Genome-Wide Identification and Expression Pattern Analysis of Polyphenol Oxidase Gene Family in Agaricus bisporus
Abstract
:1. Introduction
2. Results
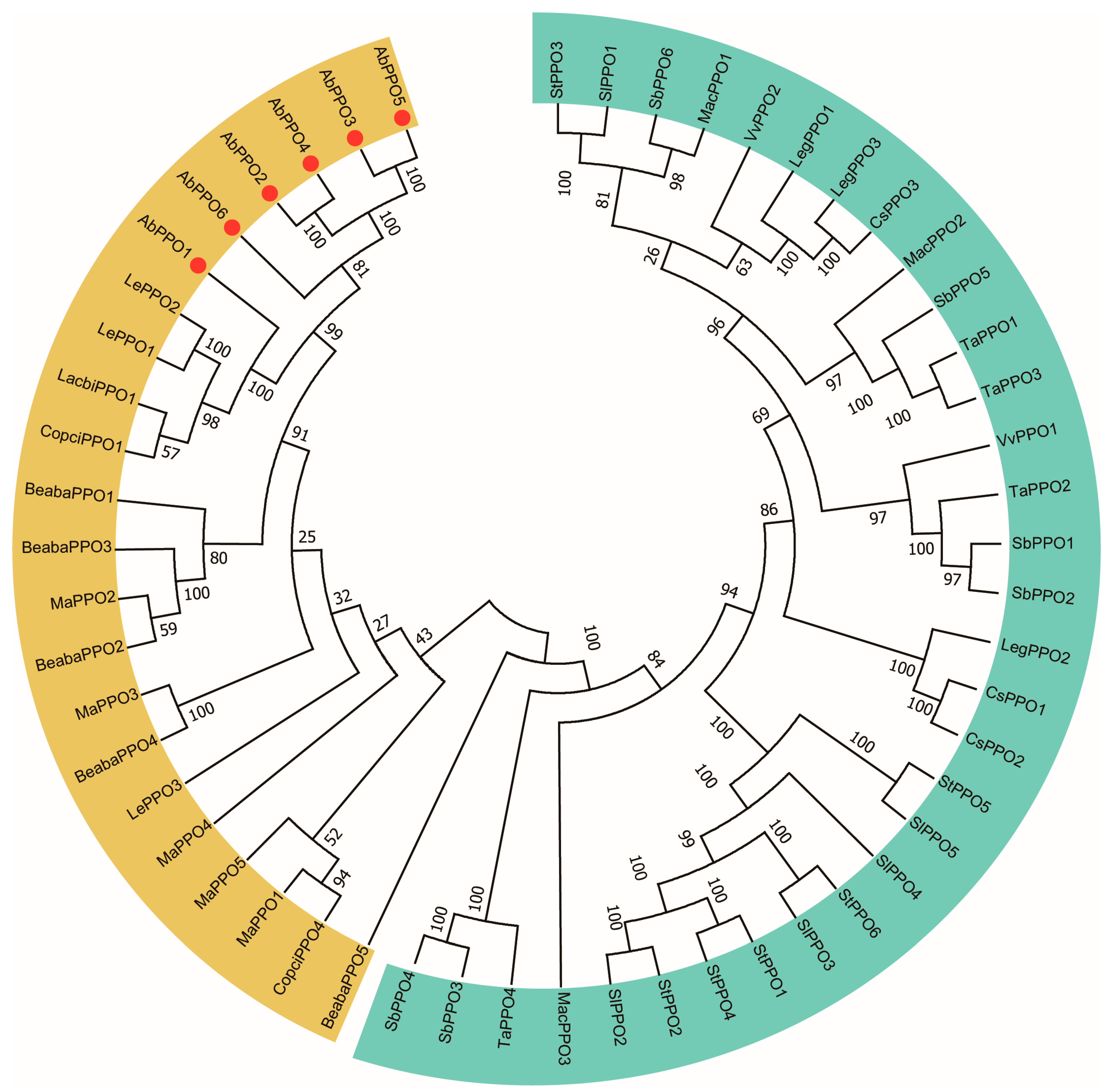
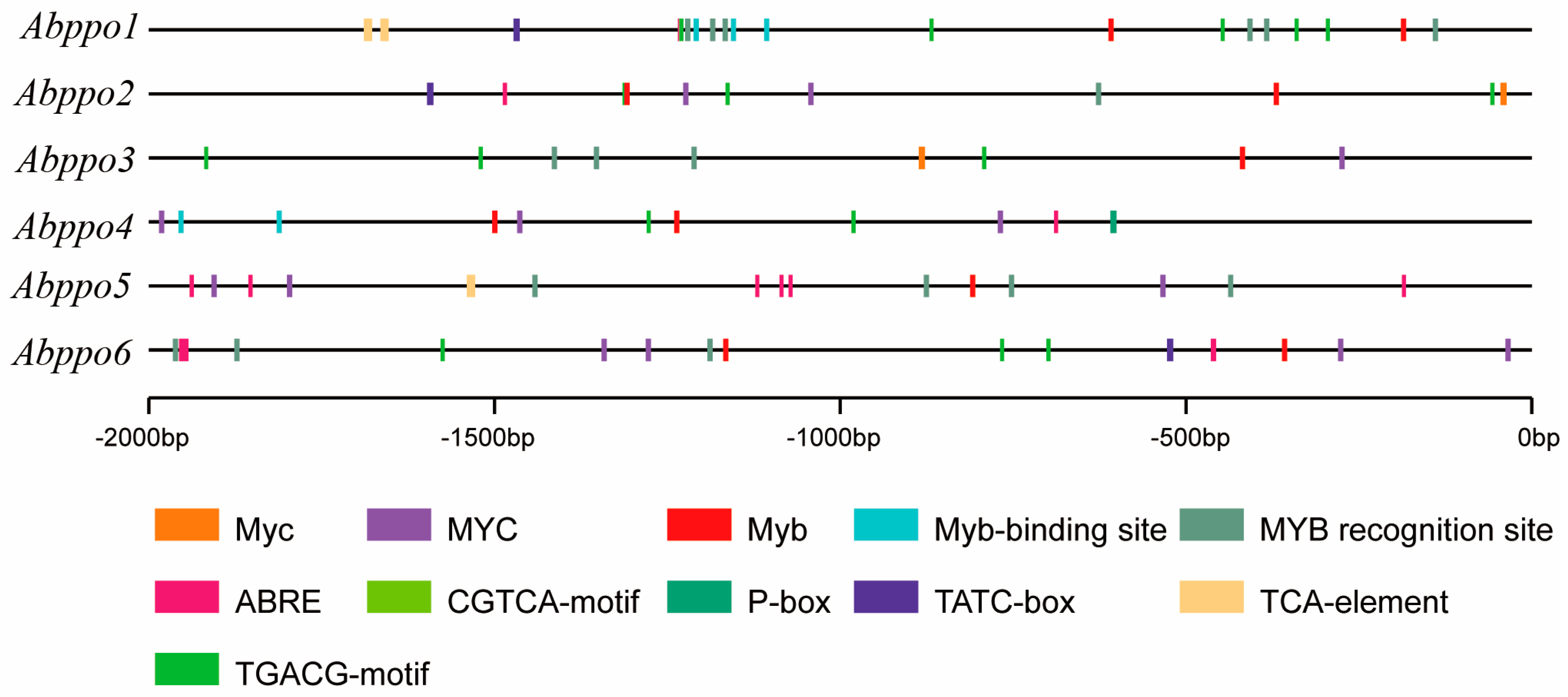
2.1. Sequence Analysis of Abppo Genes
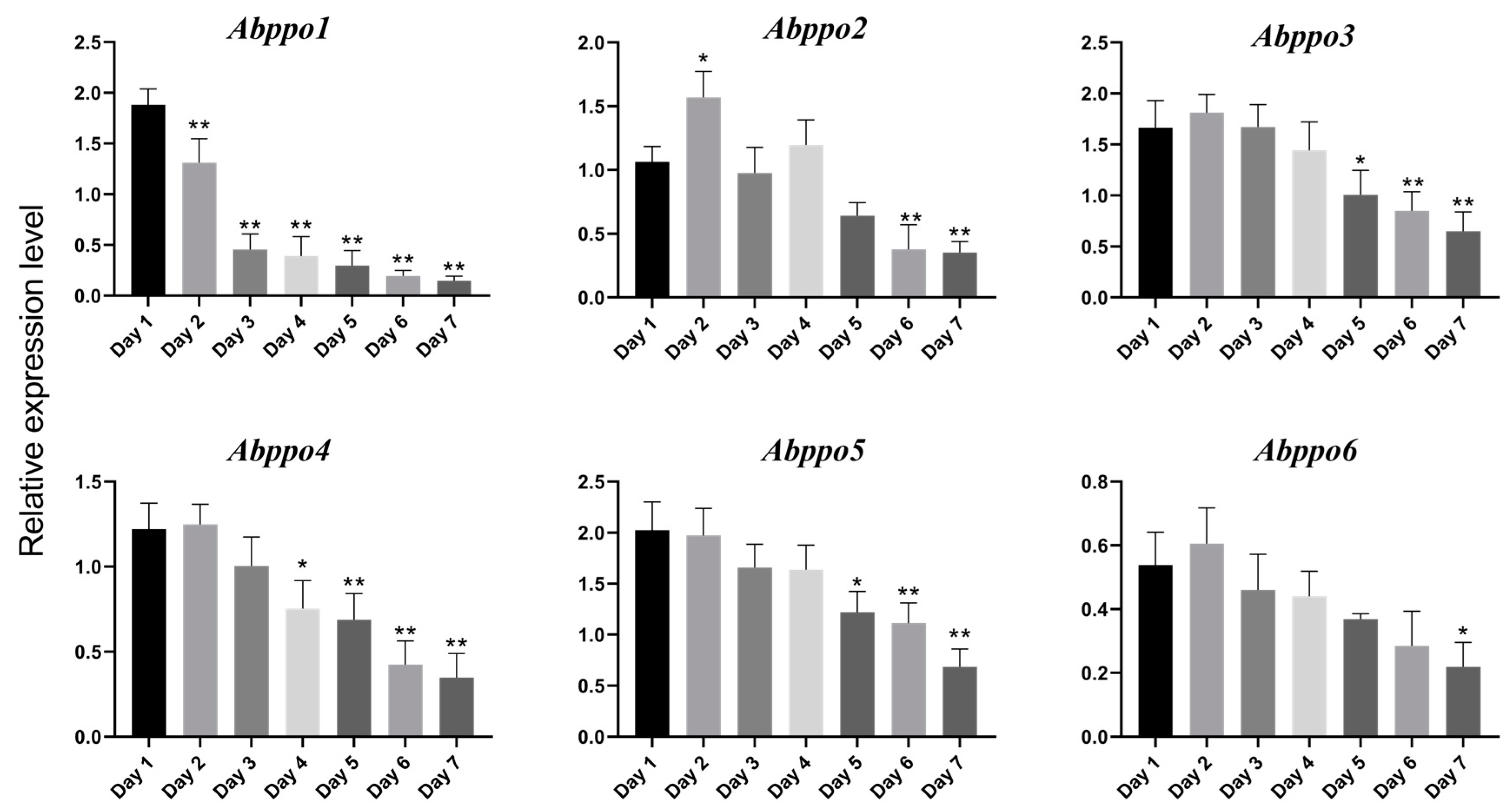
2.2. Expression Patterns of Abppo Genes
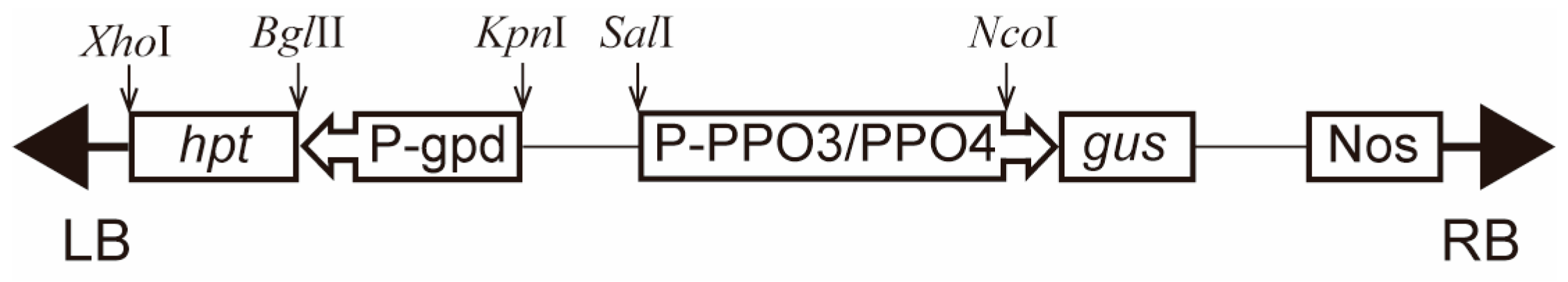
2.3. Activity Analysis of Abppo3 and Abppo4 Promoters
3. Materials and Methods
3.1. Strains and Culture Media
3.2. Plasmids and Vector Construction
3.3. Agrobacterium-Mediated Transformation of A. bisporus
3.4. Gene Bioinformatics Analysis
3.5. Cultivation of Mushroom
3.6. Sampling and RNA Extraction and Reverse Transcription
3.7. Real-Time RT-PCR Analysis
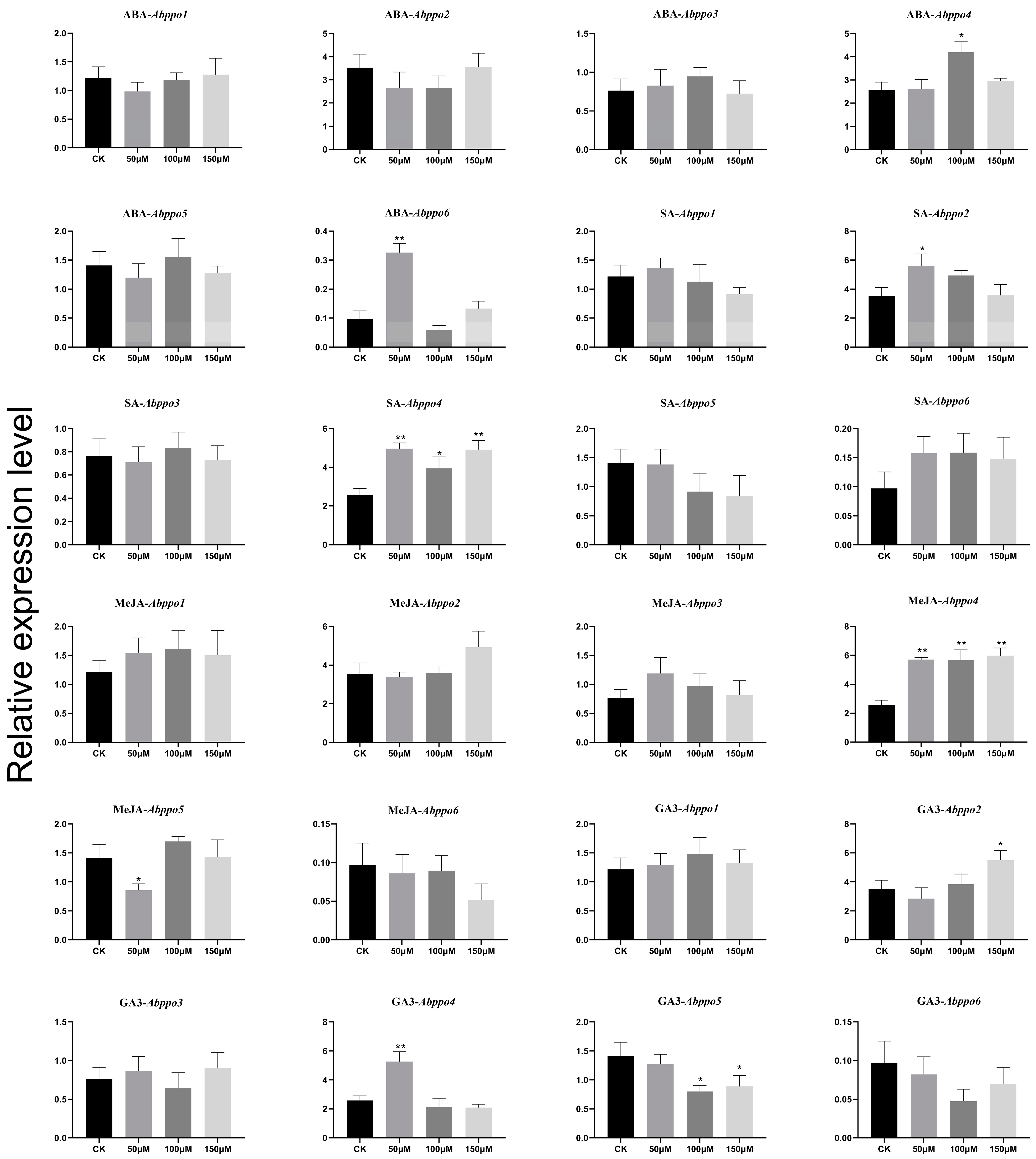
3.8. Phytohormone Induction and Histochemical Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atila, F.; Owaid, M.N.; Shariati, M.A. The nutritional and medical benefits of Agaricus bisporus: A review. J. Microbiol. Biotechnol. Food Sci. 2017, 7, 281–286. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Yáñez-Fernández, J.; Castro-Muñoz, R. Dextran/chitosan blend film fabrication for bio-packaging of mushrooms (Agaricus bisporus). J. Food Process. Preserv. 2021, 45, e15489. [Google Scholar] [CrossRef]
- Salmones, D.; Gaitan-Hernandez, R.; Mata, G. Cultivation of Mexican wild strains of Agaricus bisporus, the button mushroom, under different growth conditions in vitro and determination of their productivity. Biotechnol. Agron. Soc. Environ. 2018, 22, 45–53. [Google Scholar] [CrossRef]
