Proper Biochar Increases Maize Fine Roots and Yield via Altering Rhizosphere Bacterial Communities under Plastic Film Mulching
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
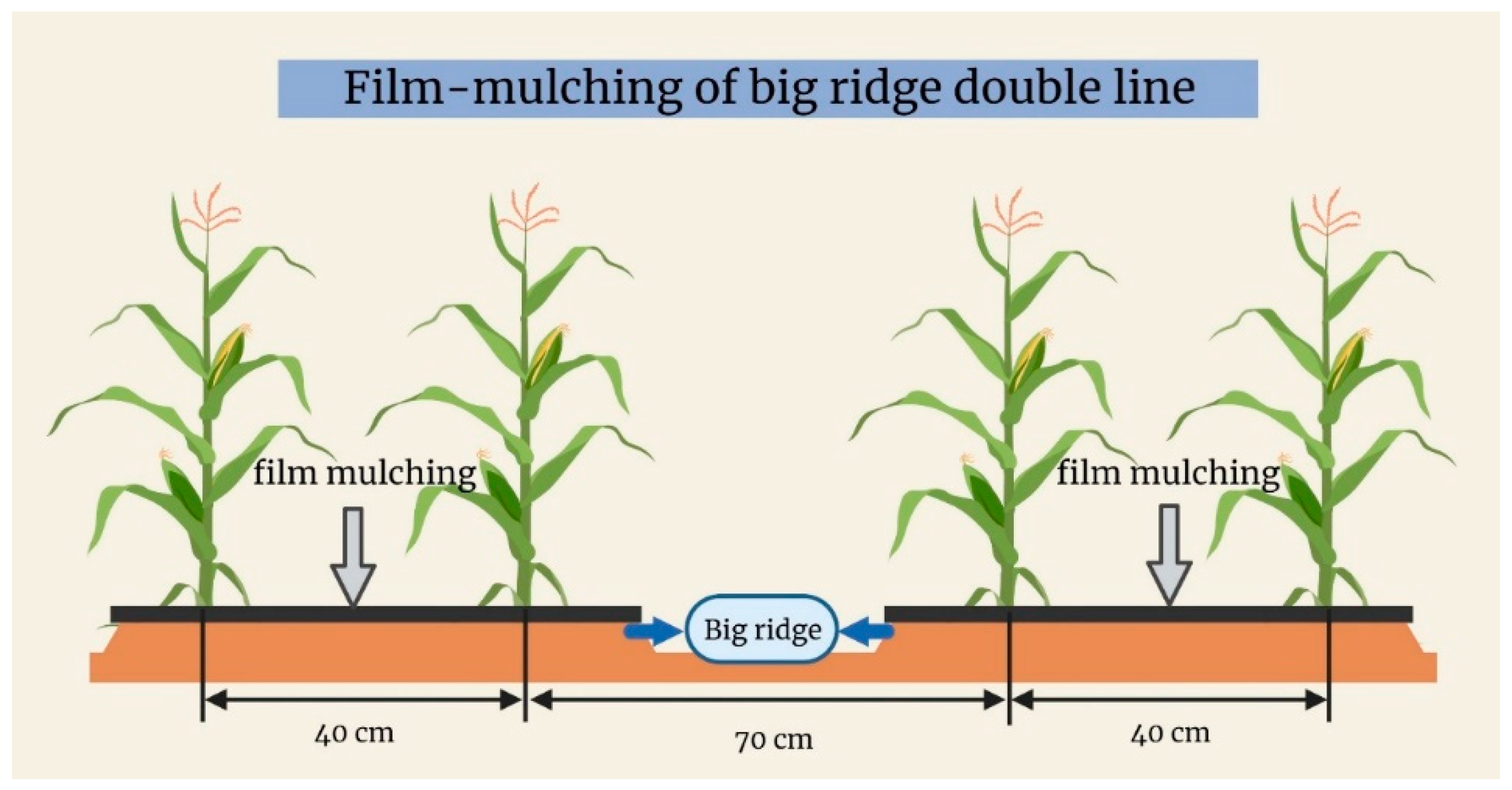
2.2. Experimental Design
2.3. Sampling and Measurements
2.3.1. Root Analysis
2.3.2. Soil Physicochemical Analysis
2.3.3. Yield Measurements
2.3.4. Bacterial Community Analysis
2.4. Statistical Analysis
3. Results
3.1. Changes in Soil Properties
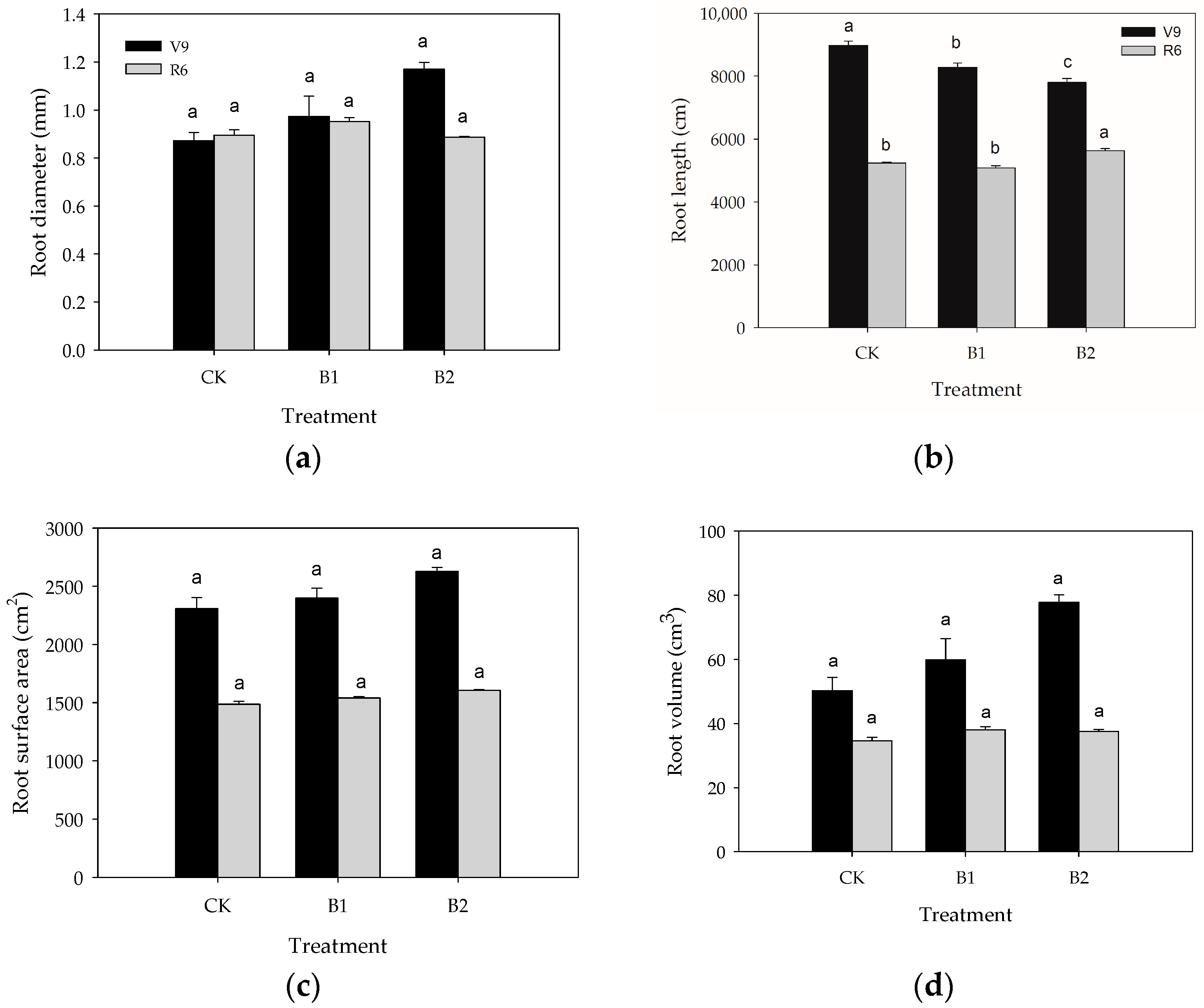
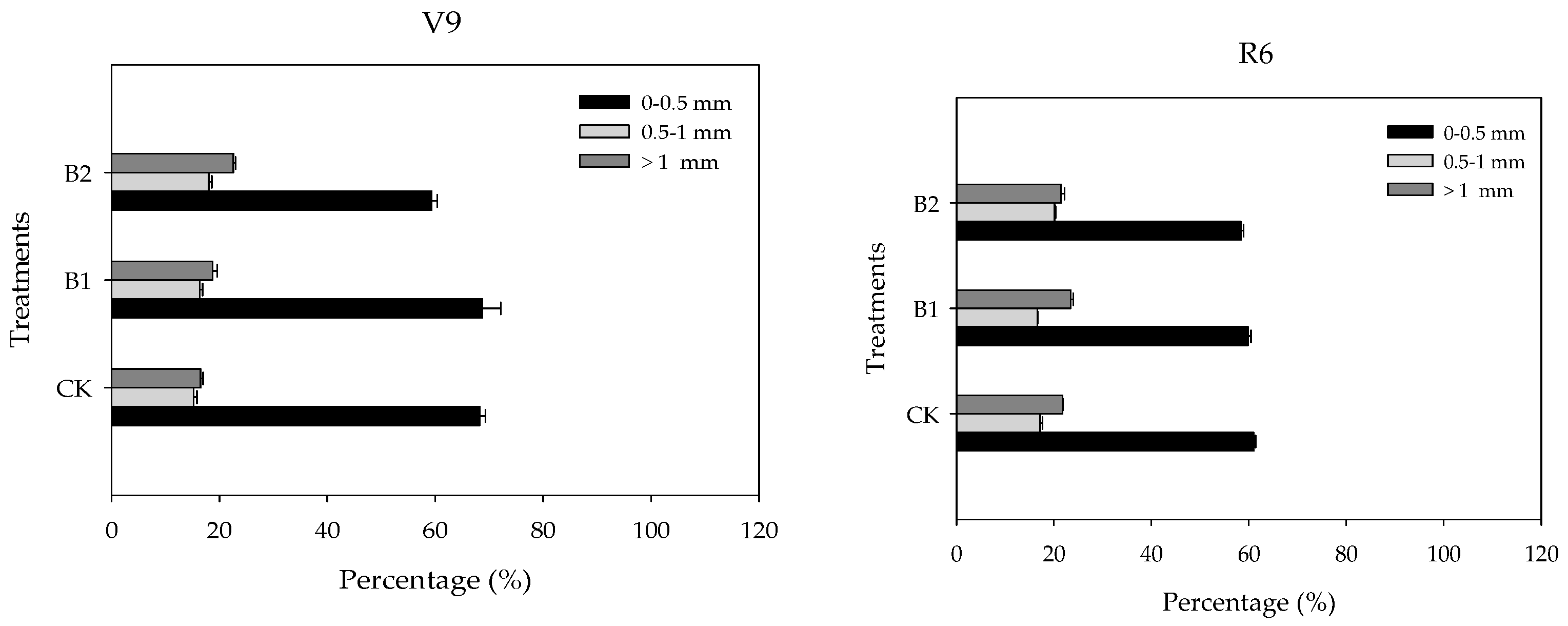
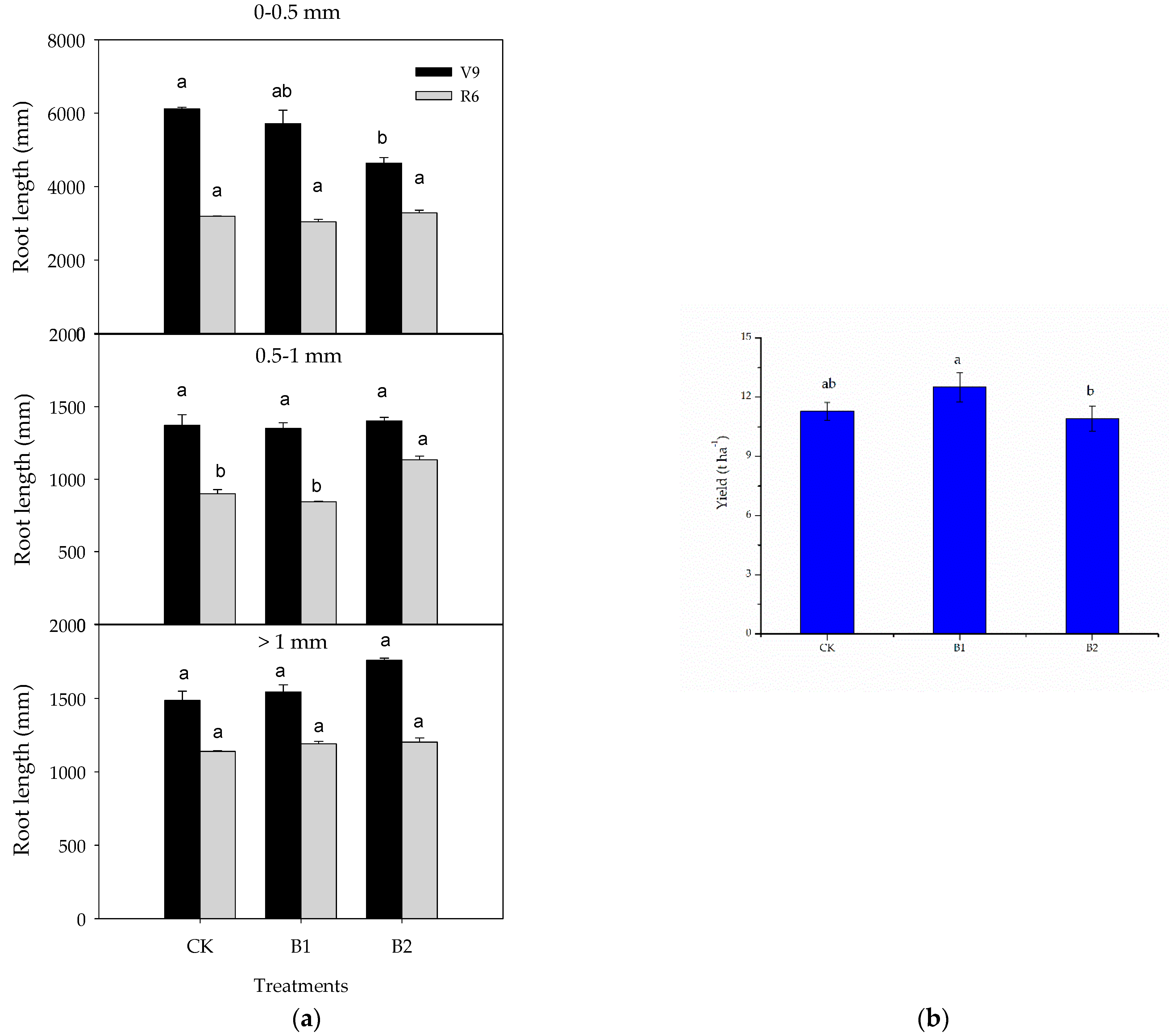
3.2. Responses of Maize Root and Yield
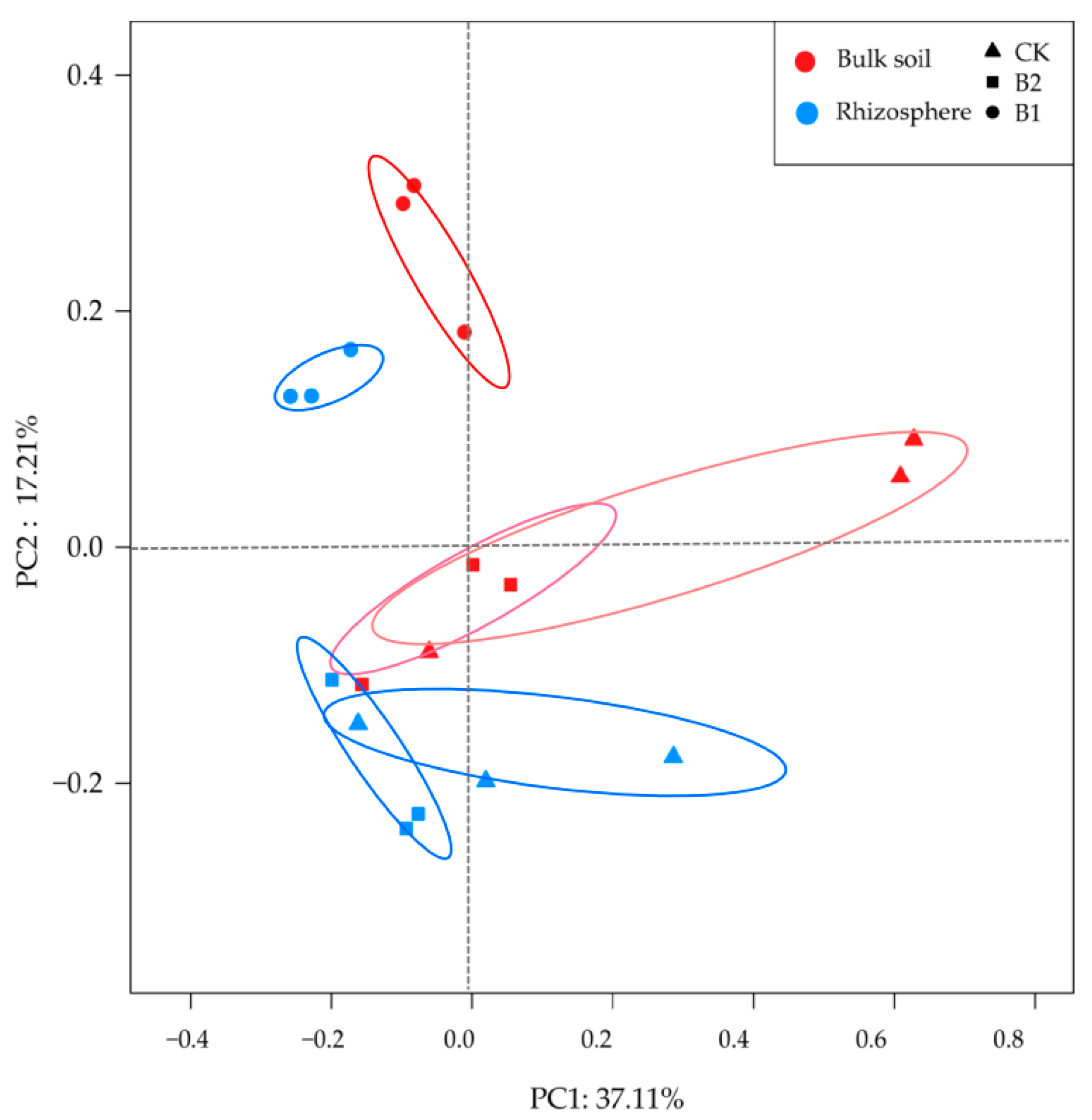
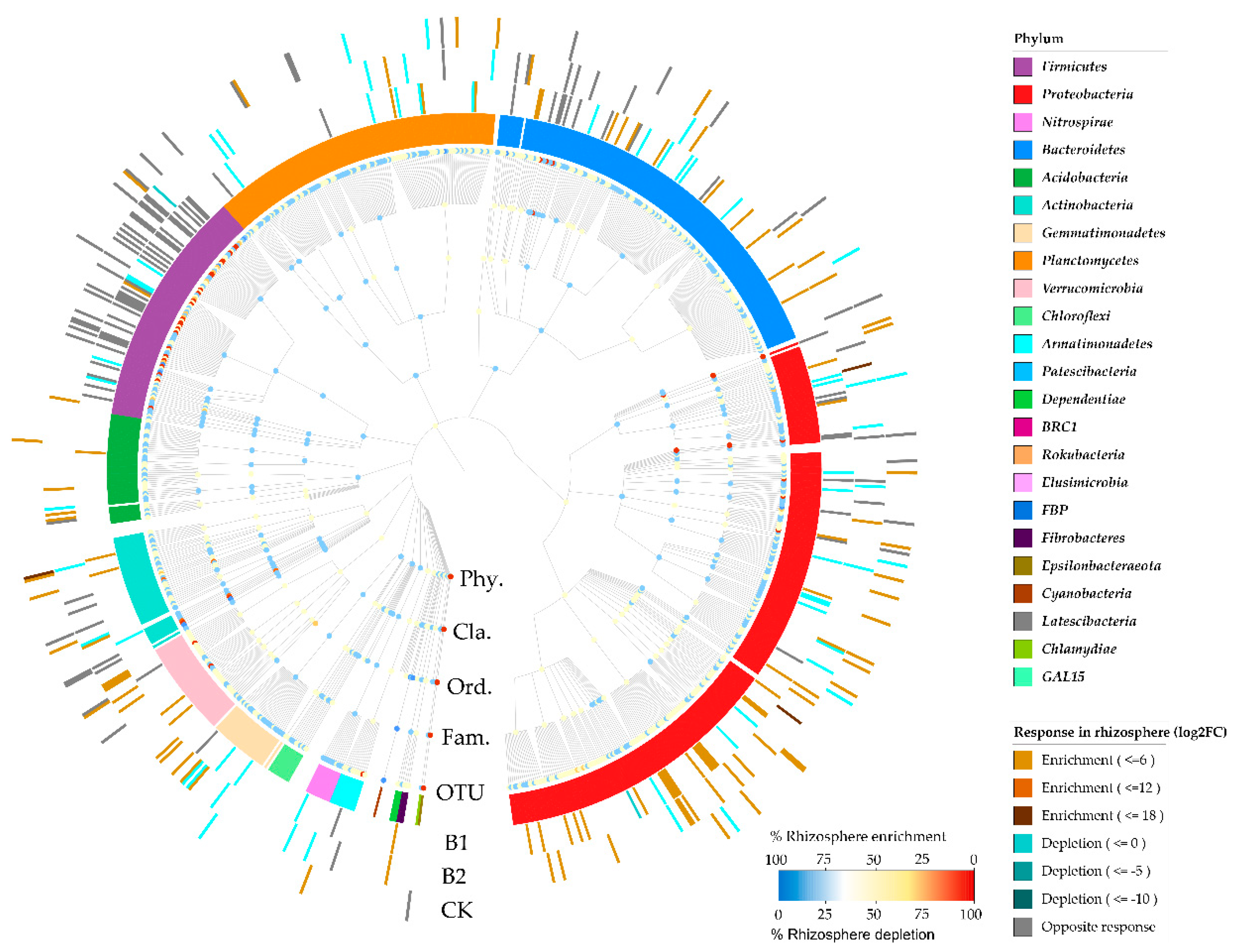
3.3. Differences in Bacterial Diversity and Community Composition
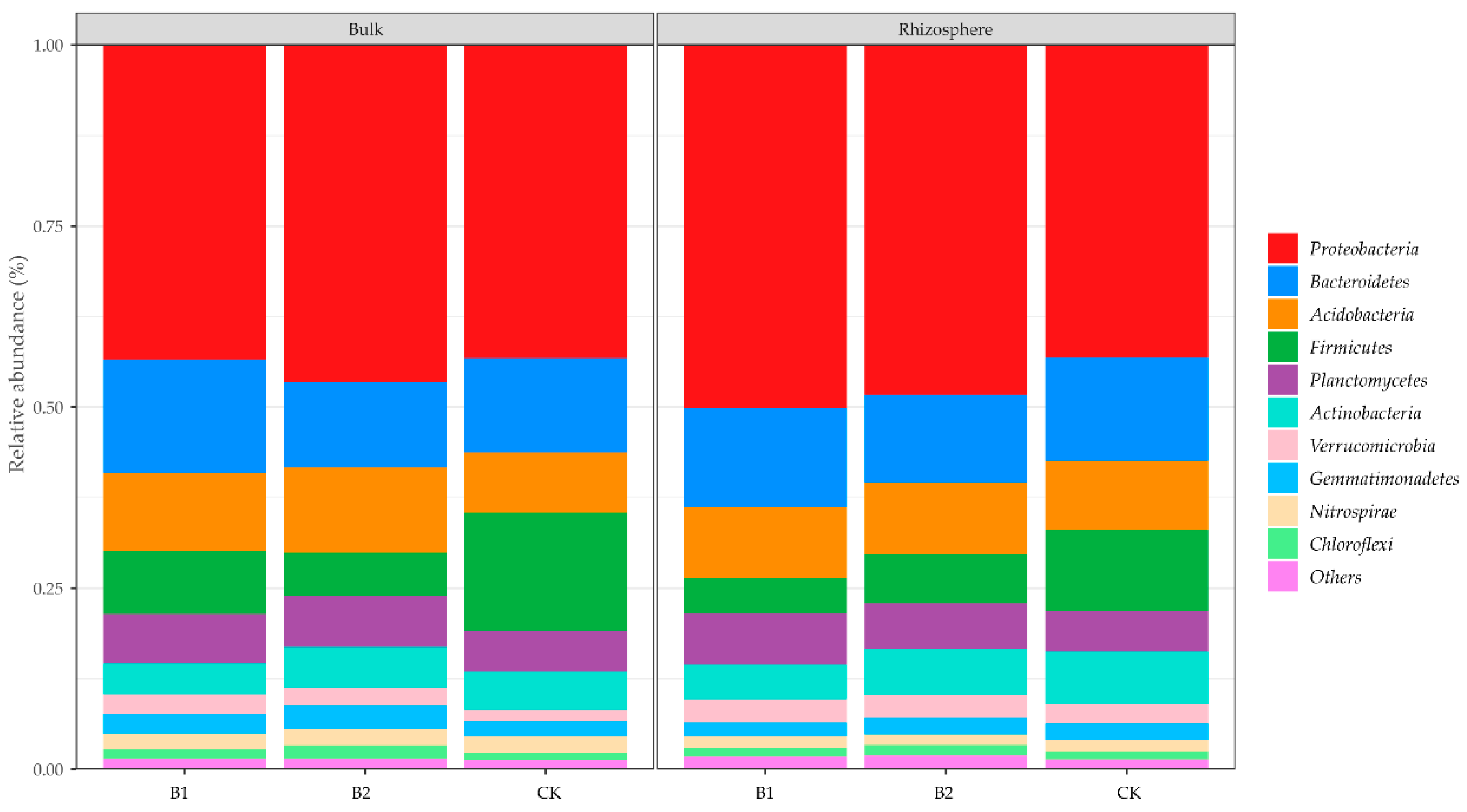
3.4. Predominant Bacterial Phyla
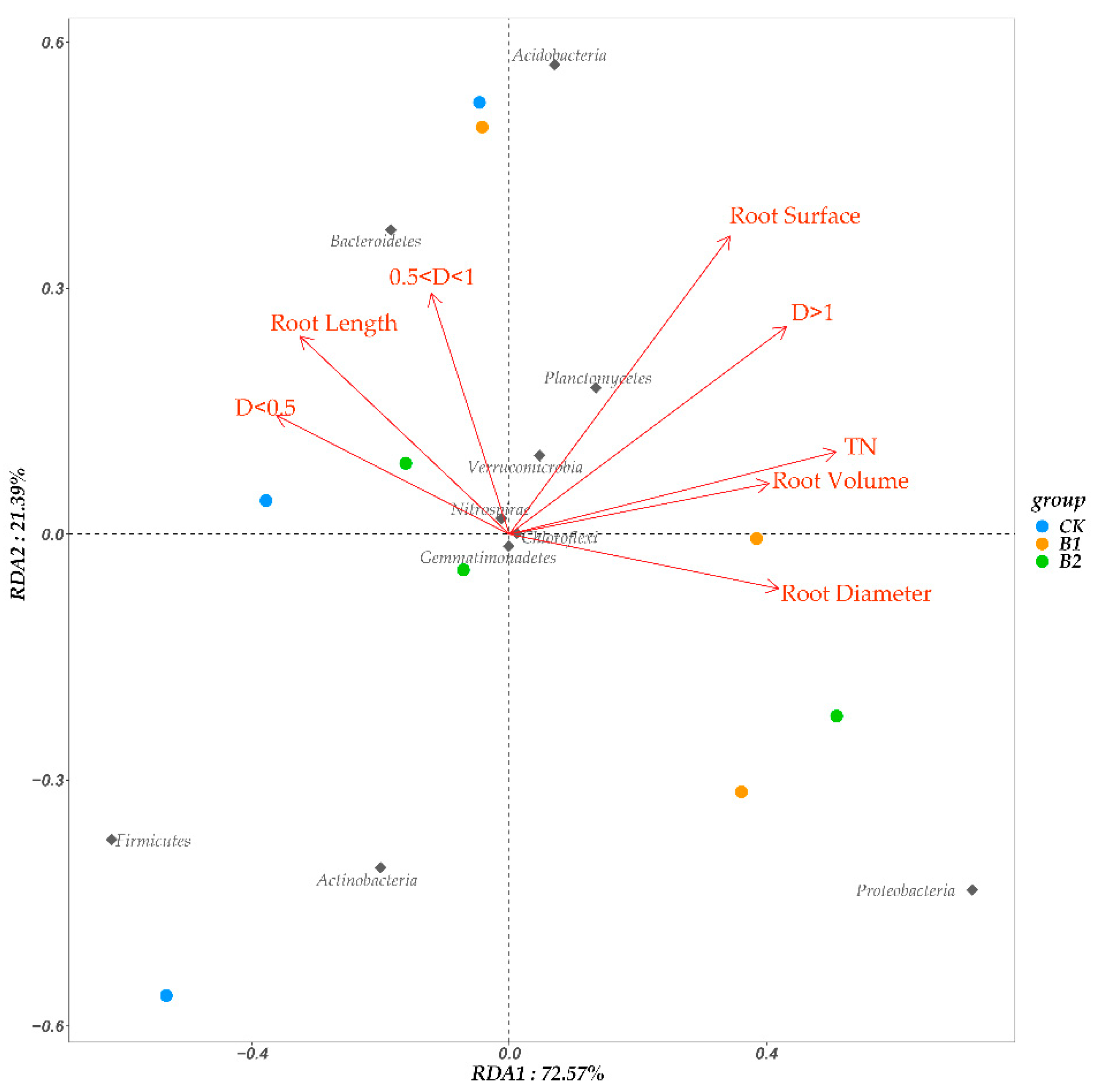
3.5. Root Morphology and Soil Properties Shaped the Rhizosphere Bacterial Community
4. Discussion
4.1. Biochar Alters the Maize Fine Root Morphology
4.2. Biochar Shapes Maize Rhizosphere Bacterial Communities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Earthscan: Burlington, VT, USA, 2009. [Google Scholar]
- Initiative, I.B. Standardized product definition and product testing guidelines for biochar that is used in soil. IBI Biochar Stand. 2012. [Google Scholar]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico–chemical properties and microbial responses in biochar–amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Usman, A.R.A.; El-Naggar, A.H.; Aly, A.A.; Ibrahim, H.M.; Elmaghraby, S.; Al-Omran, A. Conocarpus biochar as a soil amendment for reducing heavy metal availability and uptake by maize plants. Saudi J. Biol. Sci. 2015, 22, 503–511. [Google Scholar] [CrossRef]
- El-Naggar, A.H.; Usman, A.R.A.; Al-Omran, A.; Ok, Y.S.; Ahmad, M.; Al-Wabel, M.I. Carbon mineralization and nutrient availability in calcareous sandy soils amended with woody waste biochar. Chemosphere 2015, 138, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Chan, K.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Soil Res. 2008, 45, 629–634. [Google Scholar] [CrossRef]
