the farm and the quality of the organic matter, and may give a false impression of the nitrogen supply from the soil.
Figure 3 shows the nitrogen supply capacity relative to total soil nitrogen content reported by a routine foreign laboratory and the values measured in our mineralization study on the same soils, using orchard soils with organic matter contents ranging from 1.5 to 10% (Table 1). The commercial laboratory assumes an almost perfect relationship between nitrogen supply and total N content, while our data show almost no relationship between these two parameters. The pH of orchard soils varies by a factor of more than 100, ranging from very acidic to neutral (Table 1), and the origin and quality of organic matter can vary greatly from soil to soil, affecting the biological life and mineralization capacity of the soil. Other studies on Norwegian soils have shown that total N is not necessarily a good indicator of nitrogen mineralization or plant availability in the soil (see Section 4.2).
In the average of 40 laboratory reports from abroad, 1.7% of the total N in soil organic matter is mineralized during the growing season [55]. Figure 2 shows that there is a good relationship between Øien N and mineral N after mineralization. Growth experiments with grass and cereals have shown that the laboratory method can estimate plant availability well, and that about 50% of the amount measured by the method is taken up by plants in field experiments [37] and 44% in a laboratory pot experiment [28]. The rooting depth of apple trees, grass and cereals is quite similar, so the results for these crops can be considered comparable.
Mineralization measured by the Øiens method averaged 60 kg N ha−1 (extracted minus mineral N before extraction) for all soils. Assuming 50% supply and uptake by plants, which was shown in the field study by Øien [37], this corresponds to 30 kg N ha−1 and 0.48% of the total mineralized N per year. This amount is far from routine laboratory data, and is more realistic in relation to the Norwegian climate and agricultural practice. In our opinion, the KCl method of Øien and Selmer-Olsen [23] can be used as a method to obtain a good measure of mineralizable N in the soil during the growing season, including in orchard soils. This requires that the soil be analyzed quickly after sampling or frozen until analysis can be performed. Drying and storing dry soil increases the mineralization of N in the soil [26]. The current practice of storing dry soils for varying lengths of time before analysis provides an incorrect measure of plant-available N. This method is judged to be much better for predicting the plant availability of soil N, and is a rapid method compared to the time-consuming incubation methods.
Results for available nitrogen based on total nitrogen significantly overestimate plant-available N and may result in under-fertilization to achieve optimum good quality yields.
5. Conclusions
The soil in many orchards has low pH, which negatively affects the mineralization of nitrogen from organic matter. Incubation experiments show that both temperature and time have a strong effect on mineralization, but even at a temperature as low as 3 °C, mineralization is significant. A 1 °C increase in temperature in the 8–15 °C temperature range increases the amount of N mineralized by 5–7%, indicating an effect of a possible change to a warmer climate. There is no significant relationship between total nitrogen in the soil and the amount of mineralized nitrogen, most likely due to differences in the origin and quality of the organic material at the experimental sites.
Mineralized N shows a very good correlation with the soil chemical method of measuring plant-available N in the soil by extraction with potassium chloride during heating. It can be used as a method for estimating the amount of plant-available N in fresh soils, and is recommended for the most balanced nutrient supply.
The routine laboratory measurement of plant-available nitrogen based on total nitrogen without the evaluation of the quality of the organic material and the different Nordic climatic and growing conditions leads to the overestimation of plant-available nitrogen, which can result in severe under-fertilization and thus lower yields with poor quality.
Author Contributions
Conceptualization, T.K. and M.M.; methodology and formal analysis, V.Z. and T.K.; writing—original draft preparation T.K.; writing—review and editing T.K., V.Z., A.S., M.F.A., V.L. and M.M.; project administration M.M. and T.K. and funding acquisition M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Research Council of Norway. Project name TERREPLE (Precision fertilization to apple trees). Project number 281968.
Data Availability Statement
All data are stored in the available data formats at the Norwegian University of Life Sciences.