- Lin, X.H.; Sun, D.W. Research advances in browning of button mushroom (Agaricus bisporus): Affecting factors and controlling methods. Trends Food Sci. Technol. 2019, 90, 63–75. [Google Scholar] [CrossRef]
- Zhang, K.; Pu, Y.Y.; Sun, D.W. Recent advances in quality preservation of postharvest mushrooms (Agaricus bisporus): A review. Trends Food Sci. Technol. 2018, 78, 72–82. [Google Scholar] [CrossRef]
- McCord, J.D.; Kilara, A. Control of enzymatic browning in processed mushroom (Agaricus bisporus). J. Food Sci. 1983, 48, 1479–1484. [Google Scholar] [CrossRef]
- Jolivet, S.; Arpin, N.; Wichers, H.J.; Pellon, G. Agaricus bisporus browning: A review. Mycol. Res. 1998, 102, 1459–1483. [Google Scholar] [CrossRef]
- Bernaś, E. Comparison of the mechanism of enzymatic browning in frozen white and brown A. bisporus. Eur. Food Res. Technol. 2018, 244, 1239–1248. [Google Scholar] [CrossRef]
- Soulier, L.; Foret, V.; Arpin, N. Occurrence of agaritine and γ-glutaminyl-4-hydroxybenzene (GHB) in the fructifying mycelium of Agaricus bisporus. Mycol. Res. 1993, 97, 529–532. [Google Scholar] [CrossRef]
- Wu, X.; Guan, W.; Yan, R.; Lei, J.; Xu, L.; Wang, Z. Effects of UV-C on antioxidant activity, total phenolics and main phenolic compounds of the melanin biosynthesis pathway in different tissues of button mushroom. Postharvest Biol. Technol. 2016, 118, 51–58. [Google Scholar] [CrossRef]
- Gul, I.; Ahmad, M.S.; Naqvi, S.M.S.; Hussain, A.; Wali, R.; Farooqi, A.A.; Ahmed, I. Polyphenol oxidase (PPO) based biosensors for detection of phenolic compounds: A Review. J. Appl. Biol. Biotechnol. 