- Pandit, N.R.; Mulder, J.; Hale, S.E.; Martinsen, V.; Schmidt, H.P.; Cornelissen, G. Biochar improves maize growth by alleviation of nutrient stress in a moderately acidic low-input Nepalese soil. Sci. Total Environ. 2018, 625, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Prendergast-Miller, M.; Duvall, M.; Sohi, S. Biochar-root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Dawes, M.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Wang, J.; Wang, W.; Zhang, T.; Li, J. Effects of root phenotypic changes on the deep rooting of Populus euphratica seedlings under drought stresses. PeerJ 2019, 7, e6513. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gao, X.; Wu, P.; Zhao, X.; Zhang, W.; Zou, Y.; Siddique, K.H.M. Drought responses of profile plant-available water and fine-root distributions in apple (Malus pumila Mill.) orchards in a loessial, semi-arid, hilly area of China. Sci. Total Environ. 2020, 723, 137739. [Google Scholar] [CrossRef]
- Montagnoli, A.; Di Iorio, A.; Ceriani, R.M.; Scippa, G.S.; Chiatante, D. Root seasonal pattern, spatial distribution, and C: N ratio of matgrass pasture (Nardus stricta L.) in the Lombardy Prealps. Plant Biosyst. 2010, 144, 463–470. [Google Scholar] [CrossRef]
- Di Iorio, A.; Montagnoli, A.; Terzaghi, M.; Scippa, G.S.; Chiatante, D. Effect of tree density on root distribution in Fagus sylvatica stands: A semi-automatic digitising device approach to trench wall method. Trees 2013, 27, 1503–1513. [Google Scholar] [CrossRef]
- Terzaghi, M.; Montagnoli, A.; Di Iorio, A.; Scippa, G.; Chiatante, D. Fine-root carbon and nitrogen concentration of European beech (Fagus sylvatica L.) in Italy Prealps: Possible implications of coppice conversion to high forest. Front. Plant Sci. 2013, 4, 192. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.; Guo, D. Impacts of environmental factors on fine root lifespan. Front. Plant Sci. 2014, 5, 205. [Google Scholar] [CrossRef]
- Lambers, H. Growth, respiration, exudation, and symbiotic associations: The fate of carbon translocated to the roots. In Root Development and Function; Gregory, P.J., Lake, J.V., Rose, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1987; pp. 125–145. [Google Scholar]
- Hinsinger, P.; Bengough, A.G.; Vetterlein, D.; Young, I.M. Rhizosphere: Biophysics, biogeochemistry and ecological relevance. Plant Soil 2009, 321, 117–152. [Google Scholar] [CrossRef]
- Wei, X.; Razavi, B.S.; Hu, Y.; Xu, X.; Zhu, Z.; Liu, Y.; Kuzyakov, Y.; Li, Y.; Wu, J.; Ge, T. C/P stoichiometry of dying rice root defines the spatial distribution and dynamics of enzyme activities in root-detritusphere. Biol. Fertil. Soils 2019, 55, 251–263. [Google Scholar] [CrossRef]
- Zhang, Q.; Du, Z.; Lou, Y.; He, X. A one-year short-term biochar application improved carbon accumulation in large macroaggregate fractions. Catena 2015, 127, 26–31. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M. Biochar and the nitrogen cycle: Introduction. J. Environ. Qual. 2010, 39, 1218–1223. [Google Scholar] [CrossRef]