Acknowledgments
We want to thank chief engineer Oddny Gimmingsrud and master student Tijana Malesevic for valuable help to carry out the incubation experiment and laboratory analysis. We also want to thank the advisors Stine Huseby and Gaute Myren in Norwegian Advisory Service for soil sampling and useful information about the sampling sites.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salisbury, F.B.; Ross, C.W. Plant Physiology, Hormones and Plant Regulators: Auxins and Gibberellins; Wadsworth Publishing: Belmont, CA, USA, 1992. [Google Scholar]
- Faust, M. Physiology of Temperate Zone Fruit Trees; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 1989. [Google Scholar]
- Kvåle, A. Fruktdyrking, 3rd ed.; Landbruksforlaget: Oslo, Norway, 1995. [Google Scholar]
- Zekri, M.; Obreza, T. Nitrogen (N) for Citrus Trees; A Series of the Department of Soil and Water Sciences; University of Florida: Gainesville, FL, USA, 2013. [Google Scholar]
- Keeney, D.R.; Nelson, D.W. Nitrogen -Inorganic forms. In Methods of Soil Analysis. Part 2; Page, A.L., Ed.; Agronomy: Madison, WI, USA; pp. 642–698.
- Lågbu, R.; Nyborg, Å.; Svendgård-Stokke, S. Jordsmonnstatistikk i Norge; NIBIO Rapport; NIBIO: Ås, Norway, 2018; Volume 13, p. 75. [Google Scholar]
- Paulsen, K.N. Soil Qulity and Fertiliser Application in Norwegian Apple Orchards. Master’s Thesis, Norwegian University of Life Sciences (NMBU), Ås, Norway, 15 May 2019. [Google Scholar]
- Dessureault-Rompre, J.; Zebarth, B.J.; Burton, D.L.; Georgallas, A. Predicting soil nitrogen supply from soil properties. Can. J. Soil Sci. 2015, 95, 63–75. [Google Scholar] [CrossRef]
- Risch, A.C.; Zimmermann, S.; Ochoa-Hueso, R.; Schuts, M.; Frey, B.; Firn, J.L.; Fay, P.A.; Hagedorn, F.; Borer, E.T.; Seabloom, E.W.; et al. Soil net nitrogen mineralisation across global grasslands. Nat. Commun. 2019, 10, 4981. [Google Scholar] [CrossRef] [PubMed]
- Cassity-Duffey, K.; Cabrera, M.; Franklin, D.; Gaskin, J.; Kissel, D. Effect of soil texture on nitrogen mineralization from organic fertilizers in four common southeastern soils. Soil Sci. Soc. Am. J. 2020, 84, 534–542. [Google Scholar] [CrossRef]
- Zhang, B.; Jingyi, L.; Craig, F.D.; Woodley, A.L.; Yang, X. Effect of crop rotation and cropping history on net nitrogen mineralization dynamics of a clay loam soil. Can. J. Soil Sci. 2022, 102, 445–456. [Google Scholar] [CrossRef]
- Mulder, E.G. Effect of liming an acid peat soil on microbial activity. Trans. 4th Int. Congr. Soil Sci. 1950, 2, 117–121. [Google Scholar]
- Edmeades, D.C.; Judd, M.; Sarathchandra, S.U. The Effect of Lime on Nitrogen Mineralization as measured by Grass growth. Plant Soil 1981, 60, 177–186. [Google Scholar] [CrossRef]
- Adams, F.; Martin, J.B. Liming Effects on Nitrogen Use and Efficiency. In Nitrogen in Crop Production; Hauck, R.D., Ed.; ASA, CSSA, SSSA: Madison, WI, USA, 1984; pp. 417–426. [Google Scholar]
- Lyngstad, I. Effect of Liming on Mineralization of Soil Nitrogen as Measured by Plant Uptake and Nitrogen Released during Incubation. Plant Soil 1992, 144, 247–253. [Google Scholar] [CrossRef]
- Miller, K.A.; Aegerter, B.J.; Clark, N.E.; Leinfelder-Miles, M.; Miyao, E.M.; Smith, R.; Wilson, R.; Geisseler, D. Relationship Between Soil Properties and Nitrogen Mineralization in Undisturbed Soil Cores from California Agroecosystems. Commun. Soil Sci. Plant Anal. 2019, 50, 77–92. [Google Scholar] [CrossRef]
- Carroll, J.E.; Robinson, T.L. New York Integrated Fruit Production Protocol for Apples; Cornell University: New York, NY, USA, 2006; p. 31. [Google Scholar]
- Kowalczyk, W.; Wrona, D.; Przybylko, S. Effect of Nitrogen Fertilization of Apple Orchard on Soil Mineral Nitrogen Content, Yielding of the Apple Trees and Nutritional Status of Leaves and Fruits. Agriculture 2022, 12, 17. [Google Scholar] [CrossRef]
- Vangdal, E. Gjødsling til Frukthagar; NIBIO: Ås, Norway, 2017. [Google Scholar]
- NIBIO. Fertilization Handbook; Norwegian Institute of Bioeconomy Research: Ås, Norway, 2023; Available online: https://www.nibio.no/tema/jord/gjodslingshandbok (accessed on 1 January 2023).