2017, 5, 72–85. [Google Scholar]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Mayer, A.M. Polyphenol oxidases in plants-recent progress. Phytochemistry 1986, 26, 11–20. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, T.; Liu, W.; Zou, L.; Liu, C.; Terefe, N.S. Inhibitory effects of organic acids on polyphenol oxidase: From model systems to food systems. Crit. Rev. Food Sci. Nutr. 2020, 60, 3594–3621. [Google Scholar] [CrossRef]
- Hunt, M.D.; Eannetta, N.T.; Yu, H.; Newman, S.M.; Steffens, J.C. cDNA cloning and expression of potato polyphenol oxidase. Plant Mol. Biol. 1993, 21, 59–68. [Google Scholar] [CrossRef]
- Newman, S.M.; Eannetta, N.T.; Yu, H.; Prince, J.P.; Carmen de Vicente, M.; Tanksley, S.D.; Steffens, J.C. Organisation of the tomato polyphenol oxidase gene family. Plant Mol. Biol. 1993, 21, 1035–1051. [Google Scholar] [CrossRef]
- Kim, J.Y.; Seo, Y.S.; Kim, J.E.; Sung, S.K.; Song, K.J.; An, G.; Kim, W.T. Two polyphenol oxidases are differentially expressed during vegetative and reproductive development and in response to wounding in the Fuji apple. Plant Sci. 2001, 161, 1145–1152. [Google Scholar] [CrossRef]
- Queiroz, C.; Mendes Lopes, M.L.; Fialho, E.; Valente-Mesquita, V.L. Polyphenol oxidase: Characteristics and mechanisms of browning control. Food Rev. Int. 2008, 24, 361–375. [Google Scholar] [CrossRef]
- Tang, T.; Xie, X.; Ren, X.; Wang, W.; Tang, X.; Zhang, J.; Wang, Z. A difference of enzymatic browning unrelated to PPO from physiology, targeted metabolomics and gene expression analysis in Fuji apples. Postharvest Biol. Technol. 2020, 170, 111323. [Google Scholar] [CrossRef]