- Fang, W.; Song, Z.; Tao, S.; Zhang, D.; Huang, B.; Ren, L.; Cheng, H.Y.; Yan, D.D.; Li, Y.; Cao, A.C.; et al. Biochar mitigates the negative effect of chloropicrin fumigation on beneficial soil microorganisms. Sci. Total Environ. 2020, 738, 139880. [Google Scholar] [CrossRef]
- Schmidt, H.P.; Kammann, C.; Niggli, C.; Evangelou, M.W.H.; Mackie, K.A.; Abiven, S. Biochar and biochar-compost as soil amendments to a vineyard soil: Influences on plant growth, nutrient uptake, plant health and grape quality. Agric. Ecosyst. Environ. 2014, 191, 117–123. [Google Scholar] [CrossRef]
- Jones, D.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.; Murphy, D. Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Li, L.; Zheng, J.; Qu, J.; Zheng, J.; Zhang, X.; Pan, G. Consistent increase in abundance and diversity but variable change in community composition of bacteria in topsoil of rice paddy under short term biochar treatment across three sites from South China. Appl. Soil Ecol. 2015, 91, 68–79. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, Z.; Xiao, X.; Zhang, N.; Wang, X.; Yang, Z.; Xu, K.; Liang, Y. Structural changes of soil organic matter and the linkage to rhizosphere bacterial communities with biochar amendment in manure fertilized soils. Sci. Total Environ. 2019, 692, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, H.; Chen, J.; Lu, J.; Wu, W.; Liu, X.; Li, C.; Dong, Q.; Siddique, K.H.M. Biochar incorporation increases winter wheat (Triticum aestivum L.) production with significantly improving soil enzyme activities at jointing stage. CATENA 2022, 211, 105979. [Google Scholar] [CrossRef]
- Yang, X.; Wang, W.; Chen, X.; Sardans, J.; Wang, C.; Vancov, T.; Fang, Y.; Wang, S.; Yuan, X.; Llusià, J.; et al. Effects of N-enriched biochar on ecosystem greenhouse gas emissions, rice yield, and bacterial community diversity in subtropical rice paddy soils. Eur. J. Soil Biol. 2022, 113, 103440. [Google Scholar] [CrossRef]
- Li, W.; Hou, Y.; Long, M.; Wen, X.; Han, J.; Liao, Y. Long-term effects of biochar application on rhizobacteria community and winter wheat growth on the Loess Plateau in China. Geoderma 2023, 429, 116250. [Google Scholar]