- Benbi, D.K.; Richter, J. A critical review of some approaches to modelling nitrogen mineralization. Biol. Fertil. Soils 2002, 35, 168–183. [Google Scholar] [CrossRef]
- Ros, G.H.; Temminghoff, E.J.M.; Hoffland, E. Nitrogen mineralization: A review and meta-analysis of the predictive value of soil tests. Eur. J. Soil Sci. 2011, 62, 162–173. [Google Scholar] [CrossRef]
- Øien, A.; Selmer-Olsen, A.R. A Laboratory method for evaluation of available nitrogen in soil. Acta Agric. Scand. 1980, 30, 149–156. [Google Scholar] [CrossRef]
- Bærug, R.; Lyngstad, I.; Selmer-Olsen, A.R.; Øien., A. Studies on Soil Nitrogen. I. An Evaluation of Laboratory Methods for Available Nitrogen in Soils from Arable and Ley-arable Rotations. Acta Agric. Scand. 1973, 23, 173–181. [Google Scholar] [CrossRef]
- Øien, A.; Selmer-Olsen, A.R.; Bærug, R.; Lyngstad, I. Studies on Soil Nitrogen. III. Effects of Drying, Deep-freezing and Storage on Moist Soil on Nitrogen Mineralization. Acta Agric. Scand. 1974, 24, 222–226. [Google Scholar] [CrossRef]
- Cabrera, M.L.; Kissel, D.E. Potentially Mineralizable Nitrogen in Disturbed and Undisturbed Soil samples. Soil Sci. Soc. Am. J. 1988, 52, 1010–1015. [Google Scholar] [CrossRef]
- Westerplate, J.; Norwegian Agricultural Advisory Service, NLR Østafjells, Gvarv, Norway. Soil chemical analysis from Eurofins laboratory in Netherlands. Personal communication, 2017. [Google Scholar]
- Selmer-Olsen, A.R.; Øien, A.; Bærug, R.; Lyngstad, I. Evaluation of a KCI-Hydrolyzing method for available nitrogen in soil by pot experiment. Acta Agric. Scand. 1981, 31, 251–255. [Google Scholar] [CrossRef]
- Linden, B.; Lyngstad, I.; Sippola, J.; Soegaard, K.; Kjellerup, V. Nitrogen Mineralization during the Growing Season. 2. Influence of soil organic matter content, and effect on optimum nitrogen fertilization of spring barley. Swed. J. Agric. Res. 1992, 22, 49–60. [Google Scholar]
- Elonen, P. Particle-Size Analysis of Soil. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 1971. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Ed.; SSSA Book series; Wiley: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Egnér, H.; Domingo, W.R. Untersuchungen über die chemische Boden-Analyse als Grundlage für die Beurteilung des Nährstoffzustandes der Boden. Kung. Landbr. Ann. 1960, 26, 196–215. [Google Scholar]
- Institute, S. SAS for Windows, Version 9.4; SAS Support: Cary, NC, USA, 2016. [Google Scholar]
- Lourens, F. Kinoch- Volhoubare Landbou; RSA: Haifa, Israel, 1999. [Google Scholar]
- NIBIO. Agronomic Meteorological Service, 13 July 2023. Available online: https://lmt.nibio.no (accessed on 13 July 2023).