- Flurkey, W.H.; Inlow, J.K. Proteolytic processing of polyphenol oxidase from plants and fungi. J. Inorg. Biochem. 2008, 102, 2160–2170. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, R.W.; Royer, J.C.; Baller, L.M.; Kohli, Y.; Horgen, P.A.; Anderson, J. Meiotic behavior and linkage relationships in the secondarily homothallic fungus Agaricus bisporus. Genetics 1993, 133, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Morin, E.; Kohler, A.; Baker, A.R.; Foulongne-Oriol, M.; Lombard, V.; Nagye, L.G.; Ohm, R.A.; Patyshakuliyeva, A.; Brun, A.; Aerts, A.L.; et al. Genome sequence of the button mushroom Agaricus bisporus reveals mechanisms governing adaptation to a humic-rich ecological niche. Proc. Natl. Acad. Sci. USA 2012, 109, 17501–17506. [Google Scholar] [CrossRef] [PubMed]
- Wichers, H.J.; Recourt, K.; Hendriks, M.; Ebbelaar, C.E.M.; Biancone, G.; Hoeberichts, F.A.; Mooibroek, H.; Soler-Rivas, C. Cloning, expression and characterisation of two tyrosinase cDNAs from Agaricus bisporus. Appl. Microbiol. Biotechnol. 2003, 61, 336–341. [Google Scholar] [CrossRef]
- Wu, J.; Chen, H.; Gao, J.; Liu, X.; Cheng, W.; Ma, X. Cloning, characterization and expression of two new polyphenol oxidase cDNAs from Agaricus bisporus. Biotechnol. Lett. 2010, 32, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Li, N.Y.; Cai, W.M.; Jin, Q.L.; Qin, Q.P.; Ran, F.L. Molecular cloning and expression of polyphenoloxidase genes from the mushroom, Agaricus bisporus. Agric. Sci. China. 2011, 10, 185–194. [Google Scholar] [CrossRef]
- Weijn, A.; Bastiaan-Net, S.; Wichers, H.J.; Mes, J.J. Melanin biosynthesis pathway in Agaricus bisporus mushrooms. Fungal Genet. Biol. 2013, 55, 42–53. [Google Scholar] [CrossRef]