- Yuan, M.; Zhu, X.; Sun, H.; Song, J.; Li, C.; Shen, Y.; Li, S. The addition of biochar and nitrogen alters the microbial community and their cooccurrence network by affecting soil properties. Chemosphere 2023, 312, 137101. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Tong, C.; Hu, K.; Zhou, B.; Xing, S.; Mao, Y. Biochar-fertilizer interaction modifies N-sorption, enzyme activities and microbial functional abundance regulating nitrogen retention in rhizosphere soil. Sci. Total Environ. 2020, 739, 140065. [Google Scholar] [CrossRef] [PubMed]
- Sarma, B.; Gogoi, N.; Bharali, M.; Mali, P. Field evaluation of soil and wheat response to combined application of hardwood biochar and inorganic fertilizers in acidic sandy loam soil. Exp. Agric. 2017, 54, 507–519. [Google Scholar] [CrossRef]
- Noyce, G.L.; Basiliko, N.; Fulthorpe, R.; Sackett, T.E.; Thomas, S.C. Soil microbial responses over 2 years following biochar addition to a north temperate forest. Biol. Fertil. Soils 2015, 51, 649–659. [Google Scholar] [CrossRef]
- Husakova, E.; Hochholdinger, F.; Soukup, A. Lateral root development in the maize (Zea mays) lateral rootless1 mutant. Ann. Bot. 2013, 112, 417–428. [Google Scholar] [CrossRef][Green Version]
- Palta, J.A.; Yang, J. Crop root system behaviour and yield. Field Crops Res. 2014, 165, 1–4. [Google Scholar] [CrossRef]
- Dai, L.; Li, H.; Tan, F.; Zhu, N.; He, M.; Hu, G. Biochar: A potential route for recycling of phosphorus in agricultural residues. GCB Bioenergy 2016, 8, 852–858. [Google Scholar] [CrossRef]
- Kolton, M.; Harel, Y.M.; Pasternak, Z.; Graber, E.R.; Elad, Y.; Cytryn, E. Impact of biochar application to soil on the root–associated bacterial community structure of fully developed greenhouse pepper plants. Appl. Environ. Microbiol. 2011, 77, 4924–4930. [Google Scholar] [CrossRef]
- Amendola, C.; Montagnoli, A.; Terzaghi, M.; Trupiano, D.; Oliva, F.; Baronti, S.; Miglietta, F.; Chiatante, D.; Scippa, G.S. Short-term effects of biochar on grapevine fine root dynamics and arbuscular mycorrhizae production. Agric. Ecosyst. Environ. 2017, 239, 236–245. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Hernandez-Soriano, M.C.; Dalal, R.C.; Finn, D.; Menzies, N.W.; Hoeschen, C.; Mueller, C.W. Nitrogen-rich microbial products provide new organo-mineral associations for the stabilization of soil organic matter. Glob. Change Biol. 2018, 24, 1762–1770. [Google Scholar] [CrossRef]
- Miller, W.P.; Miller, D.M. A micro-pipette method for soil mechanical analysis. Commun. Soil Sci. Plant Anal. 1987, 18, 1–15. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, Y.; Zhang, W.; Sui, Y.; Jin, D.; Xin, W.; Yi, J.; He, D. Biochar prepared at different pyrolysis temperatures affects urea-nitrogen immobilization and N2O emissions in paddy fields. PeerJ 2019, 7, e7027. [Google Scholar] [CrossRef]