- Selmer-Olsen, A.R.; Øien, A.; Bærug, R.; Lyngstad, I. Studies on Soil Nitrogen. II. Anaerobic Incubation of Soil in Potassium Cloride Solution. Acta Agric. Scand. 1974, 27, 217–221. [Google Scholar] [CrossRef]
- Øien, A. Jordanalyser for vurdering av tilgjengelig nitrogen. Jord Og MyrundersøKelser 1985, 5, 259–265. [Google Scholar]
- Havelin, J.L.; Tisdale, S.L.; Nelson, W.L. Soil Fertility and Fertilizers. An Introduction to Nutrient Managment, 8th ed.; Prentice Hall: Pearson, NJ, USA, 2014. [Google Scholar]
- Licina, V.; Krogstad, T.; Simic, A.; Aksic, M.F.; Meland, M. Nutrition and Fertilizer Application to Apple Trees—A Review; NIBIO Report; NIBIO: Ås, Norway, 2021; Volume 7, pp. 1–79. [Google Scholar]
- Curtin, D.; Campbell, C.A.; Jalil, A. Effects of acidity on mineralization: pH-dependence of organic matter mineralization in weakly acidic soils. Soil Biol. Biochem. 1998, 30, 57–64. [Google Scholar] [CrossRef]
- Weil, R.R.B.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Pearson: New York, NY, USA, 2017. [Google Scholar]
- Vlassak, K. Total Soil Nitrogen and Nitrogen Mineralization. Plant Soil 1970, 32, 27–32. [Google Scholar] [CrossRef]
- Hanssen-Bauer, I.; Førland, E.J.; Haddeland, I.; Hisdal, H.; Lawrence, D.; Mayer, S.; Nesje, A.; Nilsen, J.E.Ø.; Sandven, S.; Sandø, A.B.; et al. Climate in Norway 2100—A Knowledge Base for Climate Adaption. Miljødirektoratet: Oslo, Norway, 2017; pp. 1–48. [Google Scholar]
- Vimic, A.V.; Mandic, M.V.; Aksic, M.F.; Vukicevic, K.; Meland, M. Climate potential for apple growing in Norway—Part 1: Zoning of areas with heat conditions favorable for apple growing under obsered climate change. Atmosphere 2023, 14, 993. [Google Scholar] [CrossRef]
- Matar, A.E.; Beck, D.P.; Pala, M.; Garabet, S. Nitrogen Mineralization Potentials of selected Mediterranean Soils. Commun. Soil Sci. Plant Anal. 1991, 22, 23–36. [Google Scholar] [CrossRef]
- Vigil, M.F.; Eghball, B.; Cabrera, M.L.; Jakubowski, B.R.; Davis, J.G. Accounting for seasonal nitrogen mineralization: An overview. J. Soil Water Conserv. 2002, 57, 464–469. [Google Scholar]
- Wade, J.; Horwath, W.R.; Burger, M.B. Integrating Soil Biological and Chemical Indices to Predict Net Nitrogen Mineralization across California Agricultural Systems. Soil Sci. Soc. Am. J. 2016, 80, 1675–1687. [Google Scholar] [CrossRef]
- Lazicki, P.; Geisseler, D.; Lloyd, M. Nitrogen mineralization from organic amendments is variable but predictable. J. Environ. Qual. 2020, 49, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.M.; Tabatabai, M.A. Mineralization of Nitrogen in Soils Amended with Organic wastes. J. Environ. Qual. 1986, 15, 193–198. [Google Scholar] [CrossRef]
- Masunga, R.H.; Uzokwe, V.N.; Malay, P.D.; Odeh, I.; Sing, A.; Buchan, D.; De Neve, S. Nitrogen mineralization dynamics of different valuable organic amendments commonly used in agriculture. Appl. Soil Ecol. 2016, 101, 185–193. [Google Scholar] [CrossRef]
- Pinto, R.; Brito, L.M.; Coutinho, J. Nitrogen Mineralization from Organic Amendments Predicted by Laboratory and Field Incubations. Commun. Soil Sci. Plant Anal. 2020, 51, 515–526. [Google Scholar] [CrossRef]
- Geisseler, D.; Smith, R.; Cahn, M.; Muramoto, J. Nitrogen mineralization from organic fertilizers and composts: Literature survey and model fitting. J. Environ. Qual. 2021, 50, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Skifteplan, Skifteplan Fertilization Program. Agromatic AS. 2022. Available online: www.skifteplan.no (accessed on 1 January 2023).
- Thom, T.; Hanlon, A.; Lindsay, R.; Richards, J.; Stoneman, R.; Brooks, A. Conserving Bogs. The Management Handbook, 2nd ed.; Scottish Raised Bog Conservation Project: IUCN UK Peatland Programme; Royal Soc Wildlife Trust: Nottinghamshire, UK, 2014. [Google Scholar]
- Krogstad, T. Results of 40 soil samples analysed at a commersial laboratory. Acta Agric. Scand. 2023; scientific article in progress. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).