- Hammond, J.B.; Nichols, R. Changes in respiration and soluble carbohydrates during the post-harvest storage of mushrooms (Agaricus bisporus). J. Sci. Food Agric. 1975, 26, 835–842. [Google Scholar] [CrossRef]
- Yadav, M.C.; Tripathi, Y.K.; Verma, R.N.; Upadhyay, R.C.; Dhar, B.L. Molecular breeding for development of genetically improved strains and hybrids of Agaricus bisporus. In Current Vistas in Mushroom Biology and Production; Mushroom Society of India: Solan, Hiachal Pradesh, India, 2003; pp. 261–274. [Google Scholar]
- Chen, C.; Chen, H.; He, Y.; Xia, R. TBtools, a toolkit for biologists integrating various biological data handling tools with a user-friendly interface. BioRxiv 2018, 289660. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Jefferson, R.A. Plant reporter genes: The GUS gene fusion system. In Genetic Engineering Principles and Methods; Springer: Boston, MA, USA, 1988; pp. 247–263. [Google Scholar]
- Zhang, J.; Sun, X. Recent advances in polyphenol oxidase-mediated plant stress responses. Phytochemistry 2021, 181, 112588. [Google Scholar] [CrossRef]
- Gooding, P.S.; Bird, C.; Robinson, S.P. Molecular cloning and characterisation of banana fruit polyphenol oxidase. Planta 2001, 213, 748–757. [Google Scholar] [CrossRef]
- Lynch, M.; Conery, J.S. The evolutionary fate and consequences of duplicate genes. Science 2000, 290, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, D.; Li, J.; Shao, F.; Lu, S. Characterization of the polyphenol oxidase gene family reveals a novel microRNA involved in posttranscriptional regulation of PPOs in Salvia miltiorrhiza. Sci. Rep. 2017, 7, 44622. [Google Scholar] [CrossRef]