- Guo, H.; York, L.M. Maize with fewer nodal roots allocates mass to more lateral and deep roots that improve nitrogen uptake and shoot growth. J. Exp. Bot. 2019, 70, 5299–5309. [Google Scholar] [CrossRef]
- Brennan, A.; Jiménez, E.M.; Puschenreiter, M.; Alburquerque, J.A.; Switzer, C. Effects of biochar amendment on root traits and contaminant availability of maize plants in a copper and arsenic impacted soil. Plant Soil 2014, 379, 351–360. [Google Scholar] [CrossRef]
- Sui, Y.; Gao, J.; Liu, C.; Zhang, W.; Lan, Y.; Li, S.; Meng, J.; Xu, Z.; Tang, L. Interactive effects of straw-derived biochar and N fertilization on soil C storage and rice productivity in rice paddies of Northeast China. Sci. Total Environ. 2016, 544, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Spor, A.; Hénault, C.; Bru, D.; Bizouard, F.; Jones, C.M.; Maron, P.A. Loss in microbial diversity affects nitrogen cycling in soil. ISME J. 2013, 7, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Meister, R.; Rajani, M.S.; Ruzicka, D.; Schachtman, D.P. Challenges of modifying root traits in crops for agriculture. Trends Plant Sci. 2014, 19, 779–788. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Roumet, C.; Birouste, M.; Picon-Cochard, C.; Ghestem, M.; Osman, N.; Vrignon-Brenas, S.; Cao, K.F.; Stokes, A. Root structure–function relationships in 74 species: Evidence of a root economics spectrum related to carbon economy. New Phytol. 2016, 210, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Du, G.; Tian, J.; Jiang, C.; Zhang, W. Effect of irrigation methods on root growth, root-shoot ratio and yield components of cotton by regulating the growth redundancy of root and shoot. Agric. Water Manag. 2020, 234, 106120. [Google Scholar] [CrossRef]
- Sun, C.X.; Wang, D.; Shen, X.B.; Li, C.C.; Liu, J.; Lan, T.; Wang, W.Y.; Xie, H.T.; Zhang, Y.L. Effects of biochar, compost and straw input on root exudation of maize (Zea mays L.): From function to morphology. Agric. Ecosyst. Environ. 2020, 297, 106952. [Google Scholar] [CrossRef]
- Abiven, S.; Hund, A.; Martinsen, V.; Cornelissen, G. Biochar amendment increases maize root surface areas and branching: A shovelomics study in Zambia. Plant Soil 2015, 395, 45–55. [Google Scholar] [CrossRef]
- Viger, M.; Hancock, R.D.; Miglietta, F.; Taylor, G. More plant growth but less plant defence? First global gene expression data for plants grown in soil amended with biochar. GCB Bioenergy 2015, 7, 658–672. [Google Scholar] [CrossRef]