- Hamdan, N.; Lee, C.H.; Wong, S.L.; Fauzi, C.E.N.C.A.; Zamri, N.M.A.; Lee, T.H. Prevention of enzymatic browning by natural extracts and genome-editing: A review on recent progress. Molecules 2022, 27, 1101. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.J.; Cookson, A.; Allison, G.; Sullivan, M.L.; Winters, A.L. Gene expression patterns, localization, and substrates of polyphenol oxidase in red clover (Trifolium pratense L.). J. Agric. Food Chem. 2013, 61, 7421–7430. [Google Scholar] [CrossRef]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.M.; Pavan, S.; Montemurro, C. Polyphenol oxidases in crops: Biochemical, physiological and genetic aspects. Int. J. Mol. Sci. 2017, 18, 377. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, J.; Liu, Y.; Yan, D.; Wu, H.; Li, R.; Jiang, Z.; Yang, Y.; Ren, X. Polyphenol oxidase plays a critical role in melanin formation in the fruit skin of persimmon (Diospyros kaki cv. ‘Heishi’). Food Chem. 2020, 330, 127253. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Shi, Y.J.; Zhao, Q.; Zhao, K.J.; Cui, X.L.; Chen, L.H.; Yang, H.B.; Zhang, F.; Mi, J.X.; Huang, J.L.; et al. Genome-wide investigation and expression profiling of polyphenol oxidase (PPO) family genes uncover likely functions in organ development and stress responses in Populus trichocarpa. BMC Genom. 2021, 22, 731. [Google Scholar] [CrossRef]
- Largeteau, M.L.; Latapy, C.; Minvielle, N.; Regnault-Roger, C.; Savoie, J.M. Expression of phenol oxidase and heat-shock genes during the development of Agaricus bisporus fruiting bodies, healthy and infected by Lecanicillium fungicola. Appl. Microbiol. Biotechnol. 2010, 85, 1499–1507. [Google Scholar] [CrossRef]
- Shao, P.; Wu, W.; Chen, H.; Sun, P.; Gao, H. Bilayer edible films with tunable humidity regulating property for inhibiting browning of Agaricus bisporus. Food Res. Int. 2020, 138, 109795. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, L.; Zhao, G.; Shen, C.; Yan, H.; Guan, J.; Yang, K. The effects of modified atmosphere packaging on core browning and the expression patterns of PPO and PAL genes in ‘Yali’ pears during cold storage. Food Sci. Technol. 2015, 60, 1243–1248. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, P.; Wang, B.; Tariq, P.; Zhao, F.; Zhao, M.; Wang, Q.; Yang, T.; Fang, J. Overexpression of polyphenol oxidase gene in strawberry fruit delays the fungus infection process. Plant Mol. Biol. Rep. 2016, 34, 592–606. [Google Scholar] [CrossRef]
- Tran, L.T.; Constabel, C.P. The polyphenol oxidase gene family in poplar: Phylogeny, differential expression and identification of a novel, vacuolar isoform. Planta 2011, 234, 799–813. [Google Scholar] [CrossRef]
- Aziz, E.; Batool, R.; Akhtar, W.; Rehman, S.; Gregersen, P.L.; Mahmood, T. Expression analysis of the polyphenol oxidase gene in response to signaling molecules, herbivory and wounding in antisense transgenic tobacco plants. 3 Biotech. 2019, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Rouster, J.; Leah, R.; Mundy, J.; Cameron-Mills, V. Identification of a methyl jasmonate-responsive region in the promoter of a lipoxygenase 1 gene expressed in barley grain. Plant J. 1997, 11, 513–523. [Google Scholar] [CrossRef]