- Sun, C.X.; Chen, X.; Cao, M.M.; Li, M.Q.; Zhang, Y.L. Growth and metabolic responses of maize roots to straw biochar application at different rates. Plant Soil 2017, 416, 487–502. [Google Scholar] [CrossRef]
- Müller, D.B.; Vogel, C.; Bai, Y.; Vorholt, J.A. The plant microbiota: Systems-level insights and perspectives. Annu. Rev. Genet. 2016, 50, 211–234. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Kelly, C.N.; CalderÓN, F.C.; Acosta–MartÍNez, V.; Mikha, M.M.; Benjamin, J.; Rutherford, D.W.; Rostad, C.E. Switchgrass Biochar Effects on Plant Biomass and Microbial Dynamics in Two Soils from Different Regions. Pedosphere 2015, 25, 329–342. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Xu, M.; Xia, H.; Wu, J.; Yang, G.; Zhang, X.; Peng, H.; Yu, X.; Li, L.; Xiao, H.; Qi, H. Shifts in the relative abundance of bacteria after wine-lees-derived biochar intervention in multi metal-contaminated paddy soil. Sci. Total Environ. 2017, 599–600, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Z.; Lee, X.; Tang, Y.; Zhang, Q.H. Long-term effects of biochar amendment on rhizosphere and bulk soil microbial communities in a karst region, southwest China. Appl. Soil Ecol. 2019, 140, 126–134. [Google Scholar] [CrossRef]
- Manasa, M.R.K.; Katukuri, N.R.; Nair, S.D.; Yang, H.J.; Yang, Z.M.; Rong, B.G. Role of biochar and organic substrates in enhancing the functional characteristics and microbial community in a saline soil. J. Environ. Manag. 2020, 269, 110737. [Google Scholar] [CrossRef]
- Sarfraz, R.; Yang, W.H.; Wang, S.S.; Zhou, B.Q.; Xing, S.H. Short term effects of biochar with different particle sizes on phosphorous availability and microbial communities. Chemosphere 2020, 256, 126862. [Google Scholar] [CrossRef]
- Lan, Z.M.; Chen, C.R.; Rashti, M.R.; Yang, H.; Zhang, D.K. Linking feedstock and application rate of biochars to N2O emission in a sandy loam soil: Potential mechanisms. Geoderma 2019, 337, 880–892. [Google Scholar] [CrossRef]
- Hu, L.; Cao, L.; Zhang, R. Bacterial and fungal taxon changes in soil microbial community composition induced by short-term biochar amendment in red oxidized loam soil. World J. Microbiol. Biotechnol. 2014, 30, 1085–1092. [Google Scholar] [CrossRef]
- Mueller, R.C.; Belnap, J.; Kuske, C.R. Soil bacterial and fungal community responses to nitrogen addition across soil depth and microhabitat in an arid shrubland. Front. Microbiol. 2015, 6, 891. [Google Scholar] [CrossRef]