- Shen, Q.; Ho, T.H. Functional dissection of an abscisic acid (ABA)-inducible gene reveals two independent ABA-responsive complexes each containing a G-box and a novel cis-acting element. Plant Cell. 1995, 7, 295–307. [Google Scholar]
- Vicente-Carbajosa, J.; Moose, S.P.; Parsons, R.L.; Schmidt, R.J. A maize zinc-finger protein binds the prolamin box in zein gene promoters and interacts with the basic leucine zipper transcriptional activator Opaque2. Proc. Natl. Acad. Sci. USA 1997, 94, 7685–7690. [Google Scholar] [CrossRef]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell. 2003, 15, 63–78. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Mao, J.; Zhang, L.; Zhu, C.; Qin, Q.; Li, N. Genome-Wide Identification and Expression Pattern Analysis of Polyphenol Oxidase Gene Family in Agaricus bisporus. Agronomy 2023, 13, 2534. https://doi.org/10.3390/agronomy13102534
Chen Y, Mao J, Zhang L, Zhu C, Qin Q, Li N. Genome-Wide Identification and Expression Pattern Analysis of Polyphenol Oxidase Gene Family in Agaricus bisporus. Agronomy. 2023; 13(10):2534. https://doi.org/10.3390/agronomy13102534
Chicago/Turabian StyleChen, Yanan, Jingxiu Mao, Lanlan Zhang, Changjun Zhu, Qiaoping Qin, and Nanyi Li. 2023. "Genome-Wide Identification and Expression Pattern Analysis of Polyphenol Oxidase Gene Family in Agaricus bisporus" Agronomy 13, no. 10: 2534. https://doi.org/10.3390/agronomy13102534
APA StyleChen, Y., Mao, J., Zhang, L., Zhu, C., Qin, Q., & Li, N. (2023). Genome-Wide Identification and Expression Pattern Analysis of Polyphenol Oxidase Gene Family in Agaricus bisporus. Agronomy, 13(10), 2534. https://doi.org/10.3390/agronomy13102534