- Makhalanyane, T.P.; Valverde, A.; Gunnigle, E.; Frossard, A.; Ramond, J.B.; Cowan, D.A. Microbial ecology of hot desert edaphic systems. FEMS Microbiol. Rev. 2015, 39, 203–221. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Baran, A. Effect of poultry litter biochar on soil enzymatic activity, ecotoxicity and plant growth. Appl. Soil Ecol. 2016, 105, 144–150. [Google Scholar] [CrossRef]
- Whitman, T.; Pepe-Ranney, C.; Enders, A.; Koechli, C.; Campbell, A.; Buckley, D.H.; Lehmann, J. Dynamics of microbial community composition and soil organic carbon mineralization in soil following addition of pyrogenic and fresh organic matter. ISME J. 2016, 10, 2918–2930. [Google Scholar] [CrossRef] [PubMed]
| Organic Matter | Total N | Available P | Available K | pH | |
|---|---|---|---|---|---|
| (g kg−1) | (mg kg−1) | ||||
| Sandy loam | 11.19 | 0.86 | 32.35 | 69.00 | 6.95 |
| Year | Treatment | TN (mg g−1) | C/N | BD (g cm−3) | SP (%) |
|---|---|---|---|---|---|
| 2017 | CK | 0.61 ± 0.06a | 18.57 ± 1.34a | 1.63 ± 0.02a | 38.55 ± 0.88a |
| B1 | 1.07 ± 0.26a | 12.67 ± 3.24b | 1.54 ± 0.07a | 41.96 ± 2.66a | |
| B2 | 0.89 ± 0.16a | 12.21 ± 0.62b | 1.48 ± 0.05a | 44.01 ± 2.05a | |
| 2018 | CK | 0.61 ± 0.12a | 14.86 ± 1.78a | 1.34 ± 0.07a | 49.50 ± 2.56a |
| B1 | 0.82 ± 0.11a | 14.06 ± 1.18a | 1.25 ± 0.08a | 52.83 ± 2.90a | |
| B2 | 0.80 ± 0.05a | 13.55 ± 0.54a | 1.22 ± 0.09a | 53.99 ± 3.49a | |
| Source of variation | |||||
| Treatment | * | * | ** | * | |
| Year | ns | ** | ns | ** | |
| Treatment × Year | ns | ns | ns | ns | |
| Soil Compartment | Treatment | Observed OTUs | Shannon | Simpson | Chao1 | ACE | Good’s Coverage |
|---|---|---|---|---|---|---|---|
| Rhizosphere | CK | 877 ± 27 | 5.89 ± 0.16 | 0.0060 ± 0.0023 | 921 ± 19 | 905 ± 23 | 0.9973 ± 0.0000 |
| B1 | 868 ± 13 | 5.87 ± 0.07 | 0.0058 ± 0.0008 | 908 ± 11 | 929 ± 6 | 0.9975 ± 0.0005 | |
| B2 | 903 ± 3 | 5.94 ± 0.02 | 0.0053 ± 0.0001 | 940 ± 5 | 896 ± 12 | 0.9975 ± 0.0000 | |
| Bulk | CK | 797 ± 88 | 5.63 ± 0.32 | 0.0089 ± 0.0045 | 891 ± 51 | 859 ± 61 | 0.9961 ± 0.0010 |
| B1 | 847 ± 21 | 5.87 ± 0.04 | 0.0056 ± 0.0006 | 907 ± 28 | 879 ± 30 | 0.9968 ± 0.0013 | |
| B2 | 841 ± 31 | 5.90 ± 0.09 | 0.0052 ± 0.0006 | 887 ± 32 | 889 ± 28 | 0.9970 ± 0.0006 | |
| Biochar | |||||||
| B0 | 837 ns | 5.76 ns | 0.0075 ns | 906 ns | 0.9967 ns | 882 ns | |
| B1 | 858 ns | 5.87 ns | 0.0057 ns | 907 ns | 0.9972 ns | 904 ns | |
| B2 | 872 ns | 5.92 ns | 0.0053 ns | 913 ns | 0.9972 ns | 893 ns | |
| Soil compartment | |||||||
| Rhizosphere | 883 a | 5.90 ns | 0.0057 ns | 923 a | 0.9974 a | 910 a | |
| Bulk | 829 b | 5.80 ns | 0.0066 ns | 895 b | 0.9966 b | 876 b | |
| Significance | |||||||
| Biochar | ns | ns | ns | ns | ns | ns | |
| Soil compartment | * | ns | ns | ns | ns | * | |
| Biochar × Compartment | ns | ns | ns | ns | ns | ns | |
| Bacterial Phylum | RD | RL | RS | RV | D < 0.5 | 0.5 < D < 1 | D > 1 | TN | C/N | BD | SP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteobacteria | 0.43 | −0.37 | 0.28 | 0.40 | −0.39 | −0.17 | 0.38 | 0.09 | −0.33 | −0.36 | 0.36 |
| Bacteroidetes | −0.30 | 0.54 | 0.39 | −0.10 | 0.43 | 0.52 | 0.1 | 0.60 | −0.42 | −0.27 | 0.27 |
| Acidobacteria | 0.02 | 0.09 | 0.24 | 0.09 | 0.02 | 0.18 | 0.23 | 0.38 | −0.59 | −0.53 | 0.53 |
| Firmicutes | −0.44 | 0.29 | −0.45 | −0.45 | 0.36 | 0.05 | −0.51 | −0.60 | 0.80 ** | 0.69 * | −0.69 * |
| Planctomycetes | 0.08 | −0.11 | 0.14 | 0.06 | −0.06 | −0.32 | 0.2 | 0.57 | −0.66 | −0.62 | 0.62 |
| Actinobacteria | −0.17 | −0.12 | −0.70 * | −0.33 | 0.03 | −0.30 | −0.63 | −0.29 | 0.19 | 0.24 | −0.24 |
| Verrucomicrobia | 0.46 | −0.28 | 0.46 | 0.48 | −0.37 | 0.11 | 0.47 | 0.70 * | −0.81 ** | −0.63 | 0.63 |
| Gemmatimonadetes | −0.26 | 0.08 | −0.54 | −0.38 | 0.09 | 0.11 | −0.34 | 0.13 | −0.36 | −0.38 | 0.38 |
| Nitrospirae | −0.15 | 0.45 | 0.63 | 0.07 | 0.27 | 0.58 | 0.41 | 0.03 | −0.03 | 0.15 | −0.15 |
| Chloroflexi | 0.50 | −0.70 * | −0.27 | 0.30 | −0.62 | −0.45 | 0.02 | 0.08 | −0.51 | −0.69 * | 0.69 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, Y.; Wang, Y.; Xiao, W.; Chang, C.; Zhang, S.; Zhao, H. Proper Biochar Increases Maize Fine Roots and Yield via Altering Rhizosphere Bacterial Communities under Plastic Film Mulching. Agronomy 2023, 13, 60. https://doi.org/10.3390/agronomy13010060
Sui Y, Wang Y, Xiao W, Chang C, Zhang S, Zhao H. Proper Biochar Increases Maize Fine Roots and Yield via Altering Rhizosphere Bacterial Communities under Plastic Film Mulching. Agronomy. 2023; 13(1):60. https://doi.org/10.3390/agronomy13010060
Chicago/Turabian StyleSui, Yanghui, Yanbo Wang, Wanxin Xiao, Cheng Chang, Shuping Zhang, and Haiyan Zhao. 2023. "Proper Biochar Increases Maize Fine Roots and Yield via Altering Rhizosphere Bacterial Communities under Plastic Film Mulching" Agronomy 13, no. 1: 60. https://doi.org/10.3390/agronomy13010060
APA StyleSui, Y., Wang, Y., Xiao, W., Chang, C., Zhang, S., & Zhao, H. (2023). Proper Biochar Increases Maize Fine Roots and Yield via Altering Rhizosphere Bacterial Communities under Plastic Film Mulching. Agronomy, 13(1), 60. https://doi.org/10.3390/agronomy13010060






